Abstract
The large (l) and small (s) isoforms of FREQUENCY (FRQ) are elements of interconnected feedback loops of the Neurospora circadian clock. The expression ratio of l-FRQ versus s-FRQ is regulated by thermosensitive splicing of an intron containing the initiation codon for l-FRQ. We show that this splicing is dependent on light and temperature and displays a circadian rhythm. Strains expressing only l-FRQ or s-FRQ support short and long temperature-compensated circadian rhythms, respectively. The thermosensitive expression ratio of FRQ isoforms influences period length in wt. Our data indicate that differential expression of FRQ isoforms is not required for temperature compensation but rather provides a means to fine-tune period length in response to ambient temperature.
Keywords: circadian clock, control of period length, Neurospora
1. Introduction
Most organisms display daily rhythms in biological functions. These are supported by circadian clocks that regulate expression of a large number of genes. Circadian clocks in eukaryotes are composed of interconnected positive and negative feedback loops operating on a transcriptional, translational and posttranslational level. Even under constant conditions (e.g. constant darkness), these feedback loops support a self-sustained endogenous oscillation that mimics an astronomical day. In nature, clocks are precisely synchronized to the 24 h period of the earth’s rotation by external cues such as light and temperature (reviewed in: [1 –5]).
The transcription factor White Collar Complex (WCC), consisting of WC-1 and WC-2 subunits [6], and FREQUENCY (FRQ) [7] are crucial elements of the circadian clock of Neurospora [4]. The WCC activates transcription of frq, which encodes the central regulator of the WCC. FRQ inhibits in a negative feedback loop the activity of the WCC by promoting its phosphorylation and in a positive loop it supports accumulation of high levels of WCC [8, 9, 10]. The conflicting functions of FRQ in the negative and positive loops are coordinated in temporal and spatial fashion [11]. In the course of a day FRQ is progressively phosphorylated at multiple sites, which triggers its degradation during the night [4, 5]. Towards the end of the night negative feedback is relieved and new frq RNA is synthesized. As a result frq RNA and FRQ protein display circadian rhythms in abundance. The FRQ rhythm promotes rhythmic inactivation and reactivation of the WCC, thus driving circadian outputs such as the synthesis of carotenoids and the formation of asexual spores (conidia) [17,18].
The frq gene encodes a large (l) and a small (s) isoform of FRQ protein, which start at codons 1 and 100 of the frq ORF, respectively [12]. Expression levels of l-FRQ increase significantly with increasing temperature [13, 14, 15], while levels of s-FRQ do not. FRQ abundance as well as the ratio of l-FRQ vs. s-FRQ are critical for a self-sustained rhythm [12–14]. frq RNA is transcribed by two promoters [15]. The long 5′-UTR of frq RNA contains several upstream open reading frames (uORFs) [13]. The uORFs, which are in non-consensus context for translation initiation, are more efficiently translated at lower temperatures resulting in trapping of scanning ribosomes and reduction of translation initiation at the frq ORF [14]. Accordingly, FRQ levels decrease with decreasing temperature. In addition, the l-FRQ vs. s-FRQ ratio is regulated by temperature dependent splicing of an intron encompassing the translation initiation site of l-FRQ [15, 14]. This intron is referred to as intron 2 in [15] and intron 6 (frq-I6) in [14] and hereafter in this report. The splice-sites of frq-I6 deviate from consensus and the intron is spliced with higher efficiency at lower temperatures [14]. Accordingly, the fraction of s-FRQ increases with decreasing temperature. Both thermosensitive mechanisms together, trapping of scanning ribosomes and splicing of frq-I6, regulate abundance and ratio of l- vs. s-FRQ in a temperature-dependent fashion.
