Abstract
Averaged event-related potentials (ERPs) represent sensory and cognitive processing of stimuli during wakefulness independent of behavioural responses, and reflect the underlying state of the CNS during sleep. Components measured during wakefulness which are reflective of arousal state or the automatic switching of attention are sensitive to prior sleep disruption. Components reflecting active attentional influences during the waking state appear to be preserved in a rudimentary form during REM sleep, but in a way that highlights the differences in the neurochemical environment between wakefulness and REM sleep. Certain ERP components only appear within sleep. These begin to emerge at NREM sleep onset and may reflect inhibition of information processing and thus have utility as markers of the functional status of sleep preparatory mechanisms. These large amplitude NREM components represent synchronized burst firing of large number of cortical cells and are a reflection of the nervous system’s capacity to generate delta frequency EEG activity. As such they are useful in assessing the overall integrity of the nervous system in populations not showing substantial amounts of SWS as measured using traditional criteria. While requiring care in their interpretation, ERPs nonetheless provide a rich tool to investigators interested in probing the nervous system to evaluate daytime functioning in the face of sleep disruption, the ability of the sleeping nervous system to monitor the external environment, and the ability of the nervous system to respond to stimuli in a manner consistent with the initiation or maintenance of sleep.
Keywords: P300, P3a, Deprivation, OSA, Insomnia, Narcolepsy, N350, N550, K-complex
When EEG is used as a dependent variable in sleep research, it is typically as a passive observational measure to assess some characteristic of central nervous system (CNS) arousal or activation level. Evoked potentials (or event-related potentials) 1, the small amplitude changes in the EEG that are elicited by an external physical stimulus or internal psychological event, provide a means to “probe” the extent of processing within the nervous system during sleep. In the waking state, ERPs provide data that can complement the usual EEG and behavioural measures of performance (e.g. reaction time). Specifically, they provide measures of signal processing that may be independent of motor control, motivation or even conscious awareness of the presented stimuli, depending upon the components investigated. The present review provides an overview of theoretical and methodological issues involved in using ERPs in sleep research and then turns to two broad areas in which they can be extremely useful to sleep researchers: the daytime assessment of the impact of sleep disruption; and the determination of CNS function during sleep.
Signal averaging theory
ERPs utilize a simple mathematical process to enable the distinction between the specific relevant EEG change or “response” (signal) and the irrelevant background ongoing EEG activity (noise) unrelated to the stimulus or task characteristics. This is achieved by time locked averaging of responses to stimuli under two assumptions. First, that the unrelated “noise” EEG will be distributed randomly relative to the stimuli, and thus will tend to average to zero. Second, that the “signal” EEG response has an invariant temporal relationship with the stimulus. Under these assumptions, averaging increases the signal to noise ratio (SNR) as a square root function of the number of responses averaged 1. Thus, going from 16 responses to 64 responses leads to a doubling of the SNR, with a further subsequent doubling requiring the averaging of 256 responses. The number of responses needed to resolve specific signals is, however, determined largely by the intrinsic signal-to-noise ratio (SNR). For example, components in averaged auditory brainstem responses that are 1 or 2 microvolts, but that occur in the presence of 20–30 microvolt background EEG, need many more responses to be reliably seen than averaged K-complex components that are typically at least double the background amplitude EEG.
The waveform resulting from the averaging process contains a series of positive and negative peaks (components) that are thought to reflect activity in underlying generators within the brain2. In ERPs collected during wakefulness, early components (<80 ms approximately) typically reflect sensory processing, those around 100 ms are sensitive to arousal and attention and later components are thought to reflect higher order CNS processing related to cognitive function. During sleep, later responses reflect the synchronized activity of cortical and thalamo-cortical systems in a frequency range consistent with the underlying neurophysiological state 2.
The impact of sleep disruption on daytime function
ERP methodology is particularly well-suited to the study of the effects of sleepiness and sleep deprivation (SD). The effects of sleep deprivation on performance are now well-documented (see 3 and 4 for an earlier review and a meta-analysis). The effects of SD are obviously dependent on the duration of wakefulness and on the task. SD appears to affect long and boring tasks, those that demand sustained vigilance for successful completion, more than tasks that are short and interesting. Cognitive scientists generally provide two measures of performance, the accuracy at which a task is carried out (generally measured as the hit rate) and the speed at which decisions are made (generally measured by reaction time; RT). In general, hit rate declines and RT is prolonged after periods of SD although these effects are task dependent 5.
ERP findings at times are consistent with performance measures and at times are not. Some ERP researchers treat the disassociation of the physiological and performance measures as almost an embarrassment, as if the ERP measure is somehow in error if it does not support the performance measures. In actual fact, if ERPs provided exactly the same information as the traditional performance measures, there would be no point in recording them. The use of a performance measure alone requires an inference about which of the many possible hypothetical cognitive processes might have led to the actual response (for example, attention-vigilance, memory comparison, decision-making). The advantage of ERPs is that they can provide a measurement of cognitive activity preceding, during and following the actual response, and some insight as to which cognitive factors are being affected.
