Abstract
The state of wound oxygenation is a key determinant of healing outcomes. From a diagnostic standpoint, measurements of wound oxygenation are commonly used to guide treatment planning such as amputation decision. In preventive applications, optimizing wound perfusion and providing supplemental O2 in the peri-operative period reduces the incidence of post-operative infections. Correction of wound pO2 may, by itself, trigger some healing responses. Importantly, approaches to correct wound pO2 favorably influence outcomes of other therapies such as responsiveness to growth factors and acceptance of grafts. Chronic ischemic wounds are essentially hypoxic. Primarily based on the tumor literature, hypoxia is generally viewed as being angiogenic. This is true with the condition that hypoxia be acute and mild to modest in magnitude. Extreme near-anoxic hypoxia, as commonly noted in problem wounds, is not compatible with tissue repair. Adequate wound tissue oxygenation is required but may not be sufficient to favorably influence healing outcomes. Success in wound care may be improved by a personalized health care approach. The key lies in our ability to specifically identify the key limitations of a given wound and in developing a multifaceted strategy to specifically address those limitations. In considering approaches to oxygenate the wound tissue it is important to recognize that both too little as well as too much may impede the healing process. Oxygen dosing based on the specific need of a wound therefore seems prudent. Therapeutic approaches targeting the oxygen sensing and redox signaling pathways are promising.
Keywords: hyperbaric oxygen, topical oxygen, angiogenesis, patient, redox, hypoxia, therapy, clinical
The clinical application of O2 to wound healing occurs at many levels: diagnostic, preventive and therapeutic. From a diagnostic standpoint, measurements of wound oxygenation (transcutaneous O2 measurements or TCOM) are commonly used to guide treatment planning such as amputation decision 1-6. In preventive applications, optimizing wound perfusion and providing supplemental O2 in the peri-operative period reduces the incidence of post-operative infections 7-9. Correction of wound pO2 (partial pressure of oxygen in the wound tissue) may, by itself, trigger some healing responses 10-18. More importantly, approaches to correct wound pO2 favorably influence outcomes of other therapies such as responsiveness to growth factors and acceptance of grafts 10, 19, 20. This leads to the concept of correction of wound hypoxia as adjunct to other therapeutic modalities 14, 21. Although the case for therapeutic approaches aimed at correcting wound tissue hypoxia is compelling, outcomes in the wound clinics have been inconsistent. The objective of this review article is to concisely address some of the fundamental and emergent concepts in tissue O2 sensing and response with the goal to illuminate salient complexities and perform critical analysis of what should help improve clinical outcomes in response to O2-based therapeutics.
Wound Ischemia and Hypoxia
Vascular complications commonly associated with problematic wounds are primarily responsible for wound ischemia. Limitations in the ability of the vasculature to deliver O2-rich blood to the wound tissue leads to, among other consequences, hypoxia. Hypoxia is a reduction in oxygen delivery below tissue demand, whereas ischemia is a lack of perfusion, characterized not only by hypoxia but also by insufficient nutrient supply. Hypoxia, by definition, is a relative term. It is defined by a lower tissue partial pressure of oxygen (pO2) compared to the pO2 to which the specific tissue element in question is adjusted to under healthy conditions in vivo. Depending on the magnitude, cells confronting hypoxic challenge either induce an adaptive response that includes increasing the rates of glycolysis and conserve energy or undergo cell death 22. Generally, acute mild to moderate hypoxia supports adaptation and survival. In contrast, chronic extreme hypoxia leads to tissue loss. While the tumor tissue is metabolically designed to thrive under conditions of hypoxia 23, hypoxia of the wound primarily caused by vascular limitations is intensified by coincident conditions (e.g. infection, pain, anxiety and hyperthermia) and leads to poor healing outcomes 24, 25.
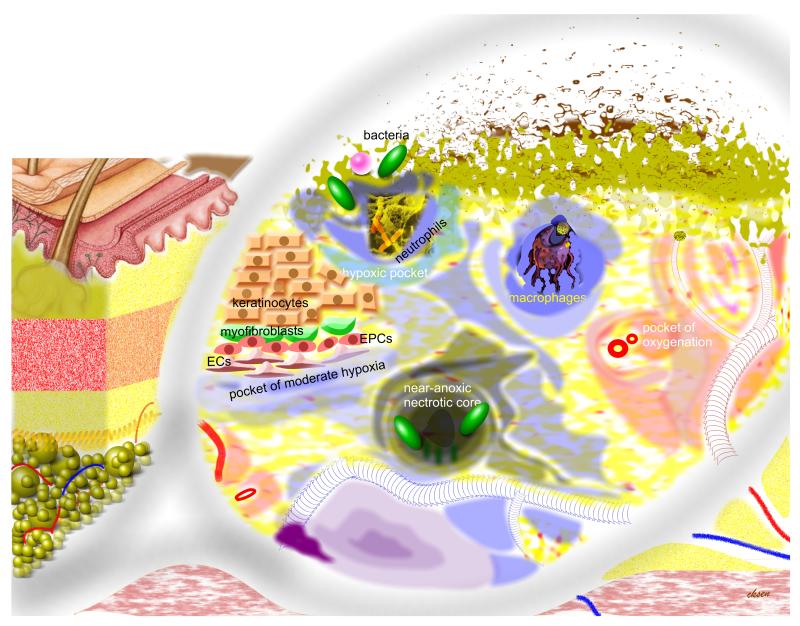
Three major factors may contribute to wound tissue hypoxia: (i) peripheral vascular diseases garroting O2 supply, (ii) increased O2 demand of the healing tissue, and (iii) generation of reactive oxygen species (ROS) by way of respiratory burst and for redox signaling (Fig. 1). Other related factors such as arterial hypoxia (e.g. pulmonary fibrosis or pneumonia, sympathetic response to pain, hypothermia, anemia caused by major blood loss, cyanotic heart disease, high altitude) may contribute to wound hypoxia as well. Depending on factors such as these, it is important to recognize that wound hypoxia may range anywhere from near-anoxia to mild-modest hypoxia 26, 27. In this context it is also important to appreciate that point measurements 28 performed in the wound tissue may not provide a complete picture of the wound tissue biology because it is likely that the magnitude of wound hypoxia is not uniformly distributed throughout the affected tissue especially in large wounds. This is most likely the case in chronic wounds presented clinically as opposed to experimental wounds which are more controlled and homogenous in nature. In any single problem wound presented in the clinic, it is likely that there are pockets of near-anoxic as well as that of different grades of hypoxia (Fig. 2). As the weakest link in the chain, tissue at the near-anoxic pockets will be vulnerable to necrosis which in turn may propagate secondary tissue damage and infection. Pockets of extreme hypoxia may be flooded with hypoxia-inducible angiogenic factors but would fail to functionally vascularize because of insufficient O2 that is necessary to fuel the repair process. Indeed, uncontrolled expression of vascular endothelial growth factor and its receptors leads to insufficient skin angiogenesis 29. Whether cells in the pockets of extreme hypoxia are O2-responsive is another concern. Even if such cells may have passed the point of no return in the survival curve, correction of tissue oxygenation is likely to help clean-up the dead or dying tissue 30 31 and replace the void with proliferating neighboring cells. Pockets of moderate or mild hypoxia are likely to be the point of origin of successful angiogenic response as long as other barriers such as infection and epigenetic alterations are kept to a minimum.
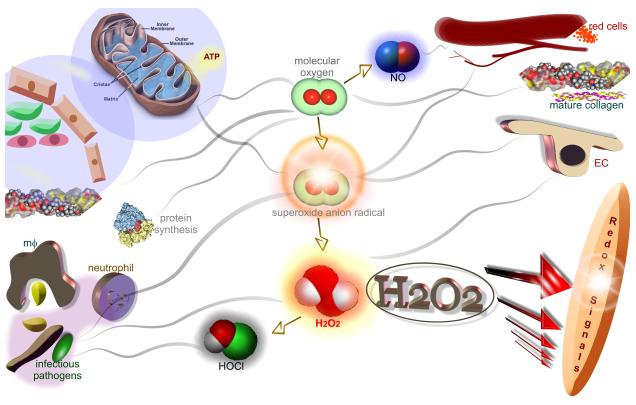
Figure 1. Significance of molecular oxygen and its derivatives in wound healing.
In its molecular form, oxygen is required for oxidative metabolism derived energy synthesis, protein synthesis and the maturation (hydroxylation) of extracellular matrices such as collagen. Molecular oxygen is also required for NO synthesis which in turn plays a key role in the regulation of vascular tone as well as in angiogenesis. In a wound setting, large amounts of molecular oxygen are partially reduced to form reactive oxygen species (ROS). ROS includes oxygen free radicals such as superoxide anion as well its non-radical derivative hydrogen peroxide (H2O2). Superoxide anion radical is the one-electron reduction product of oxygen. NADPH oxidases represent one major source of superoxide anion radicals at the wound-site. NADPH oxidases in phagocytic cells help fight infection. Superoxide anion also drives endothelial cell signaling such as required during angiogenesis. In biological tissues, superoxide anion radical rapidly dismutates to hydrogen peroxide-either spontaneously or facilitated by enzymes called superoxide dismutases. Endogenous hydrogen peroxide drives redox signaling, a molecular network of signal propagation that supports key aspects of wound healing such as cell migration, proliferation and angiogenesis. Neutrophil-derived hydrogen peroxide may be utilized by myeloperoxidase to mediate peroxidation of chloride ions resulting in the formation of hypochlorous acid (HOCl), a potent disinfectant.
Figure 2. Heterogeneous distribution of oxygen in the wound tissue: hypothetical pockets of graded levels of hypoxia.
Structures outside the illustrated magnifying glass represent the macro tissue structures. Objects under the glass represent a higher resolution. Shade of black (anoxia) or blue represents graded hypoxia. Shade of red or pink represents oxygenated tissue. Tissue around each blood vessel is dark pink in shade representing regions that are well oxygenated (oxygen-rich pockets). Bacteria and bacterial infection are presented by shades of green on the surface of the open wound.
Wound Hypoxia: The Imbalance between Limited Supply and High Demand
Limited supply: peripheral vascular diseases
Peripheral vascular disease (PVD) can affect the arteries, the veins as well as the lymph vessels. The most common and important type of PVD is peripheral arterial disease, or PAD, which affects about eight million Americans. The ankle brachial pressure index (ABPI) represents a simple non-invasive method to detect arterial insufficiency within a limb. Arterial diseases, especially those associated with diabetes, represent a major complicating factor in wound healing. PAD is the only identifiable etiology in approximately 10% of leg ulcers 32. In an ischemic limb, peripheral tissues are deprived of blood supply as PAD progresses causing tissue loss, ulcers and gangrene.
Venous insufficiency, on the other hand, is the root cause of most leg ulcers 33. Chronic venous insufficiency, characterized by the retrograde flow of blood in the lower extremity, is associated with changes in the venous wall and valves generally caused by inflammatory disorders induced by venous hypertension and associated fluid shear stress. Factors causing arterial hypoxemia may also limit O2 supply to the wound tissue. Compromised pulmonary health 34, loss of hepatic function 35, 36, hemodialysis 37, anemia 38, 39, altitude hypoxemia 40, nitroglycerin therapy 41, nasal packing 42, critical illness 43, pain 44 and hypothermia 45, 46 are some examples of conditions associated with arterial hypoxemia. Vasoconstricting drugs may contribute to tissue hypoxia as well 47.
High demand: increased demand of the healing tissue
Mitochondrial respiration is responsible for more than 90% of O2 consumption in humans. Cells utilize O2 as the final electron acceptor in the aerobic metabolism of glucose to generate ATP which fuels most active cellular processes such as during wound healing 48. Increased energy demand of the healing tissue leads to a hypermetabolic state wherein additional energy is generated from oxidative metabolism increasing the O2 demand of the healing tissue 49-52. ATP thus generated powers tissue repair. At the injury site, extracellular ATP may be contributed by platelets and other disintegrating cells. Extracellular ATP liberated during hypoxia or inflammation can either signal directly to purinergic receptors or, after phosphohydrolytic metabolism, can activate surface adenosine receptors. Purinergic signaling may influence numerous aspects of wound biology including immune response, inflammation, vascular as well as epithelial biology. ATP may be immunostimulatory or vice versa depending on extracellular concentrations as well as on expression patterns of purinergic receptors and ecto-enzymes 53. Extracellular ATP induces receptor activation in epithelial cells. ATP, released upon epithelial injury, acts as an early signal to trigger cell responses including an increase in heparin-binding EGF-like growth factor shedding, subsequent transactivation of the epidermal growth factor (EGF) receptor and its downstream signaling, resulting in wound healing 54. ATP released from the injured epithelial cells is now known to also turn on NADPH oxidases 55, the activity of which is critically required to produce the redox signals required for wound healing 19, 56, 57. Human endothelial cells are rich in purinergic receptors and therefore responsive to extracellular ATP as well 58. ATP induces endothelium dependent vasodilation 59, Both ATP as well as adenosine adenosine regulate smooth muscle and endothelial cell proliferation 60. Recognizing that hypoxia limits ATP synthesis in the ischemic wound tissue, therapeutic ATP delivery systems have been studied for their effect on wound healing 61. While these approaches may compensate for the deficiency of ATP per se in the ischemic wound tissue, they will fail to address the other essential functions of O2 and its derivatives in wound healing as discussed below.
Absolute requirements for O2 arise in several points along the angiogenic sequence. For instance, all vessels require a net or sheath of extracellular matrix, mainly collagen and proteoglycans, to guide tube formation and resist the pressures of blood flow. Conditions for collagen deposition and polymerization can be created only if molecular O2 is available to be incorporated into the structure of nascent collagen by prolyl- and lysyl hydroxylases. Without the obligatory extracellular, hydroxylated collagen, new capillary tubes assemble poorly and remain fragile 62-64. This has a convincing clinical correlate in scurvy, i.e. ascorbate deficiency. Scurvy may result from insufficient intake of ascorbate which is required for correct collagen synthesis in humans. Ascorbate is required for the post-translational hydroxylation of collagen that enables the matured collagen molecules to escape to the extracellular space and provide the necessary tensile strength 65. In scurvy, the collagenous sheath cannot form because, under ascorbate-deficient conditions, collagen cannot be hydroxylated. Consequently, new vessels fail to mature. Older vessels weaken and break, and wounds fail to heal 62. In this context it is important to recognize that the collagen hydroxylation process requires molecular oxygen. Thus, even under ascorbate-sufficient conditions collagen may fail to mature if there is insufficient supply of oxygen to the tissue. Collagen deposition proceeds in direct proportion to pO2 across the entire physiologic range, from zero to hundreds of mm Hg. The Km for O2 for this reaction is approximately 25 and the Vmax is approximately 250 mm Hg, suggesting that new vessels cannot even approach their greatest possible rate of growth unless the wound tissue pO2 is high 66. Angiogenesis is directly proportional to pO2 in injured tissues 63. Hypoxic wounds deposit collagen poorly and become infected easily, both of which are problems of considerable clinical significance 67, 68.
High demand: increased production of reactive species
Phagocytic NADPH oxidases
Sbarra and Karnovsky’s 1959 discovery of the leukocyte oxidase 69 in phagocytes came into limelight when in the late 1970s, the pioneering works of Bernard Babior that linked the explosive production of superoxide ions (O2 .- ) by leukocyte oxidase to bacterial killing 70. During phagocytosis of microbial intruders, professional phagocytes of our innate immune system increase their O2 consumption through the inducible activity of NADPH-oxidase (NOX) that generates O2.- and H2O2. These oxygen-derived metabolites give rise to yet other ROS that are potently anti-microbial but which may also cause damage by destroying surrounding tissue and cells. NADPH oxidase, catalyzing the deliberate production of ROS by cells, has been extensively investigated in phagocytes (neutrophilic and eosinophilic granulocytes, monocytes, and macrophages) 71. Exposure of these cells to any of a large number of stimuli activates a “respiratory burst”, caused by an activation of the plasma membrane bound NADPH oxidase (NADPH + 2O2 → NADP+ + 2O2.- + H+). The O2.- then rapidly dismutates to H2O2. Approximately 98% of the O2 consumed by wound neutrophils is utilized for respiratory burst 24. NADPH Oxidase supports macrophage survival 72 and enables dead cell cleansing by phagocytosis 73. Appropriate infection management may therefore spare precious O2 at the wound site which would otherwise be utilized via respiratory burst 74. Overt infection poses the risk of intensifying wound tissue hypoxia.
