Abstract
Adenovirus (Ad) vectors have been developed as human immunodeficiency-1 (HIV-1) vaccine vectors because they consistently induce immune responses in preclinical animal models and human trials. Strong promoters and codon-optimization are often used to enhance vaccine-induced HIV-1 gene expression and immunogenicity. However, if the transgene is inherently cytotoxic in the cell line used to produce the vector, and is expressed at high levels, it is difficult to rescue a stable Ad HIV-1 vaccine vector. Therefore we hypothesized that generation of Ad vaccine vectors expressing cytotoxic genes, such as HIV-1 env, would be more efficient if expression of the transgene was down regulated during Ad rescue. To test this hypothesis, a Lac repressor-operator system was applied to regulate expression of reporter luciferase and HIV-1 env transgenes during Ad rescue. The results demonstrate that during Ad rescue, constitutive expression of the Lac repressor in 293 cells reduced transgene expression levels to approximately 5% of that observed in the absence of regulation. Furthermore, Lag-regulation translated into more efficient Ad rescue compared to traditional 293 cells. Importantly, Ad vectors rescued with this system showed high levels of transgene expression when transduced into cells that lack the Lac repressor protein. The Lac-regulated system also facilitated the rescue of modified Ad vectors that have non-native receptor tropism. These tropism-modified Ad vectors infect a broader range of cell types than the unmodified Ad, which could increase their effectiveness as a vaccine vector. Overall, the Lac-regulated system described here (i) is backwards compatible with Ad vector methods that employ bacterial-mediated homologous recombination (ii) is adaptable for the engineering of tropism-modified Ad vectors and (iii) does not require co-expression of regulatory genes from the vector or the addition of exogenous chemicals to induce or repress transgene expression. This system therefore could facilitate the development of Ad-based vaccine candidates that otherwise would not be feasible to generate.
1. Introduction
1.1 Current HIV-1 vaccines
HIV-1 vaccine clinical trials are reaching into a record number of developed and under-developed countries worldwide (Kresge, 2007). This increase in testing is driven by the premise that a protective vaccine, even if only partially effective, would have enormous benefits in the lives of people affected by HIV infection and the economic costs associated with health care and productivity. A number of vaccine candidates are currently being evaluated, including plasmid DNA (pDNA), synthetic peptides, recombinant proteins, live viral vectors, and various combinations of these different components. Poxvirus- and Ad-based vectors have emerged as the most promising of the virally-vectored HIV-1 vaccines. Among these two vector types, Ad serotype 5 (Ad5)-based vaccines have consistently demonstrated the ability to induce immune responses in pre-clinical animal models and phase I/II human trials. Despite their apparent ability to elicit strong T cell responses, Ad5-based vaccines are also paradoxically the most susceptible to inhibition by naturally occurring pre-existing vector immunity, which can significantly limit its efficacy. To address this issue, several groups including our own are developing innovative Ad vectors that circumvent neutralization by pre-existing anti-Ad5 antibodies (Nab) in vaccinees (Barouch et al., 2004; Blackwell et al., 2000; de Souza et al., 2006; Fitzgerald et al., 2003; McCoy et al., 2007; Nanda et al., 2005; Thorner et al., 2006; Vanniasinkam and Ertl, 2005); nevertheless a recent study suggests that vector modification alone may not completely negate the limitations associated with pre-existing Ad5 immunity (Liu et al., 2007). Importantly however, results from the STEP/HVTN 502 HIV clinical trial have brought into question the use of Ad5-vectored HIV-1 vaccines, and perhaps virally-vectored vaccines in general, due to a lack of efficacy and the unanticipated association of pre-existing Ad5 immunity with increased acquisition of HIV-1 infection, especially in uncircumsized vaccinees (Sekaly, 2008; Steinbrook, 2007). Despite this significant setback there is still interest in Ad-based vaccines, therefore continued vector development and discovery research is highly warranted.
1.2 Recombinant Ad5 vector development
As a recombinant viral vector, Ad5 has shown utility in the context of gene therapies, immunotherapies, and vaccines (see reviews in Refs. (Barouch and Nabel, 2005; Ghosh et al., 2006; McConnell and Imperiale, 2004)). Perhaps one of the most compelling arguments for the continued use of Ad5-based therapies lies in the considerable amount of past and ongoing vector development and the growing body of information on the immune responses elicited by Ad vectors and on vector-host interactions. In this regard, Ad vector development encompasses a range of promising approaches to manipulate cell tropism (Douglas et al., 1996; Krasnykh et al., 1996; Rogers et al., 1997; Stevenson et al., 1997), afford cell- or tissue-specific transgene expression (Glasgow et al., 2006) and modulate immune responses through the expression of cytokines or costimulatory ligands (Braciak et al., 2000; Bukczynski et al., 2004; Wiethe et al., 2003). Furthermore, a considerable amount of vector development has taken place investigating Ad vectors of different serotypes. For example, human Ad serotypes 35, 41, 46 and 49 (Barouch et al., 2004; Lemiale et al., 2007; Xin et al., 2007) as well as simian, bovine and porcine Ad vectors (McCoy et al., 2007; Moffatt et al., 2000) are currently being evaluated as vaccine candidates. Similar approaches to alter vector tropism that have been employed in other Ad-based therapies and could have utility in HIV-1 vaccine design include direct genetic modification of viral capsid proteins (Kasono et al., 1999; Mercier et al., 2004) and the use of molecular bridging molecules such as antibodies (Blackwell et al., 1999; Volpers et al., 2003), single-chain antibodies (Watkins et al., 1997) and recombinant fusion proteins (Pereboev et al., 2002; Pereboev et al., 2004). Importantly, some of these tropism-modification approaches have the added value of facilitating escape from pre-existing Ad5 Nab (Blackwell et al., 2000). Overall, the ongoing vector development illustrates the flexibility that the Ad platform lends to the increasingly complex needs of HIV-1 vaccine design.
1.3 Regulation of cytotoxic transgene expression
During the development of recombinant Ad vectors, several studies have reported difficulty in rescuing the vectors themselves, which in some cases was linked to the toxicity of recombinant transgene expression. This toxicity could be mitigated by countering the toxic cellular effects and/or by regulating toxic gene expression. For example, by including a caspase inhibitor in the cell culture medium, Ad vectors that express pro-apoptotic Fas ligand (FasL) have been successfully propagated (Aoki et al., 2000). In other examples, regulated gene expression was needed to successfully rescue or propagate Ad vectors that express human tumor necrosis factor alpha (Hu et al., 1997), vesicular stomatitis virus G-protein (Yoshida et al., 1997), FasL (Rubinchik et al., 2000), rabies virus glycoprotein (Matthews et al., 1999) and the ASF/SF2 splicing factor (Edholm et al., 2001). To add to this list, we have observed that some Ad vaccine vectors that express full-length HIV env sequences are consistently difficult or impossible to rescue. The problem is exascerbated when the gene is codon-optimized and transcribed from a strong promoter. Our experience is consistent with early studies showing the cytotoxicity of Env (Sodroski et al., 1986) and a number of studies that have since described different mechanisms of Env glycoprotein cytotoxicity (Andreau et al., 2004; Perfettini et al., 2005). For example, soluble gp120 induces programmed cell death through interaction with CD4 and/or CXCR4 and CCR5 chemokine receptors (Cicala et al., 2000; Roggero et al., 2001; Trushin et al., 2007) and dysregulation of intracellular calcium homeostasis (Haughey and Mattson, 2002); the latter of which may be linked with neuronal damage and AIDS dementia. In addition, the interaction of membrane-bound gp120/41 with CD4 and co-receptor can induce syncytia formation resulting in cellular and nuclear fusion that leads to apoptosis via caspase activation and mitochondrial depolarization. It was also observed that Env glycoprotein-induced syncytia formation from Ad5-infected cells (Li et al., 2001), produces a massive amount of actin filament disruption that is likely involved in the resulting cytotoxicity (unpublished observation). Of note, proposed mechanisms of HIV-1 pathogenesis have also implicated Env glycoprotein-mediated T cell depletion of infected CD4 T cells as well as bystander killing of uninfected cells (Grossman et al., 2002; Ho et al., 1995; Meissner et al., 2006; Wei et al., 1995). The problems associated with Env toxicity also extend into HIV-1 vaccine design. For example, in a recent Phase I clinical trial using a mixture of rAd5 vectors that express HIV-1 subtype B Gag-Pol fusion protein and envelope (Env) from subtypes A, B, and C (Catanzaro et al., 2006), the investigators determined in the preclinical studies that the cytotoxicity of full-length gp160 could be reduced or eliminated by deletion of the COOH-terminal cytoplasmic domain (Chakrabarti et al., 2002). Such COOH-terminal truncations, either by design or spontaneous mutations, have also been shown to increase incorporation, surface expression and/or genetic stability of Env (Devitt et al., 2007; Wyatt et al., 2007; Yao et al., 2000).
