Abstract
The dorsolateral prefrontal cortex (dlPFC) plays an important role in working memory, including the control of memory-guided response. In this study, with 24 subjects, we used high frequency repetitive transcranial magnetic stimulation (rTMS) to evaluate the role of the dlPFC in memory-guided response to two different types of spatial working memory tasks: one requiring a recognition decision about a probe stimulus (operationalized with a yes/no button press), another requiring direct recall of the memory stimulus by moving a cursor to the remembered location. In half the trials, randomly distributed, rTMS was applied to the dlPFC and in a separate session, the superior parietal lobule (SPL), a brain area implicated in spatial working memory storage. A 10-Hz (3 sec., 110% of motor threshold) train of TMS was delivered at the onset of the response period. We found that only dlPFC rTMS significantly affected performance, with rTMS of right dlPFC decreasing accuracy on delayed-recall trials, and rTMS of left and right dlPFC decreasing and enhancing accuracy, respectively, on delayed-recognition trials. These findings confirm that the dlPFC plays an important role in memory-guided response, and suggest that the nature of this role varies depending on the processes required for making a response.
Keywords: working memory, spatial, recall, recognition, response, TMS
Introduction
There is ample evidence that the prefrontal cortex (PFC) plays an important role in working memory (Fuster, 1997; Goldman-Rakic, 1990). However, the extent of its involvement in each of the three theoretically dissociable components of the classic delay task -- stimulus presentation (encoding), delay (storage), and response -- remains a focus of active research. With respect to storage, the long-accepted view that PFC plays a critical role in storage of information in working memory has increasingly been called into question (for a review, see Postle, 2006b). For example, we previously used repetitive transcranial magnetic stimulation (rTMS) during the delay period of a spatial delayed-recognition task and found that, when targeting the dorsolateral PFC (dlPFC) in healthy subjects, the effects of rTMS on performance were minimal. Delay-period rTMS of the superior parietal lobule (SPL), in contrast, had a significant effect on performance (Hamidi, Tononi, & Postle, In Press). This is consistent with fMRI evidence suggesting an important role for SPL in the storage of spatial information (Curtis, 2006; Postle, 2006a), a function that is in line with its role in processing perception of spatial information (Awh, Anllo-Vento, & Hillyard, 2000; Mesulam, 1999). The results of Hamidi et al. (In Press) with regard to the dlPFC are supported by the literature on patients with damage to the frontal lobes, which shows that patients often perform well on tasks of simple immediate recall, but can be relatively more impaired when the task requires memory-guided actions (e.g., Knight & D’Esposito, 2003). For example, patients with large frontal lesions have intact short-term memory spans (the number of items can be recalled immediately after presentation), and thus would seem to be unimpaired at the storage, per se, of information in working memory. However, the performance of many of these same patients suffers when motor output demands are manipulated by testing memory with delayed response (D’Esposito & Postle, 1999). The current study, therefore, was designed as a prospective test of the hypothesis that the dlPFC, more so than SPL, contributes importantly to memory-guided response.
There can be, of course, many different kinds of memory-guided response. Because previous neuropsychological evidence suggests that different types of working memory tasks may be differentially supported by the dlPFC (D’Esposito & Postle, 1999; Miotto, Bullock, Polkey, & Morris, 1996), we selected to study two commonly used tasks that differ only in their response requirements: delayed-recognition and delayed-recall. This design allowed us to address the broad question about memory-guided response, as well as begin exploring the finer-grained functional organization of the dlPFC – i.e., whether different hypothesized processes may be differentially supported by this region. Recognition, which can be operationalized by requiring a same/different judgment of a probe stimulus, requires evaluation of the similarity of the item currently accessible to perception with that being held in memory, and a registration of the binary outcome of that evaluation with a yes or no button press. Thus, it emphasizes stimulus perception, decision processes, and response selection (i.e. mapping of the decision to the appropriate response choice). Recall, in which response is cued with a stimulus unrelated to what is stored in memory, requires a complete reproduction of the stored information, and consequently requires a less constrained (and typically more complex) memory-guided motor response. Therefore, it follows that accurate performance in a recall task is more reliant on the neuronal processes involved in the motor planning necessary for reproducing the remembered stimulus. In addition to the differences in motor responses, the two tasks are different in that recall relies more strongly on readout of the stored items in memory, whereas recognition requires a comparison or verification process (Cabeza, Locantore, & Anderson, 2003; Haist, Shimamura, & Squire, 1992). Furthermore, recognition can be performed with a weaker mnemonic representation of the memorandum, because the presence of the probe provides information to aid in remembering the details of the stored item (Tulving & Psotka, 1971). As expected, the long-term memory literature has long shown that normal subjects are better at performing tasks requiring recognition than those requiring recall (Rock & Engelstein, 1959).
Activity in the dlPFC is thought to play a significant role in both working memory-guided response generation and in motor planning and response execution (e.g., Hester, D’Esposito, Cole, & Garavan, 2007; Miller, Erickson, & Desimone, 1996; Rypma, Berger, & D’Esposito, 2002). This has been well studied in the long-term memory literature. For example, Donaldson and Rugg (1999) showed that in normal subjects, both recall and recognition of associated word pairs elicit frontal event-related potentials (ERPs) during retrieval, although the specific pattern of the ERPs differed. Recognition was associated with an early bilateral frontal component and a late right-sided positive ERP whereas recall was associated with only the late right-sided frontal ERP. Neuropsychological studies of patients with frontal lobe damage, however, have produced conflicting data. For example, Wheeler and Stuss (2003) found, with a task requiring memory of a list of nouns, that recognition memory was intact, whereas van Asselen et al. (2006) found that patients with frontal lobe damage were impaired on a task of spatial search (which predominantly employs recognition). Similarly, Bor et al. (2006) found that frontal lobe patients had a decreased spatial span, whereas D’Esposito and Postle (1999) found no such changes in spatial span. Furthermore, Kopelman and Stanhope (1998) found that frontal lobe patients perform at control levels in a task requiring recall of words.