The NOX of ‘professional’ phagocytic cells transfers electrons across the wall of the phagocytic vacuole, forming O2.- in the lumen. It is generally accepted that this system promotes microbial killing through the generation of ROS and through the activity of myeloperoxidase 75. In response to bacterial infection, the neutrophil NADPH oxidase assembles on phagolysosomes to catalyze the transfer of electrons from NADPH to O2, forming O2.- and derivative ROS. The active oxidase is composed of a membrane-bound cytochrome (e.g. gp91phox and p22phox) together with three cytosolic phox proteins, p40phox, p47phox, and p67phox, and the small GTPase Rac2, and is regulated through a process involving protein kinase C, MAPK, and phosphatidylinositol 3-kinase 76, 77. In the resting cell, two of the subunits, p22phox and gp91phox, are located in the membrane, and the remaining components are present in the cytosol. The electron-carrying components of the oxidase are located in gp91phox 78-81. The NADPH binding site is generally regarded to be in gp91phox as well, but there is some evidence that it may be in p67phox. The catalytic subunit gp91phox, dormant in resting cells, becomes activated by assembly with cytosolic regulatory proteins. When the oxidase is activated, p47phox is phosphorylated at specific sites, and the cytosolic components together with Rac2 migrate to the membrane to assemble the active oxidase 19. Mutations in p47phox are a cause of chronic granulomatous disease, an immune-deficient condition characterized with impaired healing response 82, 83. Rac2 mutation is another factor responsible for impaired human neutrophil NADPH oxidase function, low O2.- generation and compromised wound healing 84 . The concentration of O2 necessary to achieve half maximal ROS production (the Km) is in the range of 45-80 mmHg, with maximal ROS production at pO2 at >300 mmHg 24. Thus, the maximal effects of respiratory burst dependent wound infection management can only be achieved through the administration of supplemental O2 to attain wound pO2 levels beyond those encountered when breathing room air 85. This also explains why the state of wound tissue oxygenation is a sensitive indicator for the risk of infection in surgical patients 8, 9, 86, 87.
Oxygen free radicals and reactive derivatives: a paradigm shift and emergence of redox signaling
In the 1980s, oxygen free radicals drew much attention in biomedical research. Limitations in methodological approaches to sensitively detect and monitor the extremely short-living reactive species clouded a true appreciation of the significance of oxygen-derived free radicals and reactive species in health and disease. The paradigm that emerged was too simple to be meaningful in its complete sense. The primary identity of free radicals was that they were destructive to biological tissues, and that approaches to antagonize free radicals i.e. antioxidants are helpful 88-96. Based on this crude preliminary concept, numerous clinical trials testing the efficacy of antioxidants were hastily started and the results were understandably disappointing 97-101. Lack of consideration of a very important aspect of free radical biology which started to crystallize only in the late 1990s proved to be very expensive in many ways. Work during the mid-late 1990s led to the recognition that at very low levels oxygen-derived free radicals and derivative species such as H2O2 may serve as signaling messengers 102-104. The field of redox signaling was thus born 102, 105-107 with a dedicated international peer-reviewed Journal (www.liebertpub.com/ars). Today, the concept that reactive derivatives of O2 may serve as signaling messengers has revolutionized cell biology 108-123 and has led to the concept of redox-based clinical therapeutics 124-129.
Non-phagocytic NADPH oxidases
Given the traditional bad and ugly image of oxygen free radicals and its derivatives few would have imagined that even non-phagocytic cells of the human body have a dedicated apparatus to generate ROS. In 1999, the cloning of mox1 marked a major progress in categorically establishing the presence of distinct NADPH oxidases in non-phagocytic cells 123. Mox1 or p65Mox was described as encoding a homologue of the catalytic subunit of the O2.- -generating NADPH oxidase of phagocytes, gp91phox. Mox1 messenger RNA is expressed in colon, prostate, uterus and vascular smooth muscle, but not in peripheral blood leukocytes. Later, Mox1 was renamed as NOX1 referring to NADPH oxidase 130. Over the last years, six homologs of the cytochrome subunit of the phagocyte NADPH oxidase were found: NOX1, NOX3, NOX4, NOX5, DUOX1, and DUOX2. Together with the phagocyte NADPH oxidase itself (NOX2/gp91(phox)), the homologs are now referred to as the NOX family of NADPH oxidases. Activation mechanisms of these enzymes and tissue distribution of the different members of the family are markedly different. The physiological functions of NOX family enzymes include host defense, post-translational processing of proteins, cellular signaling, regulation of gene expression, cell differentiation and renewal of precursor cells 131-135. NOX enzymes also contribute to a wide range of pathological processes. NOX deficiency may lead to immunosuppresion, lack of otoconogenesis, or hypothyroidism. Increased NOX activity also contributes to a large number or pathologies, in particular cardiovascular diseases and neurodegeneration 136. Thus, optimal generation of O2.- is required to sustain healthy living.
Acute inflammation following injury is the site for abundant production of ROS by phagocytic NADPH oxidases. As inflammation resolves and phagocyte count at the wound site falls, several aspects of healing such as cell proliferation and migration are supported by redox signaling where low-level ROS produced by non-phagocytic oxidases serve as messenger molecules 57. The critical significance of the NADPH oxidases in wound healing is rapidly unfolding. As discussed previously, NADPH oxidase deficient mice and humans suffer from impaired healing. As an integral part of the healing response, wounding induces H2O2 production 56. This response is also conserved in plants 137. Wound fluid from healing tissues contains the highest concentration of H2O2 compared to all other bodily fluids 56, 138. Of note, selective decomposition of H2O2 at the wound site using catalase overexpression approaches impairs the healing process demonstrating the key significance of H2O2 in wound healing 56. Importantly, catalase-dependent decomposition of H2O2 generates O2 as end-product. Thus, molecular O2 is not sufficient if NADPH oxidase dependent O2-consumption and redox signaling is impaired. How redox signals may contribute to tissue repair has been recently reviewed elsewhere 57, 139 and is beyond the scope of this article. In the context of this article it is important to appreciate that redox signals are generated at the cost of tissue O2. Thus, tissue hypoxia will limit redox signaling and disable the function of several growth factors (e.g. PDGF, VEGF, KGF, IGF, TGFα) and numerous molecular mechanisms (e.g. leukocyte recruitment, cell motility, integrin function) which rely on redox signaling 57, 139, 140.
Collagen deposition provides the matrix for angiogenesis and tissue remodeling. Maturation of collagen is O2-dependent. Of the O2 dependent enzymatic processes, the rate of collagen synthesis is reflected by the rate at which prolyl hydroxylation occurs 141. Collagen synthesis is half-maximal (Km using Micahelis-Menton equation) at a pO2 of 20-25mmHg 66, 142, with Vmax at levels approaching 250 mmHg. This represents levels of O2 availability that exceeds the pO2 normally present in the wound tissue and suggests that adequate wound tissue oxygenation is crucial to support collagen synthesis and maturation. Indeed, increasing wound oxygenation results in increased collagen deposition and tensile strength 143-145.
NO synthases
NO is widely recognized as a major signaling messenger that drive numerous aspects of (patho)physiology 146-149.O2 consuming nitric-oxide synthases (NOS) catalyze NO formation from the amino acid L-arginine. The reaction of NOS with O2 is fast and takes place within several steps 150. NOS are known to catalyze more than one reaction: the NO-producing reaction is considered to be the coupled reaction, and the uncoupled reactions are those that produce ROS, such as O2.- and H2O2 151. The key significance of NO in wound healing has been reviewed elsewhere 152, 153. In the context of this article it is important to note that O2 is often the overlooked substrate in NO synthesis. To date there has been little consideration of the role of O2 tension in the regulation of nitric oxide production associated with wound healing. Tissue O2 tension is known to significantly alter endogenous NO production in articular cartilage where the tissue pO2 is comparable to that of ischemic wounds 154. The preliminary observation that hyperbaric oxygen (HBO) therapy may significantly increase local wound NO levels is therefore understandable 155. Once generated, the biological significance of NO also depends on the tissue oxygenation status 156. As NO gas based therapies are being considered for healing wounds clinically, it is important to recognize that NO can block mitochondrial function by interacting with the cytochrome c oxidase (complex IV) of the electron transport chain in a manner that is reversible and in competition with O2. Concentrations of NO too low to inhibit respiration can trigger cellular defense response mechanisms. Inhibition of mitochondrial respiration by NO at low O2 concentrations can cause so-called “metabolic hypoxia” and divert O2 towards other oxygen-dependent systems. Metabolic hypoxia refers to a state wherein although O2 is available the cell is unable to utilize it for respiration 157. Such a diversion reactivates prolyl hydroxylases and thus accounts for the prevention by NO of the stabilization of the hypoxia inducible factor (HIF). When NO inhibits mitochondrial respiration under hypoxia, it prevents mitochondria from depleting local oxygen, enabling the continued hydroxylation and degradation of HIF-1α, thus leading to a situation in which the cell may fail to register hypoxia. Furthermore, in a wound setting where O2.- production is highly active, NO is likely to generate peroxynitrite which can affect the action of key enzymes, such as mitochondrial complex I, by S-nitrosation 157. NO-based wound therapeutics should be designed in light of these complexities.
The stability of HIF, and therefore its ability to drive HIF-dependent gene transcription, is differentially regulated by NO under conditions of normoxia and hypoxia. While NO stabilizes HIF under normoxia, the effect is exactly opposite under conditions of hypoxia 158. Under conditions of normoxia, NO may attenuate the ubiquitination of HIF-1α and thus abrogate binding of pVHL to HIF-1α 159. Ubiquitination of HIF would not take place if HIF is not hydroxylated by PHDs. Indeed, NO inhibits PHD activity. Fe2+ -coordination by NO seems to be the explanation for how NO inhibits PHDs. The stabilization of HIF under normoxia is also explained by the induction of HIF-1α synthesis by NO 160. Although speculative, different redox-active products, derived from chemically distinct NO donors, use divergent transmission systems to stabilize/express HIF-1α 160. Under conditions of hypoxia, NO and its derivatives inhibit hypoxia-induced HIF-1α accumulation 158. In light of the observation that NO attenuates PHD activity under normoxia to stabilize HIF-1α, raises the question whether PHD activity is regained under conditions of hypoxia-NO co-existence. An affirmative answer to this question came from the observation that oxygen-dependent death domain of HIF-1α, which accounts for protein stability, is needed for NO and its derivatives to reverse hypoxic HIF-1α stabilization 161. Several mechanistic hypotheses have been proposed to explain how NO impairs accumulation of HIF-1α under hypoxia 158. The scenario gets even more complicated in a wound setting where both phagocytic as well as non-phagocytic NADPH oxidases generate copious amounts of superoxide anion radicals 56, 138. Furthermore, hypoxic tissues are known to generate more ROS. The HIF system has revealed an unexpectedly direct connection between molecular oxygen, superoxide and NO in achieving or attenuating responses to hypoxia. The reaction between O2.- and NO represents a primary biochemical path in vivo 162. Flux rates of NO and O2.-, as well as the presence of antioxidant enzymes, can modulate HIF-1α stabilization 158. Understanding the multiple signals, that have the potential to deliver a flexible and controlled response to hypoxia, will be critical to develop therapeutic maneuvers. Thus, a clear appreciation of the specific wound tissue redox environment 57 becomes critically important in the context of planning NO-based therapeutics.
The Normoxic Setpoint and Oxygen Sensing
Cellular O2 homeostasis is tightly maintained within a narrow range (“normoxia”) due to the risk of oxidative damage from excess O2 (hyperoxia), and of metabolic demise from insufficient O2 (hypoxia). Vast majority of the current literature focuses on the sensing of hypoxia, and the work on hyperoxic sensing is limited. Both hypoxia and hyperoxia are relative terms. They refer to a state of oxygenation that departs from the normoxic setpoint, i.e. the pO2 to which cells or tissues are adjusted to under basal conditions 163. For any given cell or tissue, normoxic setpoint represents that state of oxygenation where the cell or tissue does not report hypoxia neither do they induce hyperoxia- induced cell signaling or manifest overt oxygen toxicity. It is likely that this set-point would represent a range of pO2, the span of which might depend on the tissue in question. Any change of O2 ambience exceeding that span would result in the switching on of a hypoxic or hyperoxic response. In the finest of scales, such response would be detected in the molecular scale such as HIF-stabilization or HRE-transactivation for hypoxia and say p21-induction for hyperoxia 164, 165. In a relatively coarser scale, oxygen-sensitive changes in cellular phenotype may be noted. Of note, different organs of the body have different normoxic set-points. While the lung and arterial vasculature represent the high end, organs such as the liver have very low basal pO2. pO2 ranges from 90 to below 3 Torr in mammalian organs under normoxic conditions with arterial pO2 of about 100 Torr or ∼14%O2 166.
Hypoxia sensing
Hypoxia sensing and response is activated upon exposure to a state of oxygenation that is lower than the pO2 to which the cells or tissue is adjusted to under basal conditions. This response cascade is centrally important in coping with the challenge of O2 deficiency. Hypoxia response has been mostly studied in transformed and tumor cells. It is important to recognize that findings from such cells may not be directly applicable to non-transformed primary cells which are involved in wound healing 167. Hypoxia is a hallmark of all ischemic diseases but is also noted under several physiological processes where exposure to a dynamic state of oxygenation is an integral component. During early pregnancy, trophoblast differentiation occurs in an environment of relative low O2 tension which is essential for normal embryonic and placental development 168. O2 supply to the human embryo in the first trimester is tightly controlled, suggesting that too much O2 may interfere with development. Relative to maternal tissue pO2, embryo is normally in a state of partial hypoxia 169, 170. Thus, hypoxia sensing and response is not only implicated in ischemic disease conditions but is also required for development where a changing state of oxygenation seems to serve as a cue for successful development. Whether this is nature’s approach to quality check each healthy birth for the ability of the new-born to cope with ischemic diseases later on in their lives may be viewed as a matter of interesting speculation.
Hypoxia sensing and response mechanisms may be broadly classified into two general categories: HIF-dependent and HIF-independent. Extensive discussion of these pathways is beyond the scope of this article and the readers are referred to excellent review articles 171-173.
HIF-dependent pathways
The basic helix-loop-helix (bHLH) proteins form a large superfamily of dimeric transcriptional regulators that are found in organisms from yeast to humans and function in critical developmental processes. One basis for the evolutionary classification of bHLH proteins is the presence or absence of additional domains, of which the most common are the PAS, orange and leucine-zipper domains. PAS domains, located carboxy-terminal to the bHLH region, are 260-310 residues long and function as dimerization motifs. They allow binding with other PAS proteins, non-PAS proteins, and small molecules such as dioxin. The PAS domain is named after three proteins containing it: Drosophila Period (Per), the human aryl hydrocarbon receptor nuclear translocator (Arnt) and Drosophila Single-minded (Sim). HIFs belong to the bHLH-PAS family of environmental sensors which bind to canonical DNA sequences call hypoxia response elements (HRE) in the promoters or enhancers of target genes 174. HIF is able to direct transcription from either of two transactivation domains, each of which is regulated by distinct mechanisms. The O2-dependent asparaginyl hydroxylase factor-inhibiting HIF-1alpha (FIH-1) is a key regulator of the HIF C-terminal transactivation domain, and provides a direct link between O2 sensing and HIF-mediated transcription. Additionally, there are phosphorylation and nitrosylation events reported to modulate HIF transcriptional activity, as well as numerous transcriptional coactivators and other interacting proteins that together provide cell and tissue specificity of HIF target gene regulation 175.