Interpreting ERP components thus requires an understanding of the many complex cognitive processes that may affect them. The extent to which these ERPs will be affected by sleepiness is dependent on the extent to which the cognitive events that they reflect vary with sleepiness. Many early ERP studies of sleep deprivation have examined the late positive component, P300 (or P3b)1. The P300 is most-often elicited in the so-called “oddball” paradigm in which subjects are presented with a train of frequently occurring “standard” stimuli that change to a “target” at odd and unpredictable times. Any physical feature of the standard could be changed, for example its pitch, colour, intensity, duration, location and so forth. Indeed, the absence or omission of the standard could serve as a target stimulus. P300 thus occurs independently of the actual physical features of the target. What is therefore important is that the subject must detect it. When the detection is made, the P300 appears as a large parietal maximum positive wave, peaking at about 300 ms (thus the nomenclature, “P300”) provided the target is clearly distinguishable from the standard. When the target detection is made more difficult, the peak latency of P300 can be prolonged by several hundred ms.
The amplitude of P300 is thought to reflect the updating of an active working memory 6. The memory for the features of the standard stimulus is well-formed, because it is presented frequently. The memory for the target stimulus is less-well formed. Upon its presentation and subsequent detection, its memory representation must be revised or updated. Note that this updating process requires that the subject actively attend to the stimuli, maintain the representation of the standard in working memory through an active rehearsal process, detect stimulus change through a comparison of incoming stimulus features to existing memory representations and subsequently update memory, if necessary. A failure or deterioration of any of these cognitive activities could thus affect P300. P300 cannot be elicited if the subject ignores the stimuli or fails to detect the target.
P300 is sensitive to variation in the subject’s level of arousal. During the sleep onset period, the amplitude of P300 gradually declines and its latency becomes increasingly prolonged 7–10. Gora et al. 11 and Colrain et al. 12 have provided data indicating that this prolongation of latency is sensitive to the EEG micro state in which the stimuli are presented, with a dramatic increase in latency occurring in responses to stimuli presented during theta activity as compared to alpha activity within stage 1 sleep. While the amplitude of P300 is maximum over parietal areas of the scalp,, during the sleep onset period when the subject is still able to detect the target stimulus, the largest changes in its amplitude are apparent over frontal areas of the scalp, consistent with the frontal hypothesis of sleepiness 10, 12, 13.
The effects of sleep deprivation have often been explained by a failure to maintain sustained attention for long periods of time or perhaps because of momentary “lapses of attention”. Several studies of sleep deprivation have thus often employed the oddball task or a variation of it to test these hypotheses. In general, as expected, a decrease in the amplitude of P300 and an increase in its latency have been found 8, 14, 15. Morris et al. 16 did not however observe a decrease in its amplitude. When observed, the P300 decrement might reflect an inability to sustain attention. Of course, as indicated above, manipulation of P300 amplitude could be explained by a change in any of several different cognitive activities. However, many theorists have noted that subjects might be able to exert “compensatory effort” to sustain effort for the brief period in which the oddball paradigm was run. Alternatively, differences across studies might reflect subtle variation in task demands. Again, sleep deprivation will not affect all cognitive tasks. Thus, P300 might be affected in some studies but not in others depending on the nature of the cognitive task.
Findings of the impact of experimental sleep fragmentation (as opposed to total sleep deprivation) have been mixed. Cote et al. 17 found no changes in P300 following two nights of sleep fragmentation, although they did report significant decreases in an earlier N1 amplitude, peaking at about 100 ms. Kingshott et al. 18 found reduced P300 amplitudes following a night of sleep fragmentation at some frontal, central and temporal scalp sites but not over parietal areas where P300 (P3b) is most prominent. A major difference between the studies was that whereas the Kingshott et al.18 data were based on a single measurement taken mid-afternoon designed to coincide with the post-prandial dip in alertness, the Cote et al. 17 data were averaged across multiple recording sessions conducted at different times during the days following the fragmented nights. Interestingly the Cote et al. study failed to show P300 effects despite significant alterations in quantitative EEG (alpha/theta ratios moving in the direction of more theta activity), significant alterations in mood and significant reduction in the amplitude of the earlier N1 component of the ERP. This result again highlights the differential sensitivity of ERP measures to manipulations such as sleep fragmentation. The ERPs do reflect different underlying cognitive activities and again, some components may be affected by factors such as sleep fragmentation while others may not..
Recent studies have focused on ERP components that may reflect activity of the frontal regions. Such ERPs are therefore of particular interest for testing the hypothesis that sleep deprivation has its major effects on frontally modulated tasks. Gosselin et al. 19 have recently used a variation of the oddball task to examine the detection of novelty. Subjects were presented with frequently presented standard tones and rarely presented target tones and novel environmental sounds. They were asked to button press upon detection of the target tone. Because the novel stimuli form part of the oddball sequence (and could thus not be ignored), they also will elicit a late positive wave. This positivity is similar to the parietal-maximum classical P300 except that its scalp topography has a more fronto-central maximum and peaks earlier 20. This positivity is thus labeled the novel P3 (or P3a) 2 to distinguish it from the more parietal maximum P300 (or P3b).
