Summary
A mouse model of western equine encephalitis (WEE) was characterized for use in antiviral studies. Virus was detected in several tissues, most notably an average titer of 9.5 ± 1.1 Log10 50% cell culture infectious doses (CCID50)/g tissue in the brains of infected animals. Signs of WEE included limb weakness, paralysis, involuntary spasms or extension of limbs, clenching of paws, hunching, ruffling of fur, and eye exudates, many of which are indicative of neurological disease. The pyrazinecarboxamide derivative, T-705, was found to be active in Vero cells against WEE virus (WEEV) with an 90% effective concentration (EC90) of 49 μg/ml (selective index [SI] >20). Treatment with T-705 in this WEE mouse model resulted in significant improvement in survival and mean day to death after oral treatment administered twice a day for 7-days at a dose of 400 mg/kg/d. Virus titer in the brain was not significantly reduced, despite a 1-log reduction in average brain titer in treated animals on 4 dpi. Signs of disease were relatively mild in treated animals, but were not eliminated. Treatment with T-705 improved morbidity and mortality of WEEV-infected mice, further illustrating the broad-spectrum activity of T-705 in the treatment of RNA viruses.
T-705, a pyrazinecarboxamide derivative, has been shown to have broad-spectrum activity against several RNA viruses. Most notably, this compound is effective against influenza in cell culture as well as in animal models through inhibition of the viral polymerase enzyme (Furuta et al., 2002; Furuta et al., 2005; Sidwell et al., 2007; Takahashi et al., 2003) and is currently undergoing clinical trials for the treatment of influenza infection in man (2007). Other RNA viruses that are effectively treated by T-705 in animal models include yellow fever virus (Julander et al., 2009), West Nile virus (Morrey et al., 2008), and several bunya and arenaviruses (Gowen et al., 2007). With efficacy seen in an encephalitic West Nile virus model, it was anticipated that T-705 might also be effective in the treatment of western equine encephalitis virus (WEEV) infection in cell culture as well as in a mouse model.
WEEV is an alphavirus that causes occasional outbreaks of human disease (Calisher, 1994) and is listed as a category B agent on the NIH Biodefense and Emerging Diseases list as an agent of bioterrorism concern (Sidwell and Smee, 2003). Emphasis has been placed on finding therapeutic options for viruses on the NIH list (http://www3.niaid.nih.gov/topics/emerging/list.htm), which places value on the discovery of broad-spectrum antiviral agents. The present study is in line with this philosophy- attempting to show efficacy of the broad-spectrum antiviral T-705 against WEEV.
Hamsters, which are very susceptible to WEEV infection, were used in previous antiviral studies, but neurological signs of disease are not observed in infected hamsters, despite relatively high virus titer detected in the brain (Julander et al., 2007). While performing a titration of WEEV (VR-70, American Type Culture Collection) administered intraperitoneally (i.p.) in C57BL/6 mice (18–20 g, Charles River Laboratories) disease signs that were indicative of neurologic involvement were observed, which included limb weakness, paralysis, hunching, and fur ruffling. Challenge doses of 103.9 or 104.9 pfu/ml given i.p. resulted in high mortality (80–90%), which correlated with observation of the aforementioned disease signs. Infection of mice by s.c. injection with 104.9 pfu/ml resulted in low morbidity and mortality (1/7 showed neurologic disease signs and died shortly thereafter). It appeared that route of virus challenge had a large influence on the progression of neurological disease.
Disease parameters of 6–8 week old C57BL/6 mice infected with WEEV were evaluated to determine appropriate markers of morbidity for use in antiviral studies. Virus was detected by infectious cell culture assay in several tissues, including brain, liver, spleen, kidney, and serum of infected mice (Figure 1), but not in sham-infected controls (data not shown). While virus was detected in all of the tissues tested, brain titers reached the highest levels, had a more consistent distribution, and peaked 4 dpi, which was similar to previously published data (Liu et al., 1970). The brain is also the target organ in WEE models (Aguilar, 1970), as well as in human infection (Anderson, 1984), which underlies the potential importance of a therapeutic agent that can lower the titer in the brains of infected animals.
Figure 1.