These conflicting results are further complicated by evidence of hemispheric asymmetry during tasks of working memory (D’Esposito et al., 1998; Muri et al., 2000). The overall pattern of these findings is such that with spatial working memory right hemisphere activity dominates, whereas with verbal and other nonspatial working memory tasks the left hemisphere dominates. However, some suggest that these results are more associated with memory-related motor preparation than storage of information, per se (Hester, D’Esposito, Cole, & Garavan, 2007; Volle et al., 2005). Furthermore, in a study comparing recall versus recognition from long-term memory of verbal information, Cabeza et al. (2003) found that the left PFC is more active during recall tasks whereas the right PFC is more active during recognition tasks. They proposed that this asymmetry arises from a division of the PFC into a system involved in monitoring and verification of retrieved items (dominant during recognition) and a system associated with production of information (dominant during recall). Whether this mechanism might generalize to the spatial domain, or with recognition versus recall from working memory, is not known.
To determine the extent to which memory-guided response relies on the dlPFC, we applied rTMS to the dlPFC (Brodmann Area 9/46), as well as the SPL (Brodmann Area 7). Furthermore, comparison of the effect of rTMS on recall versus recognition let us explore the nature of response-related processes supported by the dlPFC. rTMS uses the principle of electromagnetic induction to temporarily alter processing in a specific brain area (Walsh & Rushworth, 1999). If activity of a brain area is required for accurate performance of a task, rTMS applied to that area is expected to alter performance in systematic ways (e.g., Feredoes, Tononi, & Postle, 2006; Sack & Linden, 2003). If response period rTMS to the dlPFC has a greater effect on delayed recognition trials than on delayed recall trials, then it would suggest that the dlPFC plays a greater role in the processes involved in comparison between the probe and the remembered stimuli. If response period dlPFC rTMS has a greater effect on delayed recall trials, then it follows that the dlPFC is more important in control of the motor response. To our knowledge this is the first systematic study of recall versus recognition in spatial working memory.
METHODS
Subjects
24 right-handed, young adults (14 male, mean age = 22.9 [S.D. = 4.8]) were recruited from the University of Wisconsin community. Subjects did not have any psychiatric or neurological conditions, as determined by a psychiatrist or clinical psychologist who administered a structured psychiatric diagnostic interview (MINI, Sheehan et al., 1998) and mood assessment (HAM-D, Mean = 0.25 [S.D. = 0.61], Hamilton, 1960). The study was approved by the local ethics committee. All subjects were compensated monetarily.
Behavioral Task
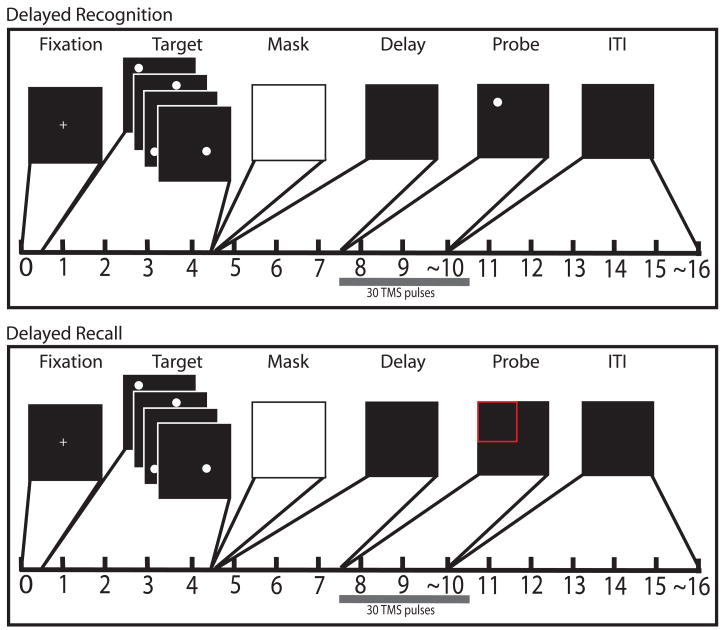
The behavioral task consisted of two different response procedures: one of recognition, another of recall. The two were randomly interleaved, and until the response period, the structure of the behavioral task was the same for both trial types: that is, subjects could not predict the trial type prior to the onset of the response stimulus (Fig. 1). This eliminated the possibility of any differential processing prior to rTMS delivery. Each trial began with a 500-ms presentation of a central fixation cross, followed by a 4-sec presentation of a target set: four white circles of 1.4° of visual angle, one located at a randomly determined location within each quadrant of the screen. Organization of the screen into quadrants was not made explicit in the target display. The targets were presented one at a time, each with a duration of 1 sec. The offset of the final target was followed by a luminance mask that was flashed on the screen for 100 ms, followed by a 3-sec delay period. During the response phase of the delayed-recognition trials, a circle identical in appearance to the targets was presented and subjects indicated with a yes/no button press (index finger/thumb) on a control stick (Attack 3, Logitech, Fremont, CA) whether its location corresponded to that of one of the four targets. The probe appeared in each of the four quadrants with p=.25, and at one of the target locations with p=.5. If the probe was non-matching, the distance from the nearest target circle location varied between 3.33 and 4.74° of visual angle. During the response phase of the delayed-recall trials, one quadrant of the screen was highlighted with a red outline, a cursor appeared at the center of the screen and subjects were to move the cursor to the location that had been occupied by the target from that quadrant. The cursor was moved with the control stick and the correct location was selected by pressing a “trigger” button (index finger) on the control stick. Prior to testing, subjects were trained on control of the cursor with the control stick. Subjects were scored on accuracy (proportion correct for recognition and error in degrees of visual angle for recall) and reaction time (RT) to the button press on both trial types. Subjects performed a total of 288 trials resulting in 96 trials per brain area targeted. Of these 96 trials, 48 were recognition trials, 48 were recall trials and orthogonally, 48 were with rTMS, 48 were without.
Figure 1.
Schematic of behavioral tasks: A) Delayed recognition B) Delayed recall. Timing of the task after the probe period is approximate because the ITI began immediately after subjects made a response.