1.4. Regulated Ad expression system
As mentioned above, in the development of vaccine vectors for evaluating HIV-1 Env immunogenicity, Ad vectors expressing full-length HIV-1 89.6 env genes have been difficult to rescue in our laboratory. By comparison, Ad vectors that express 89.6 env genes truncated at amino acid position 719 have been rescued with considerably more ease. However, in considering the potential importance of conformational neutralizing antibody epitopes that require an intact Env structure, our goal was to develop a system that facilitated the production of Ad vectors that express full-length env genes. Therefore, in this study we employed a modified Ad5 vector system to address the toxicity associated with full-length Env expression. Our approach utilizes the Lac repressor-operator model to down-regulate transgene expression during the rescue of the Ad vector. The Lac-regulated Ad vectors rescued with this system express equivalent levels of transgene in permissive cells and, in the case of the 89.6 env gene, were more efficiently rescued compared to standard Ad vectors expressing the same genes. Using this Lac-regulated system we have also rescued modified Ad vectors, such as the chimeric Ad5/3 vector described in this report, which may have application for the development of alternative Ad-based vaccine vectors. In the aggregate, the system described below may facilitate the development and evaluation of Ad-based vaccine candidates that otherwise would not have been feasible.
2. Materials and Methods
2.1. Cell lines used in the study
The AD-293 cell line (called 293-AD here), derived from HEK293 cells with improved cell adherence and plaque forming properties, was purchased from Stratagene (Cat. No. 240085; La Jolla, CA, USA). 293-IQ, derived from HEK293 cells with constitutive expression of the Lac repressor (Matthews et al., 1999), were purchased from Microbix Virology (Cat. No. PD-02-04; Toronto, Ontario, Canada). HeLa (Cat. No. CCL-2), FaDu (Cat. No. HTB-43), DU145 (Cat. No. HTB-81), MDA-MB-231 (Cat. No. HTB-26) and SKOV-3 (Cat. No. HTB-77) were purchased from the American Tissue Culture Collection. All cells were subcultured and grown in the recommended media at 37°C, 5% CO2 as described by the vendors. Growth media and sub-culturing methods are available on request.
2.2. Construction of recombinant Ad plasmids
The plasmid pShuttleB316M (Figure 1), which was used for generating the Ad vectors in this study, was constructed as follows. First, the PacI restriction enzyme site in the plasmid pDC316(io) (Microbix), which contains the Lac operator element, was destroyed by cutting with PacI and then blunted with Klenow (large fragment) DNA polymerase. This modification was necessary since a critical downstream step requires linearization of the recombinant plamsid at a different PacI site. The resulting blunt ends were then re-ligated to generate the plasmid pDC316M. Next, pDC316M was digested with Xbal (position 455) and PvuII (position 1689) and the resulting XbaI-PvuII fragment containing the Lac operator element was gel purified. In parallel, the plasmid pShuttle (Stratagene) was modified to remove two EcoRI restriction enzyme sites (at positions 379 and 2265). This step was necessary since it was planned to use the EcoRI site within the multiple cloning site of pDC316M for subsequent cloning of genes. The first EcoRI (nt 379) was removed by digesting with EcoRV (position 373) and SalI (position 389), filling-in with Klenow and re-ligating to produce pShuttleA. Next, the second EcoRI site (position 2265) was destroyed by cutting with EcoRI, filling-in with Klenow DNA polymerase and re-ligating to produce pShuttleB. The plasmid pShuttleB was then digested with BglII, filled-in with Klenow DNA polymerase to blunt the BgIII site and then digested with XbaI. The XbaI-PvuII fragment from pDC316M was then cloned into the digested pShuttleB, resulting in the final plasmid pShuttleB316M. Clones at each of the steps were screened by restriction enzyme digestion for the presence of insert and sequenced. GFP and codon-optimized HIV-1 89.6 env genes were cloned into the EcoRI and SalI sites on pShuttleB316M after PCR amplification using primers (GFP forward primer: 5′-TCAG GAATTC ATGGTG AGCAAG GGCGAG GA-3′; GFP reverse primer: 5′-GACT GTCGAC TTATCT AGATCC GGTGG ATC-3′; 89.6 Env forward primer: 5′-TCAG GAATTC ATGCGC GTGAAG GAGAAG TA -3′; 89.6 Env reverse primer: 5′-TCAG GTCGAC CTACAG CAGGAT GCGCTC CA -3′) that contained restriction enzyme sites (underlined). The GFP gene was amplified from pAdTrack-CMV (Stratagene) and codon-optimized HIV-1 89.6 env gene was amplified from pCAGGS-89.6env, a kind gift from Dr. Richard Compans (Emory University). Annotated sequences of the clones used in this study are available upon request.
Figure 1. Construction of a shuttle plasmid for generating Ad vectors containing a lac operator regulatory domain.
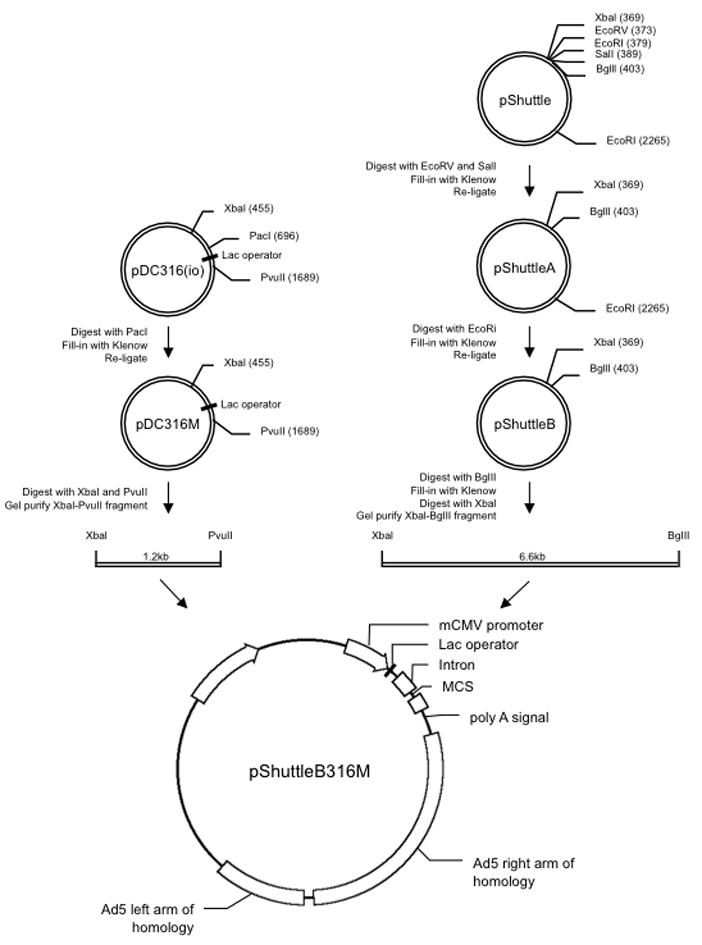
The plasmid pShuttleB316M was used for the generation of the Lac-regulated Ad vectors in this study. It was constructed by PacI restriction enzyme digestion of the plasmid pDC316 (Microbix) and then blunting with Klenow DNA polymerase. The resulting blunt ends were then re-ligated to generate the plasmid pDC316M. This modification destroyed the PacI site was necessary since this PacI site interferes with subsequent steps used to generate recombinant Ad vectors. Next, pDC316M was digested with Xbal (position 455) and PvuII (position 1686) and the resulting XbaI-PvuII fragment was gel purified. The plasmid pShuttle (Stratagene) was modified to remove the EcoRI restriction enzyme site (at position 379) by digesting with EcoRV (position 373) and SalI (position 389), filling-in with Klenow and re-ligating to produce pShuttleA. Next, a second EcoRI site (position 2665) was destroyed by cutting with EcoRI, filling-in with Klenow DNA polymerase and re-ligating to produce pShuttleB. The plasmid pShuttleB was then digested with BglII, filled-in with Klenow DNA polymerase and digested with XbaI. The XbaI-PvuII fragment from pDC316M was then cloned into the digested pShuttleB, resulting in the final plasmid pShuttleB316M. Clones at each of the steps were screened by restriction enzyme digestion and/or PCR for the presence of insert and sequenced. The gfp and HIV-1 89.6 env genes were cloned into the multiple cloning site (MCS) of pShuttleB316M, resulting in plasmids pShuttleB316M-GFP and pShuttleB316M-Env, respectively, which were subsequently recombined with pAdEasy-1 or pAd5/3Easy to produce the lac-regulated Ad vectors used in this study.