Here we have addressed functional differences of the FRQ isoforms. We show that strains expressing either l-FRQ or s-FRQ alone display fairly well temperature-compensated circadian rhythms, so that the basis of compensation in wild type does not lie in the relative levels at which the two forms are expressed. However, s-FRQ supports a longer period than l-FRQ, and in wt strains, the free-running period decreases slightly with increasing temperature, in part reflecting the expression ratio l-FRQ vs. s-FRQ. Our data suggest that the thermosensitive l-FRQ vs. s-FRQ ratio provides a molecular means to facilitate control of period length at different temperatures.
2. Materials and methods
2.1 Strains, growth conditions and racetube assays
s-frq, l-frq and wtcont., a Δfrq strain transformed with a wt frq gene, were constructed as described previously [14]. The plasmid used for mutagenesis was pBM60. It contains the frq locus and a portion of the his-3 gene, which complements histidine deficiency of the background strain by homologuous recombination. frq-ATG1mut was created by site directed mutagenesis, changing the l-FRQ translation initiation site from AAC ATG GCG to AAT ATT GCG. In frq-I6-DM the splice donor was changed from C-GTGAGT to C-ACTAGT. The frq ORFs and part of the UTRs were sequenced prior to transformation. Construction of hvc-16 was described previously [15]. Media for liquid cultures and racetubes were prepared as described [14]. Racetube evaluation was performed using the Chrono II software (Roenneberg, LMU Munich).
2.2 RT-PCR
Quantitative real-time RT-PCR was performed as described previously [14] using the same primers and fluorescent probes.
2.3 Western blot analysis
For all experiments shown 200 μg whole cell protein extract was treated with 100 units calf intestinal phosphatase (NEB) for 1 h at 37°C. Western blots were decorated with affinity purified antibodies against the C-terminus of FRQ.
3. Results
The ratio of l-FRQ to s-FRQ increases with increasing temperature due to thermosensitive splicing of frq RNA at intron 6 (frq-I6) [15, 14]. To examine whether splicing of frq-I6 is affected by the circadian clock, RNA samples obtained from light grown tissues and from circadian time courses at 22°C, 25°C and 28°C were analyzed by quantitative RT-PCR (Fig. 1A, B). At each temperature the fraction of spliced frq-I6 oscillated in circadian fashion (Fig. 1B). The mean fractions of spliced frq-I6 were about 11%, 9% and 6,4% at 22, 25 and 28°C, respectively while the amplitude of the oscillation remains relatively constant. The highest fraction of spliced frq-I6 was present in the late subjective afternoon, i.e. approximately 4 h after levels of frq RNA have reached their circadian maximum. Thus, the fraction of spliced frq-I6 is high when frq RNA levels are declining. The data indicate that the mean splicing efficiency of frq-I6 is set by temperature, while splice isoforms accumulate in a clock dependent fashion. Interestingly, at lower temperatures even the maximal fraction of spliced frq-I6 was lower in DD than in LL (Fig. 1A and B), suggesting that the abundance of spliced frq-I6 RNA is light-dependent (see below).
Fig. 1.
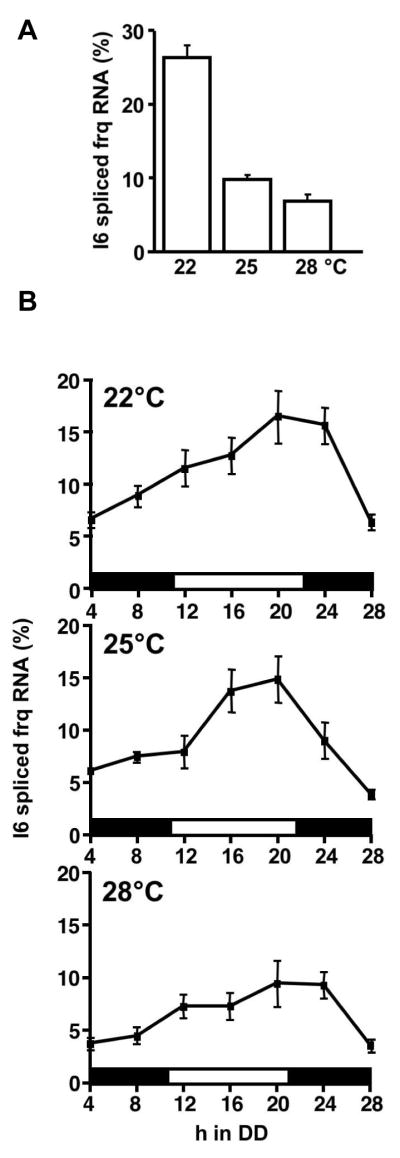
Splicing efficiency of frq-I6 is set by temperature and modulated in a circadian and temperature dependent fashion. Neurospora cultures were grown at the indicated temperatures and harvested (A) in constant light (LL) and (B) after the indicated time periods in darkness (DD). RNA was prepared and quantified by RT-PCR using fluorescent probes specific to spliced frqI6 and to total frq RNA [14]. The fraction (in %) of frq RNA spliced at I6 (spliced/total frq RNA) was plotted versus the time in DD. Black and white bars indicate subjective night and day, respectively.