HIF-1 consists of a constitutively expressed subunit HIF-1beta and an oxygen-regulated subunit HIF-1alpha (or its paralogs HIF-2alpha and HIF-3alpha). The transcriptional role of HIF is primarily dependent on the stabilization of HIF-1alpha or its paralogs under hypoxic conditions. Under O2-replete conditions HIF-1alpha is very labile 176. Molecular O2 targets HIF for degradation by post-translational hydroxylation at specific prolyl residues within the alpha subunits. Hydroxylation at two prolyl residues within the central degradation domain of HIF1-alpha increases the affinity for the von Hippel-Lindau (pVHL) E3 ligase complex by at least three orders of magnitude, thus directing HIF-alpha polypeptides for proteolytic destruction by the ubiquitin/proteasome pathway. Because the HIF hydroxylases have an absolute requirement for molecular O2 this process is suppressed in hypoxia allowing HIF-alpha to escape destruction and activate transcription.
The O2 sensitive prolyl-hydroxylase domain enzymes (PHDs) and the asparagines hydroxylase (FIH, factor inhibiting HIF) regulate the transcriptional activity of HIFs 175. The unusual high KM of PHDs for oxygen allows small changes in the oxygen supply to affect enzyme activity, which makes this system an ideal oxygen sensor. In hypoxia, FIH-1 hydroxylation of Asn803 within the C-terminal transactivation domain (TAD) does not occur and HIF-1alpha fails to form a fully active transcriptional complex. Thus, HIF prolyl hydroxylation regulates proteolytic degradation of HIF whereas HIF asparaginyl hydroxylation modulates interaction with transcriptional co-activators. These hydroxylations are catalysed by a set of non-haem Fe(II)- and 2-oxoglutarate (2-OG)-dependent dioxygenases. During catalysis, the splitting of molecular O2 is coupled to the hydroxylation of HIF and the oxidative decarboxylation of 2-OG to give succinate and CO2. The von Hippel-Lindau tumor suppressor gene product, pVHL, functions as the substrate recognition component of an E3-ubiquitin ligase, which targets the O2-sensitive alpha-subunit of hypoxia-inducible factor (HIF) for rapid proteasomal degradation under normoxic conditions and as such plays a central role in molecular O2 sensing.
Stabilization of HIF under hypoxic conditions is followed by nuclear localization where HIF may bind to DNA sequences and other transcriptional regulators to influence gene expression (Table 1). The passage of transcription factors e.g. HIF-1alpha into the nucleus through the nuclear pore complex is regulated by nuclear transport receptors. Therefore nucleocytoplasmic shuttling can regulate transcriptional activity by facilitating the cellular traffic of transcription factors between both compartments 177.
Table 1.
HIF-1 target genes
| Erythropoiesis/ Iron Metabolism |
Cell Survival/ Proliferation |
Angiogenesis | Vascular tone |
Glucose Metabolism |
Matrix Metabolism |
|---|---|---|---|---|---|
| EPO | IGF2 | VEGF | NOS2 | HK1,2 | MMPs |
| Tf | TGF-α | Leptin | HO1 | LDHA | PAR/PAI |
| Tfr | ADM | TGF-▯3 | ET1 | PKM | Coll PHD |
| Ceruloplasmin | BNip3 | EG-VEGF | ADM | PFKL | |
| NIX | α1b | PGK1 | |||
| NDRG2 | PFKFB3 | ||||
| GAPDH | |||||
| GLUT1,3 | |||||
| ENO1 | |||||
| CA-9 | |||||
| ALD-A,C | |||||
| AK-3 |
α1b, α1b-adrenergic receptor; ADM, adrenomedulin; AK, adenylate kinase; ALD, aldolase; BNip3, Bcl-2/adenovirus EIB 19kD-interacting protein 3; CA, carbonic anhydrase; Coll PHD, collagen prolylhydroxylases; EG-VEGF, endocrine gland-derived VEGF; ENO, enolase; EPO, erythropoietin; ET, endothelin; GAPDH, gylceraldehyde phosphate dehydrogenase; GLUT, glucose transporters; HK1,2, hexokinase 1,2; HO, heme oxygenase; IGF, insulin-like growth factor; LDH-A, lactate dehydrogenase-A; MMP, matrix metalloproteinases; NDRG, N-Myc downstream-regulated genes; NIX, Nip 3-like protein X; NOS, nitric oxide synthase; PAR/PAI, plasminogen activator receptors and inhibitors; PGK1, phosphoglycerate kinase 1; PFKL, phosphofructokinase L; PKM, pyruvate kinase M; TGF, transforming growth factor; TF, transferrin; Tfr, Tf receptor.
Shortly after the cloning of HIF-1alpha, a closely related protein, HIF-2 alpha [also known as endothelial PAS protein, HIF-like factor (HLF), HIF-related factor (HRF), and member of the PAS superfamily 2 (MOP2)] was identified and cloned 178. HIF-2alpha regulates erythropoietin production in adults 179. HIF-1alpha functions as an up-stream player in the p21-mediated growth arrest of keratinocytes 180. Thus, HIF may antagonize certain aspects of skin repair. Negative pressure wound therapy, known to be effective in healing wounds clinically, is known to antagonize the stabilization of HIF-1alpha 181. HIF-dependent pathways for survival and vascularization can function under conditions were hypoxia is moderate and not extreme. As long as there is a threshold level of oxygenation sufficient to sustain life, HIF-dependent survival responses may benefit wound healing 182-184. Near-anoxic hypoxia, often noted in problem wounds 26, 27, is not compatible with life or tissue repair.
HIF-independent pathways
Conservation of ATP under conditions of limited O2 supply is a HIF-independent survival response that is not compatible with the energy-demanding healing process 49. For example, HIF-independent hypoxic inhibition of protein synthesis and cell growth is mediated by: i. hypoxia-induced cellular energy depletion; ii, mTOR inhibition via the AMPK/TSC2/Rheb pathway; iii, eEF2 inhibition mediated by AMP-activated protein kinase (AMPK); and iv, induction of ER stress that leads to eIF2α inhibition 185. mTOR is a Ser/Thr kinase that integrates signals from growth factors and nutrients to increase ribosome biogenesis 186. Upon hypoxic energy starvation, AMPK phosphorylates eEF2 kinase (eEF2K) on Ser398 and activates its kinase activity187. eEF2K then phosphorylates elongation factor eEF2 at Thr56, resulting in the inhibition of peptide elongation. mRNA translation is a critical component of cell growth and proliferation that is critically supported by eIF2α. Hypoxia causes ER stress which in turn inhibits eIF2α 185. Wound healing requires protein synthesis 188-190. Hypoxia causes global downregulation of protein synthesis. Hypoxia-induced translational attenuation may be linked to ER stress and the unfolded-protein response 191. The translational efficiency of individual genes is dynamic and changes with alterations in the cellular environment 192. Whereas changes in transcription can take hours to achieve, translational regulation is rapid and reversible 193. Preferential translation of select mRNA is another hallmark of response to hypoxia. Roughly 2.5% of total cellular transcripts are preferentially translated, despite arrest of global protein synthesis, in response to sustained extreme hypoxia 194. Taken together, while all these hypoxia-responses represent important HIF-independent mechanisms of energy conservation that promote survival under low O2 conditions, they are not compatible with the formation of new tissue as required during wound healing.
Intermittent hypoxia
O2 sensing is no longer a unique property limited to chemoreceptors but is a common property of tissues 195. The classic concept of intermittent hypoxia has been markedly revised in light of our current understanding of O2 sensing. Intermittent hypoxia (IH), or periodic exposure to hypoxia interrupted by return to normoxia or less hypoxic conditions, occurs in many circumstances. Chronic intermittent hypoxia (CIH) is a common life-threatening condition that occurs in many different diseases, including sleep-disordered breathing manifested as recurrent apneas. Excessive ROS have been identified as one of the causative factors in a variety of morbidities 196. In experimental models, CIH activates ROS dependent responses that include (a) altered carotid body function, the primary chemoreceptor for sensing changes in arterial blood O2; (b) elevated blood pressure; (c) enhanced release of transmitters and neurotrophic factors; (d) altered sleep and cognitive behaviors; and (e) activation of second-messenger pathways and transcriptional factors. Considerable evidence indicates elevated ROS levels in patients experiencing CIH as a consequence of recurrent apneas 196. Recently we evaluated the prevalence of obstructive sleep apnea (OSA) in the patient population of the OSU Wound Center. Between August 15th and September 30th of 2007, 105 consecutive unscreened patients of the wound center completed a sleep screening questionnaire. In this representative sample of patients of the wound center, fifty-one percent either were diagnosed with, or were at very high risk for OSA. Forty-three percent of patients with chronic non-healing wound were deemed at high risk for OSA 197. Whether intermittent hypoxia associated with OSA in chronic wound patients complicates wound healing warrants further investigation. Results of our survey may be explained by the association that many with chronic wounds are overweight due to metabolic complications (e.g. PAD and type II diabetes), and sleep apnea is more prevalent in overweight individuals. Merit of the hypothesis that sleep disorder may complicate wound healing is supported by the extensive literature identifying OSA as a causative factor underlying vascular disorders 198, 199.
Hyperoxia sensing
O2 got its name from “Principe Oxygene”, which means the acidifying principle. “Oxy” is from Greek, and means sharp or acid; “gen” is also from Greek, and means the origin of. Taken together, oxygen means “the origin of acid”. Joseph Priestly’s (1774) “dephlogisticated air” 200 and Carl Scheele’s (1771) “fire air” were soon characterized by Antoine Lavoisier as pure respirable air 201. Within decades of the first realization that oxygen is the element of life, Brizé-Fradin 202 noted in 1808 that “vital air” or pure oxygen would soon wear life out instead of maintaining it. That oxygen may be harmful to human health was first postulated in the late 19th century with Paul Bert’s work (1878) on oxygen sickness. Paul Bert’s work is regarded as one of the cornerstones of HBO medicine 203. He concluded that to avoid harmful effects, oxygen should not be inhaled at a concentration above 60% at 1ATA. Bert’s observation was extended through Michaeli’s theoretical considerations, Gerschman’s experimental verification and finally caught the interests of biomedical scientists when in 1969 McCord & Fridovich demonstrated that a metalloenzyme produced H2O2 by combining O2.- with hydrogen 204, 205. Today, H2O2 is widely known to function as a cellular messenger 108-123. Hyperoxia-inducible molecular biomarkers have been characterized 164, 165 enabling us to detect hyperoxic insult long before overt signs of oxygen toxicity and adverse clinical symptoms are manifested 206.
Although marginal hyperoxic challenge may induce favorable responses 207, a state of tissue oxygenation that far exceeds the normoxic setpoint of a given tissue is a clear risk factor that deserves appropriate attention 208. In a wound with pockets of hypoxia ranging in magnitude from extreme to marginal (Fig. 2), the goal should be to re-establish normoxia in the worst affected hypoxic pockets without exposing other parts of the wound tissue to such high levels of pO2 that would antagonize healing by hyperoxia-induced growth arrest or simply overt oxygen toxicity. One needs to be cautious about too much of a good thing 209. Endothelial progenitor cells (EPCs) are essential in vasculogenesis and wound healing, but their circulating and wound level numbers are decreased in diabetes. Hyperoxia reverses the diabetic defect in EPC mobilization 210. Moderate hyperoxia increases the appearance of new blood vessels in wounds 11. In addition to inducing VEGF gene expression, moderate hyperoxia enhances the expression of VEGF121/165 proteins and facilitates the release of VEGF165 from cell-associated stores 211. Among the factors that may oppose wound healing, extreme hyperoxia causes growth arrest 212-215 and cell death by a mitochondria-dependent apoptosis pathway 171, 216, 217. In addition, extreme hyperoxia does pose the threat of oxidative stress 218, 219.
Tuning the normoxic setpoint
When cells grown under standard culture conditions of 20% O2 are moved to 5% O2 ambience, hypoxia is reported by way of HIF-response elements. When the same cells are maintained at 5% O2 over long periods of time the O2-sensitive molecular machinery undergoes adjustment such that the same cells no longer report hypoxia. Interestingly, if these cells are maintained under mild hyperoxic conditions, e.g. 30% O2, and then brought down to 20% O2 culture conditions they report hypoxia 163. These simple observations establish two important points: (i) that it is not the actual pO2 but the ΔpO2 that seems to matter; and (ii) that the normoxic setpoint in a cell can be reset by the adjustment of O2-sensing machinery that is capable of responding to changes in the O2 ambience. In this simplified example, the machinery is represented by the PHD family of proteins the expression of which is upregulated under conditions of hypoxia and down-regulated under conditions of hyperoxia. This is noted not only in vitro but also in vivo. Here, although the example is limited to PHDs to keep the discussion simple it is important to recognize that there are numerous other O2-sensitive functions in a cell that would contribute to its overall response to any pO2 outside the normoxic setpoint. Thus, the normoxic setpoint in a biological cell is tunable. For example, under conditions of no change in ambient O2 condition a cell may be made to report hypoxia, as measured by HIF-transactivation, simply by knock-down of the PHDs 163. In response to down-regulated PHD1, cells not only report HRE-dependent gene expression but causes metabolic adaptations lowering tissue O2 consumption 220. Conditional inactivation of PHD2 in mice is sufficient to activate a subset of HIF target genes, including erythropoietin, leading to striking increases in red blood cell production 221. Tuning of the normoxic-setpoint when the cells are exposed to modest changes in O2 ambience seems to happen physiologically perhaps as an adaptive response. Comprehension of the pathways involved in such process should help us employ pharmacological and/or genetic approaches to therapeutically adjust the normoxic setpoint on an as needed basis. For example, moderate hypoxia is known to be a robust cue to initiate the angiogenic response. One can reap the angiogenic benefits of that knowledge by adopting therapeutic approaches that would lead to suppression of PHD function resulting in HIF stabilization and HRE-dependent transactivation. Indeed, this approach is being explored for wound therapies.
Tissue Oxygenation and Wound Therapy
HIF PHD-directed Wound Therapeutics
The PHD inhibitor FG-4497 readily stabilizes HIF-1alpha and subsequently drives the expression downstream of HIF target genes. FG-4497 is helpful in colitis perhaps by benefiting wound healing at the site of inflammation 222. Extracellular matrix (ECM) is predominantly collagen, and the imino acids (Pro and HyPro) comprise 25% of collagen residues. The final step in collagen degradation is catalyzed by prolidase, the obligate peptidase for imidodipeptides with Pro and HyPro in the carboxyl terminus. Defective wound healing in patients with inherited prolidase deficiency is associated with histologic features of angiopathy suggesting that prolidase may play a role in angiogenesis. Recently it has been demonstrated that prolidase inhibits PHD activity to induce HRE-dependent transactivation and facilitate angiogenic signaling 223. HIF-specific PHD inhibitors are being tried out for their efficacy in treating wounds. It is likely that such approaches to pharmacologically stabilize HIF will facilitate responses such as generation of angiogenic factors. Whether that response translates to functionally successful angiogenesis and improvements in wound closure will depend on whether other fundamental pre-requisites such as a threshold level of tissue oxygenation is present to fuel the healing process. This is of particular concern for ischemic wounds that suffer from extreme chronic hypoxia. If hypoxia alone would have been sufficient to heal, all ischemic wounds would have undergone rapid healing. Clinical observation is exactly the opposite. The key here is to couple hypoxia-response signaling with conditions such as appropriate tissue oxygenation that could sustain the healing process. PHD inhibitors alone are not likely to yield favorable outcomes in extremely hypoxic wounds. Furthermore, it is important to note in this context that PHD inhibition may stabilize HIF but does not guarantee transcriptional function. Co-substrate and co-factor requirements for Fe(II), ascorbate, and the Krebs cycle intermediate 2-OG, and inducible changes in the cellular abundance of three closely related HIF prolyl hydroxylases (PHD1-3) provide additional interfaces with cellular O2 status that may be important in regulating the oxygen-sensitive signal. Although under conditions of acute hypoxia PHD inactivation supports tissue survival, recently it has been demonstrated that under conditions of chronic hypoxia PHD overactivation is necessary as a survival response224. Chronic ischemic tissue overactivates all three isoforms of PHD to survive224. The merit of PHD inhibition for the treatment of ischemic wounds involving chronic hypoxia warrants reconsideration in this new light.
First and foremost it needs to be borne in mind that the overarching goal of oxygen therapy should be to correct wound hypoxia. While to some extent hyperoxia may be well tolerated by tissues, it would be prudent to avoid extreme hyperoxia 225. Although oxygen toxicity may not be imminently overt, an overdose of O2 is likely to trigger molecular responses such as cell cycle arrest and epigenetic modifications 226, 227 which would oppose healing. Second, approaches to keep a wound oxygenated over a longer period of time, as opposed to a few hours usually targeted in HBO therapy, should prove to be beneficial. In response to HBO, there is no sustained change in tissue O2 tension beyond the period of treatment 228.
