Abstract
In this study the mechanism by which S-nitrosocysteine (CysNO) activates soluble guanylyl cyclase has been investigated. CysNO is the S-nitrosated derivative of the amino acid cysteine, and has previously been shown to be transported into various cell types by amino acid transport system L (L-AT). Here we show using both neuroblastoma and pulmonary artery smooth muscle cells, that CysNO stimulates cGMP formation at low concentrations, but this effect is lost at higher concentrations. Stimulation of cGMP accumulation occurs only after its transport into the cell and subsequent flavoprotein reductase-mediated metabolism to form nitric oxide (NO). Consequently, CysNO can be regarded as a cell-targeted NO releasing agent. However, CysNO also functions as an NO-independent thiol-modifying agent and can compromise cellular antioxidant defenses in a concentration-dependent manner. The observed biphasic nature of CysNO-dependent cGMP accumulation appears to be due to these two competing mechanisms. At higher concentrations, CysNO likely inactivates guanylyl cyclase through modification of an essential thiol group on the enzyme, either directly or as a result of a more generalized oxidative stress. We show here that higher concentrations of CysNO can increase cellular S-nitrosothiol content to non-physiological levels, deplete cellular glutathione (GSH) and inhibit cGMP formation in parallel. Although the inhibition of sGC by S-nitrosation has been suggested as a mechanism of nitrovasodilator tolerance, in the case of CysNO, it appears to be more a reflection of a generalized oxidative stress placed upon the cell by the non-physiological levels of intracellular S-nitrosothiol generated upon CysNO exposure.
Keywords: S-Nitrosation, cyclic guanosine monophosphate, guanylyl cyclase, amino acid transport system L, enzyme activation, enzyme inhibition
Introduction
One of the most well established functions of nitric oxide (NO) is the activation of soluble guanylyl cyclase (sGC) [1,2]. NO binds to the ferrous heme iron of sGC, triggering a conformational change in the protein which results in enzyme activation. In vascular smooth muscle cells, the increase in cyclic guanosine monoposphate (cGMP) which results from the activation of sGC, subsequently activates cGMP-dependent protein kinase and ultimately leads to a decrease in cellular Ca2+ levels and muscle relaxation. Although it has been known for many years that S-nitrosothiols can activate sGC [3] and elicit a vasodilatory response [4], and S-nitrosocysteine (CysNO) was once proposed as a candidate for the identity of endothelial derived relaxing factor [5], the mechanism by which S-nitrosothiols activate sGC has not been established. Moreover, reports of chiral specificity in the action of some S-nitrosothiols suggest that their biological activity is more complex than spontaneous NO release [6]. It is often assumed that these compounds spontaneously liberate NO in the extracellular space and that consequently, their cellular activities mimic those of NO. However, work from this and other laboratories has established the amino acid transport system L (L-AT) as a major mechanism of S-nitrosothiol delivery into cells [7–10], suggesting that the transport of such compounds into the intracellular space represents an important pathway of S-nitrosothiol action. In particular, S-nitrosothiols based on a single un-substituted amino acid, such as CysNO and S-nitrosohomocysteine, but not small peptides (S-nitrosoglutathione, S-nitrosocysteinyl glycine) or substituted amino acids (S-nitroso-N-acetyl penicillamine, S-nitroso-N-acetyl cysteine), are ligands for the L-AT and are avidly taken up by cells in culture [7]. Interestingly, it has been recently demonstrated that CysNO can also inhibit NO-dependent vessel relaxation via the direct modification of sGC thiol groups and this effect was not duplicated with other S-nitrosothiol compounds [11], suggesting that the specific uptake of CysNO by the L-AT may be responsible for both the activation and inhibition of sGC.
In this study we have examined the importance of S-nitrosothiol transport on the activation of sGC by CysNO in two discrete cell lines – human pulmonary artery smooth muscle cells (HPASMC) and SH-SY5Y neuroblastoma cells. We show that CysNO induces a biphasic response in sGC, activating the enzyme at low concentrations and inhibiting at high concentrations. The mechanism of sGC activation involves cellular uptake of CysNO by L-AT followed by intracellular reduction of CysNO and release of NO by an as yet unidentified cellular flavoprotein(s), while inhibition of sGC activity appears to involve the direct modification of sGC by CysNO. It has recently been shown that CysNO may inhibit sGC through the S-nitrosation of an essential cysteine residue and that this may be a mechanism of tolerance to other nitrovasodilators [12]. However, we only observe inhibition of sGC activity in parallel with the loss of cellular glutathione (GSH), suggesting that loss of sGC activity may occur as a result of the severe depletion of the reduced thiol pool that occurs after exposure of cells to CysNO.
Materials and Methods
Materials
L-cysteine, sodium nitrite, DTPA, diphenyleneiodonium (DPI), N-ethylmaleimide (NEM), iodine and mercury chloride were purchased from Sigma. Hepes, hydrochloric acid, methanol, potassium iodide, potassium phosphate monobasic, potassium phosphate dibasic, and sodium hydroxide were obtained from Fisher. Sulfanilamide was supplied by Aldrich. Dulbecco’s modified Eagle’s medium, penicillin/streptomycin and phosphate-buffered saline (PBS) were obtained from Gibco. Fetal bovine serum was purchased from HyClone. HPASMCs and medium 231 were supplied by Cascade Biologics. Isobutylmethylxanthine (IBMX) was purchased form Alexis Biochemicals. cGMP Direct Biotrak enzyme immunoassay (EIA) was supplied by Amersham Biosciences. Oxyhemoglobin (oxyHb) was purified from fresh human blood as previously described [13] and was reacted with NEM to block free thiols, followed by purification on G25 Sephadex column. OxyHb concentration was determined by measuring the absorbance at 577 nm (ε577 = 14.4 mM−1·· cm−1). GSNO and CysNO were synthesized as described previously [7,14,15].