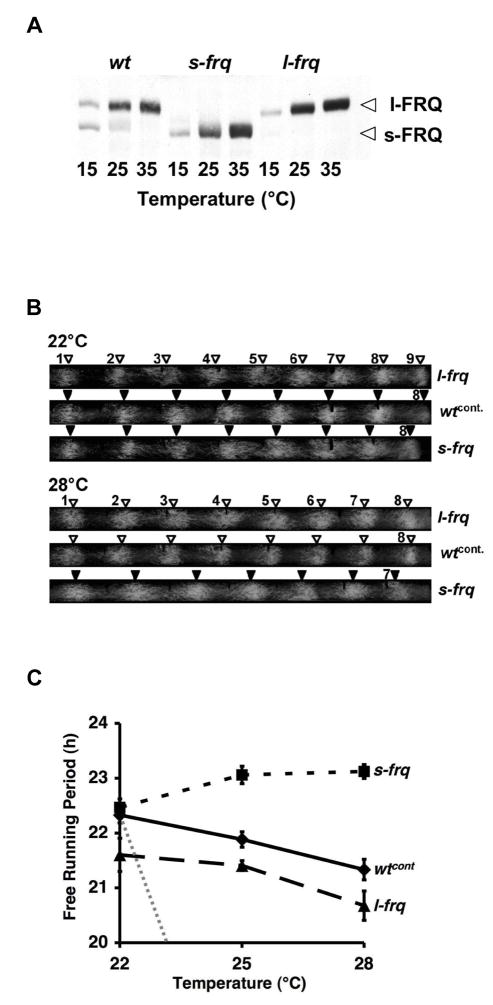
We asked whether the two FRQ isoforms differ in function. As reported previously [14], mutation of the splice sites of frq-I6 towards consensus (I6opt) and towards non-splice sites (I6mut) results in expression of s-FRQ and l-FRQ, respectively (Fig. 2A). To functionally characterize the FRQ isoforms, conidiation rhythms were analyzed under free-running conditions on race tubes at different temperatures. A comprehensive assessment revealed that rhythms in l-frq and s-frq strains are temperature-compensated with slight tendencies of s-frq and l-frq strains towards over- and undercompensation, respectively (Fig. 2B, C, Table 1). Furthermore, both strains entrain to 12 h light- 12 h dark cycles (not shown). These data indicate that thermosensitive splicing of I6 is not required for temperature compensation of the clock per se. However, the period of s-frq was at each temperature 1 to 2 h longer than the period of l-frq. Due to the large number of conidial bands evaluated (>500) the differences in period length of l-frq and s-frq at 25°C and 28°C were highly significant with p values < 4 × 10−10 in the ANOVA test (Table 1B). The period lengths of wt-strains (wt and wtcont.) were between those of s-frq and l-frq (Fig. 2B, C, Table 1A). The period decreased slightly with increasing temperature, correlating with the temperature dependent change of the expression ratio of s- vs. l-FRQ.