Because we were interested in assessing effects of rTMS on the brain hemisphere targeted, and because we wanted to minimize any effects rTMS would have on motor output, we randomly assigned subjects into two groups, one responding with their right hand, the other with their left hand. Before the experimental trials began, both groups were required to go through practice trials until they reached 75% percent accuracy during the recognition trials and were within 2.2° of visual angle from the target during the recall trials. In addition, to obtain a baseline measure of spatial working memory abilities, we measured the memory span for spatial locations in all subjects. We found no difference in spatial working memory span between subjects who received left hemisphere rTMS (mean±S.D. = 5.83±1.59) versus those that received right hemisphere rTMS (mean±S.D. = 5.83±1.47).
Anatomical MRI
Whole-brain images were acquired with a 3T scanner (GE Signa VH/I). High-resolution T1-weighted images (256 axial slices, 0.5mm × 0.5mm × 0.8mm) were obtained for all participants. This scan was used to reconstruct a 3-dimensional image of each subject’s head, which was used to target rTMS.
rTMS Session
rTMS procedures
For the rTMS session the subject was seated comfortably and his/her head was localized in space via an infrared-based frameless stereotaxy system (eXimia Navigated Brain Stimulation (NBS), Nexstim, Helsinki, Finland). The TMS coil was also fitted with infrared-reflecting beacons, thereby permitting us to accurately target specific regions of the brain.
Prior to the start of the behavioral task, resting motor threshold was determined for each subject as measured by an electromyograph (Bagnoli Handheld EMG System, Delsys, Boston, MA). We used motor threshold to calibrate the stimulation intensity for each subject by starting at 110% of motor threshold, but accounting for scalp-to-cortex distance for each brain area targeted (Stokes et al., 2005).
During rTMSpresent trials a 3-sec train of 10 Hz rTMS (30 pulses) began with the onset of the probe stimulus. TMS was delivered with an air-cooled Magstim Standard Rapid magnetic stimulator fit with a 70-mm figure 8 stimulating coil (Magstim Co., Whitland, U.K.).
rTMS Controls
We used two levels of control in this study. The first level consisted of the randomly distributed half of the trials in each block during which rTMS was not applied. This allowed us to calculate the effect of rTMS (rTMSpresent − rTMSabsent) for each block in a manner that would control for possible long-term effects of rTMS (i.e., effects that might persist longer than the stimulus train, perhaps affecting subsequent trials). The second level of control was the inclusion of a brain area that was not expected to have direct involvement in working memory -- the area representing the leg in the primary somatosensory cortex of the postcentral gyrus (PCG). This latter level of control allowed us to account for any superficial effects of rTMS on behavior, such as those associated with scalp sensations and noise, as well as for regionally nonspecific effects of rTMS of the cortex.
Target selection
Subjects were randomly assigned to receive rTMS of the left hemisphere or the right hemisphere. Three brain areas (dlPFC, PCG, and SPL) were targeted with rTMS across two 12-block sessions (4 blocks per brain area). To eliminate the effect of fatigue and to stay within the safety limits of the maximum number of stimuli per subject per day (Wasserman, 1998), each session was performed on a different day. The order of the brain areas targeted with rTMS was counterbalanced across subjects, and repeated for each subject on the second day.
Of the 24 subjects, 12 received rTMS of the left hemisphere, 12 of the right. rTMS targeting for all subjects was based on individual anatomy. The dlPFC target was identified as the middle frontal gyrus on the ventral bank of the superior frontal sulcus at the level of the sulcus frontalis medius (approximately at ±40, +45, +28 atlas coordinates, Talairach & Tournoux, 1988), a region assumed to correspond to the border of Brodmann areas 9 and 46 (Oliveri et al., 2001; Petrides, 2000). The PCG target was chosen as an area immediately posterior to the central sulcus, close to the midline. Care was taken to ensure that subjects did not experience any peripheral sensations from stimulation of the somatosensory cortex. SPL targets were chosen dorsal and medial to the intraparietal sulcus and posterior to the postcentral sulcus (corresponding to Brodmann area 7).
Data Analysis Plan
Presented below is a summary of the significant results. Full details of the results (e.g., full reporting of ANOVAs) are available in the supplementary materials. Because both accuracy and RT were dependant variables in this study we first ran a multivariate analysis of variance (MANOVA) that included 3 levels of the factor Brain Area (dlPFC, SPL, PCG), 2 levels of hemisphere of stimulation (left, right), and 2 levels of memory task (recognition, recall). We then ran follow-up univariate analyses of variance (ANOVAs) for accuracy and RT separately with the same factors. In order to allow for direct comparison between the two memory tasks, both accuracy and RT for each subject were normalized within subject to rTMSabsent levels [“rTMS effect” = (rTMSpresent − rTMSabsent)/rTMSabsent], then these “rTMS effect” scores were entered into the analyses. Therefore the factor of the presence of rTMS was also taken into account in these analyses. With all ANOVAs, if a significant effect involved the factor of Brain Area, follow-up secondary comparisons were made of each experimental brain area (dlPFC and SPL) versus the control brain area (PCG). We also analyzed data by the visual hemifield in which the probe was presented and provide these results in the supplementary materials to this study. For recognition trials, accuracy data were recorded as the proportion correct for each subject, for recall trials, accuracy was defined as the median error in degrees of visual angle measured in the response. In both memory conditions, for each subject, RT was defined as the median RT in milliseconds.
RESULTS
3-way MANOVA (Brain Area, Hemishpere, Memory Task) on normalized accuracy and RT data revealed a significant effect [p(Wilk’s∧) < 0.001]. We assessed for significant effects on accuracy and RT separately.