The most fundamental factors in wound care are fluid management, temperature management, pain control, increased arterial O2 tension, the use of appropriate sterile techniques, and administration of prophylactic antibiotics 229. In addition, numerous cellular and molecular players are required to act in concert to successfully execute wound healing 230, 231. While examining the efficacy of O2 therapy in wound healing, it is critically important to recognize that O2 cannot act in isolation. Oxygen therapy may be only expected to benefit in those cases where the remaining essential players are functional and hypoxia is the only rate limiting factor. Thus, oxygen therapy is generally recommended as an adjunct to other forms of wound care 232, 233.
HBO
Hyperbaric oxygen therapy represents an effective approach to bolster tissue O2 levels 5 and has been found to benefit wound healing under specific conditions 234-238. Importantly, HBO may potentially work synergistically with growth factors such as PDGF to improve the outcomes of ischemic wounds 20. Because PDGF requires O2-derived H2O2 for successful function, this finding is not surprising 239. HBO causes sharp elevation in tissue pO2 240, 241. The administration of two atmospheres of 100% O2 for 2h may raise tissue pO2 by 10-20 folds 242, 243 over the values under basal room air conditions. This systemic approach to oxygenate tissues seems to offer some unique potential advantages. HBO may increase bone marrow NO in vivo thereby increasing the release of EPC into circulation. EPC mobilization into circulation is triggered by hyperoxia through induction of bone marrow NO with resulting enhancement in ischemic limb perfusion and wound healing 244-246. HBO may also increase nitric oxide levels in perivascular tissues via stimulation of NOS. Exposures to 2.0 and 2.8 ATA O2 stimulated neuronal (type I) NOS (nNOS) and significantly increased steady-state NO concentration, but the mechanism for enzyme activation differed at each partial pressure. Enzyme activation at 2.0 ATA O2 appeared to be due to an altered cellular redox state. Exposure to 2.8 ATA O2, but not 2ATA O2, increased nNOS activity by enhancing nNOS association with calmodulin 247. Thus, dosing does seem to matter in HBO therapy. Yet, in the clinics HBO is applied in a standard format to all patients regardless of their individual needs. Could this be an important factor in explaining the less than satisfactory results that HBO is generally thought to have produced in clinical settings 248? When a flat dose of oxygen is provided to all wound patients, it is possible that the specific dose applied is successful in oxygenating the pockets of extreme hypoxia in some wounds. In these cases, beneficial outcomes should be expected to follow. In the same vein it may be hypothesized that for some other cases, the dose applied is excessive compared to the need of the wound. In these wound with pockets of more moderate hypoxia, the same dose of HBO may be excessive negating the beneficial effects of hypoxia. This is of outstanding interest because excessive oxygen is known to cause growth arrest and accelerate cellular senescence 249-251. Because the ability to handle oxygen toxicity is dependent on the expression of genes encoding antioxidant proteins 252-259, it is possible that in some patients pre-disposed to oxidative stress the massive increase in tissue pO2 following HBO results in molecular responses such as growth arrest 212-214, 260 which may not manifest overt signs of oxygen toxicity but does resist wound healing. Another consideration in this regard would be the observation that a large fraction of chronic wound patients suffer from malnutrition 261-265. Such individuals are also known to be pre-disposed to oxidative stress and are limited in their ability to fend against oxygen toxicity 266-268. It is therefore reasonable to propose that chronic wound patients suffering from malnutrition are pre- disposed to HBO-induced oxidative stress. Taken together, such hypotheses would explain the inconsistent outcomes reported following HBO treatment 269-272 and call for HBO dosing regimens where physicians would prescribe the target wound pO2. This approach would be consistent with the emerging concept of personalized healthcare 273 and would require the design of new HBO devices fitted with the capability of real-time mapping of wound O2 tension as can be made possible via technologies such as electron paramagnetic resonance spectroscopy 274, 275.
Topical oxygen
Studies reported during the last five years renew interest in examining the significance of topical approaches to oxygenate cutaneous wounds as adjunctive therapy 1, 14, 18, 276, 277. Topically applied O2 gas is able to modestly raise the pO2 of the superficial wound tissue 277. In cases where hypoxia of the superficial wound tissue is a key limitation, topical oxygenation should prove to be helpful. Encouraging results obtained from the use of topical O2 gas in both clinical 1, 18 as well as pre-clinical 277 settings warrant serious consideration of this approach. Recently, perfluorocarbon droplets encapsulated in aqueous continuous phase has been used as topical O2 emulsion to treat experimental wounds. Results from this double-blind in vivo study demonstrate that topical approaches to oxygenate the wound significantly enhance the rate of epithelialization of partial-thickness excisional wounds and second-degree burns. Whether the emulsion was able to increase wound tissue pO2 was not examined, however 276. Epithelial wound healing is improved by transdermal sustained-delivery treatment with 100% O2 14. A recent clinical study testing the effects of topical O2 gas application on chronic wound presented clinically reports significant improvement in wound size. Interestingly, topical oxygen treatment was associated with higher VEGF expression in the wound edge tissue 18. Pure O2 is known to induce VEGF 15, 63, 219. Findings of the study testing the effects of topical oxygen gas on chronic wounds are consistent with previous findings suggesting that topical treatment may induce wound angiogenesis 278. Randomized clinical trials testing the effects of topical oxygenation on wound outcomes are warranted.
HBO and topical oxygen approaches have several contrasting features. The systemic effects of HBO, both favorable as well as unfavorable, may not be expected with topical oxygen. Topical oxygenation can only modestly raise tissue pO2 277 and cannot match the large increases in tissue pO2 typically noted in response to HBO 242, 243. If the goal is to correct hypoxia of the superficial tissue, topical approaches should be helpful. However, if the goal is to achieve larger supraphysiological levels of tissue pO2, HBO would represent the approach of choice. An advantage of topical approaches is that they are portable and therefore applicable in a field or home setting. The cost advantage of topical oxygenation over HBO is another practical consideration 276, 279, 280.
Summary
The etiology of chronic ischemic wounds is generally multi-factorial of which hypoxia is a common factor in most cases. Primarily based on the tumor literature, hypoxia is generally viewed as being angiogenic. This is true with the condition that hypoxia be acute and mild-modest in magnitude. Extreme hypoxia, as commonly noted in problem wounds, is not compatible with life or tissue repair. Adequate wound tissue oxygenation is required but may not be sufficient to favorably influence healing outcomes. Success in wound care depends on a personalized health care approach. The key lies in our ability to specifically identify the key limitations of a given wound and in developing a multifaceted strategy to address those limitations. In considering approaches to oxygenate the wound tissue it is important to recognize that both too little as well as too much may impede the healing process. Oxygen dosing based on the specific need of a wound therefore seems prudent. Therapeutic approaches targeting the oxygen sensing and redox signaling pathways are promising as well. Investment in bringing such capabilities to clinical practice should yield lucrative returns.
Acknowledgment
Supported by NIH awards RO1 HL073087, GM 077185 and GM 069589 to CKS.
Literature Cited
- 1.Kalliainen LK, Gordillo GM, Schlanger R, Sen CK. Topical oxygen as an adjunct to wound healing: a clinical case series. Pathophysiology. 2003;9:81–87. doi: 10.1016/s0928-4680(02)00079-2. [DOI] [PubMed] [Google Scholar]
- 2.Padberg FT, Back TL, Thompson PN, Hobson RW., 2nd Transcutaneous oxygen (TcPO2) estimates probability of healing in the ischemic extremity. Journal of Surgical Research. 1996;60:365–9. doi: 10.1006/jsre.1996.0059. [DOI] [PubMed] [Google Scholar]
- 3.Kabon B, Kurz A. Optimal perioperative oxygen administration. Curr Opin Anaesthesiol. 2006;19:11–8. doi: 10.1097/01.aco.0000192775.24774.15. [DOI] [PubMed] [Google Scholar]
- 4.Niinikoski J. Hyperbaric oxygen therapy of diabetic foot ulcers, transcutaneous oxymetry in clinical decision making. Wound Repair and Regeneration. 2003;11(6):458–461. doi: 10.1046/j.1524-475x.2003.11610.x. [DOI] [PubMed] [Google Scholar]
- 5.Niinikoski JH. Clinical hyperbaric oxygen therapy, wound perfusion, and transcutaneous oximetry. World J Surg. 2004;28:307–11. doi: 10.1007/s00268-003-7401-1. [DOI] [PubMed] [Google Scholar]
- 6.Hopf HW, Rollins MD. Wounds: an overview of the role of oxygen. Antioxid Redox Signal. 2007;9:1183–92. doi: 10.1089/ars.2007.1641. [DOI] [PubMed] [Google Scholar]
- 7.Kurz A, Sessler D, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical wound infection and shorten hospitalization. NEJM. 1996;334:1209–15. doi: 10.1056/NEJM199605093341901. [DOI] [PubMed] [Google Scholar]
- 8.Grief R, Akca O, Horn E-P, Kurz A, Sessler D. Supplemental periopertive oxygen to reduce the incidence of surgical wound infection. NEJM. 2000;342:161–67. doi: 10.1056/NEJM200001203420303. [DOI] [PubMed] [Google Scholar]
- 9.Belda FJ, Aguilera L, Garcia de la Asuncion J, Alberti J, Vicente R, Ferrandiz L, Rodriguez R, Company R, Sessler DI, Aguilar G, Botello SG, Orti R. Supplemental perioperative oxygen and the risk of surgical wound infection: a randomized controlled trial. Jama. 2005;294:2035–42. doi: 10.1001/jama.294.16.2035. [DOI] [PubMed] [Google Scholar]
- 10.Nakada T, Saito Y, Chikenji M, Koda S, Higuchi M, Kawata K, Ishida S, Takahashi S, Kondo S, Kubota Y, Kubota I, Shimizu Y. Therapeutic outcome of hyperbaric oxygen and basic fibroblast growth factor on intractable skin ulcer in legs: preliminary report. Plast Reconstr Surg. 2006;117:646–51. doi: 10.1097/01.prs.0000197206.48963.60. discussion 52-3. [DOI] [PubMed] [Google Scholar]
- 11.Sheikh AY, Rollins MD, Hopf HW, Hunt TK. Hyperoxia improves microvascular perfusion in a murine wound model. Wound Repair Regen. 2005;13:303–8. doi: 10.1111/j.1067-1927.2005.130313.x. [DOI] [PubMed] [Google Scholar]
- 12.Knighton DR, Silver IA, Hunt TK. Regulation of wound-healing angiogenesis-effect of oxygen gradients and inspired oxygen concentration. Surgery. 1981;90:262–70. [PubMed] [Google Scholar]
- 13.Klemetti E, Rico-Vargas S, Mojon P. Short duration hyperbaric oxygen treatment effects blood flow in rats: pilot observations. Lab Anim. 2005;39:116–21. doi: 10.1258/0023677052886529. [DOI] [PubMed] [Google Scholar]
- 14.Said HK, Hijjawi J, Roy N, Mogford J, Mustoe T. Transdermal sustained-delivery oxygen improves epithelial healing in a rabbit ear wound model. Arch Surg. 2005;140:998–1004. doi: 10.1001/archsurg.140.10.998. [DOI] [PubMed] [Google Scholar]
- 15.Sheikh AY, Gibson JJ, Rollins MD, Hopf HW, Hussain Z, Hunt TK. Effect of hyperoxia on vascular endothelial growth factor levels in a wound model. Archives of Surgery. 2000;135:1293–7. doi: 10.1001/archsurg.135.11.1293. [DOI] [PubMed] [Google Scholar]
- 16.Chen SJ, Yu CT, Cheng YL, Yu SY, Lo HC. Effects of hyperbaric oxygen therapy on circulating interleukin-8, nitric oxide, and insulin-like growth factors in patients with type 2 diabetes mellitus. Clin Biochem. 2006 doi: 10.1016/j.clinbiochem.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Botello SA, Garcia-Granero E, Lillo R, Lopez-Mozos F, Millan M, Lledo S. Randomized clinical trial to evaluate the effects of perioperative supplemental oxygen administration on the colorectal anastomosis. Br J Surg. 2006;93:698–706. doi: 10.1002/bjs.5370. [DOI] [PubMed] [Google Scholar]
- 18.Gordillo GM, Roy S, Khanna S, Schlanger R, Khandelwal S, Phillips G, Sen CK. Topical oxygen therapy induces VEGF expression and improves closure of clinically presented chronic wounds. Clinical and Experimental Pharmacology and Physiology. 2008;35:957–64. doi: 10.1111/j.1440-1681.2008.04934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sen CK. The general case for redox control of wound repair. Wound Repair Regen. 2003;11:431–38. doi: 10.1046/j.1524-475x.2003.11607.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhao LL, Davidson JD, Wee SC, Roth SI, T.A. M. Effect of hyperbaric oxygen and growth factors on rabbit ear ischemic ulcers. Arch Surg. 1994;129:1043–9. doi: 10.1001/archsurg.1994.01420340057010. [DOI] [PubMed] [Google Scholar]
- 21.Gordillo GM, Sen CK. Revisiting the essential role of oxygen in wound healing. Am J Surg. 2003;186:259–63. doi: 10.1016/s0002-9610(03)00211-3. [DOI] [PubMed] [Google Scholar]
- 22.Taylor CT, Pouyssegur J. Oxygen, hypoxia, and stress. Ann N Y Acad Sci. 2007;1113:87–94. doi: 10.1196/annals.1391.004. [DOI] [PubMed] [Google Scholar]
- 23.Kim JW, Gao P, Dang CV. Effects of hypoxia on tumor metabolism. Cancer Metastasis Rev. 2007;26:291–8. doi: 10.1007/s10555-007-9060-4. [DOI] [PubMed] [Google Scholar]
- 24.Allen DB, Maguire JJ, Mahdavian M, Wicke C, Marcocci L, Scheuenstuhl H, Chang M, Le AX, Hopf HW, Hunt TK. Wound hypoxia and acidosis limit neutrophil bacterial killing mechanisms. Arch Surg. 1997;132:991–6. doi: 10.1001/archsurg.1997.01430330057009. [DOI] [PubMed] [Google Scholar]
- 25.Kumari R, Willing LB, Krady JK, Vannucci SJ, Simpson IA. Impaired wound healing after cerebral hypoxia-ischemia in the diabetic mouse. J Cereb Blood Flow Metab. 2006 doi: 10.1038/sj.jcbfm.9600382. [DOI] [PubMed] [Google Scholar]