Cell Culture
Human neuroblastoma cells (SH-SY5Y) obtained from the American Type Tissue Collection were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10 % fetal bovine serum, penicillin (100 units/ml), and streptomycin (100 μg/ml). HPASMCs were routinely cultured as recommended by the supplier. Cells were incubated at 37°C in a humidified atmosphere of 5 % CO2 and 95 % air. For each experiment, cells were seeded onto 6-well plates and grown until reaching 70–80 % confluence. The medium was removed by double wash with PBS. Specific compounds were added to cells in HBSS (supplemented with 10 mM Hepes). For experiments that involved cGMP measurements in HPASMCs, IBMX (1 mM), a phosphodiesterase inhibitor, was present during the treatment to prevent cGMP degradation. Cells used in experiments were between passages 4–10.
S-Nitrosothiol measurements
For detection of S-nitrosothiols, cells were washed twice with PBS, and 250 μl of lysis buffer (50 mM phosphate pH 7.4, 1mM DTPA, 50 mM NEM) was added to the cells. Cells were scraped and sonicated (550 Sonic Dismembrator from Fisher Scientific, power level 2 for 15 seconds) followed by centrifugation (12,000 × g for 5 min). The supernatant was used for measurement of S-nitrosothiols using the triiodide-dependent ozone-based chemiluminescence method as described previously [16–18]. Samples were pretreated with 10 % (vol/vol) of sulfanilamide (100 mM in 2 N HCl) to remove nitrite. HgCl2 (5 mM) was used to verify the presence of S-nitrosothiols. A standard curve was generated using GSNO.
GSH measurements
The HPLC-based SBD-F method was used to measure intracellular thiols [19]. Briefly, HPASMCs were washed twice with PBS and scraped into 200 μl of buffer (50 mM phosphate, 1mM DTPA, pH 7.4) followed by sonication and centrifugation. 25 μl of sample was mixed with 25 μl of a chilled solution of 10 % trichloroacetic acid (TCA) and centrifuged to precipitate protein. 25 μl of resulting supernatant was mixed with 75 μl of ammonium 7-fluorobenzo-2-oxa-1,3-diazole-4-sulphonate (SBD-F) (0.66 mg/ml in 2.5 M borate, pH 9.5) and incubated for 1 h at 60°C. The analysis was performed on Kromasil C-18 column (5 μm, 250 mm × 4.6 mm I.D., Alltech) using solvent A (0.1 M sodium acetate, pH 4, methanol; 98:2), solvent B (0.1 M sodium phosphate, pH 6, methanol; 95:5) and methanol. The chromatographic conditions were as follows: solvent A (100 %) graded to solvent B (100 %) between 0 and 20 min, methanol (100 %, 20), and solvent A (100 %, 30 min). GSH was detected fluorometrically (ex. 385 nm, em. 515 nm) with a 10 min retention time.
Cyclic GMP detection
cGMP production in HPASMCs and SH-SY5Y cells was measured using the cGMP Enzymeimmunoassay (EIA) Biotrak System (Amersham Bioscience). The Acetylation EIA procedure for measurement of intracellular cGMP was employed (protocol 3 from the manufacturer’s instructions). The absorption of the samples was measured on a plate reader at 450 nm, after the addition of sulphuric acid.
Results
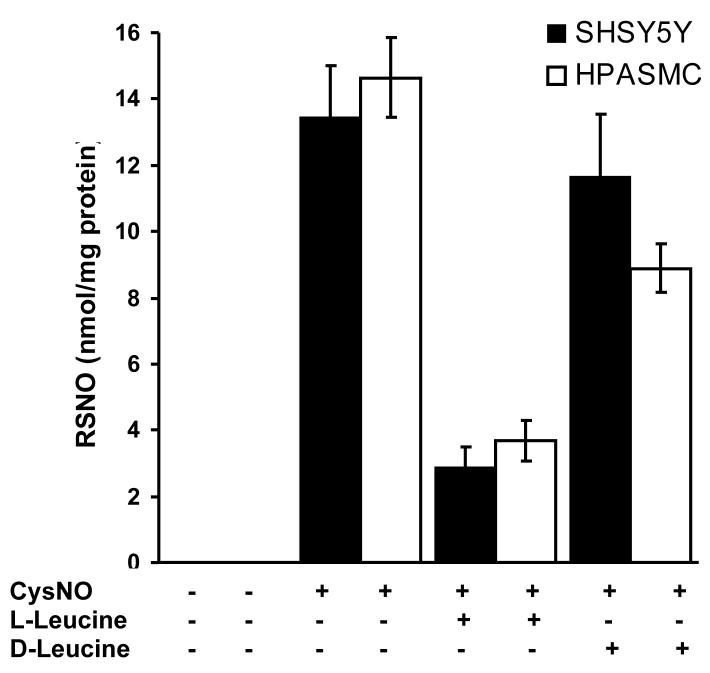
We have previously shown that RAW264.7 macrophages and bovine aortic endothelial cells take up CysNO via the L-AT system resulting in a concentration dependent increase in intracellular S-nitrosothiol (RSNO) levels [7,10,20]. In order to ascertain that the cells used in this study (HPASMC and SH-SY5Y cells) were also capable of transporting CysNO into the intracellular space, we exposed these cells to increasing concentrations of CysNO and measured intracellular RSNO levels by tri-iodide-based chemiluminescence. As shown in Figure 1, exposure of both HPASMC and SH-SY5Y cells to CysNO resulted in a dose-dependent increase in intracellular RSNO levels. At the highest CysNO concentration tested (200 μM), the intracellular RSNO levels were approximately 19 and 28 nmol RSNO/mg protein for SH-SY5Y and HPASMC, respectively. To confirm that the uptake of CysNO was due to the L-AT system, we examined the effects of both L- and D-leucine. As shown in Figure 2, L-leucine substantially inhibited CysNO uptake, whereas the D isomer was less effective. This is in agreement with the known chiral specificity of this transporter [21]. These results strongly suggest that both cell types examined contain a CysNO uptake pathway similar to that described previously in other cell types and are able to take up CysNO to generate levels of intracellular S-nitrosothiols that are 2–3 orders of magnitude higher than measured (patho)physiological levels [20,22].