2.3. Generation of Ad vectors
The viral vectors Ad5GFP, Ad5lacGFP, Ad5/3lacGFP, Ad5Env, Ad5lacEnv and Ad5/3lacEnv were generated by homologous recombination between the appropriate shuttle plasmid (above) and the Ad genomic plasmids, pAdEasy-1 (Stratagene) or pAd5/3Easy (Kanerva et al., 2002; Krasnykh et al., 1996; Mittal et al., 1993), in E. coli BJ5183. The plasmid pAd5/3Easy encodes a modified fiber gene that has a chimeric Ad5 shaft and Ad3 knob region corresponding to nts 647–1208 of Genbank accession numbers X01998 and M12411. Following homologous recombination, candidate clones were screened by PacI restriction enzyme digestion and PCR analyses and positive clones were then sequenced. PacI-digested recombinant Ad plasmids were transfected with Lipofectimine 2000 (Invitrogen, Carlsbad, CA) into 293-AD or 293-IQ cells seeded on 6-well plates. Ten to 14 days later, the virus from the transfected cells and media was collected and expanded further on 15 × T175 flasks of 293-AD cells. All viruses were purified by double centrifugation on cesium chloride gradients and subjected to dialysis as described elsewhere (Graham and Prevec, 1991). The physical titers, or total virus particles (VP), were determined spectrophotometrically by measuring the OD at 260 nm where 1 absorbance unit is equivalent to 1.1 × 1012 virus particles (Maizel et al., 1968). Viral titers were determined by a standard 50% tissue culture infectious dose (TCID50) assay using 293-AD cells. The TCID50 was converted to plaque forming units (PFU) per ml where the PFU/ml has been empirically determined to be 0.7 log less that the TCID50/ml.
2.4. Flow cytometric analyses of GFP and HIV-1 Env expression
To quantify the levels of HIV 89.6 Env or GFP expression, cells were infected at the indicated MOI and 24 h later prepared for flow cytometry. For the analyses of HIV 89.6 Env expression, the cells were harvested, washed and then permeabilized with Cytofix/Cytoperm (BD Bioscience, Cat. No. 554722) at 4°C for 30 min. After washing 3 times with Perm Wash Buffer (BD Bioscience, Cat. No. 554723), the cells were incubated with a 1:1000 dilution of T8 MAb at 4°C for 30 min. MAb T8, which reacts with the C1 region of most subtype B gp120s (Earl et al., 1994), was a kind gift from Dr. P. Earl. The cells were washed as above and then incubated with 1:200 diluted phycoerythrin (PE)-conjugated anti-mouse IgG at 4°C for 30 min. The cells were then washed, fixed with 1% formalin and analyzed on a FACSCalibur flow cytometer (BD Biosciences). Data was acquired with CellQuest software and analyzed with FlowJo version 8.3.3 software. For analysis of GFP expression, the cells were harvested with trypsin/EDTA, washed with PBS 2 times, and directly analyzed as described above.
2.5. Fluorescence analysis of GFP reporter gene expression
GFP expression was measured in live 293-AD and 293-IQ cells after being mock-infected or infected with Ad5GFP or Ad5lacGFP (MOI = 1) in 24-well plates. Forty-two h later, the cell monolayers were washed 2 times with PBS and then GFP expression was directly measured on a Synergy™ HT Multi-Detection Microplate Reader and quantified using KCJunior software version 1.3 (BioTek, Winooski, VT, USA). For some experiments 10 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) was added to the culture media 4 h before measuring GFP expression.
2.6. Microscopy
Bright field and epifluorescence microscopy was performed using a Nikon TE-200 inverted epifluorescence microscope equipped with bright field and fluorescence optics. Image capture and acquisition was performed with Spot version 4.5 software (Diagnostic Instruments, Inc.).
3. Results
3.1. Generation of a Lac-regulated Ad shuttle plasmid
Our overriding goal is to produce HIV-1 vaccine vectors that express biologically relevant antigens that will elicit protective humoral and cellular-mediated immune responses. In the process of developing Ad vaccine vectors that express codon-optimized, full-length HIV-1 env sequences, many of these vectors were difficult or impossible to rescue. Similar to others that have reported problems in rescuing Ad vectors that express toxic genes (Bruder et al., 2000; Edholm et al., 2001; Matthews et al., 1999), we reasoned that high-level expression due to strong, constitutive CMV promoter activity and codon-optimization of the full-length HIV genes was extremely cytotoxic to the producer 293-AD cell line, which precluded virus rescue. We hypothesized that regulated expression of the cytolytic transgene product in the producer cell line would allow more efficient rescue of recombinant Ad vectors. To test this hypothesis, we made a new shuttle plasmid for Ad generation that incorporates the lac operator sequence. Figure 1 diagrams the steps involved in constructing the new shuttle plasmid. The final plasmid, pShuttleB316M, contains all the necessary elements for pAdEasy-based bacterial homologous recombination. Genes of interest inserted in the multiple cloning site (MCS) are under the transcriptional control of the murine CMV (mCMV) immediate early (IE) promoter that has activity in both human and murine cells (Addison et al., 1997). A lac operon regulator sequence is located between the mCMV IE promoter and the start codon of the gene of interest, which down regulates transcription in the presence of the Lac repressor. Subsequent to generating this shuttle plasmid we cloned either the jellyfish Aequorea Victoria green fluorescent protein (GFP) or HIV-1 89.6 env gene as described in Methods.
3.2. Reporter gene expression is repressed in recombinant Ad5 vectors containing the Lac operator
Next, pShuttleB316M-GFP was used to rescue an Ad vector (called Ad5lacGFP) that expresses GFP. To determine if GFP reporter gene expression in the Ad vector was down-regulated in the presence of the Lac repressor, 293-AD and 293-IQ cells were infected with Ad5lacGFP. The 293-IQ cell line, which was derived from HEK293 cells, constitutively expresses the Lac repressor (Matthews et al., 1999). Visual observation by fluorescence microscopy showed a clear difference of GPF expression in the two cell lines (Fig. 2A). Quantification of expression demonstrated an ~95% reduction in GFP fluorescence in the 293-IQ cell line compared to the 293-AD cell line (Fig. 2B). Furthermore, the suppression of GFP expression was relieved when 10 mM IPTG was added to the cells 24 h before infection (Fig. 2C). IPTG is a common molecular mimic of allolactose that triggers transcription of the lac operator by binding and inhibiting the lac repressor. This latter finding indicates that the reduction in transgene expression is Lac repressor-mediated, since pretreatment with IPTG did not induce GFP expression in the 293-AD cells or in cells infected with a GFP-expressing Ad vector that did not have a lac operator element (i.e. Ad5GFP). Overall, these results indicate that Ad transgene expression can be regulated effectively in the Lac repressor-expressing 293-IQ cell line used for the rescue and propagation of Ad vectors.
Figure 2. Reporter gene expression is efficiency repressed in Lac-regulated Ad vectors.

293-AD and 293-IQ cells were infected with Ad5lacGFP (MOI = 1) that contains the Lac operator element. (A) Twenty-four h later the cells were visualized by light (left) and fluorescence (right) microscopy. Representative photomicrographs are shown at 20X. (B) GFP expression was measured in the infected cells in Panel A on a Synergy™ HT Multi-Detection Microplate Reader and quantified using KCJunior software version XXX (BioTek, Winooski, VT, USA). The graph shows the average amount of GFP expression from 105 cells. Error bars show the standard deviation from triplicate samples. (C) IPTG induces expression of Lac-regulated gene expression. IPTG (10 mM) was added to the culture media prior to the infection of 293-AD and 293-IQ cells with Ad5GFP or Ad5lacGFP, and then ~24 h later GFP expression was measured as in B. The percentage of IPTG-induced GFP expression for each virus/cell line combination was normalized to the uninduced (no IPTG) virus/cell line combination. The graph shows the average of quadruplicate samples and error bars represent the standard deviation.
3.3. Ad5 replication is substantially reduced by the Lac-regulated system
To characterize further the Lac-regulated Ad vector system in the absence of a cytotoxic transgene, de novo virus production was compared in 293-AD or 293-IQ cell lines infected with standard Ad5 and Lac-regulated Ad5 vectors (Fig. 3). The titer of the standard Ad5 vector (Ad5GFP) was 5-fold higher in the 293-AD cells compared to the 293-IQ cells. Moreover, there was an even greater difference (~80-fold) in the replication of the Lac-regulated Ad vector (Ad5lacGFP) in the two cells lines. The highest yielding combination was the Ad5GFP virus grown in 293-AD cells, which was ~1000-fold greater than the yield of the Lac-regulated Ad5lacGFP virus grown in 293-IQ cells. These results indicate that the 293-IQ cells, although originally derived from HEK 293 cells, may have lost some capacity for production of Ad5 viruses. Furthermore, in addition to repressing transgene expression, the lac operator element severely attenuates virus replication in the 293-IQ cells. On the basis of this later finding and similar results with the HIV-1 env-expressing Ad vector (below), it was decided to rescue the Lac-regulated vectors on 293-IQ cells and then expand subsequently the vectors on 293-AD cells.