Gosselin et al. 19 tested two independent groups, a control, non-sleep deprived (NSD) and a totally sleep deprived (TSD) group, and looked at both classical P300 and novel P3 (P3a) responses. The NSD group was tested 12 hrs following normal sleep while the TSD group was tested following 36 hrs after their last sleep period. The results, presented in Figure 1, illustrate that sleep deprivation can alter cognitive functioning in a manner far more complex than the performance data might suggest. RT to the target tones did not increase following sleep deprivation. However, target P300 latency was significantly prolonged. Response biases such as a willingness to respond rapidly prior to complete extraction of stimulus features will result in a speeded RT but will not affect P300 latency. In such cases, error rates should increase and this was observed by Gosselin et al. in the TSD group. This was especially apparent for false positives – the incorrect detection of standards as targets.
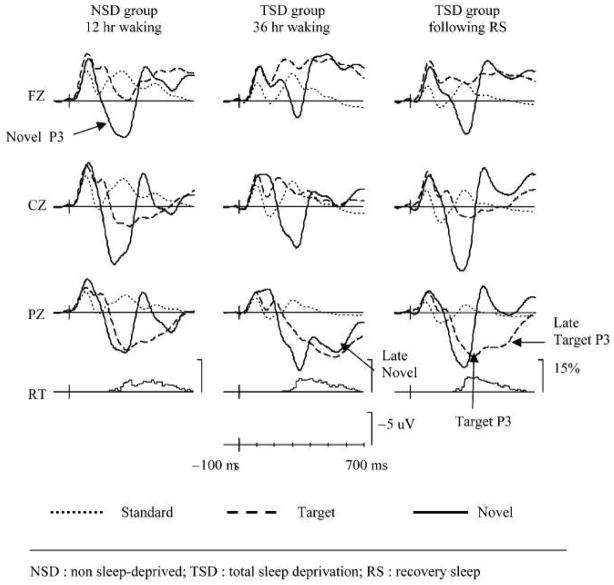
Figure 1.
Grand-average ERPs following presentation of frequently occurring standard tones (dotted line), and rarely presented target tones (dashed line) and novel, environmental sounds (solid line) are illustrated. Two different groups of subjects were tested, control subjects who were not sleep deprived (NSD) and a totally sleep deprived (TSD) group. The TSD group was also recorded following recovery sleep (RS). From 19 with permission. Negativity at the scalp relative to the reference is traced as an upward deflection in this and all other figures.
The novel, rare stimulus elicited a large novel P3a over fronto-central scalp in the control group (Figure 1). This peaked much earlier than the classic P300 following detection of the target.. In agreement with the hypothesis that sleep deprivation mainly affects frontal tasks, the frontal dispersion of the novel P3 was markedly attenuated in the TSD group. Frontal impairment is also associated with the inability to withhold an inappropriate response 21. A second large, late positivity was also observed following presentation of both the novel and target stimuli. Multiple P3s have been reported when subjects and cognitive task requires a two-stage decision 22, 23. It is possible that subjects might have attempted to encode and perhaps name the environmental stimuli following TSD. Alternatively, the TSD subjects may have needed to make the effortful decision that the novel sounds were also not target stimuli. The target P300 was also affected by sleep deprivation. Consistent with other studies, its amplitude was attenuated and it’s peaked prolonged.
The Gosselin et al. 19 study reinforces the view that the effects of sleep deprivation are highly dependent on task demands. Moreover, while a task may be successfully completed, the strategy used to arrive at the decision may vary. Thus, it is possible that measures of performance may not vary following sleep deprivation. This does not necessarily imply that the strategy used to arrive at a decision was the same. For example, tasks that may require minimal attentional resources might require considerable effort following sleep deprivation.
The relationship between ERP measures of attentional processing and normal variations in sleep quality was recently evaluated by Salmi et al. 24. The authors employed an oddball task consisting of rapidly presented standard stimuli and at rare and unpredictable times a deviant that either varied in its duration or pitch. Subjects watched a video and ignored the auditory stimuli. In the control (non sleep-deprived) condition, the deviant stimuli elicited a small negativity, the Mismatch Negativity (MMN) and a later P3a. The MMN is proposed to reflect detection of stimulus deviance and this process occurs independently of attention (i.e., pre-attentively). The P3a has been proposed to reflect a switching of attention from the task at hand to the otherwise unattended auditory stimuli, allowing its features to be available to consciousness. This may be affected by task demands and thus the availability of attentional resources. Using 72 hour actigraphy to monitor sleep, and multiple ERP recording sessions during the day, the authors were able to show significant correlations between P3a amplitude and sleep variables such as sleep onset latency (where the values ranged between1.25 and 26.4 minutes) and sleep efficiency (where the values ranged between 76% and 99%). The P3a declined in amplitude with increasing sleepiness. Thus, the ability to automatically switch attention from ongoing cognition demands to relevant input may be compromised by increasing sleepiness. The switching of attention when appropriate is also thought to be a function of the frontal lobe. No relationships were observed between sleep variables and the mismatch negativity (MMN), an ERP phenomenon thought to index automatic stimulus detection mechanisms 25. The authors concluded that automatic stimulus detection is relatively robust to the impact of the previous night’s sleep quality.