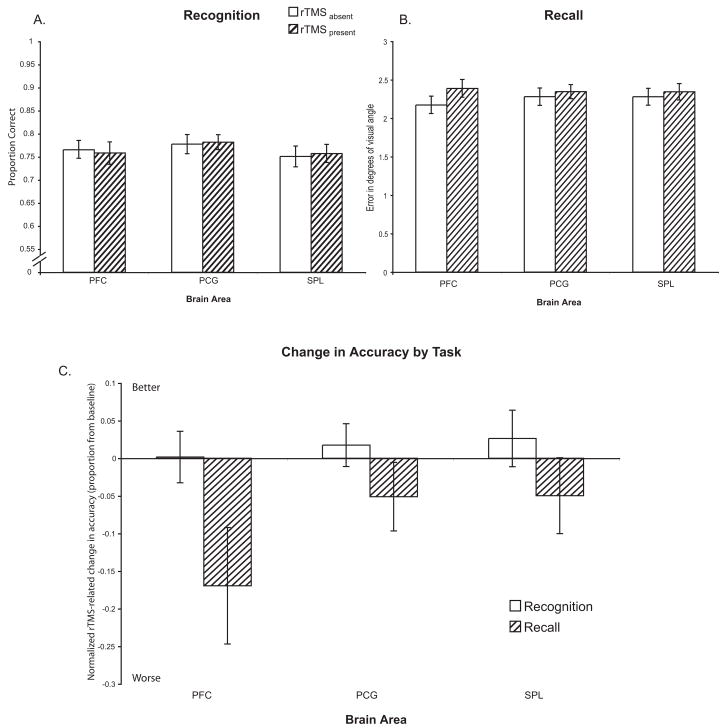
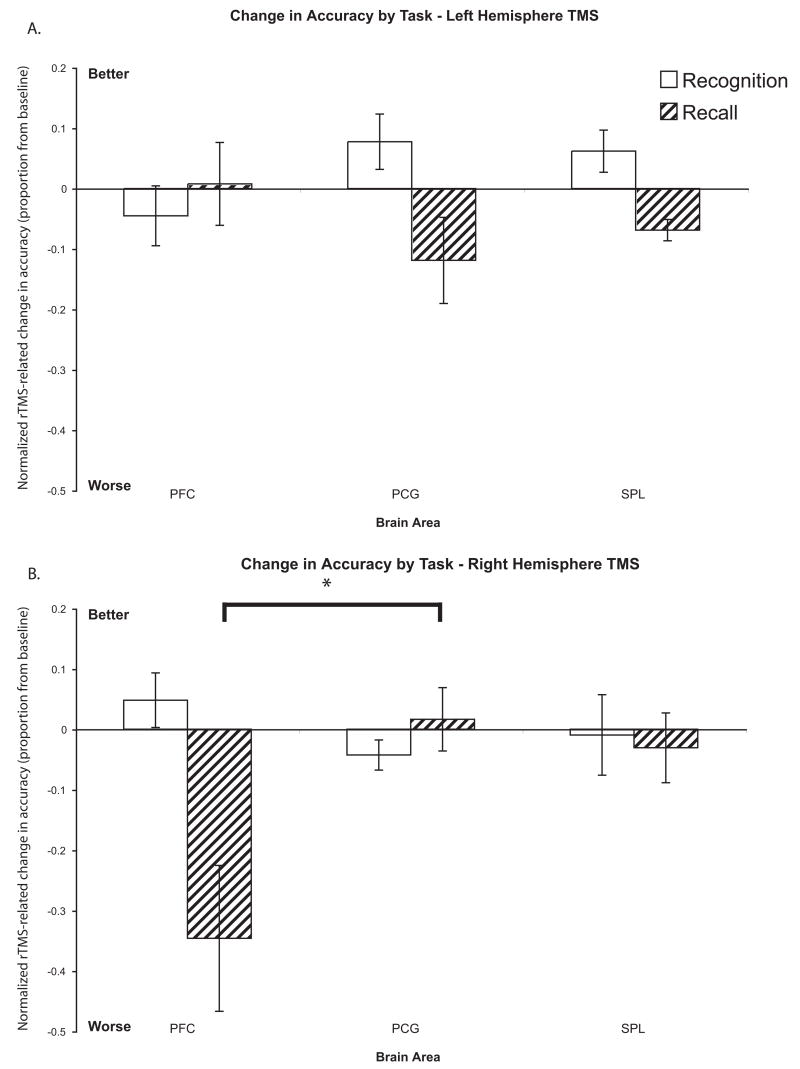
There was an overall trend of rTMS decreasing accuracy during recall trials (rTMSabsent (mean±S.D.) = 2.25±0.42° error vs. rTMSpresent = 2.36±0.46° error; t(23) = 1.59; n.s.; Fig. 3A), but no such trend for recognition trials (rTMSabsent (mean±S.D.) = 76.5±7.1% correct vs. rTMSpresent = 76.6±6.7% correct; t(23) = 0.09; n.s.; Fig. 3B). There was no difference in baseline (rTMSabsent) accuracy between subjects who received left hemisphere vs. right hemisphere rTMS for either recall [t(23) = 0.53; n.s.] or recognition [t(23) = 0.27; n.s.]. 3-way ANOVA (Brain Area, Hemisphere, Memory Task) on the normalized data found a main effect of Memory Task [F(1,22) = 10.16; p<0.005], reflecting the regionally-nonspecific effect of rTMS decreasing accuracy on recall trials more than on recognition trials [t(23) = 3.06; p<0.01]. There were no main effects of Brain Area or Hemisphere, as well as no 2-way interactions (all Fs < 1.88). However, we did find a significant 3-way interaction of Brain Area × Memory Task × Hemisphere [F(2,44) = 10.77; p<0.0005]. Follow-up with post-hoc tests on recall trials revealed that rTMS only to the right dlPFC led to a large decrease in accuracy. Pairwise comparison of the effect of right dlPFC rTMS versus right PCG rTMS was significant [t(22) = 2.54; p<0.05; See Fig. 4B]. With recognition trials, the opposite pattern was true: compared to its effect on the PCG, rTMS to the right dlPFC led to a relative improvement in accuracy whereas rTMS to the left dlPFC led to a relative decrement in accuracy [t(22) = 2.46; p<0.05; Fig. 4A and 4B].
Figure 3.
Accuracy: A) Delayed-recognition task B) Delayed-recall task. C) Change in accuracy with rTMS in both trial types normalized to the rTMSabsent condition. There were no regionally-specific significant effects. However, there was a trend towards a larger rTMS-induced decrease in accuracy in recall trial versus recognition trials when the dlPFC was targeted [t(23) = 1.87; p=0.07].
Figure 4.