Figure 3. Lac-regulated Ad vectors amplify more efficiently on 293-AD compared to 293-IQ cells.

293-AD and 293-IQ cells were infected (MOI = 1) with standard Ad5 or lac-regulated Ad5 vectors that express GFP. The de novo virus in the cell and medium fractions were pooled and the titers (pfu/ml) were determined using a TCID50 assay.
3.4. Repression of HIV-1 env gene expression facilitates efficient recombinant Ad rescue
Next we determined if suppression of cytotoxic transgene expression allowed for more efficient de novo rescue of Ad vectors that express the HIV-1 env gene. We have previously rescued Ad vectors that express truncated, codon-optimized HIV-1 Env, but not full-length versions of the same gene. This difference is likely due to the intrinsic toxicity of the cytoplasmic C-terminal domain of the Env protein. Therefore, a full-length, codon-optimized 89.6 HIV-1 env gene was cloned into the pShuttleB316M vector followed by homologous recombination with pAdEasy-1 in E. coli. The resulting recombinant Ad plasmid, pAd5lacEnv, or a control Ad plasmid that expresses the gfp gene (above) was then transfected into 293-AD or 293-IQ cells. Ten days later the amounts of Ad viruses produced from both cell lines was quantified using a TCID50 assay (Fig. 4). Both 293-AD and 293-IQ cell lines rescued GFP-expressing Ad vectors at comparable levels (left two panels). However, as expected based on multiple previous attempts, the 293-AD cells rescued no detectable levels of Env-expressing Ad vectors (top right panel). Conversely, Env-expressing Ad vectors were rescued from the 293-IQ cells (bottom right panel), with an average yield of 7.1 × 103 plaque forming units per 1.0 μg plasmid DNA transfected. These results demonstrated that the utility of the Lac-regulated system is most pronounced when rescuing Ad vectors that express cytotoxic gene projects like HIV env, since apparently non-toxic genes such as gfp are rescued from both cell lines.
Figure 4. An Ad vector that expresses a cytopathic HIV-1 env gene are efficiently rescued on 293-IQ cells and propagated more efficiently on 293-AD cells.
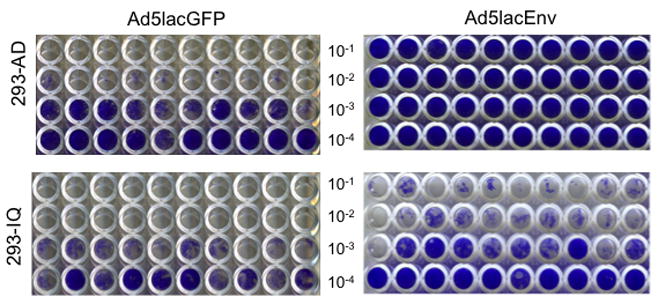
The plasmid pShuttleB316M containing either gfp or full-length, codon-optimized HIV-1 89.6 env genes was recombined with pAdEasy-1 in E. coli BJ5183. Positive recombinants were transfected into 293-AD or 293-IQ cells and then 14 days later the medium/cell fractions were collected and subject to 3 freeze-thaw cycles. The titers of rescued virus in the medium/cell lysates were then determined using a standardized 96-well TCID50 assay on 293-AD cells. Ten days later the titers were calculated and the wells were stained with crystal violet and photographed. Clear and partially blue wells indicate cytopathic effect due to virus replication; whereas solid blue wells indicate that no virus was rescued, resulting in no cytopathic effect or virus replication.
3.5. Ad-mediated Env gene is efficiently expressed in the absence of Lac repression
As mentioned above we have previously attempted to generate Ad vectors that express full-length, codon-optimized env genes, however the majority of the time we were unable to rescue the virus. On other attempts Ad vectors were successfully rescued, however we later discovered that HIV env gene expression was inactivated by deletion of all or part of the open reading frame. We therefore tested our newly generated lac-regulated env-expressing Ad vectors for Env expression. Both 293-AD and 293-IQ cells were infected (MOI = 0.5, 2.0 or 5.0) with the Ad5lacEnv virus or a negative-control Ad5Luc virus that expresses luciferase. Twenty-four h later the cells were labeled intracellularly with anti-Env antibody and analyzed by flow cytometry (Figure 5). The 293-AD cells showed Env expression at all the MOIs tested. In contrast, there was efficient down-regulation of Env expression in the 293-IQ cells at the two lower MOIs (0.5 and 2.0); however this down-regulation of Env expression in the 293-IQ could be titrated away using a higher MOI (i.e, 5.0). Calculation of the MFIs (i.e., experimental virus MFI minus control virus MFI) showed repression of Env expression in the 293-IQ cells relative to the 293-AD cells of approximately 10-, 13- and 7-fold at 0.5, 2.0 and 5.0 MOIs, respectively (Table 1). In summary, these results demonstrated that the lac-regulated Ad vector that we rescued does express HIV-1 Env in the absence of regulation. Moreover, Env expression was repressed in the 293-IQ cells, however this repression could be overcome by using higher MOIs. Most importantly, HIV-1 Env is efficiently expressed in the absence of the Lac repressor.
Figure 5. Lac-regulated Ad vectors express the full-length, codon-optimized HIV-1 89.6 env gene.
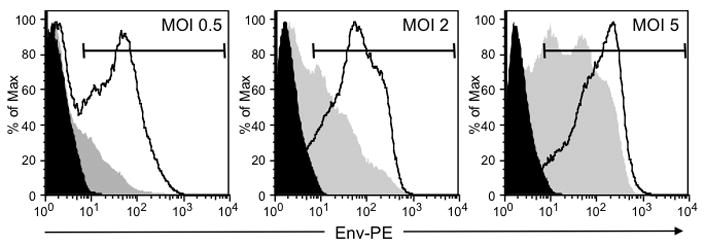
293-AD and 293-IQ cells were infected (MOI = 0.5, 2.0 or 5.0) with Ad5lacEnv or negative-control Ad5Luc that expresses luciferase. Twenty-four h later, the cells were harvested and labeled intracellularly with the anti-Env monoclonal antibody T8 and PE-conjugated anti-mouse IgG. The labeled cells were then washed, fixed with 1% formalin and analyzed on a FACSCalibur flow cytometer (BD Biosciences). Data was acquired with CellQuest software and analyzed with FlowJo version 8.3.3 software. Representative histograms from 3 separate experiments show 293-AD cells infected with Ad5Luc (solid black), 293-AD cells infected with Ad5lacEnv (black lines) and 293-IQ cells infected with Ad5lacEnv (solid gray). The markers show the gates used to quantify the MFI (see Table 1) for each sample.
Table 1.
Expression levels of HIV-1 Env in 293-AD and 293-IQ cell lines at different MOIs
| Sample | MOI1 | MFI2 |
|---|---|---|
| 293-AD + Ad5Luc | 0.5 | 1.6 |
| 293-IQ + AdlacEnv | 0.5 | 2.3 |
| 293-AD + AdlacEnv | 0.5 | 12.0 |
|
| ||
| 293-AD + Ad5Luc | 2 | 1.7 |
| 293-IQ + AdlacEnv | 2 | 6.4 |
| 293-AD + AdlacEnv | 2 | 77.6 |
|
| ||
| 293-AD + Ad5Luc | 5 | 1.9 |
| 293-IQ + AdlacEnv | 5 | 15.0 |
| 293-AD + AdlacEnv | 5 | 97.6 |
MOI = multiplicity of infection
MFI = mean fluorescence intensity.
3.6. Tropism-modified Ad vectors can be rescued with the Lac-regulated system
An underlying purpose for developing this particular Ad vector system was to use previously developed modified pAdEasy-based reagents. One such example is a pAdEasy-based plasmid, called pAd5/3Easy, which has the Ad5 knob region replaced with that of the adenovirus type 3 (Ad3). Ad vectors made with the plasmid pAd5/3Easy have tropism for the Ad3 receptor, resulting in enhanced infectivity on a variety of cell lines and primary cell types (Kanerva et al., 2002; Kawakami et al., 2003; Komarova et al., 2006; Tekant et al., 2005). To test the utility of our lac-regulated plasmid for generating tropism-modified Ad vectors, we performed homologous recombination with pShuttleB316M-Env and pAd5/3Easy. The resultant virus that was rescued, Ad5/3lacEnv, was then analyzed for HIV-1 Env expression by flow cytometry. We observed comparable levels of Env expression between Ad5lacEnv and Ad5/3lacEnv on 293-AD, HeLa and MDA-231 cells (Figure 6, top three panels; Table 2). However, on DU 145, FaDu and SKOV-3 cells we observed low levels of Env expression by the Ad5lacEnv vector; whereas high levels of Env expression was detected with Ad5/3lacEnv in these cell lines (Figure 6, bottom three panels; Table 2). One likely explanation for the differences of infection efficiency between the two Ad vectors is due to the relative levels of expression of the native Ad5 and Ad3 receptors (Short et al., 2006; Sirena et al., 2004) on the different cell lines. Importantly, these results demonstrate that our lac-regulated system is applicable to the development of tropism-modified Ad vector, which have the potential of infecting a broader range of cells types (compared to Ad5). In context of HIV-1 vaccine development, tropism-modified Ad vectors may also be valuable as stand-alone vaccine modalities or in combination with other vaccine vectors
Figure 6. Lac-regulated chimeric Ad5 and Ad5/3 vectors express HIV-1 Env in a range of cell types representing different tissues.