Another recent application of ERP methodology to the testing of frontal functions is apparent in studies of error detection. Error rates often increase as a result of sleep deprivation. Why they increase is however not well understood. It is possible that subjects do not detect the fact that they have committed errors. Or, as is common with frontal lobe patients, they may be unable to take corrective, remedial action even if they are aware that an error has been committed. A negative-going ERP, maximum over centro-frontal areas of the scalp is apparent 50–100 ms following an error response. This response is thus called the error-related negativity (“ERN” or “Ne”) 26 and is followed by a later positive potential, Pe. Like the novel P3, the ERN also appears to reflect activity in the prefrontal and anterior cingulate cortices 27, perhaps sharing a common function, the need to detect and inhibit inappropriate responses. The ERN was much larger following an incorrect than a correct response. Of the three studies that have studied extended wakefulness effects on the ERN, two have indicated a decreased ERN with sleep deprivation 28, 29 (see Figure 2). No ERN effect was seen by Murphy and Segalowitz 30 in an extended wakefulness protocol where subjects were tested after approximately 20 hours of wakefulness (at three hours after their regular bed-time). It would thus appear that ERN is only impacted when substantial amounts of sleep are lost. Scheffers et al. 29 point out that detection of errors can be associated with remedial actions including attempts to inhibit error, to correct it immediately or to make adjustment in response strategies and thus avoid future error. The ERN may reflect error detection 26 or perhaps conflict monitoring following an error 31. While the attenuation of the ERN following sleep deprivation may reflect an inability to detect error, it may also be related to the fact that more errors are being made. The ERN also decreases as the number of errors increases 32. In the Tsai et al. study 28, the number of errors actually decreased after sleep deprivation, although more than half of the subjects did maintain similar accuracy rates under both sleep conditions. Nevertheless, the ERN was still reduced following sleep deprivation even though error rates were not. The decrease in the ERN could not therefore simply be explained by an increase in the number of errors. The attenuated ERN may thus be related to the failure to detect that an error had been committed. When the sleep-deprived subjects made an error, they often appeared to be unable to correct it thus making another error on the subsequent trial. These results are consistent with findings that a consequence of increased sleepiness is an inability to monitor performance 33.
Figure 2.
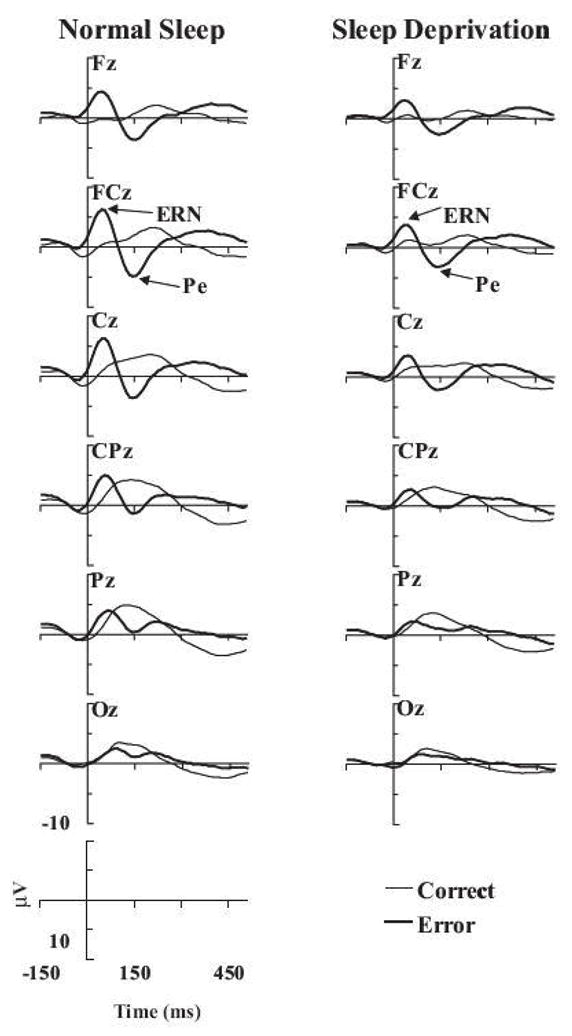
The error-related negativity (ERN) following normal sleep and sleep deprivation. Trials were time-locked to the response and then averaged. The grand average of the trials in which correct (thin line) and incorrect-error decisions (thick line) were made is illustrated. From 28, with permission.