Fig. 2.
l-FRQ to s-FRQ determines the length of free running period. (A) Western blot analysis of protein extracts from cultures grown in LL at the indicated temperatures. Protein extracts were treated with phosphatase prior to SDS PAGE. Optimizing the splice sites of intron 6 (s-frq) results in the expression of s-FRQ while mutation of the splice sequences (l-frq) results in expression of l-FRQ. The temperature dependence of expression levels of FRQ is not affected by mutation of frq-I6. (B) Typical racetubes of s-frq, l-frq and the corresponding wt (wtcont.) at 22°C and at 28°C. Conidial bands are indicated by arrow heads and numbered. The growth rate of the strains was identical. (C) l-frq and s-frq support considerably well temperature compensated short and long period rhythms, respectively. The period lengt of wt is between that l-frq and s-frq. Data represent assessment of the temperature dependence of the free-running period of l-frq, s-frq, and wtcont. from 183 racetubes, each with an average of 5 conidial bands (see also Table 1A). The dotted line represents a hypothetical uncompensated reaction with a Q10 of 2.
Table 1.
| Table 1A Free-running periods of wt and mutant strains at the indicated temperatures. For s-frq, l-frq and wtcont the results from ≥3 independent racetube experiments are summarized. n = number of racetubes evaluated per data point. In average 5 conidial bands were evaluated per racetube. | |||||||
|---|---|---|---|---|---|---|---|
| 22°C | 25°C | 28°C | Q10 | ||||
| τ (hr) | SEM | τ (hr) | SEM | τ (hr) | SEM | ||
| wtcont. | 22.32 | ±0.14 (n=22) | 21.88 | ±0.14 (n=26) | 21.33 | ±0.19 (n=17) | 1.08 |
| wt | 22.44 | ±0.08 (n=4) | 21.88 | ±0.07 (n=4) | 21.64 | ±0.01 (n=4) | 1.06 |
| l-frq | 21.60 | ±0.30 (n=13) | 21.41 | ±0.09 (n=31) | 20.68 | ±0.27 (n=15) | 1.07 |
| s-frq | 22.47 | ±0.15 (n=20) | 23.06 | ±0.16 (n=20) | 23.12 | ±0.13 (n=19) | 0.95 |
| frq-I6-DM | 21.36 | ±0.21 (n=14) | 21.66 | ±0.09 (n=21) | 20.40 | ±0.23 (n=10) | 1.08 |
| frq- ATG1mut | 23.54 | ±0.07 (n=10) | 23.33 | ±0.27 (n=5) | 22.81 | ±0.30 (n=8) | 1.05 |
| Table 1B Significance of differences in period length between the indicated strains at different temperatures (see Fig. 2C and Table 1A). p-values of ANOVA test shown. | |||
|---|---|---|---|
| 22°C | 25°C | 28°C | |
| wtcont.: s-frq | 0.5099 | 1.5939E-06 | 2.2801E-09 |
| wtcont.: l-frq | 3.2171E-03 | 4.8717E-03 | 0.0474 |
| s-frq: l-frq | 8.5717E-03 | 5.1327E-13 | 4.0358E-10 |
| wtcont.: frq-ATG1mut | 4.8702E-06 | 2.2182E-04 | 2.4636E-04 |
| wtcont.: wt | 0.7408 | 0.9933 | 0.4420 |
| l-frq: frq-I6-DM | 0.5068 | 0.0649 | 0.4536 |
In summary, the period of strains expressing a single FRQ isoform is temperature compensated, indicating that expression of both FRQ isoforms is not required for temperature compensation per se. Rather, the temperature dependent expression ratio of l-FRQ vs. s-FRQ appears to modulate the period in wt strains in a systematic fashion and may thus serve to fine-tune the circadian oscillation in response to different ambient temperatures.