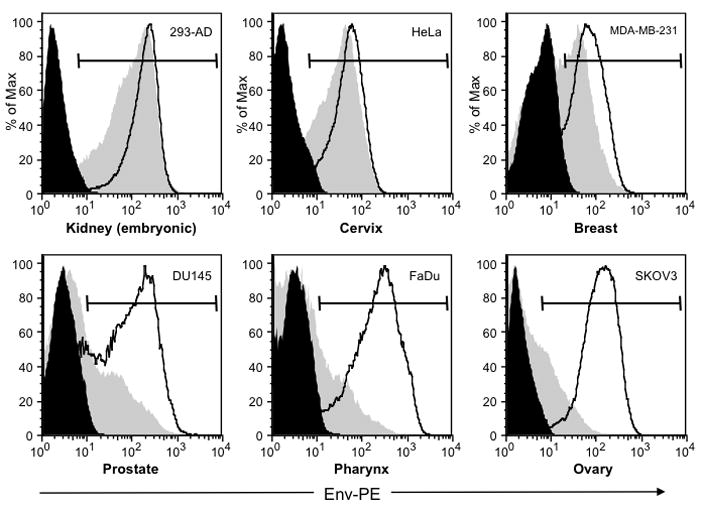
A panel of cell lines representing 6 different human tissues were infected with Ad5/3lac Env, Ad5lacEnv or Ad5Luc (MOI = 5). Twenty-four h later, the cells were labeled intracellularly with the anti-Env monoclonal antibody T8 and PE-conjugated anti-mouse IgG. The labeled cells were then washed, fixed with 1% formalin and analyzed by flow cytometry as described in Figure 5. Representative histograms from 3 separate experiments show cells infected with Ad5Luc (solid black), Ad5/3lacEnv (solid gray) and Ad5lacEnv (black lines). The markers show the gates used to quantify the MFI (see Table 2) for each sample.
Table 2.
Expression levels of HIV-1 Env in cell lines from different tissue origins
| Tissue origin (cell line) | Virus | MFI1 | Fold increase2 |
|---|---|---|---|
| Embryonic kidney (293-AD) | Ad5 | 97.6 | - |
| Ad5/3 | 200 | 2 | |
|
| |||
| Cervical (HeLa) | Ad5 | 23 | - |
| Ad5/3 | 47.5 | 2 | |
|
| |||
| Breast (MDA-MB-231) | Ad5 | 13.5 | - |
| Ad5/3 | 47.6 | 3.5 | |
|
| |||
| Prostate (DU 145) | Ad5 | 4.7 | - |
| Ad5/3 | 54 | 11.5 | |
|
| |||
| Pharynx (FaDu) | Ad5 | 2.4 | - |
| Ad5/3 | 147 | 61 | |
|
| |||
| Ovary (SKOV-3) | Ad5 | 2.4 | - |
| Ad5/3 | 113 | 47 | |
Based on gated data in Figure 6
Ad5/3 MFI divided by Ad5 MFI
4. Discussion
As one of the great paradigms of gene expression and whose finding later led to a Nobel Prize, the lac operon was the first genetic regulatory mechanism to be characterized (Jacob and Monod, 1961) and has since been adapted to numerous applications. In this study we employed the lac repressor-operator for generating Ad vectors with regulated transgene expression. In doing so we were able to rescue Ad vectors that express high levels of the full-length HIV-1 89.6 env gene, which could not be rescued previously using a conventional Ad vector system. It is important to note that two other Ad vector systems have been reported that utilize the lac repressor-operator model: Edholm et. al. (Edholm et al., 2001) employed the lac repressor-operator model to reduce the basal activity of progesterone-antagonist induced promoters and similarly Matthews et. al. (Edholm et al., 2001; Matthews et al., 1999) used it to modulate murine CMV promoter activity. Compared to these two systems, one major difference is that our system uses bacterial homologous recombination (He et al., 1998) rather than recombination in mammalian cells (e.g., HEK293 or 911 cells) to generate recombinant Ad vectors. The disadvantages of homologous recombination in mammalian cells include (i) low efficiency, (ii) the need for repeated rounds of plaque purification, (ii) and the long time period required for generation of virus stocks. As a result, the bacterial recombination method has become increasingly used and preferred by many adenoviral investigators. Moreover, an important byproduct of bacterial homologous recombination is the generation of a plasmid intermediate of the recombinant Ad that serves as a convenient and replenishable source of the recombinant viral vector. Even more importantly, this plasmid intermediate can be used for further molecular remodeling of the Ad genome. One example of the use of these intermediate recombinant Ad plasmids is in the development of the chimeric Ad5/3 used in this study.
Although the lac-regulated system was necessary for the rescue of the env-expressing Ad5 vectors (Figure 4), it is interesting that the subsequent expansion of Ad5lacEnv did not require regulated expression. In fact the expansion of lac-regulated Ad vectors was severely attenuated when propagated on the 293-IQ cells compared to the 293-AD cells (Figure 3 and data not shown). This was an unanticipated finding since we expected the Env-expressing vector to replicate poorly in the 293-AD cells -consistent with the inability to rescue in that cell line. An explanation for the differences in rescue efficiency versus propagation efficiency is not obvious. It is possible that the rescue of Ad5 vectors, which is preceded by recombinant plasmid DNA transfection, involves steps or efficiencies that are different than those during a recombinant Ad5 infection. For example, in a comparison of Lipofectamine- and Ad5-mediated transfection (Hama et al., 2006), it has been reported that largest difference between the two delivery methods occurs at the level of nuclear transcription efficiency and not during cellular uptake or intracellular distribution/trafficking. Further investigation is needed to fully understand the underlying mechanism(s) that attribute to the observed differences. We also observed that repression of env expression was overcome by increasing MOIs on the 293-IQ cells (Figure 5). This is probably due to saturation of the available Lac repressor molecules by increased Ad genome copies. Although saturation of the repressor protein did not preclude virus rescue, it is possible that tighter control under a wider range of genome copy numbers would be favorable. In this regard, we have begun to investigate the use of humanized Lac repressor and/or synthetic lac operator elements in the rescue cell line.
As mentioned above, we also used our lac-regulated Ad system to generate a chimeric, tropism-modified vector (i.e, Ad5/3). As shown in this study (Figure 6) and elsewhere, such tropism-modified Ad vectors can be designed to have higher infectivity and broader tissue tropism compared to standard Ad5 vectors (Blackwell et al., 2000; Blackwell et al., 1999; Kawakami et al., 2003; Pereboev et al., 2004). Although the mechanism for the higher efficiency of the infection of the cells with the chimeric Ad5/3 vector shown in Figure 6 is not completely clear; one possibility is that they express higher levels of the Ad3 receptor, CD46 (Sirena et al., 2004), which is a membrane cofactor protein expressed on nucleated cells. A flow cytometric analysis demonstrated that the cells used in this study do in fact express intermediate-to-high levels of the CD46 receptor (data not shown). We have previously shown that the kinetics of an Ad5/3 infection is faster than that of Ad5, which is partly defined by the receptor binding step as well as later steps in the virus replication cycle (Kawakami et al., 2003). In addition to its ability to infect a broader range of cells with higher efficiency, the chimeric Ad5/3 is also highly resistant to Ad5 NAbs (data not shown). This combination of features could make the chimeric Ad5/3 a good stand-alone vaccine vector and/or useful in heterologous prime-boost vaccination regimens. Our lab is currently investigating how differences in Ad5 and Ad5/3 infections could be exploited in the context of a vaccine. Some of the potentially important consequences of modified Ad vectors include the ability to use lower, safer doses to achieve comparable vaccination efficiency and the ability to escape pre-existing Ad5 immunity in the vaccinee populations. In addition, vector modifications can be engineered to restrict tropism in situations where infection of specific cellular targets is desirable (Hidaka et al.). For example, we have shown previously that murine dendritic cells, which are inefficiently infected by Ad5, are highly infectable with an Ad5 vector that targets the CD40 receptor (Pereboev et al., 2002; Pereboev et al., 2004). Using a skin explant model, de Gruifl el. al (de Gruijl et al., 2002) went on to demonstrate that ~44% of the cells transduced by the CD40-targeted Ad vector were CD1a-positive DCs and/or Langerhans cells, whereas >99% of the cells transduced by the untargeted Ad5 vectors were CD1a negative.