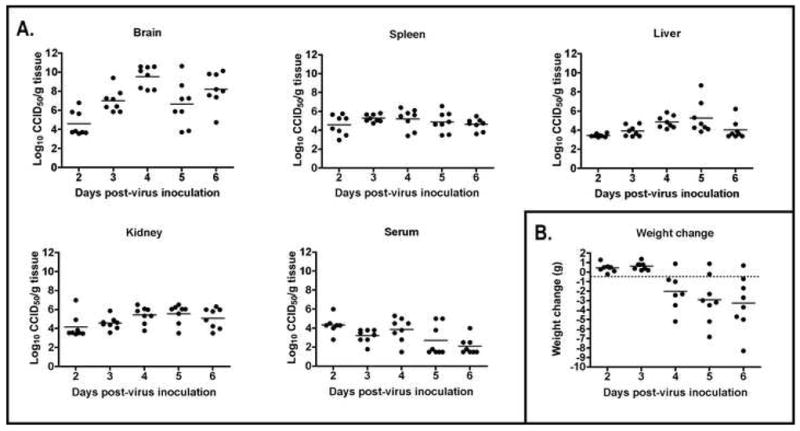
A) Titer, measured as log10 50% cell culture infectious doses (CCID50)/g tissue or ml serum, of WEEV in various tissues taken from mice on 2, 3, 4, 5, and 6 days post-virus infection (dpi) with 103.9 pfu/ml WEEV. B) Time-course weight change (g) of mice infected with WEEV.
An average mortality rate of 78% (range 76–100%) was observed in mice infected with WEEV in four separate experiments. The mean day to death of these animals was 5.6 ± 1.2 dpi, which was around 1 day longer than mean day to death of hamsters infected with the same virus strain (Julander et al., 2007). Significant (P<0.01) weight loss was observed beginning 4 days post-virus injection (dpi), which continued through 6 dpi (Figure 1).
Ninety percent of a group of 10 mice showed disease signs, which included limb weakness, paralysis, ruffled fur, and hunching (Table 1). More serious disease signs were generally followed by death, and included lying prone, involuntary extension of limbs, labored breath, lack of motility, and exudates from the eyes (data not shown). With high mortality, weight loss during later disease course, measurable signs of disease, high virus titers in the brain, and a moderate infection interval around 6 days, this mouse model of WEE appeared to be suitable for antiviral studies.
Table 1.
Occurrence of major disease signs in mice infected with western equine encephalitis virus.
| Symptom | Timinga | Affected/totalb | # multiple symptoms/# affectedc |
|---|---|---|---|
| Limb weakness | 4 dpi | 8/10 | 6/8 |
| Paralysis | 3–7 dpi | 7/10 | 7/7 |
| Lacrimation | 5–7 dpi | 3/10 | 3/3 |
| Lying prone | 3–7 dpi | 6/10 | 6/6 |
| Death | 4–8 dpi | 10/10 | N/A |
Time when disease parameter was observed
Number of animals displaying the disease sign per total animals infected
Number of animals that displayed other signs of disease per number affected with the original symptom
To evaluate the utility of this model for antiviral testing, the broad-spectrum compound T-705 (Toyama Chemical Co., Tokyo, Japan) was selected. Initial tests to determine the activity of this compound against WEEV were conducted with various half-log concentrations of T-705 between 1000 and 3.2 μg/ml in 96-well flat-bottomed microplates plated with Vero cells (ATCC CCL-81) and read after 3 days incubation at 37°C. T-705 had a 90% effective concentration (EC90) against WEEV of 49 μg/ml (312 μM) as determined by virus yield assay. The 50 % cytotoxic concentration (CC50) was > 1000 μg/ml (6.4 mM), generating a selective index of >20. Activity was confirmed in a second cell culture experiment that yielded similar results (data not shown).
To determine the efficacy of this compound in mice, T-705 was administered twice a day (bid) by oral gavage for 8 days beginning 4 h prior to virus challenge. Mice treated with 400 mg/kg/d of T-705 had a significant improvement in survival and mean day to death as compared with that of placebo-treated mice (Figure 2). Despite significant improvement in the survival curve, 60% of mice treated with T-705 died of WEE as compared with 80% mortality in the placebo-treated infection control group.
Figure 2.
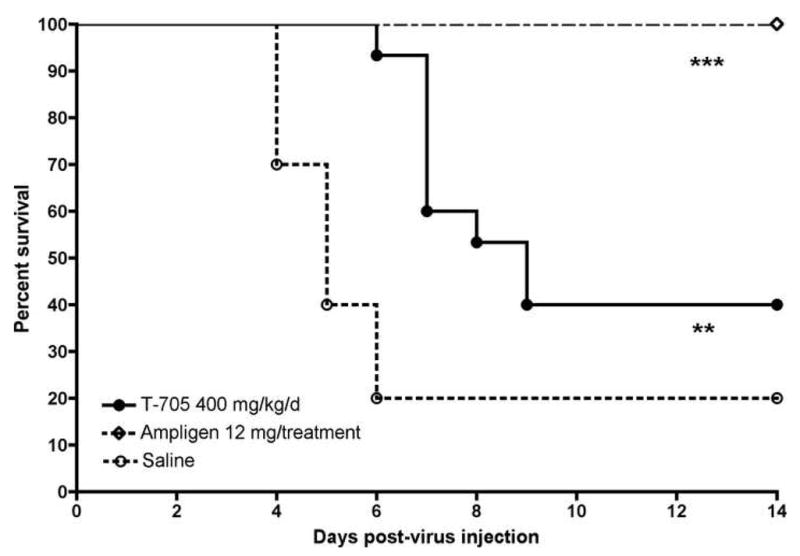
Survival of C57BL/6 mice infected with WEEV and treated twice a day for 8 days with T-705 given p.o [n=15], or −4 h and 2 dpi with ampligen given i.p. [n=10] (***P<0.001, **P<0.01, as compared with placebo-treated controls [n=20], as determined by Wilcoxon log-rank survival analysis).