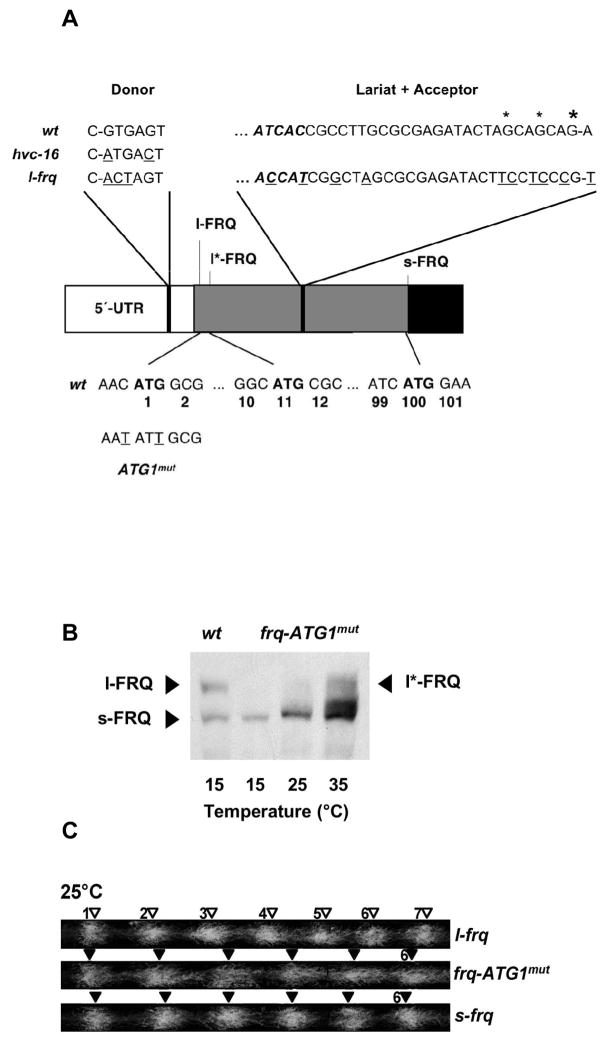
In order to generate the s-frq and l-frq strains, splice-donor, -branch (Lariat) and -acceptor sites of frq-I6 had been mutated [14]. The Lariat sequence and acceptor site of frq-I6 are located in the coding sequence of l-FRQ (Fig. 3A). Although the amino acid sequence of FRQ was not altered by the mutations, the RNA sequence contained 10 exchanges in the ORF (Fig. 3A). To address whether the period length was indeed affected by the FRQ isoforms rather than by the sequence alterations per se that were introduced to create the mutant frq alleles, we generated and analyzed additional strains that expressed only a single FRQ isoform due to different means. An additional s-FRQ expressing strain was constructed by mutating the translation initiation site of l-FRQ (Fig. 3A). Splice sites of frq-I6 were not affected in the mutant frq-ATG1mut gene. The corresponding frq-ATG1mut strain expressed predominantly s-FRQ at the three temperatures investigated (Fig. 3B). At 35°C low levels of a longer FRQ isoform, l*-FRQ, were detected, which could correspond to an inefficient translation initiation at AUG2 of the frq ORF (Fig. 3A). Since only a very minor fraction of frq-I6 is spliced above 30°C [14], the high levels of s-FRQ indicate that ribosomes were efficiently scanning through the non-consensus translation initiation site at AUG2 (Fig. 3A). frq-ATG1mut displayed a long period rhythm on racetubes, very similar to that of the s-frq strain generated by optimization of the I6-splice sites (Fig. 3C; Table 1A). In contrast to the slightly over-compensated period of s-frq, the period length of frq-ATG1mut decreased slightly with rising temperature. This effect might be due to the low levels of the l*-FRQ isoform (initiating at AUG2), which may, similar to the l-FRQ isoform, support a shorter rhythm. Thus, the data confirm that s-FRQ supports a longer free-running rhythm.