HIV-1 Env is the primary target for Nabs. However, due to the high degree of sequence variability in Env, conformational masking, and glycan shielding (Kwong et al., 2002; Wei et al., 2003) Wei, Kwong/Wyatt only a handful of NAbs have been described that have relatively broad neutralizing activity (Binley et al., 2004; Burton et al., 2004). In an effort to design immunogens for eliciting Env Nabs, we observed that full-length Env-expressing Ad vaccine vectors were problematic to consistently rescue. Although one option was to use COOH-terminally truncated Env immunogens, a compelling argument can be made that an effective vaccine will need to mimic the antigenic structure of the oligomeric Env complex found on the HIV-1 particles, which could be influenced by sequences in gp41 (Blish et al., 2008; Edwards et al., 2002). In this regard, a number of studies suggest that the conformation of the HIV-1 antigens used to elicit vaccine responses will play a significant role in their ability to elicit Nab with broad activity. For example, one recent study used a conformationally intact clade B wild-type gp120 or denatured gp120 protein to absorb NAbs present in HIV-1 sera (Li et al., 2007). Adsorption with the wild-type gp120, but not denatured gp120, removed neutralizing activity against clade A, B and C viruses, which mapped to a conformational epitope of the CD4-binding site. These results highlight the importance of having robust vaccine vectors systems that can facilitate efficient screening of multiple full-length Env immunogens, which was the basis developing the Lac-regulated Ad vector system described here. We are presently using this Ad vector system to immunize animals with panels of newly transmitted subtype C Envs and those from the chronically infected index case to define the determinants of immunogenicity in the context of the full-length, authentic Env structure. Thus, the regulated Ad system, combined with novel Env immunogens, could lead to the discovery of new avenues of inducing a broad NAb response through vaccination.
Acknowledgments
This research was supported by the Immunology and Developmental Cores of the Emory Center for AIDS Research (P30 AI050409), the Yerkes National Primate Research Center base grant (RR-00165) and NIH NIAID grants (R01AI069987 and R21AI076080).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addison CL, Hitt M, Kunsken D, Graham FL. Comparison of the human versus murine cytomegalovirus immediate early gene promoters for transgene expression by adenoviral vectors. J Gen Virol. 1997;78 (Pt 7):1653–61. doi: 10.1099/0022-1317-78-7-1653. [DOI] [PubMed] [Google Scholar]
- Andreau K, Perfettini JL, Castedo M, Metivier D, Scott V, Pierron G, Kroemer G. Contagious apoptosis facilitated by the HIV-1 envelope: fusion-induced cell-to-cell transmission of a lethal signal. J Cell Sci. 2004;117:5643–53. doi: 10.1242/jcs.01486. [DOI] [PubMed] [Google Scholar]
- Aoki K, Akyurek LM, San H, Leung K, Parmacek MS, Nabel EG, Nabel GJ. Restricted expression of an adenoviral vector encoding Fas ligand (CD95L) enhances safety for cancer gene therapy. Mol Ther. 2000;1:555–65. doi: 10.1006/mthe.2000.0076. [DOI] [PubMed] [Google Scholar]
- Barouch DH, Nabel GJ. Adenovirus vector-based vaccines for human immunodeficiency virus type 1. Hum Gene Ther. 2005;16:149–56. doi: 10.1089/hum.2005.16.149. [DOI] [PubMed] [Google Scholar]
- Barouch DH, Pau MG, Custers JH, Koudstaal W, Kostense S, Havenga MJ, Truitt DM, Sumida SM, Kishko MG, Arthur JC, Korioth-Schmitz B, Newberg MH, Gorgone DA, Lifton MA, Panicali DL, Nabel GJ, Letvin NL, Goudsmit J. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol. 2004;172:6290–7. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- Binley JM, Wrin T, Korber B, Zwick MB, Wang M, Chappey C, Stiegler G, Kunert R, Zolla-Pazner S, Katinger H, Petropoulos CJ, Burton DR. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J Virol. 2004;78:13232–52. doi: 10.1128/JVI.78.23.13232-13252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell JL, Li H, Gomez-Navarro J, Dmitriev I, Krasnykh V, Richter CA, Shaw DR, Alvarez RD, Curiel DT, Strong TV. Using a tropism-modified adenoviral vector to circumvent inhibitory factors in ascites fluid. Hum Gene Ther. 2000;11:1657–69. doi: 10.1089/10430340050111313. [DOI] [PubMed] [Google Scholar]
- Blackwell JL, Miller CR, Douglas JT, Li H, Reynolds PN, Carroll WR, Peters GE, Strong TV, Curiel DT. Retargeting to EGFR enhances adenovirus infection efficiency of squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 1999;125:856–63. doi: 10.1001/archotol.125.8.856. [DOI] [PubMed] [Google Scholar]
- Blish CA, Nguyen MA, Overbaugh J. Enhancing exposure of HIV-1 neutralization epitopes through mutations in gp41. PLoS Med. 2008;5:e9. doi: 10.1371/journal.pmed.0050009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braciak TA, Gallichan WS, Graham FL, Richards CD, Ramsay AJ, Rosenthal KL, Gauldie J. Recombinant adenovirus vectors expressing interleukin-5 and -6 specifically enhance mucosal immunoglobulin A responses in the lung. Immunology. 2000;101:388–96. doi: 10.1046/j.1365-2567.2000.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder JT, Appiah A, Kirkman WM, 3rd, Chen P, Tian J, Reddy D, Brough DE, Lizonova A, Kovesdi I. Improved production of adenovirus vectors expressing apoptotic transgenes. Hum Gene Ther. 2000;11:139–49. doi: 10.1089/10430340050016229. [DOI] [PubMed] [Google Scholar]
- Bukczynski J, Wen T, Ellefsen K, Gauldie J, Watts TH. Costimulatory ligand 4-1BBL (CD137L) as an efficient adjuvant for human antiviral cytotoxic T cell responses. Proc Natl Acad Sci U S A. 2004;101:1291–6. doi: 10.1073/pnas.0306567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, Moore JP, Nabel GJ, Sodroski J, Wilson IA, Wyatt RT. HIV vaccine design and the neutralizing antibody problem. Nat Immunol. 2004;5:233–6. doi: 10.1038/ni0304-233. [DOI] [PubMed] [Google Scholar]
- Catanzaro AT, Koup RA, Roederer M, Bailer RT, Enama ME, Moodie Z, Gu L, Martin JE, Novik L, Chakrabarti BK, Butman BT, Gall JG, King CR, Andrews CA, Sheets R, Gomez PL, Mascola JR, Nabel GJ, Graham BS. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J Infect Dis. 2006;194:1638–49. doi: 10.1086/509258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti BK, Kong WP, Wu BY, Yang ZY, Friborg J, Ling X, King SR, Montefiori DC, Nabel GJ. Modifications of the human immunodeficiency virus envelope glycoprotein enhance immunogenicity for genetic immunization. J Virol. 2002;76:5357–68. doi: 10.1128/JVI.76.11.5357-5368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicala C, Arthos J, Rubbert A, Selig S, Wildt K, Cohen OJ, Fauci AS. HIV-1 envelope induces activation of caspase-3 and cleavage of focal adhesion kinase in primary human CD4(+) T cells. Proc Natl Acad Sci U S A. 2000;97:1178–83. doi: 10.1073/pnas.97.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gruijl TD, Luykx-de Bakker SA, Tillman BW, van den Eertwegh AJ, Buter J, Lougheed SM, van der Bij GJ, Safer AM, Haisma HJ, Curiel DT, Scheper RJ, Pinedo HM, Gerritsen WR. Prolonged maturation and enhanced transduction of dendritic cells migrated from human skin explants after in situ delivery of CD40-targeted adenoviral vectors. J Immunol. 2002;169:5322–31. doi: 10.4049/jimmunol.169.9.5322. [DOI] [PubMed] [Google Scholar]
- de Souza AP, Haut LH, Silva R, Ferreira SI, Zanetti CR, Ertl HC, Pinto AR. Genital CD8(+) T cell response to HIV-1 gag in mice immunized by mucosal routes with a recombinant simian adenovirus. Vaccine. 2006 doi: 10.1016/j.vaccine.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Devitt G, Emerson V, Holtkotte D, Pfeiffer T, Pisch T, Bosch V. Incorporation of chimeric HIV-SIV-Env and modified HIV-Env proteins into HIV pseudovirions. Virology. 2007;361:465–71. doi: 10.1016/j.virol.2006.11.029. [DOI] [PubMed] [Google Scholar]