Treatment with T-705 resulted in a 1-log drop in mean infectious cell culture titers on 4 dpi in the brains of infected mice, although the difference was not statistically significant (Figure 3). A significant 3-log drop in brain titer was observed in hamsters infected with WNV and treated with T-705, suggesting that this compound can cause significant reductions in brain titers of flaviviruses (Morrey et al., 2008), but it is unknown whether this drop is a result of direct inhibition of virus in the brain or from an earlier reduction of peripheral virus that ultimately results in lower levels of virus infecting the brain. The 1-log10, non-significant reduction in virus titer in the brains of treated mice may reflect a more widespread systemic reduction in virus titer. Generally, WEEV-infected mice that display signs of neurological disease would succumb to infection. Several mice treated with T-705, however, showed signs of brain disease, but subsequently recovered, suggesting the possibility of amelioration of disease by T-705 despite neurological involvement.
Figure 3.
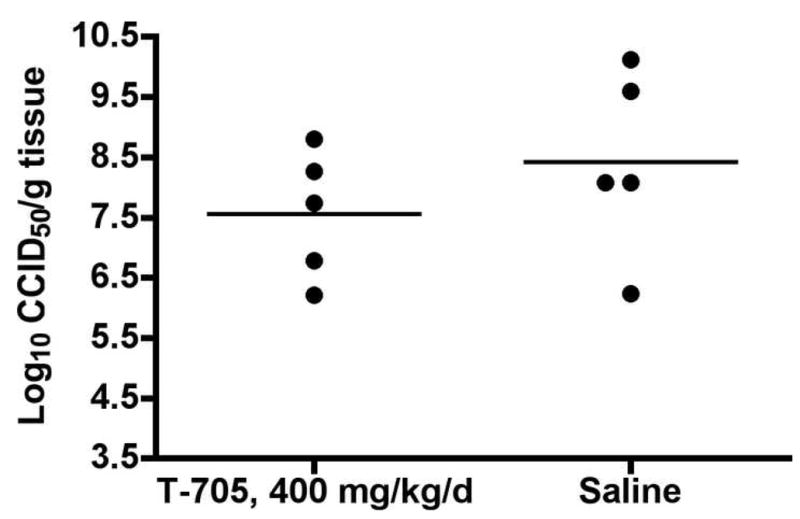
Brain titers of C57BL/6 mice infected with WEEV and treated twice a day for 7 days with T-705 administered orally at a dose of 400 mg/kg/d. Lines represent mean titers for each group (P = 0.3287 as determined by one-way Student’s t-test).
The immunomodulatory double-stranded RNA, ampligen (HEMISPHERx, Philadelphia, PA), was included as a positive control. A dose of 12 mg/kg of ampligen was administered i.p. −4 h and 2 dpi. Complete survival was observed in mice treated with ampligen (Figure 2). These results are similar to those from previously published studies reporting the treatment of WEE with double stranded RNA, including dsRNA extracted from rice dwarf virus and poly I:C (Takehara, 1977), as well as treatment with both free and liposome encapsulated poly ICLC (Wong et al., 2005). Neurological disease signs were also observed, but were generally mild limb weakness or temporary paralysis and all mice that exhibited signs recovered (data not shown).
From these studies, this mouse model appears to be suitable for antiviral studies in the development of therapies for human WEEV-infection. With significant titers in the brains of infected mice, significant weight change, observable disease signs, and high mortality, antiviral compounds can be evaluated with these parameters to gauge efficacy. T-705 showed moderate efficacy, although treatment was initiated just prior to virus challenge. Future studies, therefore, should be conducted to further evaluate this compound in the treatment of WEE, specifically evaluating the effect of treatment initiated after virus infection.
Acknowledgments
We thank Kristiina Shafer, Luci Wandersee, and Jeremy Strange for their work. This work was supported by Contract NO1-AI-15435 and Contract NO1-AI-30048 from the Virology Branch, NIAID, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Justin G. Julander, Institute for Antiviral Research, Utah State University, 5600 Old Main Hill, Logan, UT. 84322-5600. Ph (435)-797-7215, Fax (435)-797-3959, e-mail justin.julander@cc.usu.edu.