Fig. 3.
frq-ATG1mut expresses predominantly s-FRQ and supports a long period circadian rhythm. (A) Schematic outline of the translation initiation sites of FRQ isoforms and intron 6. Top: wt donor, lariat (bold italic) and acceptor sites are shown above the outline. The large asterisk indicates the sequenced acceptor site, the small asterisks indicate putative cryptic acceptors. Corresponding sequences mutated in hvc-16 and l-frq are also shown, sequence alterations are underlined. Bottom: Sequence context around the initiation sites of l-FRQ, l*-FRQ and s-FRQ. Numbers below the sequence refer to codons of l-FRQ. The initiation ATGs of l*-FRQ and s-FRQ correspond to codons 11 and 100 of l-FRQ, respectively. The sequence alterations introduced in the frq-ATG1mut allele are shown.
(B) Western blot analysis of FRQ levels and isoforms expressed in frq-ATG1mut at the indicated temperatures in constant light. (C) Racetube assays revealing that the free-running periods of frq-ATG1mut and s-frq are similar (see also Table 1A).
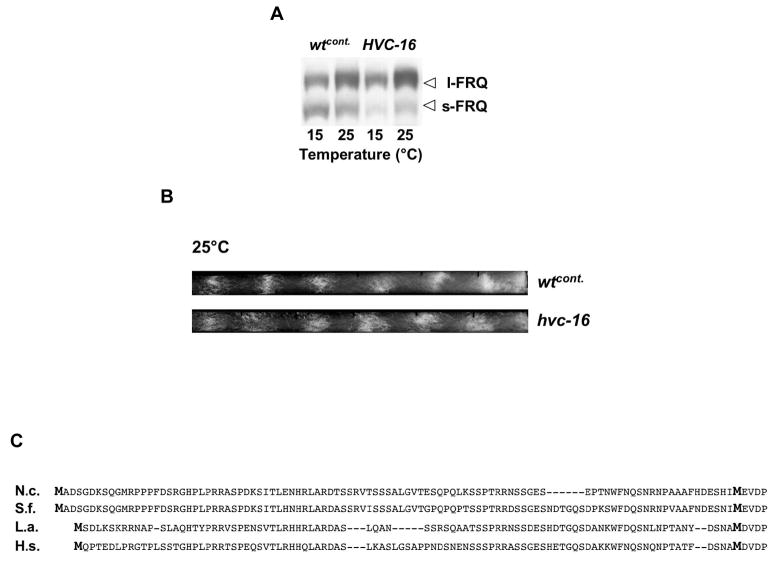
To show that the short period of the l-frq strain is due to l-FRQ rather than to the sequence alterations in the transcript we analyzed two additional strains where only the splice donor of frq-I6 was mutated. These strains did not carry sequence alterations in the ORF. In the first strain, hvc16 [15], the splice donor had been mutated from C-GTGAGT to C-ATGACT and in the second strain, frq-I6-DM, the donor was changed to C-ACTAGT. Both strains expressed predominantly l-FRQ but low levels of s-FRQ were still detectable, especially if examined in the light (Fig. 4A and data not shown). The free running period of hvc16 was very similar to that of wt (Fig. 4B, [15]) but was different from the period of the s-FRQ expressing strains, s-frq and frq-ATG1mut at 25°C and 28°C (data not shown). The free-running period of frq-I6-DM was at all temperatures similar to the l-frq period (Table 1A). One interpretation is that the donor-only mutations allow expression of low levels of s-FRQ that can affect period length. The differences between hvc16 and frq-I6-DM may be due to the low but different levels of s-FRQ that are still expressed in these strains.
Fig. 4.
(A) l- and s-FRQ expressed in hvc-16 in constant light. Western blot analysis of protein extracts from cultures grown in LL at the indicated temperatures. Protein extracts were treated with phosphatase prior to SDS PAGE. At 15 and 25°C low levels of s-FRQ can still be detected in the donor mutant hvc-16. (B) Racetube assay revealing that the free-running periods of hvc16 (21.7) and wtcont (21.5) are similar at 25°C (n=6). (C) Evolutionary conservation of s-FRQ in filamentous fungi. Alignment of the N-terminal part of the amino acid sequence of FRQ proteins of N.c., Neurospora crassa; S.f., Sordaria fimicola; L.a., Leptosphaeria australiensis; H.s., Hypocrea spinulosa. Methionine residues (M) are printed in bold.