Accuracy by hemisphere. Change in accuracy with rTMS in both trial types normalized to the rTMSabsent condition when rTMS targeted the A) left hemisphere and B) right hemisphere. With recognition trials, although the pairwise comparisons of the effect of rTMS of the left dlPFC versus that of the left PCG [t(11) = −1.65; n.s.] and the effect of rTMS of the right dlPFC versus that of the right PCG [t(11) = 1.84; p=0.09] did not reach significance, the relative change in accuracy (measured as the difference in accuracy between PCG rTMS and dlPFC rTMS) between rTMS of the left dlPFC versus that of the right dlPFC was significant [t(22) = 2.40; p<0.05]. For recall trials, the pairwise comparison of the effect of right dlPFC rTMS compared to the effect of right PCG rTMS was significant [t(11) = 3.71; p<0.005], as was the comparison of the relative changes in accuracy between right dlPFC and left dlPFC rTMS [t(22) = 2.54; p<0.05]. * − p<0.05
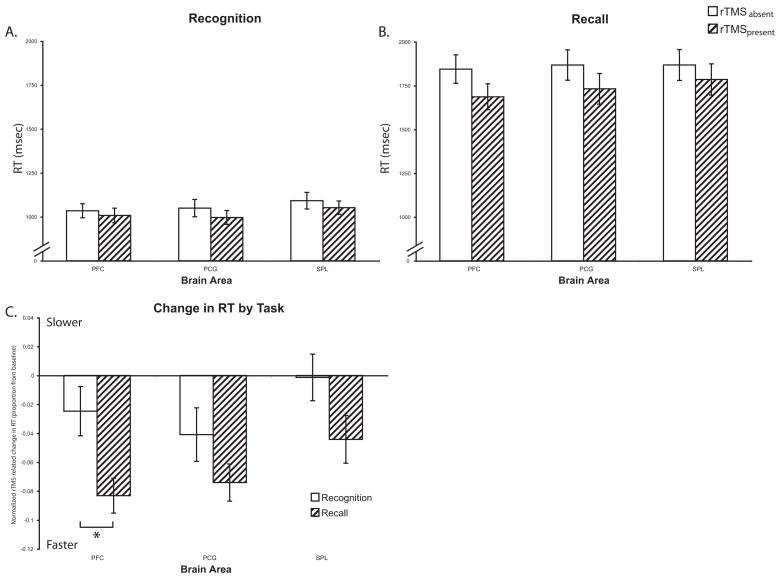
With RT, overall, subjects were slower in performing the recall vs. recognition task (recognition (mean±S.D.) = 1017±179 ms vs. recall = 1781±410 ms; t(23) = 52.82; p<0.0001). Virtually all responses were made during the 3-sec train of rTMS. rTMS had a general effect of speeding responses in both recognition (rTMSabsent (mean±S.D.) = 1031±191 ms vs. rTMSpresent = 1002±174 ms; t(23) = 2.61; p<0.05; Fig. 2A) and recall trials (rTMSabsent (mean±S.D.) = 1839±412 ms vs. rTMSpresent = 1724±412 ms; t(23) = 13.48; p<0.0001; Fig. 2B). There was no difference in baseline (rTMSabsent) RT between subjects who received left hemisphere vs. right hemisphere rTMS for either recall [t(23) = 0.06; n.s.] or recognition [t(23) = 0.04; n.s.]. ANOVA on normalized data revealed a main effect of Memory Task [F(1,22) = 10.84; p<0.005] with rTMS producing a greater speeding of RT in the recall trials. There were no other effects or interactions (all Fs < 2.47).
Figure 2.
Reaction Time: A) Delayed-recognition task B) Delayed-recall task. C) Change in accuracy with rTMS in both trial types normalized to the rTMSabsent condition. In recognition trials, PCG rTMS trended towards a greater decrease in RT compared to SPL rTMS [t(23) = 1.87; p=0.07]. Comparison of the effect of rTMS across trial types revealed that dlPFC rTMS led to a greater decrease in RT in recall versus recognition trials [t(23) = 3.44; p<0.005]. There was a similar trend with SPL rTMS [t(23) = 1.72; p=0.10]. * − p<0.05
DISCUSSION
dlPFC required for response formation in both delayed-recognition and delayed-recall
In this study we assessed the role of the dlPFC and SPL in producing a spatial memory-contingent response to two types of response cues: recognition and recall. With both types of tasks we found that rTMS to the dlPFC during the response period resulted in changes in accuracy, a finding that fits well with current accounts of dlPFC function in memory-guided response (Machens, Romo, & Brody, 2005; Passingham & Rowe, 2002; Postle, 2006b).
The major difference between the effects of rTMS on recall versus recognition trials was the direction of change in accuracy. rTMS to the right dlPFC resulted in a marginal improvement in accuracy in recognition trials, whereas it resulted in a significant decrement in accuracy when delivered during recall trials. The reason behind the opposite effects is unclear. A possible reason may be that for recall cues, the right dlPFC may be required for producing a response whereas for recognition probes, right dlPFC activity somehow interferes with response formation. Several groups have provided evidence that one role of the dlPFC during tasks of working memory is to contribute to preparation and execution of the appropriate motor response (e.g., Curtis, Rao, & D’Esposito, 2004; Pochon et al., 2001). For example, Pochon, et al. (2001) have shown that memory-guided response is associated with greater dlPFC activity when a complex motor response is required, compared to a simple yes/no buttonpress. This hypothesis is also supported by a study by Rollnik and colleagues (2000) in which they found that rTMS to the PFC results in a decrease in the amplitude of motor evoked potentials. Furthermore, the presence of anatomical connections between the dlPFC and supplementary motor area (e.g., Bates & Goldman-Rakic, 1993) support a functional role of the dlPFC in motor control. It is certainly the case that the motor response required by the recall cue is less constrained, and thus that its demands on motor execution, per se, are greater than the button press required by the recognition probe. Thus, the fact that rTMS to the dlPFC during recall trials results in a decrement in accuracy may be due to disruption of the preparation and/or execution of the memory-guided motor response required to move the cursor to the appropriate location.
One alternative explanation of the recall results could be that rTMS induced a speed-accuracy tradeoff. However, although there was a speeding of response accompanying the large decrement in accuracy, a speed-accuracy tradeoff is unlikely because a similar speeding of response was present with rTMS of PCG and of SPL, but without concomitant decreases in accuracy.