Figure 1. CysNO transport in SH-SY5Y cells and HPASMC.
SH-SY5Y cells and HPASMC were treated with various concentrations of CysNO for 60 minutes in HBSS. Intracellular S-nitrosothiol levels were measured by chemiluminescence. Data were normalized to protein concentration and represent mean ± SEM (n = 3).
Figure 2. Transport of CysNO via L-AT.
SH-SY5Y cells and HPASMC were treated with CysNO (80 μM) in the presence or absence of L- and D-leucine (8 mM) for 60 minutes in HBSS. Intracellular S-nitrosothiol levels were measured by chemiluminescence. Data were normalized to protein concentration and represent mean ± SEM (n = 3).
The concentration dependence of sGC activation in response to CysNO treatment is shown in Figure 3A for both cell lines. The presence of a phosphodiesterase inhibitor (IBMX) was determined to be essential in order to observe robust cGMP accumulation in HPASMC but not SH-SY5Y cells. CysNO exhibited a biphasic concentration-dependent effect on cGMP production in both cell lines. In both cases, CysNO at levels up to 20 μM, caused a dose-dependent accumulation of cGMP. At higher concentrations of CysNO, the levels of intracellular cGMP declined, again in a concentration-dependent manner. A concentration of 120 μM CysNO resulted in very little activation of sGC. The inhibition of sGC observed at higher levels of CysNO is in good agreement with recent studies [11,12].
Figure 3. Activation of sGC in SH-SY5Y cells and HPASMC and GSH levels in HPASMC.
A. Cells were incubated with various concentrations of CysNO in HBSS for 60 minutes. IBMX (1mM) was present during incubation of HPASMC to inhibit phosphodiesterases. cGMP levels were measured with an EIA kit. Results represent the amount of cGMP produced by cells in a single well of a 6-well plate. The data represent mean ± SEM (n=3). B. HPASMC were treated with various concentrations of CysNO for 60 minutes in HBSS. GSH levels in cell lysates were determined by HPLC. Data were normalized to protein concentration and represent mean ± SEM (n=3).
We have previously demonstrated that exposure of bovine aortic endothelial cells to CysNO can deplete cellular GSH levels in a concentration-dependent manner [10]. We therefore examined how exposure of HPASMCs to CysNO affects their intracellular GSH pool. The measured levels of intracellular GSH are plotted as a function of CysNO concentration in Figure 3B. At low concentrations, CysNO actually increased GSH levels, while at concentrations above 40 μM, CysNO substantially depleted GSH in a concentration-dependent manner. Following treatment with 200 μM CysNO, less than 10 % of the original GSH level remained. A comparison of Figures 3A and B indicates that the decline in cGMP production upon CysNO treatment is accompanied by significant GSH loss.
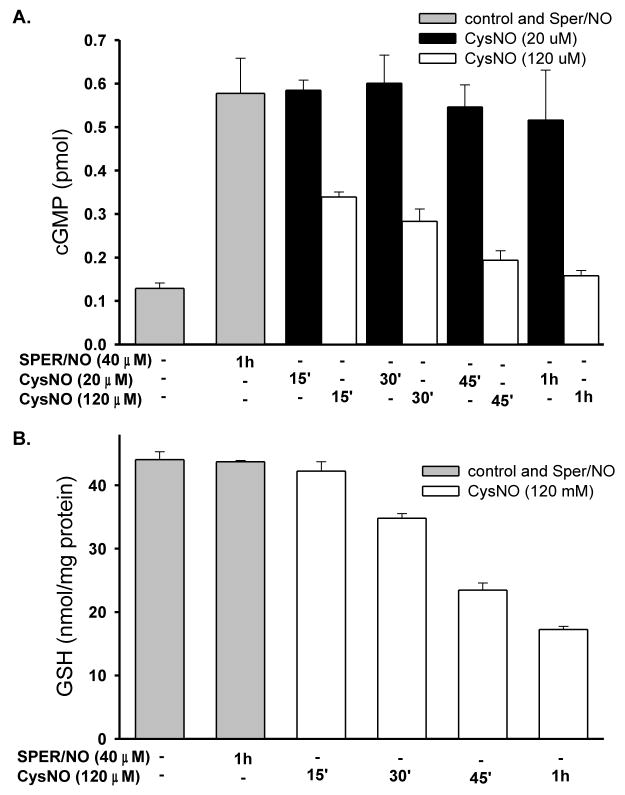
The time course of cGMP accumulation in HPASMC in response to two different doses of CysNO, 20 μM and 120 μM is shown in Figure 4A. Activation of sGC by CysNO (20 μM) occurred within 15 minutes and the level of cGMP did not change over 1 h, whereas administration of CysNO at 120 μM resulted in an initial increase and a time dependent-decrease in cGMP accumulation. Consistent with dose-dependency data, 20 μM CysNO led to higher cGMP accumulation than 120 μM CysNO. Interestingly, cellular GSH levels after incubation with CysNO (120 μM) exhibited a time-dependent depletion of GSH (Figure 4B). This is again consistent with the observation that inhibition of sGC by CysNO is related to or occurs concomitantly with a decrease in the GSH level.