4. Discussion
FRQ is a central component of positive and negative feedback loops of the circadian clock of Neurospora. Expression of FRQ is regulated in a considerably complex fashion [16]. frq is rhythmically transcribed by two promoters, Pprox and Pdist that are both controlled by WCC [15]. Intron 6 of frq RNA (frq-I6) is spliced in a thermosensitive manner leading to RNA species that encode l-FRQ and s-FRQ, respectively [15, 14]. We show here that the fraction of spliced frq-I6 is dependent on light, temperature, and on the circadian time. These observations could be accounted for if Pprox transcripts were spliced with higher efficiency than Pdist transcripts. Pprox transcripts are low in DD but efficiently induced by light [15]. Accordingly, overall splicing of frq-I6 would be more efficient in LL. Pdist transcripts display a high amplitude rhythm in darkness. When Pdist transcripts decrease in a circadian manner the fraction of Pprox transcripts, and thus overall splicing of frq-I6, would increase.
In addition to the regulation of the l-FRQ vs. s-FRQ ratio by splicing of frq-I6, the abundance of both isoforms is controlled in a thermosensitive fashion at the level of translation [14]. These elaborate mechanisms obviously control the expression of l-FRQ and s-FRQ and the question arises why this might be so important. Overall levels of FRQ have been shown to be crucial for the amplitude of circadian oscillations [7, 9, 13, 14] but what might be the function of the isoforms? The initiation codon of s-FRQ is conserved in frq genes of other filamentous fungi (Fig. 4C) along with the pattern of splicing [15], suggesting that l- and s-FRQ isoforms fulfill non-redundant functions. We show here that the free-running period of wt strains, though fairly well temperature compensated, decreases slightly with increasing temperature. l-frq supports a shorter and s-frq a longer rhythm than wt. However, the period length is slightly undercompensated in l-frq and slightly overcompensated in s-frq, suggesting the same molecular mechanisms for temperature compensation (e.g. protein phosphorylation and/or turnover) affect the two FRQ isoforms in different manner. This antidromic behavior might become more pronounced when the mechanism for temperature compensation is challenged by unfavorable environmental conditions. In a wt strain, expressing both isoforms, the temperature-dependent change in period length reflects in part the thermosensitive expression ratio of the FRQ isoforms. Accordingly, the period in wt would be less affected by a partial loss of the temperature compensating mechanism than it would in strains expressing only one FRQ isoform. Thus, the ratio of l-FRQ and s-FRQ isoforms is not required for temperature compensation per se but rather provides a means to control and fine-tune period length in response to ambient temperature.