Daytime function in patients with sleep pathology
Patients with sleep pathology such as obstructive sleep apnea syndrome (OSAS) also experience sleep fragmentation. Studies of patients during wakefulness have however reported mixed results, with some evidence for an increased P300 latency to visual 34–37 and auditory stimuli,36, 38, 39 although no effect on auditory P300 latency has also been reported 34, 35, 40. Some studies have 38, 39 and some have not 34–36, 40 found OSAS patients to have reduced auditory P300 amplitudes, although there is one report of significant correlations between P300 amplitude and respiratory disturbance index, % stage 1 sleep and sleep onset latency in the maintenance of wakefulness test.41 Likewise, improved nocturnal sleep following effective CPAP treatment has,38, 39 and has not,35, 37 been associated with decreased P300 latency relative to that seen prior to treatment. Neither of the studies that have evaluated respiratory somatosensory P300 in OSAS patients found a significant effect on amplitude or latency of the response relative to controls 40, 42.
Interestingly, a recent study Gosselin et al.43 used a similar method to Salmi et al.24 to determine the impact of OSAS on involuntary attention capture as indexed by the MMN and P3a following presentation of very rare, unattended deviant stimuli. They found no differences in MMN, but did report decreased P3a amplitude in the OSAS group who had similar sleep architecture, but significantly more micro arousals and sleep state change than the controls. Taken together with the Salmi et al. 24 finding, these data would seem to indicate that measures of automatic attentional switching such as P3a may be more sensitive to the sleep quality alterations seen in OSAS, and may be a useful measure of treatment effects.
Insomnia represents a complex challenge in terms of the investigation of daytime CNS function. As with OSAS, it is associated sleep fragmentation, increased WASO and decreases in the total amount and efficiency of sleep, all of which would be expected to lead to feelings of fatigue and sleepiness during the day. On the other hand, there is growing evidence in support of a “hyper-aroused” CNS underlying at least a good proportion of those suffering from primary psychophysiological insomnia. Thus, depending on the theoretical model, it is possible to predict both decreases (increased sleepiness or hypo arousal) or increases (hyper arousal) in N1 amplitude. The amplitude of N1 is large in the waking state. Its amplitude does decrease dramatically at sleep onset and is difficult to discern during NREM sleep. P300 is theoretically more straightforward as decreased sleep should impact negatively on the allocation of neural resources associated with attention. As will be seen, however, the data are not so easily explained.
Only a handful of studies have been conducted using ERPs to evaluate daytime functioning in insomnia, some of them looking at insomnia as a symptom in other disorders. For example, Bruder et al. 44 investigated 25 patients with depression and 27 controls and found significant correlations between P300 latency and the insomnia items on the Hamilton depression rating scale. They interpreted the finding as evidence of the slowing of cognitive processing in a subgroup of depressed patients with sleep disturbances. Anderer and colleagues 45 studied 49 post-menopausal women with insomnia related to menopause in a HRT treatment study. Pre-treatment, patients showed a significantly delayed P3 latency and significantly reduced P300 amplitude relative to controls, especially at frontal sites. LORETA analysis (a method used to mathematically explain the scalp distribution of an ERP by its underlying intracranial sources) showed that insomniacs had significantly reduced N1, P2, N2 and P300 source strength 46. The authors’ interpretation was that insomnia was associated with lower levels of “energetic” sources available for automatic and selective information processing.
Regestien et al. 47 found P1-N1 amplitudes to be significantly larger in 20 primary insomniacs than matched controls, and significantly correlated with hyper arousal scores from a subjective questionnaire scale. They interpreted this as evidence for insomniacs having a hyper aroused CNS during wakefulness. However, Szelenberger and Niemewicz 48 reported significantly smaller current source density values using LORETA to estimate sources for N1, N2 and P3 components in insomniacs. Specifically their 14 insomniacs had significantly less activity in the orbito-frontal, medial pre-frontal and anterior cingulated cortices, and the severity of the insomnia correlated significantly with control-insomnia differences in the regions.
More recently three studies have been published that have sought to relate sleep quality in insomnia to daytime measures of ERPs. DeVoto and colleagues 49 compared small numbers of college students with and without insomnia symptoms using two weeks of nightly sleep logs. The insomniacs attended the laboratory for ERP assessment once after a perceived good night’s sleep and once after a night perceived as bad. Controls were assessed after two randomly selected nights. P300 amplitude at Fz was similar for both sessions in the control subjects (8.8 vs. 8.5 μV) and these values were similar to that collected following the poor night in insomniacs (9.2 μV). The recording following the good night in the insomniacs however yielded a smaller P300 at Fz (6.9 μV). No night or group effects emerged for the other sites. The authors interpreted the data as showing an enhanced frontal P300 following a poor night’s sleep with this finding being supportive of a hypothesis of cortical hyper arousal. Given the similarity between control and patient data for all but the poor night of sleep in the insomniacs, this would appear to be a questionable interpretation.
In a second study 50, the same group compared seven college students with insomnia symptoms to seven good sleeping students. All had morning and evening assessments in their homes with overnight actigraphy. Data were compared between the best and worst nights based on the actigraph’s sleep efficiency measures. Unfortunately, despite having both pre- and post-sleep data, averages were collapsed across both sessions. As with their previous study, there were no significant night or group effects on P3 amplitude at Cz or Pz. At Fz, good sleepers had slightly smaller P3 amplitudes around the poorer night of sleep (7.3 μV vs. 8.5 μV). The insomniacs had the opposite pattern with slightly larger values around the poorer night (10.5 μV vs. 9.5 μV). The finding was again interpreted as being supportive of a frontal hyper arousal in the insomniacs that was present around the night of poorer sleep.