Figure 4. Time-dependence of cGMP accumulation and GSH depletion in HPASMC.
A. Cells were incubated with SPER/NO (40 μM) or CysNO (20 μM or 120 μM) in HBSS for indicated times. IBMX (1mM) was present during incubation to inhibit phosphodiesterases. cGMP levels were measured with an EIA kit. Results represent the amount of cGMP produced by cells in a single well of a 6-well plate. The data represent mean ± SEM (n=3). B. Cells were incubated with SPER/NO (40 μM) or CysNO (120 μM) in HBSS for indicated times. GSH levels in cell lysates were determined by HPLC. Data were normalized to protein concentration and represent mean ± SEM (n=3).
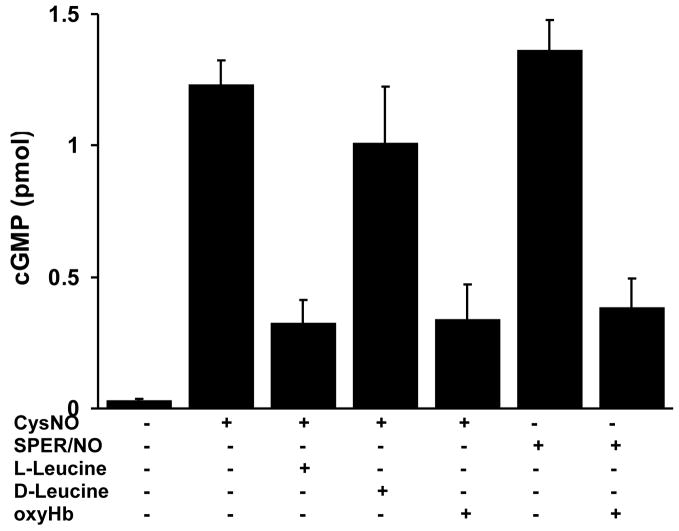
The mechanism by which CysNO activates sGC was examined in HPASMC. L-leucine, but not D-leucine, inhibited CysNO-dependent cGMP formation (Figure 5), indicating that the activation of sGC was largely dependent upon the transport of CysNO into the intracellular space by the L-AT and not upon the release of NO in the extracellular space. To determine whether CysNO activates sGC directly or if the intracellular release of NO is required, we examined the effect of oxyhemoglobin (oxyHb) on sGC activation. OxyHb is an avid NO scavenger and reacts with NO with a rate constant in excess of 107 M−1s−1, converting it to nitrate. It has been previously shown that extracellular oxyHb or erythrocytes can antagonize NO-dependent modifications of both iron and thiol targets due to freely diffusible nature of NO [22,23]. As expected, oxyHb suppressed the ability of the NO donor molecule SPER/NO to activate sGC (Figure 5). Surprisingly, oxyHb also inhibited the ability of CysNO to activate sGC, indicating that the mechanism of sGC activation involves both the uptake of CysNO and the intracellular metabolism of CysNO to form NO.
Figure 5. Effects of L-AT competitors and NO scavenger on cGMP production in HPASMC.
HPASMC were incubated with indicated compounds CysNO (20 μM), L-leucine (8mM), D-leucine (8mM), oxyHb (100 μM), SPER/NO (40 μM) in HBSS for 60 minutes. IBMX (1mM) was present during incubation to inhibit phosphodiesterases. cGMP levels were measured with an EIA kit. Results represent the amount of cGMP produced by cells in a single well of a 6-well plate. The data represent mean ± SEM (n=3).