- 26.Wattel F, Mathieu D, Coget JM, Billard V. Hyperbaric oxygen therapy in chronic vascular wound management. Angiology. 1990;41:59–65. doi: 10.1177/000331979004100109. [DOI] [PubMed] [Google Scholar]
- 27.Kalani M, Brismar K, Fagrell B, Ostergren J, Jorneskog G. Transcutaneous oxygen tension and toe blood pressure as predictors for outcome of diabetic foot ulcers. Diabetes Care. 1999;22:147–51. doi: 10.2337/diacare.22.1.147. [DOI] [PubMed] [Google Scholar]
- 28.McPhail R, Cooper LT, Hodge DO, Cabanel ME, Rooke TW. Transcutaneous partial pressure of oxygen after surgical wounds. Vasc Med. 2004;9:125–7. doi: 10.1191/1358863x04vm539oa. [DOI] [PubMed] [Google Scholar]
- 29.Distler O, Distler JH, Scheid A, Acker T, Hirth A, Rethage J, Michel BA, Gay RE, Muller-Ladner U, Matucci-Cerinic M, Plate KH, Gassmann M, Gay S. Uncontrolled expression of vascular endothelial growth factor and its receptors leads to insufficient skin angiogenesis in patients with systemic sclerosis. Circ Res. 2004;95:109–16. doi: 10.1161/01.RES.0000134644.89917.96. [DOI] [PubMed] [Google Scholar]
- 30.van der Goes A, Brouwer J, Hoekstra K, Roos D, van den Berg TK, Dijkstra CD. Reactive oxygen species are required for the phagocytosis of myelin by macrophages. J Neuroimmunol. 1998;92:67–75. doi: 10.1016/s0165-5728(98)00175-1. [DOI] [PubMed] [Google Scholar]
- 31.Leeper-Woodford SK, Mills JW. Phagocytosis and ATP levels in alveolar macrophages during acute hypoxia. Am J Respir Cell Mol Biol. 1992;6:326–34. doi: 10.1165/ajrcmb/6.3.326. [DOI] [PubMed] [Google Scholar]
- 32.Hafner J, Schaad I, Schneider E, Seifert B, Burg G, Cassina PC. Leg ulcers in peripheral arterial disease (arterial leg ulcers): impaired wound healing above the threshold of chronic critical limb ischemia. J Am Acad Dermatol. 2000;43:1001–8. doi: 10.1067/mjd.2000.108375. [DOI] [PubMed] [Google Scholar]
- 33.Chen WY, Rogers AA. Recent insights into the causes of chronic leg ulceration in venous diseases and implications on other types of chronic wounds. Wound Repair Regen. 2007;15:434–49. doi: 10.1111/j.1524-475X.2007.00250.x. [DOI] [PubMed] [Google Scholar]
- 34.Ingram RH., Jr. Arterial oxygenation differences with carbon dioxide-induced versus voluntary increases in minute ventilation in chronic airway obstruction. Am Rev Respir Dis. 1977;116:181–6. doi: 10.1164/arrd.1977.116.2.181. [DOI] [PubMed] [Google Scholar]
- 35.Krowka MJ. Pathophysiology of arterial hypoxemia in advanced liver disease. Liver Transpl Surg. 1996;2:308–12. doi: 10.1002/lt.500020412. [DOI] [PubMed] [Google Scholar]
- 36.Furukawa T, Hara N, Yasumoto K, Inokuchi K. Arterial hypoxemia in patients with hepatic cirrhosis. Am J Med Sci. 1984;287:10–3. doi: 10.1097/00000441-198405000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Romaldini H, Rodriguez-Roisin R, Lopez FA, Ziegler TW, Bencowitz HZ, Wagner PD. The mechanisms of arterial hypoxemia during hemodialysis. Am Rev Respir Dis. 1984;129:780–4. doi: 10.1164/arrd.1984.129.5.780. [DOI] [PubMed] [Google Scholar]
- 38.Ballas SK, Park CH. Severe hypoxemia secondary to acute sternal infarction in sickle cell anemia. J Nucl Med. 1991;32:1617–8. [PubMed] [Google Scholar]
- 39.Farfel Z, Freimark D, Mayan H, Gafni J. Spurious hypoglycemia, hyperkalemia and hypoxemia in chronic hemolytic anemia. Isr J Med Sci. 1990;26:606–10. [PubMed] [Google Scholar]
- 40.Apte NM, Karnad DR. Altitude hypoxemia and the arterial-to-alveolar oxygen ratio. Ann Intern Med. 1990;112:547–8. doi: 10.7326/0003-4819-112-7-547. [DOI] [PubMed] [Google Scholar]
- 41.Naschitz JE, Kuhnreich E, Yeshurun D. Arterial hypoxemia following the administration of sublingual nitroglycerin in patients with ischemic heart disease and pneumonia. Respiration. 1981;41:202–7. doi: 10.1159/000194380. [DOI] [PubMed] [Google Scholar]
- 42.Lin YT, Orkin LR. Arterial hypoxemia in patients with anterior and posterior nasal packings. Laryngoscope. 1979;89:140–4. doi: 10.1288/00005537-197901000-00015. [DOI] [PubMed] [Google Scholar]
- 43.Giovannini I, Boldrini G, Sganga G, Castiglioni G, Castagneto M. Quantification of the determinants of arterial hypoxemia in critically ill patients. Crit Care Med. 1983;11:644–5. doi: 10.1097/00003246-198308000-00011. [DOI] [PubMed] [Google Scholar]
- 44.Birklein F, Weber M, Neundorfer B. Increased skin lactate in complex regional pain syndrome: evidence for tissue hypoxia? Neurology. 2000;55:1213–5. doi: 10.1212/wnl.55.8.1213. [DOI] [PubMed] [Google Scholar]
- 45.Wetterberg T, Sjoberg T, Steen S. Effects of hypothermia in hypercapnia and hypercapnic hypoxemia. Acta Anaesthesiol Scand. 1993;37:296–302. doi: 10.1111/j.1399-6576.1993.tb03718.x. [DOI] [PubMed] [Google Scholar]
- 46.Wetterberg T, Sjoberg T, Steen S. Effects of hypothermia with and without buffering in hypercapnia and hypercapnic hypoxemia. Acta Anaesthesiol Scand. 1994;38:293–9. doi: 10.1111/j.1399-6576.1994.tb03892.x. [DOI] [PubMed] [Google Scholar]
- 47.Weissmann N, Sommer N, Schermuly RT, Ghofrani HA, Seeger W, Grimminger F. Oxygen sensors in hypoxic pulmonary vasoconstriction. Cardiovasc Res. 2006;71:620–9. doi: 10.1016/j.cardiores.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 48.Ichioka S, Ando T, Shibata M, Sekiya N, Nakatsuka T. Oxygen Consumption of Keloids and Hypertrophic Scars. Ann Plast Surg. 2008;60:194–97. doi: 10.1097/SAP.0b013e318053ec1d. [DOI] [PubMed] [Google Scholar]
- 49.Gupta A, Raghubir R. Energy metabolism in the granulation tissue of diabetic rats during cutaneous wound healing. Mol Cell Biochem. 2005;270:71–7. doi: 10.1007/s11010-005-5258-3. [DOI] [PubMed] [Google Scholar]
- 50.Hohn DC, Ponce B, Burton RW, Hunt TK. Antimicrobial systems of the surgical wound. I. A comparison of oxidative metabolism and microbicidal capacity of phagocytes from wounds and from peripheral blood. Am J Surg. 1977;133:597–600. doi: 10.1016/0002-9610(77)90018-6. [DOI] [PubMed] [Google Scholar]
- 51.Matsuda T, Clark N, Hariyani GD, Bryant RS, Hanumadass ML, Kagan RJ. The effect of burn wound size on resting energy expenditure. J Trauma. 1987;27:115–8. doi: 10.1097/00005373-198702000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Im MJ, Hoopes JE. Energy metabolism in healing skin wounds. J Surg Res. 1970;10:459–64. doi: 10.1016/0022-4804(70)90070-3. [DOI] [PubMed] [Google Scholar]
- 53.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 54.Yin J, Xu K, Zhang J, Kumar A, Yu FS. Wound-induced ATP release and EGF receptor activation in epithelial cells. J Cell Sci. 2007;120:815–25. doi: 10.1242/jcs.03389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wesley UV, Bove PF, Hristova M, McCarthy S, van der Vliet A. Airway epithelial cell migration and wound repair by ATP-mediated activation of dual oxidase 1. J Biol Chem. 2007;282:3213–20. doi: 10.1074/jbc.M606533200. [DOI] [PubMed] [Google Scholar]
- 56.Roy S, Khanna S, Nallu K, Hunt TK, Sen CK. Dermal wound healing is subject to redox control. Mol Ther. 2006;13:211–20. doi: 10.1016/j.ymthe.2005.07.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sen CK, Roy S. Redox signals in wound healing. Biochim Biophys Acta. 2008;1780:1348–61. doi: 10.1016/j.bbagen.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olanrewaju HA, Qin W, Feoktistov I, Scemama JL, Mustafa SJ. Adenosine A(2A) and A(2B) receptors in cultured human and porcine coronary artery endothelial cells. Am J Physiol Heart Circ Physiol. 2000;279:H650–6. doi: 10.1152/ajpheart.2000.279.2.H650. [DOI] [PubMed] [Google Scholar]
- 59.Harrington LS, Evans RJ, Wray J, Norling L, Swales KE, Vial C, Ali F, Carrier MJ, Mitchell JA. Purinergic 2×1 receptors mediate endothelial dependent vasodilation to ATP. Mol Pharmacol. 2007;72:1132–6. doi: 10.1124/mol.107.037325. [DOI] [PubMed] [Google Scholar]
- 60.Burnstock G. Dual control of vascular tone and remodelling by ATP released from nerves and endothelial cells. Pharmacol Rep. 2008;60:12–20. [PubMed] [Google Scholar]
- 61.Chiang B, Essick E, Ehringer W, Murphree S, Hauck MA, Li M, Chien S. Enhancing skin wound healing by direct delivery of intracellular adenosine triphosphate. Am J Surg. 2007;193:213–8. doi: 10.1016/j.amjsurg.2006.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berthod F, Germain L, Tremblay N, Auger FA. Extracellular matrix deposition by fibroblasts is necessary to promote capillary-like tube formation in vitro. J Cell Physiol. 2006;207:491–8. doi: 10.1002/jcp.20584. [DOI] [PubMed] [Google Scholar]
- 63.Hopf HW, Gibson JJ, Angeles AP, Constant JS, Feng JJ, Rollins MD, Hussain M Zamirul, Hunt TK. Hyperoxia and angiogenesis. Wound Repair Regen. 2005;13:558–64. doi: 10.1111/j.1524-475X.2005.00078.x. [DOI] [PubMed] [Google Scholar]
- 64.Hunt TK, Aslam RS, Beckert S, Wagner S, Ghani QP, Hussain MZ, Roy S, Sen CK. Aerobically Derived Lactate Stimulates Revascularization and Tissue Repair via Redox Mechanisms. Antioxid Redox Signal. 2007;9:1115–24. doi: 10.1089/ars.2007.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mussini E, Hutton JJ, Jr., Udenfriend S. Collagen proline hydroxylase in wound healing, granuloma formation, scurvy, and growth. Science. 1967;157:927–9. doi: 10.1126/science.157.3791.927. [DOI] [PubMed] [Google Scholar]
- 66.Myllyla R, Tuderman L, Kivirikko K. Mechanism of the prolyl hydroxlase reaction.2. Kinetic analysis of the reaction sequence. Eur J Biochem. 1977;80:349–57. doi: 10.1111/j.1432-1033.1977.tb11889.x. [DOI] [PubMed] [Google Scholar]
- 67.Hunt TK, Zederfeldt B, Goldstick TK. Oxygen and healing. American Journal of Surgery. 1969;118:521–5. doi: 10.1016/0002-9610(69)90174-3. [DOI] [PubMed] [Google Scholar]
- 68.Jonsson K, Jensen J, Goodson W, Scheuenstuhl H, West J, Hopf H, Hunt T. Tissue oxygenation, anemia, and perfusion in relation to wound healing in surgical patients. Annals of Surgery. 1991;214:605–13. doi: 10.1097/00000658-199111000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sbarra AJ, Karnovsky ML. The biological basis of phagocytosis. 1: Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. Journal of Biological Chemistry. 1959;234:1355. [PubMed] [Google Scholar]
- 70.Babior BM. Oxygen-dependent microbial killing by phagocytes (first of two parts) N Engl J Med. 1978;298:659–68. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- 71.Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–31. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Y, Zeigler MM, Lam GK, Hunter MG, Eubank TD, Khramtsov VV, Tridandapani S, Sen CK, Marsh CB. The role of the NADPH oxidase complex, p38 MAPK, and Akt in regulating human monocyte/macrophage survival. Am J Respir Cell Mol Biol. 2007;36:68–77. doi: 10.1165/rcmb.2006-0165OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brown JR, Goldblatt D, Buddle J, Morton L, Thrasher AJ. Diminished production of anti-inflammatory mediators during neutrophil apoptosis and macrophage phagocytosis in chronic granulomatous disease (CGD) J Leukoc Biol. 2003;73:591–9. doi: 10.1189/jlb.1202599. [DOI] [PubMed] [Google Scholar]
- 74.Knighton DR, Halliday B, Hunt TK. Oxygen as an antibiotic: a comparison of the effects of inspired oxygen concentration and antibiotic administration on in vivo bacterial clearance. Arch Surg. 1986:121. doi: 10.1001/archsurg.1986.01400020077009. [DOI] [PubMed] [Google Scholar]
- 75.Segal AW. How superoxide production by neutrophil leukocytes kills microbes. Novartis Found Symp. 2006;279:92–8. discussion 98-100, 216-9. [PubMed] [Google Scholar]
- 76.Bissonnette SA, Glazier CM, Stewart MQ, Brown GE, Ellson CD, Yaffe MB. Phosphatidylinositol 3-Phosphate-dependent and -independent Functions of p40phox in Activation of the Neutrophil NADPH Oxidase. J Biol Chem. 2008;283:2108–19. doi: 10.1074/jbc.M706639200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dang PM, Stensballe A, Boussetta T, Raad H, Dewas C, Kroviarski Y, Hayem G, Jensen ON, Gougerot-Pocidalo MA, El-Benna J. A specific p47phox -serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. J Clin Invest. 2006;116:2033–43. doi: 10.1172/JCI27544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 79.Griendling KK. NADPH oxidases: new regulators of old functions. Antioxid Redox Signal. 2006;8:1443–5. doi: 10.1089/ars.2006.8.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takeya R, Sumimoto H. Regulation of novel superoxide-producing NAD(P)H oxidases. Antioxid Redox Signal. 2006;8:1523–32. doi: 10.1089/ars.2006.8.1523. [DOI] [PubMed] [Google Scholar]
- 81.Ushio-Fukai M. VEGF signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal. 2007;9:731–9. doi: 10.1089/ars.2007.1556. [DOI] [PubMed] [Google Scholar]
- 82.Eckert JW, Abramson SL, Starke J, Brandt ML. The surgical implications of chronic granulomatous disease. American Journal of Surgery. 1995;169:320–3. doi: 10.1016/S0002-9610(99)80167-6. [DOI] [PubMed] [Google Scholar]
- 83.Kume A, Dinauer MC. Gene therapy for chronic granulomatous disease. Journal of Laboratory & Clinical Medicine. 2000;135:122–8. doi: 10.1067/mlc.2000.104458. [DOI] [PubMed] [Google Scholar]
- 84.Ambruso DR, Knall C, Abell AN, Panepinto J, Kurkchubasche A, Thurman G, Gonzalez-Aller C, Hiester A, deBoer M, Harbeck RJ, Oyer R, Johnson GL, Roos D. Human neutrophil immunodeficiency syndrome is associated with an inhibitory Rac2 mutation. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:4654–9. doi: 10.1073/pnas.080074897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wattel F, Mathieu D. [Oxygen and wound healing] Bull Acad Natl Med. 2005;189:853–64. discussion 64-5. [PubMed] [Google Scholar]
- 86.Hopf H, Hunt T, West J, Blomquist P, Goodson W, Jensen A, Jonsson K, Paty P, Rabkin J, Upton R, vonSmitten K, Whitney J. Wound tissue oxygen tension predicts the risk of wound infection in surgical patients. Archives of Surgery. 1997;132:997–1004. doi: 10.1001/archsurg.1997.01430330063010. [DOI] [PubMed] [Google Scholar]
- 87.Hopf HW, Hunt TK, Rosen N. Supplemental oxygen and risk of surgical site infection. Jama. 2004;291:1956. doi: 10.1001/jama.291.16.1956-a. author reply 58-9. [DOI] [PubMed] [Google Scholar]
- 88.Cadet JL. Free radical mechanisms in the central nervous system: an overview. Int J Neurosci. 1988;40:13–8. doi: 10.3109/00207458808985722. [DOI] [PubMed] [Google Scholar]