Donald F. Smee, Institute for Antiviral Research, Utah State University.
John D. Morrey, Institute for Antiviral Research, Utah State University.
Yousuke Furuta, Toyama Chemical Company, Ltd., 3-2-5 Nishishinjuku, Shinjuku-ku, Tokyo 187-0023, Japan.
References
- Aguilar MJ. Pathological changes in brain and other target organs of infant and weanling mice after infection with non-neuroadapted western equine encephalitis virus. Infect Immun. 1970;2:533–542. doi: 10.1128/iai.2.5.533-542.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA. Focal neurologic signs in western equine encephalitis. Can Med Assoc J. 1984;130:1019–1021. [PMC free article] [PubMed] [Google Scholar]
- Anonymous. Toyama starts U.S. trials of polymerase inhibitor. RxTrials Institute Drug Pipeline Alert. 2007 http://www.fdanews.com/newsletter/article?articleId=91489&issueId=9890.
- Calisher CH. Medically important arboviruses of the United States and Canada. Clin Microbiol Rev. 1994;7:89–116. doi: 10.1128/cmr.7.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Takahashi K, Fukuda Y, Kuno M, Kamiyama T, Kozaki K, Nomura N, Egawa H, Minami S, Watanabe Y, Narita H, Shiraki K. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob Agents Chemother. 2002;46:977–981. doi: 10.1128/AAC.46.4.977-981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Takahashi K, Kuno-Maekawa M, Sangawa H, Uehara S, Kozaki K, Nomura N, Egawa H, Shiraki K. Mechanism of action of T-705 against influenza virus. Antimicrob Agents Chemother. 2005;49:981–986. doi: 10.1128/AAC.49.3.981-986.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen BB, Wong MH, Jung KH, Sanders AB, Mendenhall M, Bailey KW, Furuta Y, Sidwell RW. In vitro and in vivo activities of T-705 against arenavirus and bunyavirus infections. Antimicrob Agents Chemother. 2007;51:3168–3176. doi: 10.1128/AAC.00356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julander JG, Shafer K, Smee DF, Morrey JD, Furuta Y. Activity of T-705 in a hamster model of yellow fever virus infection in comparison with that of a chemically related compound, T-1106. Antimicrob Agents Chemother. 2009;53:202–209. doi: 10.1128/AAC.01074-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julander JG, Siddharthan V, Blatt LM, Schafer K, Sidwell RW, Morrey JD. Effect of exogenous interferon and an interferon inducer on western equine encephalitis virus disease in a hamster model. Virology. 2007;360:454–460. doi: 10.1016/j.virol.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Voth DW, Rodina P, Shauf LR, Gonzalez G. A comparative study of the pathogenesis of western equine and eastern equine encephalomyelitis viral infections in mice by intracerebral and subcutaneous inoculations. J Infect Dis. 1970;122:53–63. doi: 10.1093/infdis/122.1-2.53. [DOI] [PubMed] [Google Scholar]
- Morrey JD, Taro BS, Siddharthan V, Wang H, Smee DF, Christensen AJ, Furuta Y. Efficacy of orally administered T-705 pyrazine analog on lethal West Nile virus infection in rodents. Antiviral Res. 2008;80:377–379. doi: 10.1016/j.antiviral.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidwell RW, Barnard DL, Day CW, Smee DF, Bailey KW, Wong MH, Morrey JD, Furuta Y. Efficacy of orally administered T-705 on lethal avian influenza A (H5N1) virus infections in mice. Antimicrob Agents Chemother. 2007;51:845–851. doi: 10.1128/AAC.01051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidwell RW, Smee DF. Viruses of the Bunya- and Togaviridae families: potential as bioterrorism agents and means of control. Antiviral Res. 2003;57:101–111. doi: 10.1016/s0166-3542(02)00203-6. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Furuta Y, Fukuda Y, Kuno M, Kamiyama T, Kozaki K, Nomura N, Egawa H, Minami S, Shiraki K. In vitro and in vivo activities of T-705 and oseltamivir against influenza virus. Antivir Chem Chemother. 2003;14:235–241. doi: 10.1177/095632020301400502. [DOI] [PubMed] [Google Scholar]
- Takehara M. Antiviral effects of double-stranded RNA from rice dwarf virus on infection of mice with western equine encephalitis virus. Microbiol Immunol. 1977;21:309–315. doi: 10.1111/j.1348-0421.1977.tb00292.x. [DOI] [PubMed] [Google Scholar]
- Wong JP, Nagata LP, Christopher ME, Salazar AM, Dale RM. Prophylaxis of acute respiratory virus infections using nucleic acid-based drugs. Vaccine. 2005;23:2266–2268. doi: 10.1016/j.vaccine.2005.01.037. [DOI] [PubMed] [Google Scholar]