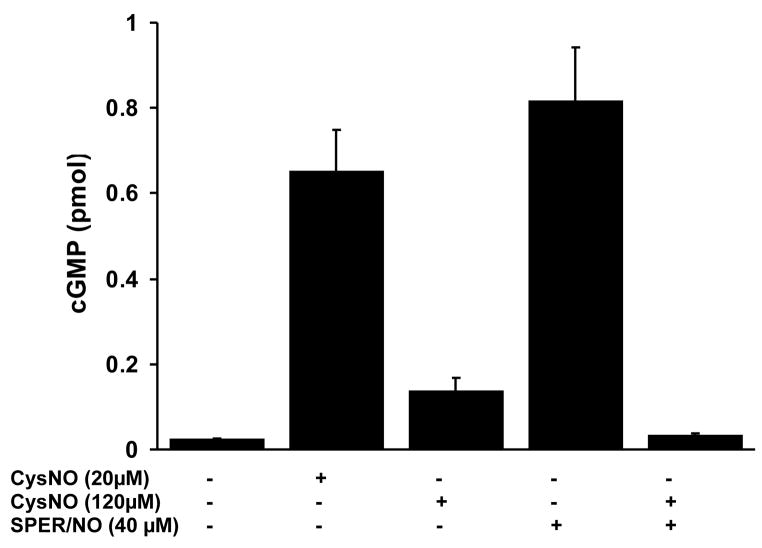
As mentioned above, cGMP levels exhibit a biphasic response to CysNO exposure. To examine if high concentrations of CysNO directly inhibit sGC activity, or inhibit CysNO-dependent cGMP formation upstream of sGC, we treated cells with 120 μM CysNO in either the absence or the presence of SPER/NO. As shown in Figure 6, while SPER/NO (40 μM) or CysNO (20 μM) alone caused a robust activation of sGC activity, CysNO at high concentration (120 μM) only resulted in a small increase in cGMP, in agreement with Figure 3. Importantly, a combination of CysNO (120 μM) and SPER/NO failed to activate cGMP formation. This suggests that CysNO-dependent inhibition of sGC occurs directly at the level of this enzyme and not as a result of inhibition of CysNO metabolism or some other up-stream event.
Figure 6. CysNO-dependent inhibition of sGC.
HPASMC were incubated with indicated compounds in HBSS for 60 minutes. IBMX (1mM) was present during incubation to inhibit phosphodiesterases. cGMP levels were measured with an EIA kit. Results represent the amount of cGMP produced by cells in a single well of a 6-well plate. The data represent mean ± SEM (n=3).
The most likely mechanism of CysNO metabolism to form NO is one-electron reduction (Equation 1) and flavoprotein reductase enzymes are known to facilitate this
| [1] |
chemistry. To determine if CysNO is metabolized by this class of enzyme, the effect of the non-specific flavoprotein reductase inhibitor diphenyleneiodonium (DPI) was examined. DPI substantially inhibited cGMP formation from CysNO, but had no effect on SPER/NO-dependent cGMP formation (Figure 7). This suggests that at least one step in the process responsible for CysNO reduction involves a flavoprotein reductase activity.
Figure 7. Effect of DPI on cGMP production in HPASMC.
HPASMC were incubated with indicated compounds in HBSS for 60 minutes. IBMX (1mM) was present during incubation to inhibit phosphodiesterases. cGMP levels were measured with an EIA kit. Results represent the amount of cGMP produced by cells in a single well of a 6-well plate. The data represent mean ± SEM (n=3).
Discussion
In this study we have examined the mechanism by which CysNO activates sGC in both the SH-SY5Y neuroblastoma cell line and in HPASMCs. These cell lines were chosen as they both contain sGC, but are of diverse origin. We show that CysNO-dependent cGMP formation exhibits a biphasic response in both cell types. CysNO levels up to 20 μM stimulate cGMP formation, whereas levels above 20 μM diminish cGMP formation. Temporally, 20 μM CysNO results in rapid and sustained activation of sGS, whereas 120 μM CysNO again shows a biphasic response with an initial increase and slow decrease in cGMP level.
The activation of cGMP formation observed at lower levels of CysNO was inhibited by both L-leucine and oxyHb. L-Leucine is a ligand for the L-AT system [21], and we have previously demonstrated that it will inhibit the intracellular accumulation of S-nitrosothiols in cells exposed to CysNO [7,10]. We show in Figure 1 that both cell lines used in this study respond in an analogous way to all other cell lines we have so far investigated in that the uptake of CysNO is inhibited by L-leucine and to a much lesser extent by D-leucine. This indicates that L-AT-dependent CysNO uptake is active in both the SH-SY5Y and HPASMC cells. Recent siRNA studies of both L-AT1 and L-AT2 have confirmed the importance of this transporter in transmembrane S-nitrosothiol transport [9]. Cysteine is a poor substrate for the L-AT system, but the S-nitrosation of the cysteine thiol appears to confer enough hydrophobicity on the amino acid to make it a strong ligand for this transporter system. The fact that CysNO-dependent cGMP formation was inhibited by L-leucine indicates that the transport of CysNO to the interior of the cell is an absolute requirement for the activation of sGC. This was not the case for the spontaneous NO donor SPER/NO, which liberates NO in the extracellular space. Interestingly, CysNO-mediated cGMP formation was also inhibited by oxyHb suggesting that CysNO does not directly activate cGMP but requires prior metabolism to form NO. As NO is a freely diffusible molecule and extracellular NO generated from SPER/NO is clearly able to activate sGC, these data indicate that very little CysNO spontaneously decays to NO in the extracellular space. It should be noted that these experiments were performed in HBSS in the presence of the metal ion chelator DTPA to specifically minimize metal ion-dependent CysNO decay. Of great interest, these data suggest that cells have the ability to reduce intracellular S-nitrosothiols to form NO allowing the activation of NO-dependent pathways. The mechanisms by which NO activates sGC are relatively well established and involve binding to an open coordination site of the ferrous heme group of sGC, which displaces a proximal histidine from the heme iron resulting in a conformational activation of enzyme activity. Recent evidence suggests heme-NO bound sGC only accounts for tonic activation whereas a second non-heme binding site is present for transient activation in response to acute stimuli [24]. However, other investigators did not observe such behavior in cellular systems and suggest only a single ligand binding site is physiologically relevant [25]. The mechanism for intracellular CysNO reduction is currently unknown, but appears to involve the activity of a flavoprotein reductase enzyme as it can be inhibited by the non-specific flavoprotein inhibitor DPI. Previous work has shown that glutathione-dependent formaldehyde dehydrogenase has the ability to reduce S-nitrosoglutathione in cells in an NADH-dependent manner [26,27]. More recently it has been suggested that carbonyl reductase 1 can act as an NADPH-dependent GSNO reductase [28], however, in bovine aortic endothelial cells, we only detected an NADH-dependent activity [10]. Regardless of the enzyme, these reductases generate hydroxylamine or ammonia but not NO, and therefore cannot be responsible for CysNO-dependent sGC activation. Tissue homogenates have been shown to possess the ability to metabolize S-nitrosothiols to nitrate, suggesting NO as an intermediate, by a mechanism independent of metal ions and cellular thiols, but inhibitable by potassium ferricyanide [29].