Acknowledgments
This work was supported by grants from Deutsche Forschungsgemeinschaft BR 1375-1 and SFB 638 and by The Fonds der Chemischen Industrie to MB and from the National Institutes of Health to J.C.D (GM34985 and GM68087) and to J.C.D. and J.J.L. (GM83336).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liu Y. Molecular mechanisms of entrainment in the Neurospora circadian clock. J Biol Rhythms. 2003;18(3):195–205. doi: 10.1177/0748730403018003002. [DOI] [PubMed] [Google Scholar]
- 2.Roenneberg T, Merrow M. The network of time: understanding the molecular circadian system. Curr Biol. 2003;13:R198–207. doi: 10.1016/s0960-9822(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 3.Gachon F, Nagoshi E, Brown SA, Ripperger J, Schibler U. The mammalian circadian timing system: from gene expression to physiology. Chromosoma. 2004;113:103–112. doi: 10.1007/s00412-004-0296-2. [DOI] [PubMed] [Google Scholar]
- 4.Dunlap JC, Loros JJ. The Neurospora circadian system. J Biol Rhythms. 2004;19:414–424. doi: 10.1177/0748730404269116. [DOI] [PubMed] [Google Scholar]
- 5.Brunner M, Schafmeier T. Transcriptional and post-transcriptional regulation of the circadian clock of cyanobacteria and Neurospora. Genes Dev. 2006;20:1061–1074. doi: 10.1101/gad.1410406. [DOI] [PubMed] [Google Scholar]
- 6.Talora C, Franchi L, Linden H, Ballario P, Macino G. Role of a white collar-1-white collar-2 complex in blue-light signal transduction. EMBO J. 1999;18:4961–4968. doi: 10.1093/emboj/18.18.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aronson BD, Johnson KA, Loros JJ, Dunlap JC. Negative feedback defining a circadian clock: autoregulation of the clock gene frequency. Science. 1994;263:1578–1584. doi: 10.1126/science.8128244. [DOI] [PubMed] [Google Scholar]
- 8.Lee K, Loros JJ, Dunlap JC. Interconnected feedback loops in the Neurospora circadian system. Science. 2000;289:107–110. doi: 10.1126/science.289.5476.107. [DOI] [PubMed] [Google Scholar]
- 9.Cheng P, Yang Y, Liu Y. Interlocked feedback loops contribute to the robustness of the Neurospora circadian clock. Proc Natl Acad Sci USA. 2001;98:7408–7413. doi: 10.1073/pnas.121170298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schafmeier T, Haase A, Káldi K, Scholz J, Fuchs M, Brunner M. Transcriptional feedback of Neurospora circadian clock gene by phosphorylation-dependent inactivation of its transcription factor. Cell. 2005;122:235–246. doi: 10.1016/j.cell.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 11.Schafmeier T, Káldi K, Diernfellner A, Mohr C, Brunner M. Phosphorylation dependent maturation of Neurospora circadian clock protein from a nuclear repressor towards a cytoplasmic activator. Genes Dev. 2006;20:297–306. doi: 10.1101/gad.360906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garceau NY, Liu Y, Loros JJ, Dunlap J. Alternative initiation of translation and time-specific phosphorylation yield multiple forms of the essential clock protein FREQUENCY. Cell. 1997;89:469–476. doi: 10.1016/s0092-8674(00)80227-5. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Garceau N, Loros JJ, Dunlap JC. Thermally regulated translational control of FRQ mediates aspects of temperature responses in the Neurospora circadian clock. Cell. 1997;89:477–486. doi: 10.1016/s0092-8674(00)80228-7. [DOI] [PubMed] [Google Scholar]
- 14.Diernfellner ACR, Schafmeier T, Merrow MW, Brunner M. Molecular mechanism of temperature-sensing by the circadian clock of Neurospora crassa. Genes Dev. 2005;19:1968–1973. doi: 10.1101/gad.345905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colot HV, Loros JJ, Dunlap J. Temperature-modulated alternative splicing and promoter use in the circadian clock gene frequency. Mol Biol Cell. 2005;16:5563–5571. doi: 10.1091/mbc.E05-08-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunner M, Diernfellner A. How temperature affects the circadian clock of Neurospora crassa. Chronobiol Int. 2006;23(1–2):81–90. doi: 10.1080/07420520500545805. [DOI] [PubMed] [Google Scholar]
- 17.Correa A, Lewis ZA, Greene AV, March IJ, Gomer RH, Bell-Pedersen D. Multiple oscillators regulate circadian gene expression in Neurospora. Proc Natl Acad Sci U S A. 2003;100(23):13597–602. doi: 10.1073/pnas.2233734100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong W, Tang X, Yu Y, Griffith J, Nilsen R, Choi D, Baldwin J, Hilton L, Kelps K, Mcguire J, Morgan R, Smith M, Case M, Arnold J, Schuttler HB, Wang Q, Liu J, Reeves J, Logan D. Systems biology of the neurospora biological clock. IET Syst Biol. 2007;1(5):257–65. doi: 10.1049/iet-syb:20060080. [DOI] [PubMed] [Google Scholar]