The findings from the two papers from the DeVoto laboratory are difficult to interpret. First, the active oddball paradigm is designed to elicit a parietally maximal P3b. The frontal maximum P3a, thought to index attention capture, is best studied using a passive (ignore) condition. Second, the underlying assumption that an increase in frontal P300 amplitude is indicative of increased arousal is not borne out the by literature. For example, Cote et al’s17 failure to find a decreased P300 amplitude following sleep fragmentation, despite observed EEG changes in alpha theta ratios clearly indicating decreased cortical arousal. Third, the use of college students rather than patient populations limits the generalizability of the findings. Finally the collapsing of data from the pre- and post-sleep periods makes it impossible to assess the differential impact of pre-sleep status from that of poor quality sleep on post-sleep function.
Sforza et al. 51 overcame many of these limitations in their study of 15 insomniacs, 45 patients with sleep disordered breathing and 13 controls. They conducted laboratory based ERP assessment pre- and post-sleep, assessed using polysomnography. They reported that the pre-sleep ERPs did not differ between the three groups for N1, P2 or P3 amplitude; however P2 and P3 latencies were longer at Cz and Pz in the SDB group. There were thus no pre-sleep differences between insomniacs and controls. They compared the evening-morning ERP difference with PSG variables using Spearman’s correlation analysis. In the insomnia group, N1 amplitude differences were significantly negatively correlated with the amount of stage 1 sleep, the number of stage changes and the number of awakenings during the night, all of which led to a smaller N1 in the morning relative to that seen pre-sleep. No effects were seen for P3 nor were any ERP differences apparent when the insomnia group was split into those with a good versus a poor night’s sleep based on subjective ratings (as per Devoto et al. 49). Sforza’s data highlight the utility of pre- and post-sleep measures, and potential usefulness of N1, a component known to be both sensitive to arousal and attention as a measure of cortical function in insomniacs.
On balance it would seem that Sforza’s 51 finding of decreased N1 amplitude together with the findings of decreased N1 source strength from both Anderer et al.46 and Szelenberger and Niemcewicz 48 would seem to counter the increase in P1-N1 amplitude reported by Regestien and argue for insomniacs having a decreased rather than an increased cortical arousal level, although these data were collected during stable wakefulness and not in the sleep onset period. Several studies have now reported a decrease in the amplitude of N1 during sleep onset in normal control subjects and within definitive NREM sleep 7, 8, 11, 12, 42, 52–56 Additionally, the increase in P3 latency reported by Bruder et al. 44 and Anderer et al. 45, the decreased P3 amplitude reported by Anderer et al. 45 and the decreased P3 source strength reported by Szelenberger and Niemcewicz 48 would seem to provide support for the hypothesis of a compromised attentional system associated with insomnia symptomatology.
Some labs have used ERPs to assess the impact of Narcolepsy on cognitive processing. In a series studies, Broughton’s group evaluated early ERP components and the P300 in untreated narcoleptic patients. Broughton et al. 57, reported Narcolepsy to be associated with decreased N1, P2 and N2 amplitudes. Aguirre and Broughton 58 assessed P300 prior to each of five MSLT sessions across a day. Patients showed the expected MSLT differences to controls and were also subjectively sleepier when measured with the Stanford Sleepiness Scale (SSS). P300 amplitude was smaller in Narcoleptics, despite a larger N1. Finally, Broughton et al. 59 sought to correlate P300 measures with MSLT sleep onset latency and to assess the utility of P300 as a diagnostic measure for Narcolepsy. Again Narcoleptics showed substantially and significantly decreased P3 amplitudes, however, the authors concluded that the MSLT was nonetheless better at discriminating patients from controls.
More recently, two other groups have assessed ERPs in Narcolepsy. Sangal et al. 60 evaluated visual and auditory ERPS in Narcoleptics undergoing a controlled trial of modafinil. Interestingly, P300 amplitude and latency discriminated between those who did and did not respond to treatment. Specifically, responders had a delayed P300 latency to visual stimuli and smaller P300 amplitude to auditory targets than non-responders. Naumann et al. 61 reported a contradictory finding of increased P300 amplitude in patients, particularly at frontal sites. Unfortunately, this is the only study to use treated patients, and the authors also reported no differences in subjective sleepiness between the groups. While the medications being used by the patients were not listed, presumably they included stimulants, and thus it is likely that what was being measured was the impact of CNS stimulant medication on ERPs rather than the impact of Narcolepsy. On balance it would thus appear that Narcolepsy is associated with decreased P300 amplitude and possibly increased P300 latency, both reflective of the increased level of daytime sleepiness.