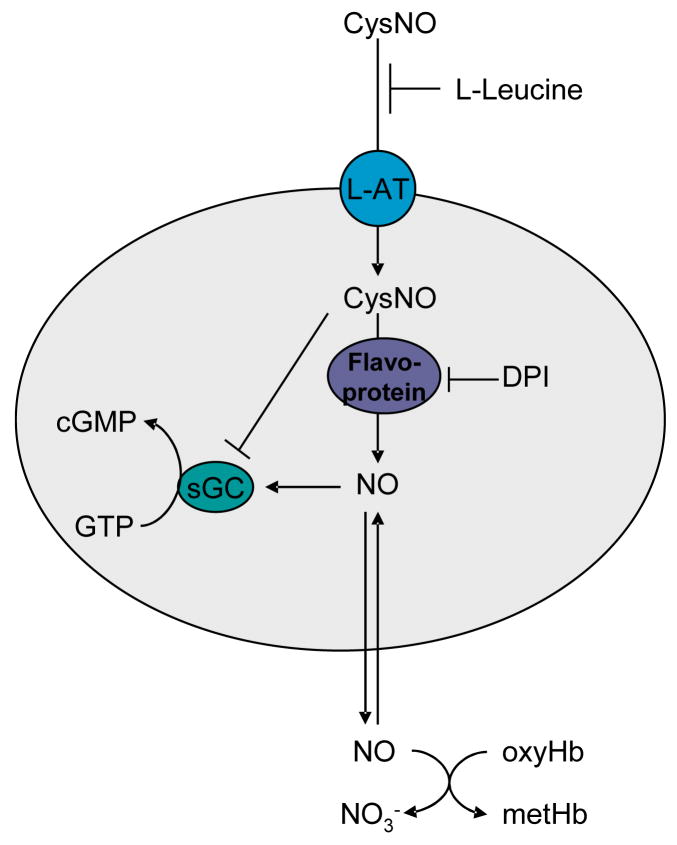
As shown in Figure 8, the model that best fits our data involves the uptake of CysNO via the L-AT system, followed by intracellular reduction of CysNO, or some other secondary S-nitrosothiol that is formed via transnitrosation, to liberate NO which can then proceed to bind to, and activate sGC.
Figure 8.
Model for activation and inhibition of sGC by CysNO.
At higher concentrations of CysNO, we observed diminished cGMP formation, consistent with the recent work of Sayed et. al. [11,12], that was due to direct inhibition at the level of sGC. This latter conclusion was drawn from the fact that cGMP formation could not be stimulated by SPER/NO in cells that had been co-treated with CysNO. It has been proposed that CysNO is able to directly inactivate sGC via the S-nitrosation of a protein thiol [11]. Although our data support such a mechanism, it is crucially important to assess protein S-nitrosation and other thiol modifications in the context of cellular changes in thiol redox state. The higher levels of CysNO that inhibit sGC activity also decrease cellular glutathione levels, and this is seen in both concentration- and time-dependent studies. In addition, sGC inhibition only occurs at total intracellular S-nitrosothiol levels in the nmol/mg range that are 1–2 orders of magnitude higher than those observed under (patho)physiological conditions [30]. Thus, sGC inactivation takes place under conditions when antioxidant defenses are depleted and additional proteins will be subjected to oxidative modifications that would not typically be modified if these systems were intact. Consequently, the perceived tolerance to CysNO-dependent vasodilation needs to be assessed in the context of total cellular redox status and the total level of thiol modification within the cell or tissue. The L-AT-dependent CysNO uptake mechanism appears avid, and exposure of cells to relatively low levels of CysNO may generate a significant oxidative stress on the cell due to the rapid concentration of extracellular CysNO into the small volume of the cell [10].
The data presented here give credence to the hypothesis that CysNO may represent a paracrine modulator of vascular tone as mechanisms of uptake and sGC activation are present within vascular smooth muscle cells. It is likely that the pharmacological activity of S-nitrosothiols is mediated by such processes, and this is likely the basis of the chiral specificity observed with some effects of S-nitrosothiols in vivo [6]. In addition, the ability of inhaled NO to elicit vascular effects may occur through the stabilization of the vasodilatory activity in the form of an S-nitrosothiol. What remains unclear is whether this represents a physiological process. The formation of S-nitrosothiols in specific locations or cell types, such as in the red blood cell through the nitrite reductase activity of deoxyhemoglobin [31–33], may represent a pool of S-nitrosothiols that can be transferred to the vasculature to elicit a vasodilatory response. Of interest in this regard is the increase in red blood cell S-nitrosothiol content in the presence of nitrite [34] and the observation that the vasodilatory ability of red cells from septicemic animals is correlated to their S-nitrosothiol content [35].