There are some caveats to be aware of with studies using rTMS. For example, one cannot always assume a disruptive effect of rTMS on neural activity (e.g., Silvanto & Muggleton, 2008; Théoret, Kobayashi, Valero-Cabré, & Pascual-Leone, 2003). The effect of rTMS has been shown to be dependant on the immediate state of the brain (e.g., Thut et al., 2003). Although, in this study, it is not possible to determine the physiological mechanisms behind the behavioral changes observed with dlPFC rTMS, the fact that dlPFC rTMS significantly affected behavior in both recall and recognition tasks shows that the dlPFC clearly plays an important role in both tasks. Furthermore, if the physiological effect of rTMS is state-dependant, then the opposite effects on behavior seen with response period dlPFC rTMS are likely due to inherent neurophysiological differences in the brain mechanisms supporting the two tasks. Another possible explanation for the differential effects of rTMS across tasks may be that the functional effects of rTMS are in fact at a brain area distant to the site of stimulation. Indeed, a study combining high frequency rTMS and 14C 2-deoxyglucose radiolabeling in cats not only found strong decreases in local metabolic activity, but also discovered decreases in metabolism at distant, anatomically connected brain areas (Valero-Cabré, Payne, Rushmore, Lomber, & Pascual-Leone, 2005). Nonetheless, the regional specificity of our effects suggests that there is something specific about the dlPFC or its connectivity that is functionally relevant to performance of our tasks. Furthermore, the opposite effects of rTMS on behavior in these two tasks suggest that this pattern of connectivity plays a functionally different role across the two tasks in this study. Without further experimentation, it will not be possible to distinguish between these possibilities.
Effects of rTMS are hemisphere-specific
We also found that rTMS of the right dlPFC specifically affected recall accuracy. That there is hemispheric asymmetry in the level of activity in the dlPFC during memory-guided response is well established. For example, Hester, et al. (2007) showed, with a task requiring working memory of a list of letters, that left dlPFC activity is associated with producing a memory-guided motor response. Although it has not been systematically studied, there is a general trend that the right dlPFC shows greater activity during tasks of spatial working memory (e.g., with an oculomotor delayed-matching to sample task, Curtis, Rao, & D’Esposito, 2004), whereas the left dlPFC shows greater activity when remembering non-spatial items (e.g., Schumacher & Jiang, 2003). Additionally, several previous studies of human frontal lobe lesions have shown that damage specifically to the right frontal cortex leads to decrements in spatial working memory performance (e.g., Bor, Duncan, Lee, Parr, & Owen, 2006; Clark et al., 2007; Miotto, Bullock, Polkey, & Morris, 1996; van Asselen et al., 2006). Numerous studies have also provided evidence for right hemisphere dominance of spatial attention (e.g., Mesulam, 1999). Our recall results are in line with these findings.
In contrast to recall, however, recognition memory declined with rTMS of left dlPFC (and improved with rTMS of the right). Although we did not predict this pattern of results a priori, there are two precedents that may account for it, one empirical and one theoretical. Empirically, Miotto et al. (1996) showed that specifically in patients with right hemisphere PFC lesions, spatial working memory performance was greatly affected with the addition of a requirement for the use of strategy. Thus, it may be that our two tasks were differentially reliant on the use of strategy. Theoretically, Kosslyn et al. (1989) have suggested that the right hemisphere is preferentially engaged in processing metric spatial relationships (e.g., how far a stimulus is from the center of the screen), whereas the left hemisphere more efficiently processes categorical spatial relationships (e.g., whether a stimulus is more or less than X mm from the center of the screen). Our results fit within this framework if one considers that recall, which requires exact reproduction of the spatial coordinates of the stored stimulus, requires the use of metric information about the stored items, whereas recognition requires a categorical decision (same or different).
On the surface, our results appear the opposite of those reported by Cabeza et al. (2003), who found with PET that the left hemisphere dominates during recall, and the right during recognition. However, there are many differences between these two studies. First, Cabeza et al. study verbal long-term memory, whereas this study tested spatial working memory. Second, Cabeza et al. studied task-related changes in blood flow, whereas the present study examined the effects of rTMS on performance. Finally, it is worth reiterating that the implication of these results for lateralization of function should be made with caution. For example, studies of the motor system (e.g., Baumer et al., 2006) have revealed strong evidence for interhemispheric effects of TMS. Thus, it is possible that our finding of a specific effect of rTMS of right dlPFC may, in fact, have been due to functional changes in the left hemisphere. It has also been suggested that the effect of rTMS can be due to its effect on the balance between activities of corresponding cortical areas in opposite hemispheres (Théoret, Kobayashi, Valero-Cabré, & Pascual-Leone, 2003).
Parietal lobe not essential for response formation
Although previous studies have provided evidence for parietal activation during the response period of spatial working memory tasks (e.g., Narayanan et al., 2005; Schluppeck, Curtis, Glimcher, & Heeger, 2006), we found that, compared to that of the control area, rTMS of the SPL did not affect accuracy or RT on our test of spatial delayed response. Although, there is ample evidence that the SPL plays an important role in storage of spatial information (Hamidi, Tononi, & Postle, In Press; Postle, 2006b; Srimal & Curtis, 2008), based on our findings, it is not essential in producing memory-guided response.
Conclusions
We applied rTMS during the response phase of delayed recognition and delayed recall of locations. We found that the dlPFC was differentially involved across the two response types, with rTMS of right dlPFC leading to an improvement in accuracy during delayed-recognition trials and leading to a decrement in accuracy during delayed-recall trials. Furthermore, we found that for delayed recognition rTMS to the left dlPFC led to a decrement in accuracy. Along with our previous finding that delay-period rTMS to the dlPFC does not affect task performance (Hamidi, Tononi, & Postle, In Press), these results suggest that the dlPFC’s contributions to working memory performance are more important for response formation and execution, than for storage itself.