- 89.Clark IA, Cowden WB, Hunt NH. Free radical-induced pathology. Med Res Rev. 1985;5:297–332. doi: 10.1002/med.2610050303. [DOI] [PubMed] [Google Scholar]
- 90.Comporti M. Three models of free radical-induced cell injury. Chem Biol Interact. 1989;72:1–56. doi: 10.1016/0009-2797(89)90016-1. [DOI] [PubMed] [Google Scholar]
- 91.Dormandy TL. Free-radical pathology and medicine. A review. J R Coll Physicians Lond. 1989;23:221–7. [PMC free article] [PubMed] [Google Scholar]
- 92.Harman D. Free radical theory of aging: history. Exs. 1992;62:1–10. doi: 10.1007/978-3-0348-7460-1_1. [DOI] [PubMed] [Google Scholar]
- 93.Machlin LJ, Bendich A. Free radical tissue damage: protective role of antioxidant nutrients. Faseb J. 1987;1:441–5. [PubMed] [Google Scholar]
- 94.Muller DP. Free radical problems of the newborn. Proc Nutr Soc. 1987;46:69–75. doi: 10.1079/pns19870009. [DOI] [PubMed] [Google Scholar]
- 95.Simpson PJ, Mickelson JK, Lucchesi BR. Free radical scavengers in myocardial ischemia. Fed Proc. 1987;46:2413–21. [PubMed] [Google Scholar]
- 96.Slater TF. Free-radical mechanisms in tissue injury. Biochem J. 1984;222:1–15. doi: 10.1042/bj2220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chylack LT, Jr., Brown NP, Bron A, Hurst M, Kopcke W, Thien U, Schalch W. The Roche European American Cataract Trial (REACT): a randomized clinical trial to investigate the efficacy of an oral antioxidant micronutrient mixture to slow progression of age-related cataract. Ophthalmic Epidemiol. 2002;9:49–80. doi: 10.1076/opep.9.1.49.1717. [DOI] [PubMed] [Google Scholar]
- 98.Greenberg ER, Baron JA, Tosteson TD, Freeman DH, Jr., Beck GJ, Bond JH, Colacchio TA, Coller JA, Frankl HD, Haile RW, et al. A clinical trial of antioxidant vitamins to prevent colorectal adenoma. Polyp Prevention Study Group. N Engl J Med. 1994;331:141–7. doi: 10.1056/NEJM199407213310301. [DOI] [PubMed] [Google Scholar]
- 99.Jha P, Flather M, Lonn E, Farkouh M, Yusuf S. The antioxidant vitamins and cardiovascular disease. A critical review of epidemiologic and clinical trial data. Ann Intern Med. 1995;123:860–72. doi: 10.7326/0003-4819-123-11-199512010-00009. [DOI] [PubMed] [Google Scholar]
- 100.Kaugars GE, Silverman S, Jr., Lovas JG, Brandt RB, Riley WT, Dao Q, Singh VN, Gallo J. A clinical trial of antioxidant supplements in the treatment of oral leukoplakia. Oral Surg Oral Med Oral Pathol. 1994;78:462–8. doi: 10.1016/0030-4220(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 101.Marchioli R, Schweiger C, Levantesi G, Tavazzi L, Valagussa F. Antioxidant vitamins and prevention of cardiovascular disease: epidemiological and clinical trial data. Lipids. 2001;36(Suppl):S53–63. doi: 10.1007/s11745-001-0683-y. [DOI] [PubMed] [Google Scholar]
- 102.Sen CK. Redox signaling and the emerging therapeutic potential of thiol antioxidants. Biochem Pharmacol. 1998;55:1747–58. doi: 10.1016/s0006-2952(97)00672-2. [DOI] [PubMed] [Google Scholar]
- 103.Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. Faseb J. 1996;10:709–20. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- 104.Sen CK. Cellular thiols and redox-regulated signal transduction. Current Topics in Cellular Regulation. 2000;36:1–30. doi: 10.1016/s0070-2137(01)80001-7. [DOI] [PubMed] [Google Scholar]
- 105.Demple B. Redox signaling and gene control in the Escherichia coli soxRS oxidative stress regulon--a review. Gene. 1996;179:53–7. doi: 10.1016/s0378-1119(96)00329-0. [DOI] [PubMed] [Google Scholar]
- 106.Powis G, Gasdaska JR, Baker A. Redox signaling and the control of cell growth and death. Adv Pharmacol. 1997;38:329–59. doi: 10.1016/s1054-3589(08)60990-4. [DOI] [PubMed] [Google Scholar]
- 107.Stamler JS. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994;78:931–6. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 108.Alvarez ME, Pennell RI, Meijer PJ, Ishikawa A, Dixon RA, Lamb C. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell. 1998;92:773–84. doi: 10.1016/s0092-8674(00)81405-1. [DOI] [PubMed] [Google Scholar]
- 109.Georgiou G. How to flip the (redox) switch. Cell. 2002;111:607–10. doi: 10.1016/s0092-8674(02)01165-0. [DOI] [PubMed] [Google Scholar]
- 110.Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, Moura IC, Lennon-Dumenil AM, Seabra MC, Raposo G, Amigorena S. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126:205–18. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 111.Singh DK, Kumar D, Siddiqui Z, Basu SK, Kumar V, Rao KV. The strength of receptor signaling is centrally controlled through a cooperative loop between Ca2+ and an oxidant signal. Cell. 2005;121:281–93. doi: 10.1016/j.cell.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 112.Tonks NK. Redox redux: revisiting PTPs and the control of cell signaling. Cell. 2005;121:667–70. doi: 10.1016/j.cell.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 113.Stone JR, Yang S. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal. 2006;8:243–70. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- 114.Hajnoczky G, Hoek JB. Cell signaling. Mitochondrial longevity pathways. Science. 2007;315:607–9. doi: 10.1126/science.1138825. [DOI] [PubMed] [Google Scholar]
- 115.Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–2. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 116.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–3. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 117.Shibata Y, Branicky R, Landaverde IO, Hekimi S. Redox regulation of germline and vulval development in Caenorhabditis elegans. Science. 2003;302:1779–82. doi: 10.1126/science.1087167. [DOI] [PubMed] [Google Scholar]
- 118.Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–9. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- 119.Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, Davies JM, Dolan L. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–6. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 120.Fox GC, Shafiq M, Briggs DC, Knowles PP, Collister M, Didmon MJ, Makrantoni V, Dickinson RJ, Hanrahan S, Totty N, Stark MJ, Keyse SM, McDonald NQ. Redox-mediated substrate recognition by Sdp1 defines a new group of tyrosine phosphatases. Nature. 2007;447:487–92. doi: 10.1038/nature05804. [DOI] [PubMed] [Google Scholar]
- 121.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–8. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 122.Lee JW, Helmann JD. The PerR transcription factor senses H2O2 by metal-catalysed histidine oxidation. Nature. 2006;440:363–7. doi: 10.1038/nature04537. [DOI] [PubMed] [Google Scholar]
- 123.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 124.Cabello CM, Bair Iii WB, Wondrak GT. Experimental therapeutics: Targeting the redox Achilles heel of cancer. Curr Opin Investig Drugs. 2007;8:1022–37. [PubMed] [Google Scholar]
- 125.Hoshino Y, Mishima M. Redox-Based Therapeutics for Lung Diseases. Antioxid Redox Signal. 2008 doi: 10.1089/ars.2007.1961. [DOI] [PubMed] [Google Scholar]
- 126.Agostinelli E, Tempera G, Molinari A, Salvi M, Battaglia V, Toninello A, Arancia G. The physiological role of biogenic amines redox reactions in mitochondria. New perspectives in cancer therapy. Amino Acids. 2007;33:175–87. doi: 10.1007/s00726-007-0510-7. [DOI] [PubMed] [Google Scholar]
- 127.Friedlich AL, Beal MF. Prospects for redox-based therapy in neurodegenerative diseases. Neurotox Res. 2000;2:229–37. doi: 10.1007/BF03033796. [DOI] [PubMed] [Google Scholar]
- 128.Pennington JD, Jacobs KM, Sun L, Bar-Sela G, Mishra M, Gius D. Thioredoxin and thioredoxin reductase as redox-sensitive molecular targets for cancer therapy. Curr Pharm Des. 2007;13:3368–77. [PubMed] [Google Scholar]
- 129.Pennington JD, Wang TJ, Nguyen P, Sun L, Bisht K, Smart D, Gius D. Redox-sensitive signaling factors as a novel molecular targets for cancer therapy. Drug Resist Updat. 2005;8:322–30. doi: 10.1016/j.drup.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 130.Arnold RS, Shi J, Murad E, Whalen AM, Sun CQ, Polavarapu R, Parthasarathy S, Petros JA, Lambeth JD. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5550–55. doi: 10.1073/pnas.101505898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mofarrahi M, Brandes RP, Gorlach A, Hanze J, Terada LS, Quinn MT, Mayaki D, Petrof B, Hussain SN. Regulation of proliferation of skeletal muscle precursor cells by NADPH oxidase. Antioxid Redox Signal. 2008;10:559–74. doi: 10.1089/ars.2007.1792. [DOI] [PubMed] [Google Scholar]
- 132.Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10:1343–74. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Alom-Ruiz SP, Anilkumar N, Shah AM. Reactive oxygen species and endothelial activation. Antioxid Redox Signal. 2008;10:1089–100. doi: 10.1089/ars.2007.2007. [DOI] [PubMed] [Google Scholar]
- 134.Naka K, Muraguchi T, Hoshii T, Hirao A. Regulation of Reactive Oxygen Species and Genomic Stability in Hematopoietic Stem Cells. Antioxid Redox Signal. 2008 doi: 10.1089/ars.2008.2114. [DOI] [PubMed] [Google Scholar]
- 135.Kulkarni AC, Kuppusamy P, Parinandi N. Oxygen, the lead actor in the pathophysiologic drama: enactment of the trinity of normoxia, hypoxia, and hyperoxia in disease and therapy. Antioxid Redox Signal. 2007;9:1717–30. doi: 10.1089/ars.2007.1724. [DOI] [PubMed] [Google Scholar]
- 136.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 137.Angelini R, Tisi A, Rea G, Chen MM, Botta M, Federico R, Cona A. Involvement of polyamine oxidase in wound healing. Plant Physiol. 2008;146:162–77. doi: 10.1104/pp.107.108902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ojha N, Roy S, He G, Biswas S, Velayutham M, Khanna S, Kuppusamy P, Zweier JL, Sen CK. Assessment of wound-site redox environment and the significance of Rac2 in cutaneous healing. Free Radic Biol Med. 2007 doi: 10.1016/j.freeradbiomed.2007.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Roy S, Khanna S, Sen CK. Redox regulation of the VEGF signaling path and tissue vascularization: Hydrogen peroxide, the common link between physical exercise and cutaneous wound healing. Free Radic Biol Med. 2008;44:180–92. doi: 10.1016/j.freeradbiomed.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 140.Roy S, Khanna S, Rink C, Biswas S, Sen CK. Characterization of the acute temporal changes in excisional murine cutaneous wound inflammation by screening of the wound-edge transcriptome. Physiol Genomics. 2008 doi: 10.1152/physiolgenomics.00045.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Prockop D, Kivirikko K, Tuderman L, Guzman N. The biosynthesis of collagen and its disorders (part 1) NEJM. 1979;301:13–23. doi: 10.1056/NEJM197907053010104. [DOI] [PubMed] [Google Scholar]
- 142.Hutton J, Tappel A, Undenfried S. Cofactor and substrate requirements of collagen proline hydroxylase. Arch Biochem Biophys. 1967;118:231–40. [Google Scholar]
- 143.Niinikoski J. Effect of oxygen supply on wound healing and formation of experimental granulation tissue. Acta Physiol Scand. 1970;78:1–72. [PubMed] [Google Scholar]
- 144.Hunt T, Pai M. The effect of varying ambient oxygen tensions on wound metabolism and collagen synthesis. Surgery Gynecology and Obstetrics. 1972;135:561–67. [PubMed] [Google Scholar]
- 145.Stephens F, Hunt T. Effect of Changes in inspired oxygen and carbon dioxide tensions on wound tensile strength. Annals of Surgery. 1971;173:515. doi: 10.1097/00000658-197104000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Buerk DG. Nitric oxide regulation of microvascular oxygen. Antioxid Redox Signal. 2007;9:829–43. doi: 10.1089/ars.2007.1551. [DOI] [PubMed] [Google Scholar]
- 147.Chen K, Pittman RN, Popel AS. Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxid Redox Signal. 2008;10:1185–98. doi: 10.1089/ars.2007.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Knott AB, Bossy-Wetzel E. Nitric Oxide in Health and Disease of the Nervous System. Antioxid Redox Signal. 2008 doi: 10.1089/ars.2008.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xia Y. Superoxide generation from nitric oxide synthases. Antioxid Redox Signal. 2007;9:1773–8. doi: 10.1089/ars.2007.1733. [DOI] [PubMed] [Google Scholar]
- 150.Marchal S, Gorren AC, Andersson KK, Lange R. Hunting oxygen complexes of nitric oxide synthase at low temperature and high pressure. Biochem Biophys Res Commun. 2005;338:529–35. doi: 10.1016/j.bbrc.2005.08.090. [DOI] [PubMed] [Google Scholar]
- 151.Stuehr D, Pou S, Rosen GM. Oxygen reduction by nitric-oxide synthases. J Biol Chem. 2001;276:14533–6. doi: 10.1074/jbc.R100011200. [DOI] [PubMed] [Google Scholar]
- 152.Isenberg JS, Ridnour LA, Espey MG, Wink DA, Roberts DD. Nitric oxide in wound-healing. Microsurgery. 2005;25:442–51. doi: 10.1002/micr.20168. [DOI] [PubMed] [Google Scholar]
- 153.Rizk M, Witte MB, Barbul A. Nitric oxide and wound healing. World J Surg. 2004;28:301–6. doi: 10.1007/s00268-003-7396-7. [DOI] [PubMed] [Google Scholar]
- 154.Fermor B, Christensen SE, Youn I, Cernanec JM, Davies CM, Weinberg JB. Oxygen, nitric oxide and articular cartilage. Eur Cell Mater. 2007;13:56–65. doi: 10.22203/ecm.v013a06. discussion 65. [DOI] [PubMed] [Google Scholar]
- 155.Boykin JV, Jr., Baylis C. Hyperbaric oxygen therapy mediates increased nitric oxide production associated with wound healing: a preliminary study. Adv Skin Wound Care. 2007;20:382–8. doi: 10.1097/01.ASW.0000280198.81130.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Landar A, Darley-Usmar VM. Evidence for oxygen as the master regulator of the responsiveness of soluble guanylate cyclase and cytochrome c oxidase to nitric oxide. Biochem J. 2007;405:e3–4. doi: 10.1042/BJ20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Galkin A, Higgs A, Moncada S. Nitric oxide and hypoxia. Essays Biochem. 2007;43:29–42. doi: 10.1042/BSE0430029. [DOI] [PubMed] [Google Scholar]