- Douglas JT, Rogers BE, Rosenfeld ME, Michael SI, Feng M, Curiel DT. Targeted gene delivery by tropism-modified adenoviral vectors. Nat Biotechnol. 1996;14:1574–8. doi: 10.1038/nbt1196-1574. [DOI] [PubMed] [Google Scholar]
- Earl PL, Broder CC, Long D, Lee SA, Peterson J, Chakrabarti S, Doms RW, Moss B. Native oligomeric human immunodeficiency virus type 1 envelope glycoprotein elicits diverse monoclonal antibody reactivities. J Virol. 1994;68:3015–26. doi: 10.1128/jvi.68.5.3015-3026.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edholm D, Molin M, Bajak E, Akusjarvi G. Adenovirus vector designed for expression of toxic proteins. J Virol. 2001;75:9579–84. doi: 10.1128/JVI.75.20.9579-9584.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards TG, Wyss S, Reeves JD, Zolla-Pazner S, Hoxie JA, Doms RW, Baribaud F. Truncation of the cytoplasmic domain induces exposure of conserved regions in the ectodomain of human immunodeficiency virus type 1 envelope protein. J Virol. 2002;76:2683–91. doi: 10.1128/JVI.76.6.2683-2691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JC, Gao GP, Reyes-Sandoval A, Pavlakis GN, Xiang ZQ, Wlazlo AP, Giles-Davis W, Wilson JM, Ertl HC. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J Immunol. 2003;170:1416–22. doi: 10.4049/jimmunol.170.3.1416. [DOI] [PubMed] [Google Scholar]
- Ghosh SS, Gopinath P, Ramesh A. Adenoviral vectors: a promising tool for gene therapy. Appl Biochem Biotechnol. 2006;133:9–29. doi: 10.1385/abab:133:1:9. [DOI] [PubMed] [Google Scholar]
- Glasgow JN, Everts M, Curiel DT. Transductional targeting of adenovirus vectors for gene therapy. Cancer Gene Ther. 2006;13:830–44. doi: 10.1038/sj.cgt.7700928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F, Prevec L. Manipulation of adenovirus vectors. In: Murray EJ, Walker JM, editors. Molecular Biology, Gene Transfer and Expression Techniques. Vol. 7. Humana Press; Clinton, NJ: 1991. pp. 109–128. [DOI] [PubMed] [Google Scholar]
- Grossman Z, Meier-Schellersheim M, Sousa AE, Victorino RM, Paul WE. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat Med. 2002;8:319–23. doi: 10.1038/nm0402-319. [DOI] [PubMed] [Google Scholar]
- Hama S, Akita H, Ito R, Mizuguchi H, Hayakawa T, Harashima H. Quantitative comparison of intracellular trafficking and nuclear transcription between adenoviral and lipoplex systems. Mol Ther. 2006;13:786–94. doi: 10.1016/j.ymthe.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Mattson MP. Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins Tat and gp120. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S55–61. doi: 10.1097/00126334-200210012-00005. [DOI] [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–14. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka Y, Hayashi Y, Fisfalen ME, Suzuki S, Takeda T, Refetoff S, DeGroot LJ. Expression of thyroid peroxidase in EBV-transformed B cell lines using adenovirus. Thyroid. 1996;6:23–8. doi: 10.1089/thy.1996.6.23. [DOI] [PubMed] [Google Scholar]
- Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–6. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- Hu SX, Ji W, Zhou Y, Logothetis C, Xu HJ. Development of an adenovirus vector with tetracycline-regulatable human tumor necrosis factor alpha gene expression. Cancer Res. 1997;57:3339–43. [PubMed] [Google Scholar]
- Jacob F, Monod J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961;3:318–56. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Kanerva A, Mikheeva GV, Krasnykh V, Coolidge CJ, Lam JT, Mahasreshti PJ, Barker SD, Straughn M, Barnes MN, Alvarez RD, Hemminki A, Curiel DT. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin Cancer Res. 2002;8:275–80. [PubMed] [Google Scholar]
- Kasono K, Blackwell JL, Douglas JT, Dmitriev I, Strong TV, Reynolds P, Kropf DA, Carroll WR, Peters GE, Bucy RP, Curiel DT, Krasnykh V. Selective gene delivery to head and neck cancer cells via an integrin targeted adenoviral vector. Clin Cancer Res. 1999;5:2571–9. [PubMed] [Google Scholar]
- Kawakami Y, Li H, Lam J, Krasnykh V, Curiel DT, Blackwell JL. Substitution of the Adenovirus Serotype 5 Knob with a Serotype 3 Knob Enhances Multiple Steps in Virus Replication. Cancer Res. 2003;63:1262–9. [PubMed] [Google Scholar]
- Komarova S, Kawakami Y, Stoff-Khalili MA, Curiel DT, Pereboeva L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol Cancer Ther. 2006;5:755–66. doi: 10.1158/1535-7163.MCT-05-0334. [DOI] [PubMed] [Google Scholar]
- Krasnykh VN, Mikheeva GV, Douglas JT, Curiel DT. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J Virol. 1996;70:6839–46. doi: 10.1128/jvi.70.10.6839-6846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kresge KJ. AIDS Vaccine Trials: ‘06 Year In Review, VAX. Vol. 5. IAVI Report Publication; 2007. [Google Scholar]
- Kwong PD, Doyle ML, Casper DJ, Cicala C, Leavitt SA, Majeed S, Steenbeke TD, Venturi M, Chaiken I, Fung M, Katinger H, Parren PW, Robinson J, Van Ryk D, Wang L, Burton DR, Freire E, Wyatt R, Sodroski J, Hendrickson WA, Arthos J. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 2002;420:678–82. doi: 10.1038/nature01188. [DOI] [PubMed] [Google Scholar]
- Lemiale F, Haddada H, Nabel GJ, Brough DE, King CR, Gall JG. Novel adenovirus vaccine vectors based on the enteric-tropic serotype 41. Vaccine. 2007;25:2074–84. doi: 10.1016/j.vaccine.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Haviv YS, Derdeyn CA, Lam J, Coolidge C, Hunter E, Curiel DT, Blackwell JL. Human immunodeficiency virus type 1-mediated syncytium formation is compatible with adenovirus replication and facilitates efficient dispersion of viral gene products and de novo-synthesized virus particles. Hum Gene Ther. 2001;12:2159–2169. doi: 10.1089/10430340152710504. [DOI] [PubMed] [Google Scholar]
- Li Y, Migueles SA, Welcher B, Svehla K, Phogat A, Louder MK, Wu X, Shaw GM, Connors M, Wyatt RT, Mascola JR. Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat Med. 2007;13:1032–4. doi: 10.1038/nm1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XL, Yu SQ, Feng X, Wang XL, Liu HM, Zhang XM, Li HX, Zhou L, Li ZL, Zheng Y. Immunogenicity of a chimeric adenovirus type 5 vector with type 35 fiber containing HIV-1 gag in mice. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2007;21:5–7. [PubMed] [Google Scholar]
- Maizel JV, Jr, White DO, Scharff MD. The polypeptides of adenovirus. II. Soluble proteins, cores, top components and the structure of the virion. Virology. 1968;36:126–36. doi: 10.1016/0042-6822(68)90122-0. [DOI] [PubMed] [Google Scholar]
- Matthews DA, Cummings D, Evelegh C, Graham FL, Prevec L. Development and use of a 293 cell line expressing lac repressor for the rescue of recombinant adenoviruses expressing high levels of rabies virus glycoprotein. J Gen Virol. 1999;80 (Pt 2):345–53. doi: 10.1099/0022-1317-80-2-345. [DOI] [PubMed] [Google Scholar]
- McConnell MJ, Imperiale MJ. Biology of adenovirus and its use as a vector for gene therapy. Hum Gene Ther. 2004;15:1022–33. doi: 10.1089/hum.2004.15.1022. [DOI] [PubMed] [Google Scholar]
- McCoy K, Tatsis N, Korioth-Schmitz B, Lasaro MO, Hensley SE, Shih-Wen L, Li Y, Giles-Davis W, Cun A, Zhou D, Xiang Z, Letvin NL, Ertl HC. The effect of pre-existing immunity to antigens of adenovirus of the human serotype 5 on immune responses of nonhuman primates to vaccine regimens based on human or chimpanzee-derived adenovirus vectors. J Virol. 2007 doi: 10.1128/JVI.02497-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner EG, Zhang L, Jiang S, Su L. Fusion-induced apoptosis contributes to thymocyte depletion by a pathogenic human immunodeficiency virus type 1 envelope in the human thymus. J Virol. 2006;80:11019–30. doi: 10.1128/JVI.01382-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier GT, Campbell JA, Chappell JD, Stehle T, Dermody TS, Barry MA. A chimeric adenovirus vector encoding reovirus attachment protein sigma1 targets cells expressing junctional adhesion molecule 1. Proc Natl Acad Sci U S A. 2004;101:6188–93. doi: 10.1073/pnas.0400542101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal SK, McDermott MR, Johnson DC, Prevec L, Graham FL. Monitoring foreign gene expression by a human adenovirus-based vector using the firefly luciferase gene as a reporter. Virus Res. 1993;28:67–90. doi: 10.1016/0168-1702(93)90090-a. [DOI] [PubMed] [Google Scholar]