Acknowledgments
This study was supported by grants MH078705 (M.H.) and MH064498 (B.R.P.) of the National Institute of Mental Health and by NARSAD (G.T.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Awh E, Anllo-Vento L, Hillyard SA. The Role of Spatial Selective Attention in Working Memory for Locations: Evidence from Event-Related Potentials. J Cogn Neurosci. 2000;12(5):840–847. doi: 10.1162/089892900562444. [DOI] [PubMed] [Google Scholar]
- Bates JF, Goldman-Rakic PS. Prefrontal connections of medial motor areas in the rhesus monkey. Journal of Comparative Neurology. 1993;336(2):211–228. doi: 10.1002/cne.903360205. [DOI] [PubMed] [Google Scholar]
- Baumer T, Bock F, Koch G, Lange R, Rothwell JC, Siebner HR, et al. Magnetic stimulation of human premotor or motor cortex produces interhemispheric facilitation through distinct pathways. J Physiol. 2006;572(3):857–868. doi: 10.1113/jphysiol.2006.104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bor D, Duncan J, Lee ACH, Parr A, Owen AM. Frontal lobe involvement in spatial span: Converging studies of normal and impaired function. Neuropsychologia. 2006;44(2):229–237. doi: 10.1016/j.neuropsychologia.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Locantore JK, Anderson ND. Lateralization of prefrontal activity during episodic memory retrieval: Evidence for the production-monitoring hypothesis. Journal of Cognitive Neuroscience. 2003;15(2):249–259. doi: 10.1162/089892903321208187. [DOI] [PubMed] [Google Scholar]
- Clark L, Blackwell AD, Aron AR, Turner DC, Dowson J, Robbins TW, et al. Association Between Response Inhibition and Working Memory in Adult ADHD: A Link to Right Frontal Cortex Pathology? Biological Psychiatry. 2007;61(12):1395–1401. doi: 10.1016/j.biopsych.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Curtis CE. Prefrontal and parietal contributions to spatial working memory. Neuroscience. 2006;139(1):173–180. doi: 10.1016/j.neuroscience.2005.04.070. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Rao VY, D’Esposito M. Maintenance of Spatial and Motor Codes during Oculomotor Delayed Response Tasks. Journal of Neuroscience. 2004;24(16):3944–3952. doi: 10.1523/JNEUROSCI.5640-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Cognitive Brain Research. 1998;7(1):1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR. The dependence of span and delayed-response performance on prefrontal cortex. Neuropsychologia. 1999;37(11):1303–1315. doi: 10.1016/s0028-3932(99)00021-4. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Rugg MD. Event-related potential studies of associative recognition and recall: electrophysiological evidence for context dependent retrieval processes. Cognitive Brain Research. 1999;8(1):1–16. doi: 10.1016/s0926-6410(98)00051-2. [DOI] [PubMed] [Google Scholar]
- Feredoes E, Tononi G, Postle BR. Direct evidence for a prefrontal contribution to the control of proactive interference in verbal working memory. PNAS. 2006;103(51):19530–19534. doi: 10.1073/pnas.0604509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. The Prefrontal Cortex. 3. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- Goldman-Rakic P. Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates. Progress in Brain Research. 1990;85:325–336. doi: 10.1016/s0079-6123(08)62688-6. [DOI] [PubMed] [Google Scholar]
- Haist F, Shimamura AP, Squire LR. On the relationship between recall and recognition memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1992;18(4):691–702. doi: 10.1037//0278-7393.18.4.691. [DOI] [PubMed] [Google Scholar]
- Hamidi M, Tononi G, Postle BR. Evaluating frontal and parietal contributions to spatial working memory with repetitive transcranial magnetic stimulation. Brain Research. doi: 10.1016/j.brainres.2008.07.008. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, D’Esposito M, Cole MW, Garavan H. Neural mechanisms for response selection: comparing selection of responses and items from working memory. NeuroImage. 2007;34(1):446–454. doi: 10.1016/j.neuroimage.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Knight RT, D’Esposito M. Lateral Prefrontal Syndrome: A Disorder of Executive Control. In: D’Esposito M, editor. Neurological Foundations of Cognitive Neuroscience. Cambridge, MA: MIT Press; 2003. [Google Scholar]
- Kopelman MD, Stanhope N. Recall and recognition memory in patients with focal frontal, temporal lobe and diencephalic lesions. Neuropsychologia. 1998;36(8):785–796. doi: 10.1016/s0028-3932(97)00167-x. [DOI] [PubMed] [Google Scholar]
- Kosslyn S, Koenig O, Barrett A, Cave C, Tang J, Gabrieli JDE. Evidence for two types of spatial representations: Hemispheric specialization for categorical and coordinate relations. Journal of Experimental Psychology. 1989;15:723–735. doi: 10.1037//0096-1523.15.4.723. [DOI] [PubMed] [Google Scholar]
- Machens CK, Romo R, Brody CD. Flexible Control of Mutual Inhibition: A Neural Model of Two-Interval Discrimination. Science. 2005;307(5712):1121–1124. doi: 10.1126/science.1104171. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philosophical Transactions of the Royal Society B: Biological Sciences. 1999;354(1387):1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural Mechanisms of Visual Working Memory in Prefrontal Cortex of the Macaque. Journal of Neuroscience. 1996;16(16):5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto EC, Bullock P, Polkey CE, Morris RG. Spatial working memory and strategy formation in patients with frontal lobe excisions. Cortex. 1996;32:613–630. doi: 10.1016/s0010-9452(96)80034-7. [DOI] [PubMed] [Google Scholar]