ERPs during sleep
Sleep represents a section of the arousal continuum in which unconsciousness can be rapidly reversed. Humans and animals engage in a series of behaviors (some as basic as closing eye lids) designed to minimize sensory input and thus maximize uninterrupted sleep. A commonly held view is that information processing continues throughout sleep “as evidenced by the fact that the sleeper will reliably be aroused by increasingly intense stimuli or the familiar sound of the alarm clock…however the sudden call of one’s own name will often quickly arouse the sleeper” 55, p228). An alternative view is that thalamic gating of sensory afferents is specifically designed to minimize the likelihood of sensory information reaching the cortex, and that only rarely will stimuli be sufficiently intense or coincidental with fluctuations in arousal level to be available for cortical processing. Thus a person may often respond to their own name, but also often will not. Even highly intense, long duration stimuli (for example, fire alarms) may fail to interrupt sleep.
Study of information processing during sleep is limited by the lack of the ability of subjects to make verbal or motor responses to presented information. The researcher thus has little access to the essentially private mental state of the sleeper. ERPs provide a unique window into the brain to assess the extent to which information is processed in different sleep states. As will be discussed below, ERPs recorded during sleep can also provide a functional measure of CNS integrity, highlight differences between different sleep stages, and provide a metric to compare normal from pathological aging.
This section is designed to highlight some potential uses of sleep ERP assessment rather than to be a review of studies of ERPs during sleep, as several such reviews have been published over the past several years 62–66. By way of introduction, NREM sleep ERPs consist of a series of large amplitude components that appear to be produced regardless of the physical characteristics used to elicit them. These are a P2 at around 200 ms, an N350, between 250 and 400 ms, an N550 between 500 and 800 ms and P900 between 800 and 1300 ms or so. P2 is also easily elicited in the waking state, as part of the N1-P2 “vertex” potential. The later, very large amplitude ERPs are however unique to sleep. They cannot be elicited in the waking state. The N550 and P900 form part of the very large amplitude stimulus-elicited K-complex. The N350 is large in averaged K-complexes, but also appears early in the sleep onset period when the K-complex cannot be elicited. The N350 appears to reflect activation of the generator responsible for vertex sharp waves. P2 and N350 are also prominent in REM sleep, and as will be highlighted below, there is debate as to the extent to whether the P300 can also be elicited in REM sleep.
The N350 and N550 are easily the largest response that can be recorded in the human subject and are much larger than any ERP that can be recorded in the waking state. The volume conduction of components of this magnitude to the scalp can only be explained by a massive synchronized firing of large numbers of presumably cortical cells. Thus their presence, latency and most importantly, amplitude, all provide functional measures of CNS integrity, as disruption of white matter will lead to decreased synchronization. Disruption of gray matter functioning should also affect the amplitude of the resulting as a result of summation of synchronized activity. N350 and N550 have been hypothesized to relate to sleep protection processes, with the earlier N350 thought to reflect the output of an active inhibitory process designed to facilitate sleep onset, and the later N550 a reflection of the evoked K-complex being a precursor to the delta waves produced in SWS 2, 67. Less work has been done on the P900 (see Bastien et al. 66) or the P2 (see Crowley and Colrain 68).
ERPs as a measure of rudimentary information processing during sleep
In terms of information processing, ERPs have been used most effectively to assess the degree to which external stimuli are processed in an “awake-like” (conscious) manner during REM sleep, when muscle paralysis prohibits any form of a motor signalling of awareness of the external environment. Several studies have focussed on whether or not a P300 is present during REM and NREM sleep. Cote 55 outlined the conditions for a component to be considered as equivalent to the waking P300. It should be target (or deviant)specific, have a scalp distribution that is maximum over parietal sites, peak at approximately 300 ms and have an amplitude that varies inversely with the probability of target presentation. When assessing NREM sleep, she also highlights the need to assess components in averages from which K-complex responses are excluded. The large N350-N550 may obscure the small amplitude P300. The K-complex and its associated components (N350 and N550) have also been evaluated as potential markers of information processing in NREM sleep, and other components associated with information processing during wakefulness (N1, mismatch negativity (MMN), N400 etc) have also been studied during sleep 24, 25, 43, 64, 69–75.
In Cote’s review 55 she concludes that there is no compelling evidence for the presence of a P300 in NREM sleep. It would seem on balance that this is a reasonable conclusion and that many of the studies identifying “P300” in sleep are in fact referring to a later P450 that may largely reflect partial repolarization between the active N350 and N550 components or even a later P900, which is probably the repolarization following the K-complex. Neither “behaves” in a manner consistent with them being related to a P300.
Two labs have however reported a P300 to the presentation of a subject’s own name in stage 2 sleep 76–78. Bastuji et al. 63 hypothesized that the intrinsic (psychological) relevance of the stimulus and its physical obtrusiveness both influence the probability of an infrequently presented stimulus receiving sufficient cognitive processing to generate a P300. They argue that a P300 will be elicited upon presentation of one’s own name because of its high level of intrinsic significance. This may be a unique exception. For example, a brief airway obstruction, a stimulus having exceedingly high intrinsic significance, elicits a large P300 in the waking state 79 but does not do so during NREM sleep 54.