- 158.Brune B, Zhou J. Nitric oxide and superoxide: interference with hypoxic signaling. Cardiovasc Res. 2007;75:275–82. doi: 10.1016/j.cardiores.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 159.Metzen E, Zhou J, Jelkmann W, Fandrey J, Brune B. Nitric oxide impairs normoxic degradation of HIF-1alpha by inhibition of prolyl hydroxylases. Mol Biol Cell. 2003;14:3470–81. doi: 10.1091/mbc.E02-12-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kasuno K, Takabuchi S, Fukuda K, Kizaka-Kondoh S, Yodoi J, Adachi T, Semenza GL, Hirota K. Nitric oxide induces hypoxia-inducible factor 1 activation that is dependent on MAPK and phosphatidylinositol 3-kinase signaling. J Biol Chem. 2004;279:2550–8. doi: 10.1074/jbc.M308197200. [DOI] [PubMed] [Google Scholar]
- 161.Huang LE, Willmore WG, Gu J, Goldberg MA, Bunn HF. Inhibition of hypoxia-inducible factor 1 activation by carbon monoxide and nitric oxide. Implications for oxygen sensing and signaling. J Biol Chem. 1999;274:9038–44. doi: 10.1074/jbc.274.13.9038. [DOI] [PubMed] [Google Scholar]
- 162.Wellman TL, Jenkins J, Penar PL, Tranmer B, Zahr R, Lounsbury KM. Nitric oxide and reactive oxygen species exert opposing effects on the stability of hypoxia-inducible factor-1alpha (HIF-1alpha) in explants of human pial arteries. Faseb J. 2004;18:379–81. doi: 10.1096/fj.03-0143fje. [DOI] [PubMed] [Google Scholar]
- 163.Khanna S, Roy S, Maurer M, Ratan RR, Sen CK. Oxygen-sensitive reset of hypoxia-inducible factor transactivation response: prolyl hydroxylases tune the biological normoxic set point. Free Radic Biol Med. 2006;40:2147–54. doi: 10.1016/j.freeradbiomed.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Roy S, Khanna S, Bickerstaff A, Subramanian SV, Atalay M, Bierl M, Pendyala S, Levy D, Sharma N, Venojarvi M, Strauch AR, Orosz CG, Sen CK. Oxygen sensing by primary cardiac fibroblasts: a key role of p21Waf1/Cip1/Sdi1. Circulation Research. 2003;92:264–71. doi: 10.1161/01.res.0000056770.30922.e6. [DOI] [PubMed] [Google Scholar]
- 165.Roy S, Khanna S, Wallace WA, Lappalainen J, Rink C, Cardounel AJ, Zweier JL, Sen CK. Characterization of perceived hyperoxia in isolated primary cardiac fibroblasts and in the reoxygenated heart. Journal of Biological Chemistry. 2003;278:47129–35. doi: 10.1074/jbc.M308703200. [DOI] [PubMed] [Google Scholar]
- 166.Porwol T, Ehleben W, Brand V, Acker H. Tissue oxygen sensor function of NADPH oxidase isoforms, an unusual cytochrome aa3 and reactive oxygen species. Respiration Physiology. 2001;128:331–48. doi: 10.1016/s0034-5687(01)00310-3. [DOI] [PubMed] [Google Scholar]
- 167.Connolly E, Braunstein S, Formenti S, Schneider RJ. Hypoxia inhibits protein synthesis through a 4E-BP1 and elongation factor 2 kinase pathway controlled by mTOR and uncoupled in breast cancer cells. Mol Cell Biol. 2006;26:3955–65. doi: 10.1128/MCB.26.10.3955-3965.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Caniggia I, Winter JL. Adriana and Luisa Castellucci Award lecture 2001. Hypoxia inducible factor-1: oxygen regulation of trophoblast differentiation in normal and pre-eclamptic pregnancies--a review. Placenta. 2002;23(Suppl A):S47–57. doi: 10.1053/plac.2002.0815. [DOI] [PubMed] [Google Scholar]
- 169.Fisher SA, Burggren WW. Role of hypoxia in the evolution and development of the cardiovascular system. Antioxid Redox Signal. 2007;9:1339–52. doi: 10.1089/ars.2007.1704. [DOI] [PubMed] [Google Scholar]
- 170.Webster WS, Abela D. The effect of hypoxia in development. Birth Defects Res C Embryo Today. 2007;81:215–28. doi: 10.1002/bdrc.20102. [DOI] [PubMed] [Google Scholar]
- 171.Gerstner B, Sifringer M, Dzietko M, Schuller A, Lee J, Simons S, Obladen M, Volpe JJ, Rosenberg PA, Felderhoff-Mueser U. Estradiol attenuates hyperoxia-induced cell death in the developing white matter. Ann Neurol. 2007;61:562–73. doi: 10.1002/ana.21118. [DOI] [PubMed] [Google Scholar]
- 172.Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007;2007:cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- 173.Semenza GL, Prabhakar NR. HIF-1-dependent respiratory, cardiovascular, and redox responses to chronic intermittent hypoxia. Antioxid Redox Signal. 2007;9:1391–6. doi: 10.1089/ars.2007.1691. [DOI] [PubMed] [Google Scholar]
- 174.Kewley RJ, Whitelaw ML, Chapman-Smith A. The mammalian basic helix-loop-helix/PAS family of transcriptional regulators. Int J Biochem Cell Biol. 2004;36:189–204. doi: 10.1016/s1357-2725(03)00211-5. [DOI] [PubMed] [Google Scholar]
- 175.Lisy K, Peet DJ. Turn me on: regulating HIF transcriptional activity. Cell Death Differ. 2008 doi: 10.1038/sj.cdd.4402315. [DOI] [PubMed] [Google Scholar]
- 176.Coleman ML, Ratcliffe PJ. Oxygen sensing and hypoxia-induced responses. Essays Biochem. 2007;43:1–15. doi: 10.1042/BSE0430001. [DOI] [PubMed] [Google Scholar]
- 177.Depping R, Steinhoff A, Schindler SG, Friedrich B, Fagerlund R, Metzen E, Hartmann E, Kohler M. Nuclear translocation of hypoxia-inducible factors (HIFs): Involvement of the classical importin alpha/beta pathway. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbamcr.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 178.Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci U S A. 1997;94:4273–8. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Percy MJ, Furlow PW, Lucas GS, Li X, Lappin TR, McMullin MF, Lee FS. A gain-of-function mutation in the HIF2A gene in familial erythrocytosis. N Engl J Med. 2008;358:162–8. doi: 10.1056/NEJMoa073123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cho YS, Bae JM, Chun YS, Chung JH, Jeon YK, Kim IS, Kim MS, Park JW. HIF-1alpha controls keratinocyte proliferation by up-regulating p21(WAF1/Cip1) Biochim Biophys Acta. 2008;1783:323–33. doi: 10.1016/j.bbamcr.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 181.Grimm A, Dimmler A, Stange S, Labanaris A, Sauer R, Grabenbauer G, Horch RE. Expression of HIF-1 alpha in irradiated tissue is altered by topical negative-pressure therapy. Strahlenther Onkol. 2007;183:144–9. doi: 10.1007/s00066-007-1560-1. [DOI] [PubMed] [Google Scholar]
- 182.Li W, Li Y, Guan S, Fan J, Cheng CF, Bright AM, Chinn C, Chen M, Woodley DT. Extracellular heat shock protein-90alpha: linking hypoxia to skin cell motility and wound healing. Embo J. 2007;26:1221–33. doi: 10.1038/sj.emboj.7601579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Vihanto MM, Plock J, Erni D, Frey BM, Frey FJ, Huynh-Do U. Hypoxia up-regulates expression of Eph receptors and ephrins in mouse skin. Faseb J. 2005;19:1689–91. doi: 10.1096/fj.04-3647fje. [DOI] [PubMed] [Google Scholar]
- 184.Mace KA, Yu DH, Paydar KZ, Boudreau N, Young DM. Sustained expression of Hif-1alpha in the diabetic environment promotes angiogenesis and cutaneous wound repair. Wound Repair Regen. 2007;15:636–45. doi: 10.1111/j.1524-475X.2007.00278.x. [DOI] [PubMed] [Google Scholar]
- 185.Liu L, Cash TP, Jones RG, Keith B, Thompson CB, Simon MC. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol Cell. 2006;21:521–31. doi: 10.1016/j.molcel.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Proud CG. Amino acids and mTOR signalling in anabolic function. Biochem Soc Trans. 2007;35:1187–90. doi: 10.1042/BST0351187. [DOI] [PubMed] [Google Scholar]
- 187.Browne GJ, Finn SG, Proud CG. Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. J Biol Chem. 2004;279:12220–31. doi: 10.1074/jbc.M309773200. [DOI] [PubMed] [Google Scholar]
- 188.Emery PW, Sanderson P. Effect of dietary restriction on protein synthesis and wound healing after surgery in the rat. Clin Sci (Lond) 1995;89:383–8. doi: 10.1042/cs0890383. [DOI] [PubMed] [Google Scholar]
- 189.Zhang XJ, Chinkes DL, Cox RA, Wolfe RR. The flow phase of wound metabolism is characterized by stimulated protein synthesis rather than cell proliferation. J Surg Res. 2006;135:61–7. doi: 10.1016/j.jss.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 190.Zieske JD, Gipson IK. Protein synthesis during corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 1986;27:1–7. [PubMed] [Google Scholar]
- 191.Koumenis C, Naczki C, Koritzinsky M, Rastani S, Diehl A, Sonenberg N, Koromilas A, Wouters BG. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol Cell Biol. 2002;22:7405–16. doi: 10.1128/MCB.22.21.7405-7416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Koritzinsky M, Magagnin MG, Beucken T, Seigneuric R, Savelkouls K, Dostie J, Pyronnet S, Kaufman RJ, Weppler SA, Voncken JW, Lambin P, Koumenis C, Sonenberg N, Wouters BG. Gene expression during acute and prolonged hypoxia is regulated by distinct mechanisms of translational control. Embo J. 2006;25:1114–25. doi: 10.1038/sj.emboj.7600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gebauer F, Hentze MW. Molecular mechanisms of translational control. Nat Rev Mol Cell Biol. 2004;5:827–35. doi: 10.1038/nrm1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Blais JD, Filipenko V, Bi M, Harding HP, Ron D, Koumenis C, Wouters BG, Bell JC. Activating transcription factor 4 is translationally regulated by hypoxic stress. Mol Cell Biol. 2004;24:7469–82. doi: 10.1128/MCB.24.17.7469-7482.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fitzgerald RS, Shirahata M, Balbir A, Grossman CE. Oxygen sensing in the carotid body and its relation to heart failure. Antioxid Redox Signal. 2007;9:745–9. doi: 10.1089/ars.2007.1546. [DOI] [PubMed] [Google Scholar]
- 196.Prabhakar NR, Kumar GK, Nanduri J, Semenza GL. ROS signaling in systemic and cellular responses to chronic intermittent hypoxia. Antioxid Redox Signal. 2007;9:1397–403. doi: 10.1089/ars.2007.1732. [DOI] [PubMed] [Google Scholar]
- 197.Khayat RN, Schutzman SJ, Patt BT, Roy S, Gordillo GM, Schlanger R, Lambert L, Rose K, Gnyawali U, Coston A, Sen CK. Prevalence of obstructive sleep apnea in patients of an academic wound center (Conference Abstract) Wound Repair and Regeneration. 2008 in press. [Google Scholar]
- 198.Jelic S, Padeletti M, Kawut SM, Higgins C, Canfield SM, Onat D, Colombo PC, Basner RC, Factor P, LeJemtel TH. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation. 2008;117:2270–8. doi: 10.1161/CIRCULATIONAHA.107.741512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lopez-Jimenez F, Kuniyoshi FH Sert, Gami A, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. Chest. 2008;133:793–804. doi: 10.1378/chest.07-0800. [DOI] [PubMed] [Google Scholar]
- 200.Priestly J. Experiments and observations on different kinds of air. J. Johnson in St. Paul’s Church-yard; London: 1775. p. Section III. [Google Scholar]
- 201.Lavoisier A. Memoir on the combustion of candles in atmospheric air and in respirable air. Paris: 1777. edited by Sciences, FAd. [Google Scholar]
- 202.Brize-Fradin . La chimie pneumatique appliquee aux travaux sous l’eau. Paris: 1808. [Google Scholar]
- 203.Bert P. La Pression Barometrique. English Translation in 1943 by M.Hitchcock and A. Hitchcock. College Book Company; Columbus, OH: 1878. [Google Scholar]
- 204.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–55. [PubMed] [Google Scholar]
- 205.McCord JM, Fridovich I. The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J Biol Chem. 1969;244:6056–63. [PubMed] [Google Scholar]
- 206.Tibbles PM, Edelsberg JS. Hyperbaric-oxygen therapy. N Engl J Med. 1996;334:1642–8. doi: 10.1056/NEJM199606203342506. [DOI] [PubMed] [Google Scholar]
- 207.Shin HK, Dunn AK, Jones PB, Boas DA, Lo EH, Moskowitz MA, Ayata C. Normobaric hyperoxia improves cerebral blood flow and oxygenation, and inhibits peri-infarct depolarizations in experimental focal ischaemia. Brain. 2007;130:1631–42. doi: 10.1093/brain/awm071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Brahimi-Horn MC, Pouyssegur J. Oxygen, a source of life and stress. FEBS Lett. 2007;581:3582–91. doi: 10.1016/j.febslet.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 209.Prince LS. Hyperoxia and EGFL7: saving cells from too much of a good thing. Am J Physiol Lung Cell Mol Physiol. 2008;294:L15–6. doi: 10.1152/ajplung.00455.2007. [DOI] [PubMed] [Google Scholar]
- 210.Gallagher KA, Liu ZJ, Xiao M, Chen H, Goldstein LJ, Buerk DG, Nedeau A, Thom SR, Velazquez OC. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117:1249–59. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Shenberger JS, Zhang L, Powell RJ, Barchowsky A. Hyperoxia enhances VEGF release from A549 cells via post-transcriptional processes. Free Radic Biol Med. 2007;43:844–52. doi: 10.1016/j.freeradbiomed.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Das KC, Dashnamoorthy R. Hyperoxia activates the ATR-Chk1 pathway and phosphorylates p53 at multiple sites. Am J Physiol Lung Cell Mol Physiol. 2004;286:L87–97. doi: 10.1152/ajplung.00203.2002. [DOI] [PubMed] [Google Scholar]
- 213.Gehen SC, Vitiello PF, Bambara RA, Keng PC, O’Reilly MA. Downregulation of PCNA potentiates p21-mediated growth inhibition in response to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2007;292:L716–24. doi: 10.1152/ajplung.00135.2006. [DOI] [PubMed] [Google Scholar]
- 214.McGrath SA. Induction of p21WAF/CIP1 during hyperoxia. Am J Respir Cell Mol Biol. 1998;18:179–87. doi: 10.1165/ajrcmb.18.2.2964m. [DOI] [PubMed] [Google Scholar]
- 215.Rancourt RC, Keng PC, Helt CE, O’Reilly MA. The role of p21(CIP1/WAF1) in growth of epithelial cells exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2001;280:L617–26. doi: 10.1152/ajplung.2001.280.4.L617. [DOI] [PubMed] [Google Scholar]
- 216.Xu D, Perez RE, Ekekezie II, Navarro A, Truog WE. Epidermal growth factor-like domain 7 protects endothelial cells from hyperoxia-induced cell death. Am J Physiol Lung Cell Mol Physiol. 2008;294:L17–23. doi: 10.1152/ajplung.00178.2007. [DOI] [PubMed] [Google Scholar]
- 217.Wang X, Wang Y, Kim HP, Choi AM, Ryter SW. FLIP inhibits endothelial cell apoptosis during hyperoxia by suppressing Bax. Free Radic Biol Med. 2007;42:1599–609. doi: 10.1016/j.freeradbiomed.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 218.Loiseaux-Meunier MN, Bedu M, Gentou C, Pepin D, Coudert J, Caillaud D. Oxygen toxicity: simultaneous measure of pentane and malondialdehyde in humans exposed to hyperoxia. Biomed Pharmacother. 2001;55:163–9. doi: 10.1016/s0753-3322(01)00042-7. [DOI] [PubMed] [Google Scholar]
- 219.Patel V, Chivukala I, Roy S, Khanna S, He G, Ojha N, Mehrotra A, Dias LM, Hunt TK, Sen CK. Oxygen: From the benefits of inducing VEGF expression to managing the risk of hyperbaric stress. Antioxidants & Redox Signaling. 2005;7:1377–87. doi: 10.1089/ars.2005.7.1377. [DOI] [PubMed] [Google Scholar]