In conclusion CysNO requires both transmembrane transport and intracellular metabolism in order to release NO and activate sGC. CysNO metabolism occurs via the activity of an as yet unidentified flavoprotein reductase. At higher concentrations CysNO directly inhibits sGC, most likely through the modification/S-nitrosation of essential thiol residues. However, such inhibition only takes place in the context of glutathione depletion and the accumulation of high and non-physiological concentrations of protein S-nitrosothiols. These data provide a mechanism by which extracellular S-nitrosothiols can elicit vasodilation by targeted NO delivery to the smooth muscle cell.
Acknowledgments
This work was support by grant GM55792 from the National Institute of General Medicine.
List of Abbreviations
- CysNO
S-nitrosocysteine
- RSNO
S-nitrosothiols
- sGC
soluble guanylyl cyclase
- cGMP
cyclic guanosine monophosphate
- L-AT
amino acid transport system L
- oxyHb
oxyhemoglobin
- DPI
diphenyleneiodonium
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Waldman SA, Murad F. Biochemical mechanisms underlying vascular smooth muscle relaxation: the guanylate cyclase-cyclic GMP system. J Cardiovasc Pharmacol. 1988;12(Suppl 5):S115–S118. [PubMed] [Google Scholar]
- 2.Denninger JW, Marletta MA. Guanylate cyclase and the .NO/cGMP signaling pathway. Biochim Biophys Acta. 1999;1411:334–350. doi: 10.1016/s0005-2728(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 3.Ignarro LJ, Edwards JC, Gruetter DY, Barry BK, Gruetter CA. Possible involvement of S-nitrosothiols in the activation of guanylate cyclase by nitroso compounds. FEBS Lett. 1980;110:275–278. doi: 10.1016/0014-5793(80)80091-3. [DOI] [PubMed] [Google Scholar]
- 4.Kowaluk EA, Poliszczuk R, Fung HL. Tolerance to relaxation in rat aorta: comparison of an S-nitrosothiol with nitroglycerin. Eur J Pharmacol. 1987;144:379–383. doi: 10.1016/0014-2999(87)90392-x. [DOI] [PubMed] [Google Scholar]
- 5.Bates JN, Harrison DG, Myers PR, Minor RL. EDRF: nitrosylated compound or authentic nitric oxide. Basic Res Cardiol. 1991;86(Suppl 2):17–26. doi: 10.1007/978-3-642-72461-9_3. [DOI] [PubMed] [Google Scholar]
- 6.Davisson RL, Travis MD, Bates JN, Lewis SJ. Hemodynamic effects of L- and D-S-nitrosocysteine in the rat. Stereoselective S-nitrosothiol recognition sites. Circ Res. 1996;79:256–262. doi: 10.1161/01.res.79.2.256. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Hogg N. The mechanism of transmembrane S-nitrosothiol transport. Proc Natl Acad Sci USA. 2004;101:7891–7896. doi: 10.1073/pnas.0401167101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satoh S, Kimura T, Toda M, Maekawa M, Ono S, Narita H, Miyazaki H, Murayama T, Nomura Y. Involvment of L-type-like aminoacid transporters in S-nitrosocystiene-stimulated noradrenaline release in the rat hippocampus. J Neurochem. 1997;69:2197–2205. doi: 10.1046/j.1471-4159.1997.69052197.x. [DOI] [PubMed] [Google Scholar]
- 9.Li S, Whorton AR. Identification of Stereoselective Transporters for S-Nitroso-L-cysteine: Role of LAT1 and LAT2 in biological activity of S-nitrosothiols. J Biol Chem. 2005;280:20102–20110. doi: 10.1074/jbc.M413164200. [DOI] [PubMed] [Google Scholar]
- 10.Broniowska KA, Zhang Y, Hogg N. Requirement of transmembrane transport for S-nitrosocysteine-dependent modification of intracellular thiols. J Biol Chem. 2006;281:33835–33841. doi: 10.1074/jbc.M603248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sayed N, Kim DD, Fioramonti X, Iwahashi T, Duran WN, Beuve A. Nitroglycerin-Induced S-nitrosylation and Desensitization of Soluble Guanylyl Cyclase Contribute to Nitrate Tolerance. Circ Res. 2008;103:606–614. doi: 10.1161/CIRCRESAHA.108.175133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sayed N, Baskaran P, Ma X, van den Akker F, Beuve A. Desensitization of soluble guanylyl cyclase, the NO receptor, by S-nitrosylation. Proc Natl Acad Sci USA. 2007;104:12312–12317. doi: 10.1073/pnas.0703944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antonini E, Brunori M. Hemoglobin and myoglobin in their reactions with ligands. North Holland Publishing Company; 1971. [Google Scholar]
- 14.Hart TW. Some observations concerning the S-nitroso and S-phenylsulphonyl derivatives of L-cysteine and glutathione. Tetrahedron Lett. 1985;26:2013–2026. [Google Scholar]
- 15.Field L, Dilts RV, Ravichandran R, Lanhert PG, Carnahan PG. An unusually stable thionitrite from N-acetyl-DL-penicillamine. X-Ray, crystal and molecular structure of 2-(acetylamino)-2-carboxy-1,1-dimethylethyl thionitrite. J Chem Soc Chem Commun. 1978:249–250. [Google Scholar]
- 16.Samouilov A, Zweier JL. Development of chemiluminescence-based methods for specific quantitation of nitrosylated thiols. Anal Biochem. 1998;258:322–330. doi: 10.1006/abio.1998.2609. [DOI] [PubMed] [Google Scholar]