- Muri RM, Gaymard B, Rivaud S, Vermersch AI, Hess CW, Pierrot-Deseilligny C. Hemispheric asymmetry in cortical control of memory-guided saccades. A transcranial magnetic stimulation study. Neuropsychologia. 2000;38(8):1105–1111. doi: 10.1016/s0028-3932(00)00030-0. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Prabhakaran V, Bunge SA, Christoff K, Fine EM, Gabrieli JDE. The Role of the Prefrontal Cortex in the Maintenance of Verbal Working Memory: An Event-Related fMRI Analysis. Neuropsychology. 2005;19(2):223–232. doi: 10.1037/0894-4105.19.2.223. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Turriziani P, Carlesimo GA, Koch G, Tomaiuolo F, Panella M, et al. Parieto-frontal Interactions in Visual-object and Visual-spatial Working Memory: Evidence from Transcranial Magnetic Stimulation. Cerebral Cortex. 2001;11(7):606–618. doi: 10.1093/cercor/11.7.606. [DOI] [PubMed] [Google Scholar]
- Passingham D, Rowe JB. Dorsal prefrontal cortex: maintenance in memory or attentional selection? In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. Oxford: Oxford University Press; 2002. pp. 221–232. [Google Scholar]
- Petrides M. Frontal Lobes and Memory. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. 2. Vol. 2. Amsterdam: Elsevier; 2000. pp. 67–84. [Google Scholar]
- Pochon JB, Levy R, Poline JB, Crozier S, Lehericy S, Pillon B, et al. The Role of Dorsolateral Prefrontal Cortex in the Preparation of Forthcoming Actions: an fMRI Study. Cerebral Cortex. 2001;11(3):260–266. doi: 10.1093/cercor/11.3.260. [DOI] [PubMed] [Google Scholar]
- Postle BR. Distraction-resistant sustained activity during delayed recognition of locations. NeuroImage. 2006a;30(3):950–962. doi: 10.1016/j.neuroimage.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Postle BR. Working Memory as an Emergent Propertry of the Mind and Brain. Neuroscience. 2006b;139(1):23–38. doi: 10.1016/j.neuroscience.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock I, Engelstein P. A Study of Memory for Visual Form. The American Journal of Psychology. 1959;72(2):221–229. [Google Scholar]
- Rollnik JD, Schubert M, Dengler R. Subthreshold prefrontal repetitive transcranial magnetic stimulation reduces motor cortex excitability. Muscle & Nerve. 2000;23(1):112–114. doi: 10.1002/(sici)1097-4598(200001)23:1<112::aid-mus15>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, D’Esposito M. The Influence of Working-Memory Demand and Subject Performance on Prefrontal Cortical Activity. Journal of Cognitive Neuroscience. 2002;14(5):721–731. doi: 10.1162/08989290260138627. [DOI] [PubMed] [Google Scholar]
- Sack AT, Linden DEJ. Combining transcranial magnetic stimulation and functional imaging in cognitive brain research: possibilities and limitations. Brain Research Reviews. 2003;43(1):41–56. doi: 10.1016/s0165-0173(03)00191-7. [DOI] [PubMed] [Google Scholar]
- Schluppeck D, Curtis CE, Glimcher PW, Heeger DJ. Sustained Activity in Topographic Areas of Human Posterior Parietal Cortex during Memory-Guided Saccades. Journal of Neuroscience. 2006;26(19):5098–5108. doi: 10.1523/JNEUROSCI.5330-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher EH, Jiang Y. Neural Mechanisms for Response Selection: Representation Specific or Modality Independent? Journal of Cognitive Neuroscience. 2003;15(8):1077–1079. doi: 10.1162/089892903322598058. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(S20):22–33. [PubMed] [Google Scholar]
- Silvanto J, Muggleton NG. New light through old windows: Moving beyond the “virtual lesion” approach to transcranial magnetic stimulation. NeuroImage. 2008;39(2):549–552. doi: 10.1016/j.neuroimage.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Srimal R, Curtis CE. Persistent neural activity during the maintenance of spatial position in working memory. NeuroImage. 2008;39(1):455–468. doi: 10.1016/j.neuroimage.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes MG, Chambers CD, Gould IC, Henderson TR, Janko NE, Allen NB, et al. A simple metric for scaling motor threshold based on scalp-cortex distance: Application to studies using transcranial magnetic stimulation. Journal of Neurophysiology. 2005;94(6):4520–4527. doi: 10.1152/jn.00067.2005. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Stuttgart, Germany: Thieme; 1988. [Google Scholar]
- Théoret H, Kobayashi M, Valero-Cabré A, Pascual-Leone A. Exploring paradoxical functional facilitation with TMS. Supplements to Clinical neurophysiology. 2003;56:211–219. doi: 10.1016/s1567-424x(09)70224-7. [DOI] [PubMed] [Google Scholar]
- Thut G, Northoff G, Ives JR, Kamitani Y, Pfennig A, Kampmann F, et al. Effects of single-pulse transcranial magnetic stimulation (TMS) on functional brain activity: a combined event-related TMS and evoked potential study. Clinical Neurophysiology. 2003;114(11):2071–2080. doi: 10.1016/s1388-2457(03)00205-0. [DOI] [PubMed] [Google Scholar]
- Tulving E, Psotka J. Retroactive Inhibition in Free Recall: Inaccessibility of Information Available in the Memory Store. Journal of Experimental Psychology. 1971;87(1):1–8. [Google Scholar]
- Valero-Cabré A, Payne BR, Rushmore J, Lomber SG, Pascual-Leone A. Impact of repetitive transcranial magnetic stimulation of the parietal cortex on metabolic brain activity: a 14C-2DG tracing study in the cat. Experimental Brain Research. 2005;163(1):1–12. doi: 10.1007/s00221-004-2140-6. [DOI] [PubMed] [Google Scholar]
- van Asselen M, Kessels RPC, Neggers SFW, Kappelle LJ, Frijns CJM, Postma A. Brain areas involved in spatial working memory. Neuropsychologia. 2006;44(7):1185–1194. doi: 10.1016/j.neuropsychologia.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Volle E, Pochon JB, Lehericy S, Pillon B, Dubois B, Levy R. Specific cerebral networks for maintenance and response organization within working memory as evidenced by the ‘double delay/double response’ paradigm. Cerebral Cortex. 2005;15(7):1064–1074. doi: 10.1093/cercor/bhh207. [DOI] [PubMed] [Google Scholar]
- Walsh V, Rushworth M. A primer of magnetic stimulation as a tool for neuropsychology. Neuropsychologia. 1999;37(2):125–135. [PubMed] [Google Scholar]
- Wasserman E. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalography and Clinical Neurophysiology. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Stuss DT. Remembering and knowing in patients with frontal lobe injuries. Cortex. 2003;39(4–5):827–846. doi: 10.1016/s0010-9452(08)70866-9. [DOI] [PubMed] [Google Scholar]