- 220.Aragones J, Schneider M, Van Geyte K, Fraisl P, Dresselaers T, Mazzone M, Dirkx R, Zacchigna S, Lemieux H, Jeoung NH, Lambrechts D, Bishop T, Lafuste P, Diez-Juan A, Harten SK, Van Noten P, De Bock K, Willam C, Tjwa M, Grosfeld A, Navet R, Moons L, Vandendriessche T, Deroose C, Wijeyekoon B, Nuyts J, Jordan B, Silasi-Mansat R, Lupu F, Dewerchin M, Pugh C, Salmon P, Mortelmans L, Gallez B, Gorus F, Buyse J, Sluse F, Harris RA, Gnaiger E, Hespel P, Van Hecke P, Schuit F, Van Veldhoven P, Ratcliffe P, Baes M, Maxwell P, Carmeliet P. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet. 2008 doi: 10.1038/ng.2007.62. [DOI] [PubMed] [Google Scholar]
- 221.Minamishima YA, Moslehi J, Bardeesy N, Cullen D, Bronson RT, Kaelin WG., Jr. Somatic inactivation of the PHD2 prolyl hydroxylase causes polycythemia and congestive heart failure. Blood. 2007 doi: 10.1182/blood-2007-10-117812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134:145–55. doi: 10.1053/j.gastro.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Surazynski A, Donald SP, Cooper SK, Whiteside MA, Salnikow K, Liu Y, Phang JM. Extracellular matrix and HIF-1 signaling: The role of prolidase. Int J Cancer. 2007;122:1435–40. doi: 10.1002/ijc.23263. [DOI] [PubMed] [Google Scholar]
- 224.Ginouves A, Ilc K, Macias N, Pouyssegur J, Berra E. PHDs overactivation during chronic hypoxia “desensitizes” HIFalpha and protects cells from necrosis. Proc Natl Acad Sci U S A. 2008;105:4745–50. doi: 10.1073/pnas.0705680105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Jacobson JM, Michael JR, Meyers RA, Bradley MB, Sciuto AM, Gurtner GH. Hyperbaric oxygen toxicity: role of thromboxane. J Appl Physiol. 1992;72:416–22. doi: 10.1152/jappl.1992.72.2.416. [DOI] [PubMed] [Google Scholar]
- 226.Gericke GS. Reactive oxygen species and related haem pathway components as possible epigenetic modifiers in neurobehavioural pathology. Med Hypotheses. 2006;66:92–9. doi: 10.1016/j.mehy.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 227.Islam KN, Mendelson CR. Permissive effects of oxygen on cyclic AMP and interleukin-1 stimulation of surfactant protein A gene expression are mediated by epigenetic mechanisms. Mol Cell Biol. 2006;26:2901–12. doi: 10.1128/MCB.26.8.2901-2912.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Siddiqui A, Davidson JD, Mustoe TA. Ischemic tissue oxygen capacitance after hyperbaric oxygen therapy: a new physiologic concept. Plast Reconstr Surg. 1997;99:148–55. doi: 10.1097/00006534-199701000-00023. [DOI] [PubMed] [Google Scholar]
- 229.Ueno C, Hunt TK, Hopf HW. Using physiology to improve surgical wound outcomes. Plast Reconstr Surg. 2006;117:59S–71S. doi: 10.1097/01.prs.0000225438.86758.21. [DOI] [PubMed] [Google Scholar]
- 230.Roh C, Lyle S. Cutaneous stem cells and wound healing. Pediatr Res. 2006;59:100R–3R. doi: 10.1203/01.pdr.0000203572.51876.ba. [DOI] [PubMed] [Google Scholar]
- 231.Schafer M, Werner S. Transcriptional control of wound repair. Annu Rev Cell Dev Biol. 2007;23:69–92. doi: 10.1146/annurev.cellbio.23.090506.123609. [DOI] [PubMed] [Google Scholar]
- 232.Blessey A, Eubanks A. Hyperbaric oxygen is an important adjunct therapy. Crit Care Nurse. 1996;16:14–5. [PubMed] [Google Scholar]
- 233.Shafer MR. Use of hyperbaric oxygen as adjunct therapy to surgical debridement of complicated wounds. Semin Perioper Nurs. 1993;2:256–62. [PubMed] [Google Scholar]
- 234.Thackham JA, McElwain DL, Long RJ. The use of hyperbaric oxygen therapy to treat chronic wounds: A review. Wound Repair Regen. 2008;16:321–30. doi: 10.1111/j.1524-475X.2008.00372.x. [DOI] [PubMed] [Google Scholar]
- 235.Kessler L, Bilbault P, Ortega F, Grasso C, Passemard R, Stephan D, Pinget M, Schneider F. Hyperbaric oxygenation accelerates the healing rate of nonischemic chronic diabetic foot ulcers: a prospective randomized study. Diabetes Care. 2003;26:2378–82. doi: 10.2337/diacare.26.8.2378. [DOI] [PubMed] [Google Scholar]
- 236.Barnes RC. Point: hyperbaric oxygen is beneficial for diabetic foot wounds. Clin Infect Dis. 2006;43:188–92. doi: 10.1086/505207. [DOI] [PubMed] [Google Scholar]
- 237.Gajendrareddy PK, Sen CK, Horan MP, Marucha PT. Hyperbaric oxygen therapy ameliorates stress-impaired dermal wound healing. Brain Behavior and Immunity. 2005;19:217–22. doi: 10.1016/j.bbi.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 238.Liu ZJ, Velazquez OC. Hyperoxia, Endothelial Progenitor Cell Mobilization, and Diabetic Wound Healing. Antioxid Redox Signal. 2008 doi: 10.1089/ars.2008.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–9. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 240.Korhonen K, Kuttila K, Niinikoski J. Subcutaneous tissue oxygen and carbon dioxide tensions during hyperbaric oxygenation: an experimental study in rats. Eur J Surg. 1999;165:885–90. doi: 10.1080/11024159950189401. [DOI] [PubMed] [Google Scholar]
- 241.Thomas PS, Hakim TS, Trang LQ, Hosain SI, Camporesi EM. The synergistic effect of sympathectomy and hyperbaric oxygen exposure on transcutaneous PO2 in healthy volunteers. Anesth Analg. 1999;88:67–71. doi: 10.1097/00000539-199901000-00013. [DOI] [PubMed] [Google Scholar]
- 242.Wallyn CR, Jampol LM, Goldberg MF, Zanetti CL. The use of hyperbaric oxygen therapy in the treatment of sickle cell hyphema. Invest Ophthalmol Vis Sci. 1985;26:1155–8. [PubMed] [Google Scholar]
- 243.Mathieu D, editor. Handbook of hyperbaric medicine. Springer; New York: 2006. p. 812. [Google Scholar]
- 244.Goldstein LJ, Gallagher KA, Bauer SM, Bauer RJ, Baireddy V, Liu ZJ, Buerk DG, Thom SR, Velazquez OC. Endothelial progenitor cell release into circulation is triggered by hyperoxia-induced increases in bone marrow nitric oxide. Stem Cells. 2006;24:2309–18. doi: 10.1634/stemcells.2006-0010. [DOI] [PubMed] [Google Scholar]
- 245.Gallagher KA, Goldstein LJ, Thom SR, Velazquez OC. Hyperbaric oxygen and bone marrow-derived endothelial progenitor cells in diabetic wound healing. Vascular. 2006;14:328–37. doi: 10.2310/6670.2006.00057. [DOI] [PubMed] [Google Scholar]
- 246.Thom SR, Bhopale VM, Velazquez OC, Goldstein LJ, Thom LH, Buerk DG. Stem cell mobilization by hyperbaric oxygen. Am J Physiol Heart Circ Physiol. 2006;290:H1378–86. doi: 10.1152/ajpheart.00888.2005. [DOI] [PubMed] [Google Scholar]
- 247.Thom SR, Fisher D, Zhang J, Bhopale VM, Ohnishi ST, Kotake Y, Ohnishi T, Buerk DG. Stimulation of perivascular nitric oxide synthesis by oxygen. Am J Physiol Heart Circ Physiol. 2003;284:H1230–9. doi: 10.1152/ajpheart.01043.2002. [DOI] [PubMed] [Google Scholar]
- 248.Berendt AR. Counterpoint: hyperbaric oxygen for diabetic foot wounds is not effective. Clin Infect Dis. 2006;43:193–8. doi: 10.1086/505223. [DOI] [PubMed] [Google Scholar]
- 249.Packer L, Fuehr K. Low oxygen concentration extends the lifespan of cultured human diploid cells. Nature. 1977;267:423–5. doi: 10.1038/267423a0. [DOI] [PubMed] [Google Scholar]
- 250.Betts DH, Perrault SD, King WA. Low oxygen delays fibroblast senescence despite shorter telomeres. Biogerontology. 2008;9:19–31. doi: 10.1007/s10522-007-9113-7. [DOI] [PubMed] [Google Scholar]
- 251.Oh S, Lee E, Lee J, Lim Y, Kim J, Woo S. Comparison of the effects of 40% oxygen and two atmospheric absolute air pressure conditions on stress-induced premature senescence of normal human diploid fibroblasts. Cell Stress Chaperones. 2008 doi: 10.1007/s12192-008-0041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ukkola O, Erkkila PH, Savolainen MJ, Kesaniemi YA. Lack of association between polymorphisms of catalase, copper-zinc superoxide dismutase (SOD), extracellular SOD and endothelial nitric oxide synthase genes and macroangiopathy in patients with type 2 diabetes mellitus. J Intern Med. 2001;249:451–9. doi: 10.1046/j.1365-2796.2001.00828.x. [DOI] [PubMed] [Google Scholar]
- 253.Foster CB, Aswath K, Chanock SJ, McKay HF, Peters U. Polymorphism analysis of six selenoprotein genes: support for a selective sweep at the glutathione peroxidase 1 locus (3p21) in Asian populations. BMC Genet. 2006;7:56. doi: 10.1186/1471-2156-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Higasa S, Tsujimura M, Hiraoka M, Nakayama K, Yanagisawa Y, Iwamoto S, Kagawa Y. Polymorphism of glutathione S-transferase P1 gene affects human vitamin C metabolism. Biochem Biophys Res Commun. 2007;364:708–13. doi: 10.1016/j.bbrc.2007.10.076. [DOI] [PubMed] [Google Scholar]
- 255.Matsuzawa D, Hashimoto K, Shimizu E, Fujisaki M, Iyo M. Functional polymorphism of the glutathione peroxidase 1 gene is associated with personality traits in healthy subjects. Neuropsychobiology. 2005;52:68–70. doi: 10.1159/000086607. [DOI] [PubMed] [Google Scholar]
- 256.Paiva L, Marcos R, Creus A, Coggan M, Oakley AJ, Board PG. Polymorphism of glutathione transferase Omega 1 in a population exposed to a high environmental arsenic burden. Pharmacogenet Genomics. 2008;18:1–10. doi: 10.1097/FPC.0b013e3282f29663. [DOI] [PubMed] [Google Scholar]
- 257.Hudson VM. Rethinking cystic fibrosis pathology: the critical role of abnormal reduced glutathione (GSH) transport caused by CFTR mutation. Free Radic Biol Med. 2001;30:1440–61. doi: 10.1016/s0891-5849(01)00530-5. [DOI] [PubMed] [Google Scholar]
- 258.Ihara Y, Nobukuni K, Takata H, Hayabara T. Oxidative stress and metal content in blood and cerebrospinal fluid of amyotrophic lateral sclerosis patients with and without a Cu, Zn-superoxide dismutase mutation. Neurol Res. 2005;27:105–8. doi: 10.1179/016164105X18430. [DOI] [PubMed] [Google Scholar]
- 259.Shibata N, Hirano A, Yamamoto T, Kato Y, Kobayashi M. Superoxide dismutase-1 mutation-related neurotoxicity in familial amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:143–61. doi: 10.1080/14660820050515151. [DOI] [PubMed] [Google Scholar]
- 260.Rancourt RC, Hayes DD, Chess PR, Keng PC, O’Reilly MA. Growth arrest in G1 protects against oxygen-induced DNA damage and cell death. J Cell Physiol. 2002;193:26–36. doi: 10.1002/jcp.10146. [DOI] [PubMed] [Google Scholar]
- 261.Anderson B. Nutrition and wound healing: the necessity of assessment. Br J Nurs. 2005;14:S30, S32, S34. doi: 10.12968/bjon.2005.14.Sup5.19955. passim. [DOI] [PubMed] [Google Scholar]
- 262.Campos AC, Groth AK, Branco AB. Assessment and nutritional aspects of wound healing. Curr Opin Clin Nutr Metab Care. 2008;11:281–8. doi: 10.1097/MCO.0b013e3282fbd35a. [DOI] [PubMed] [Google Scholar]
- 263.Edmonds J. Nutrition and wound healing: putting theory into practice. Br J Community Nurs. 2007;12:S31–4. [PubMed] [Google Scholar]
- 264.Langemo D, Anderson J, Hanson D, Hunter S, Thompson P, Posthauer ME. Nutritional considerations in wound care. Adv Skin Wound Care. 2006;19:297–8. 300, 03. doi: 10.1097/00129334-200607000-00007. [DOI] [PubMed] [Google Scholar]
- 265.Posthauer ME. The role of nutrition in wound care. Adv Skin Wound Care. 2006;19:43–52. doi: 10.1097/00129334-200601000-00015. quiz 53-4. [DOI] [PubMed] [Google Scholar]
- 266.Bobyn PJ, Corbett D, Saucier DM, Noyan-Ashraf MH, Juurlink BH, Paterson PG. Protein-energy malnutrition impairs functional outcome in global ischemia. Exp Neurol. 2005;196:308–15. doi: 10.1016/j.expneurol.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 267.Feoli AM, Siqueira IR, Almeida L, Tramontina AC, Vanzella C, Sbaraini S, Schweigert ID, Netto CA, Perry ML, Goncalves CA. Effects of protein malnutrition on oxidative status in rat brain. Nutrition. 2006;22:160–5. doi: 10.1016/j.nut.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 268.Golden MH. The development of concepts of malnutrition. J Nutr. 2002;132:2117S–22S. doi: 10.1093/jn/132.7.2117S. [DOI] [PubMed] [Google Scholar]
- 269.Bello YM, Phillips TJ. Adjunctive therapies for wound healing. Jama. 2000;284:40–1. [PubMed] [Google Scholar]
- 270.Bello YM, Phillips TJ. Recent advances in wound healing. Jama. 2000;283:716–8. doi: 10.1001/jama.283.6.716. [DOI] [PubMed] [Google Scholar]
- 271.Wang C, Schwaitzberg S, Berliner E, Zarin DA, Lau J. Hyperbaric oxygen for treating wounds: a systematic review of the literature. Arch Surg. 2003;138:272–9. doi: 10.1001/archsurg.138.3.272. discussion 80. [DOI] [PubMed] [Google Scholar]
- 272.D’Souza J, Goru J, Goru S, Brown J, Vaughan ED, Rogers SN. The influence of hyperbaric oxygen on the outcome of patients treated for osteoradionecrosis: 8 year study. Int J Oral Maxillofac Surg. 2007;36:783–7. doi: 10.1016/j.ijom.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 273.Aspinall MG, Hamermesh RG. Realizing the promise of personalized medicine. Harv Bus Rev. 2007;85:108–17. 65. [PubMed] [Google Scholar]
- 274.Hama Y, Matsumoto K, Murugesan R, Subramanian S, Devasahayam N, Koscielniak JW, Hyodo F, Cook JA, Mitchell JB, Krishna MC. Continuous wave EPR oximetric imaging at 300 MHz using radiofrequency power saturation effects. Antioxid Redox Signal. 2007;9:1709–16. doi: 10.1089/ars.2007.1720. [DOI] [PubMed] [Google Scholar]
- 275.Vikram DS, Zweier JL, Kuppusamy P. Methods for noninvasive imaging of tissue hypoxia. Antioxid Redox Signal. 2007;9:1745–56. doi: 10.1089/ars.2007.1717. [DOI] [PubMed] [Google Scholar]
- 276.Davis SC, Cazzaniga AL, Ricotti C, Zalesky P, Hsu LC, Creech J, Eaglstein WH, Mertz PM. Topical oxygen emulsion: a novel wound therapy. Arch Dermatol. 2007;143:1252–6. doi: 10.1001/archderm.143.10.1252. [DOI] [PubMed] [Google Scholar]
- 277.Fries RB, Wallace WA, Roy S, Kuppusamy P, Bergdall V, Gordillo GM, Melvin WS, Sen CK. Dermal excisional wound healing in pigs following treatment with topically applied pure oxygen. Mutat Res. 2005;579:172–81. doi: 10.1016/j.mrfmmm.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 278.Heng MC, Harker J, Csathy G, Marshall C, Brazier J, Sumampong S, Gomez E Paterno. Angiogenesis in necrotic ulcers treated with hyperbaric oxygen. Ostomy Wound Manage. 2000;46:18–28. 30–2. [PubMed] [Google Scholar]
- 279.Ciaravino ME, Friedell ML, Kammerlocher TC. Is hyperbaric oxygen a useful adjunct in the management of problem lower extremity wounds? Ann Vasc Surg. 1996;10:558–62. doi: 10.1007/BF02000444. [DOI] [PubMed] [Google Scholar]
- 280.Heng MC, Harker J, Bardakjian VB, Ayvazian H. Enhanced healing and cost-effectiveness of low-pressure oxygen therapy in healing necrotic wounds: a feasibility study of technology transfer. Ostomy Wound Manage. 2000;46:52–60. 62. [PubMed] [Google Scholar]