- 17.Rassaf T, Bryan NS, Kelm M, Feelisch M. Concomitant presence of N-nitroso and S-nitroso proteins in human plasma. Free Radic Biol Med. 2002;33:1590–1596. doi: 10.1016/s0891-5849(02)01183-8. [DOI] [PubMed] [Google Scholar]
- 18.Yang BK, Vivas EX, Reiter CD, Gladwin MT. Methodologies for the sensitive and specific measurement of S-nitrosothiols, iron-nitrosyls, and nitrite in biological samples. Free Radic Res. 2003;37:1–10. doi: 10.1080/1071576021000033112. [DOI] [PubMed] [Google Scholar]
- 19.Toyo'oka T, Imai K. High-performance liquid chromatography and fluorometric detection of biologically important thiols, derivatized with ammonium 7-fluorobenzo-2-oxa-1,3-diazole-4-sulphonate (SBD-F) J Chromatogr A. 1983;282:495–500. doi: 10.1016/s0021-9673(00)91626-1. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Hogg N. S-Nitrosothiols:Cellular formation and transport. Free Radic Biol Med. 2005;38:831–838. doi: 10.1016/j.freeradbiomed.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Segawa H, Fukasawa Y, Miyamoto K, Takeda E, Endou H, Kanai Y. Identifaction and functional characterization of a Na+ -independent neutral amin acid transporter with broad substrate selectivity. J Biol Chem. 1999;274:19745–19751. doi: 10.1074/jbc.274.28.19745. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Hogg N. The Formation and Stability of S-Nitrosothiols in RAW 264.7 Cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L467–L474. doi: 10.1152/ajplung.00350.2003. [DOI] [PubMed] [Google Scholar]
- 23.Stadler J, Bergonia HA, Di Silvio M, Sweetland MA, Billiar TR, Simmons RL, Lancaster JR., Jr Nonheme iron-nitrosyl complex formation in rat hepatocytes:Detection by electron paramagnetic resonance spectroscopy. Arch Biochem Biophys. 1993;302:4–11. doi: 10.1006/abbi.1993.1173. [DOI] [PubMed] [Google Scholar]
- 24.Cary SPL, Winger JA, Marletta MA. Tonic and acute nitric oxide signaling through soluble guanylate cyclase is mediated by nonheme nitric oxide, ATP, and GTP. Proc Natl Acad Sci USA. 2005;102:13064–13069. doi: 10.1073/pnas.0506289102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy B, Garthwaite J. Nitric oxide activation of guanylyl cyclase in cells revisited. Proc Natl Acad Sci USA. 2006;103:12185–12190. doi: 10.1073/pnas.0602544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen DE, Belka GK, Du Bois GC. S-Nitrosoglutathione is a substrate for rat alcohol dehydrogenase class III isoenzyme. Biochem J. 1998;331:659–668. doi: 10.1042/bj3310659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L, Hausladen A, Zeng M, Que L, Heitman J, Stamler JS. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature. 2001;410:490–494. doi: 10.1038/35068596. [DOI] [PubMed] [Google Scholar]
- 28.Bateman RL, Rauh D, Tavshanjian B, Shokat KM. Human carbonyl reductase 1 is an S-nitrosoglutathione reductase. J Biol Chem. 2008:M807125200. doi: 10.1074/jbc.M807125200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mani AR, Ebrahimkhani MR, Ippolito S, Ollosson R, Moore KP. Metalloprotein-dependent decomposition of S-nitrosothiols: Studies on the stabilization and measurement of S-nitrosothiols in tissues. Free Radic Biol Med. 2006;40:1654–1663. doi: 10.1016/j.freeradbiomed.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Bryan NS, Rassaf T, Maloney RE, Rodriguez CM, Saijo F, Rodriguez JR, Feelisch M. Cellular targets and mechanisms of nitros(yl)ation: An insight into their nature and kinetics in vivo. Proc Natl Acad Sci USA. 2004;101:4308–4313. doi: 10.1073/pnas.0306706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basu S, Grubina R, Huang J, Conradie J, Huang Z, Jeffers A, Jiang A, He X, Azarov I, Seibert R, Mehta A, Patel R, King SB, Hogg N, Ghosh A, Gladwin MT, Kim-Shapiro DB. Catalytic generation of N2O3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nature Chemical Biology. 2007;3:785–794. doi: 10.1038/nchembio.2007.46. [DOI] [PubMed] [Google Scholar]
- 32.Nagababu E, Ramasamy S, Rifkind JM. S-Nitrosohemoglobin: A mechanism for its formation in conjunction with nitrite reduction by deoxyhemoglobin. Nitric Oxide. 2006;15:20–9. doi: 10.1016/j.niox.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Angelo M, Singel DJ, Stamler JS. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc Natl Acad Sci USA. 2006;103:8366–8371. doi: 10.1073/pnas.0600942103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, III, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 35.Crawford JH, Chacko BK, Pruitt HM, Piknova B, Hogg N, Patel RP. Transduction of NO-bioactivity by the Red blood cell in Sepsis: Novel mechanisms of vasodilation during acute inflammatory disease. Blood. 2004:2004–03. doi: 10.1182/blood-2004-03-0880. [DOI] [PubMed] [Google Scholar]