- Moffatt S, Hays J, HogenEsch H, Mittal SK. Circumvention of vector-specific neutralizing antibody response by alternating use of human and non-human adenoviruses: implications in gene therapy. Virology. 2000;272:159–67. doi: 10.1006/viro.2000.0350. [DOI] [PubMed] [Google Scholar]
- Nanda A, Lynch DM, Goudsmit J, Lemckert AA, Ewald BA, Sumida SM, Truitt DM, Abbink P, Kishko MG, Gorgone DA, Lifton MA, Shen L, Carville A, Mansfield KG, Havenga MJ, Barouch DH. Immunogenicity of recombinant fiber-chimeric adenovirus serotype 35 vector-based vaccines in mice and rhesus monkeys. J Virol. 2005;79:14161–8. doi: 10.1128/JVI.79.22.14161-14168.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereboev AV, Asiedu CK, Kawakami Y, Dong SS, Blackwell JL, Kashentseva EA, Triozzi PL, Aldrich WA, Curiel DT, Thomas JM, Dmitriev IP. Coxsackievirus-adenovirus receptor genetically fused to anti-human CD40 scFv enhances adenoviral transduction of dendritic cells. Gene Ther. 2002;9:1189–93. doi: 10.1038/sj.gt.3301767. [DOI] [PubMed] [Google Scholar]
- Pereboev AV, Nagle JM, Shakhmatov MA, Matthews QL, Kawakami Y, Curiel DT, Blackwell JL. Enhanced gene transfer to mouse dendritic cells using adenoviral vectors coated with a novel adapter molecule. Mol Ther. 2004;9:712–20. doi: 10.1016/j.ymthe.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Perfettini JL, Castedo M, Roumier T, Andreau K, Nardacci R, Piacentini M, Kroemer G. Mechanisms of apoptosis induction by the HIV-1 envelope. Cell Death Differ. 2005;12(Suppl 1):916–23. doi: 10.1038/sj.cdd.4401584. [DOI] [PubMed] [Google Scholar]
- Rogers BE, Douglas JT, Ahlem C, Buchsbaum DJ, Frincke J, Curiel DT. Use of a novel cross-linking method to modify adenovirus tropism. Gene Ther. 1997;4:1387–92. doi: 10.1038/sj.gt.3300541. [DOI] [PubMed] [Google Scholar]
- Roggero R, Robert-Hebmann V, Harrington S, Roland J, Vergne L, Jaleco S, Devaux C, Biard-Piechaczyk M. Binding of human immunodeficiency virus type 1 gp120 to CXCR4 induces mitochondrial transmembrane depolarization and cytochrome c-mediated apoptosis independently of Fas signaling. J Virol. 2001;75:7637–50. doi: 10.1128/JVI.75.16.7637-7650.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinchik S, Ding R, Qiu AJ, Zhang F, Dong J. Adenoviral vector which delivers FasL-GFP fusion protein regulated by the tet-inducible expression system. Gene Ther. 2000;7:875–85. doi: 10.1038/sj.gt.3301172. [DOI] [PubMed] [Google Scholar]
- Sekaly RP. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J Exp Med. 2008;205:7–12. doi: 10.1084/jem.20072681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short JJ, Vasu C, Holterman MJ, Curiel DT, Pereboev A. Members of adenovirus species B utilize CD80 and CD86 as cellular attachment receptors. Virus Res. 2006;122:144–53. doi: 10.1016/j.virusres.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirena D, Lilienfeld B, Eisenhut M, Kalin S, Boucke K, Beerli RR, Vogt L, Ruedl C, Bachmann MF, Greber UF, Hemmi S. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J Virol. 2004;78:4454–62. doi: 10.1128/JVI.78.9.4454-4462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodroski J, Goh WC, Rosen C, Campbell K, Haseltine WA. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. Nature. 1986;322:470–4. doi: 10.1038/322470a0. [DOI] [PubMed] [Google Scholar]
- Steinbrook R. One step forward, two steps back--will there ever be an AIDS vaccine? N Engl J Med. 2007;357:2653–5. doi: 10.1056/NEJMp0708117. [DOI] [PubMed] [Google Scholar]
- Stevenson SC, Rollence M, Marshall-Neff J, McClelland A. Selective targeting of human cells by a chimeric adenovirus vector containing a modified fiber protein. J Virol. 1997;71:4782–90. doi: 10.1128/jvi.71.6.4782-4790.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekant Y, Davydova J, Ramirez PJ, Curiel DT, Vickers SM, Yamamoto M. Oncolytic adenoviral therapy in gallbladder carcinoma. Surgery. 2005;137:527–35. doi: 10.1016/j.surg.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Thorner AR, Lemckert AA, Goudsmit J, Lynch DM, Ewald BA, Denholtz M, Havenga MJ, Barouch DH. Circumventing Vector Cross-Reactivity Enhances the Immunogenicity of Heterologous Recombinant Adenovirus Prime-Boost Vaccine Regimens. J Virol. 2006 doi: 10.1128/JVI.01749-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trushin SA, Algeciras-Schimnich A, Vlahakis SR, Bren GD, Warren S, Schnepple DJ, Badley AD. Glycoprotein 120 binding to CXCR4 causes p38-dependent primary T cell death that is facilitated by, but does not require cell-associated CD4. J Immunol. 2007;178:4846–53. doi: 10.4049/jimmunol.178.8.4846. [DOI] [PubMed] [Google Scholar]
- Vanniasinkam T, Ertl HC. Adenoviral gene delivery for HIV-1 vaccination. Curr Gene Ther. 2005;5:203–12. doi: 10.2174/1566523053544236. [DOI] [PubMed] [Google Scholar]
- Volpers C, Thirion C, Biermann V, Hussmann S, Kewes H, Dunant P, von der Mark H, Herrmann A, Kochanek S, Lochmuller H. Antibody-mediated targeting of an adenovirus vector modified to contain a synthetic immunoglobulin g-binding domain in the capsid. J Virol. 2003;77:2093–104. doi: 10.1128/JVI.77.3.2093-2104.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins SJ, Mesyanzhinov VV, Kurochkina LP, Hawkins RE. The ‘adenobody’ approach to viral targeting: specific and enhanced adenoviral gene delivery. Gene Ther. 1997;4:1004–12. doi: 10.1038/sj.gt.3300511. [DOI] [PubMed] [Google Scholar]
- Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–12. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- Wei X, Ghosh SK, Taylor ME, Johnson VA, Emini EA, Deutsch P, Lifson JD, Bonhoeffer S, Nowak MA, Hahn BH, Saag MS, Shaw GM. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–22. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- Wiethe C, Dittmar K, Doan T, Lindenmaier W, Tindle R. Enhanced effector and memory CTL responses generated by incorporation of receptor activator of NF-kappa B (RANK)/RANK ligand costimulatory molecules into dendritic cell immunogens expressing a human tumor-specific antigen. J Immunol. 2003;171:4121–30. doi: 10.4049/jimmunol.171.8.4121. [DOI] [PubMed] [Google Scholar]
- Wyatt LS, Belyakov IM, Earl PL, Berzofsky JA, Moss B. Enhanced cell surface expression, immunogenicity and genetic stability resulting from a spontaneous truncation of HIV Env expressed by a recombinant MVA. Virology. 2007 doi: 10.1016/j.virol.2007.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin KQ, Sekimoto Y, Takahashi T, Mizuguchi H, Ichino M, Yoshida A, Okuda K. Chimeric adenovirus 5/35 vector containing the clade C HIV gag gene induces a cross-reactive immune response against HIV. Vaccine. 2007;25:3809–15. doi: 10.1016/j.vaccine.2007.01.117. [DOI] [PubMed] [Google Scholar]
- Yao QZ, Kuhlmann FM, Eller R, Compans RW, Chen CY. Production and characterization of simian-human immunodeficiency virus-like particles. Aids Research and Human Retroviruses. 2000;16:227–236. doi: 10.1089/088922200309322. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Emi N, Hamada H. VSV-G-pseudotyped retroviral packaging through adenovirus-mediated inducible gene expression. Biochem Biophys Res Commun. 1997;232:379–82. doi: 10.1006/bbrc.1996.5976. [DOI] [PubMed] [Google Scholar]