Takahara et al. 80 recently used ERPs to investigate the extent to which voluntary attention can be directed to relevant events during REM sleep. They further separated the processing of stimuli during tonic and phasic REM sleep periods. Subjects were studied on two separate nights. On one night (attentive condition) subjects were asked to try to attend to stimuli during sleep and discriminate between rare 2000 Hz and common 1000 Hz stimuli and make a finger lift response to the rare stimuli. On the other night (passive condition) they were asked to ignore the stimuli.
During wakefulness the rare target stimuli produced the expected P300 response maximal at parietal scalp sites. A P400 was seen in the tonic REM sleep averages and was larger in the attentive condition. Within the attentive condition P400 was larger to the rare tones than to the common tones. Its scalp topography was more occipital than that of the waking P300, and it was not observed in phasic REM sleep. Similar components with an occipitally shifted scalp topography have been previously reported in REM sleep by other groups 9. Infrequently presented stimuli may also elicit a large vertex maximum N350 in REM sleep, although its amplitude is typically smaller than that observed in NREM sleep. Nevertheless, this negativity will probably overlap both temporally and spatially with the P300.
The authors interpreted the presence of the P400 (presumed to the equivalent of a delayed P300 during wakefulness) as evidence of the ability to direct voluntary attention to the auditory channel during tonic REM sleep. While they did not comment on the absence of the component during phasic REM sleep periods, their data are probably reflective of some inhibition of processing or gating of external stimuli occurring during the periods associated with eye movement bursts. Sallinen et al. 81 reported a similar result but cautiously noted that tonic REM sleep can be difficult to distinguish from stage 1 sleep. Some intrusion of stage 1 epochs into the averages may account for some of the observed difference between the tonic and the unambiguously REM-associated phasic data. Cote & Campbell 82 failed to observe P300 differences elicited by high intensity (100 dB) deviant stimuli during tonic and phasic REM activity. Cote et al. 56 also reported a P300 at Pz during REM sleep but its anterior dispersion to central and frontal sites was absent. The REM sleep P300 was only reliably seen when the deviant stimuli were very rare and very loud. If the loud, deviant stimuli occurred more frequently, the P300 could not be elicited. Similarly, it could not be elicited if the deviant stimulus represented a pitch change from the standard even if it occurred very rarely.
The scalp topography of an ERP component can be a used to mathematically predict underlying brain source mechanisms. In a recent review of the source generators of P300, Linden 83 concluded that it is produced by activation of a widespread network involving the posterior cingulate cortex/superior parietal cortex, the inferior parietal lobule at the temporo-parietal junction, inferior frontal gyrus/insular cortex and the anterior cingulate cortex. On the other hand, the P3a elicited by unattended deviants has more fronto-central and less parietal scalp distribution. This is explained by activation of a similar network, but without the contribution of posterior cingulate and with less involvement of anterior cingulate and inferior frontal gyrus/insular cortices. The absence of a frontal component during REM sleep might thus reflect a difference in the balance of these generator structures relative to wakefulness. Alternatively, as mentioned an overlapping large central maximum negativity, the N350, unique to sleep may reflect the activation of additional sources, perhaps in an attempt to protect sleep. Determination of the validity of any source model will undoubtedly require additional confirmatory evidence perhaps from patient data, the fMRI or higher density EEG/MEG recordings. Bastuji et al. 63 hypothesized that the lack of a frontal element in the REM sleep P300 may reflect underlying neurochemical differences between wakefulness and REM sleep, with REM sleep being associated with the selective inhibition of frontally projecting nor-adrenergic neurons from the brain stem.
The mismatch negativity (MMN) represents the output of a change detector system. Almost any physical change to the standard stimulus will elicit the MMN. The MMN has been reported to occur to frequency deviants during REM sleep 84, 85 not during NREM sleep 56. Nevertheless, the MMN that can be recorded during REM sleep is only elicited by large frequency differences between the standard and the deviant. 71. A large deviant will also elicit a larger N1 than that observed following presentation of the standard. Although an N1 is difficult to elicit in NREM 86 several labs have recorded an N1 during REM sleep 64. Thus, there is some debate about whether the enhanced negativity that is observed following presentation of a large deviant reflects a true MMN or rather reflects an enhanced deviant N1. Kisley et al. 87 assessed sensory gating in REM sleep using the mid-latency P50 component of the auditory evoked response. Sensory gating is usually assessed by presenting two auditory stimuli in very close temporal proximity. During wakefulness, the typical finding in healthy subjects is attenuation of the P50 to the second stimulus relative to that observed following presentation of the first stimulus. Schizophrenics do not demonstrate this phenomenon. Kisley et al. 87 reported that control subjects show strong inhibition to the second stimulus during REM sleep. Remarkably, schizophrenics continued to show a failure of suppression of the second P50 during REM sleep.
N350 as a possible indicator of active inhibition of sensory processing
N350 appears in stage 1 NREM sleep during the transition from a drowsy wakefulness to stable sleep. It cannot be recorded during wakefulness. Ogilvie et al.7 and Harsh et al. 8 reported that it generally first appeared when subjects failed to make a behavioral response to external stimuli. Thus, N350 emerges when the subject is no longer able to signal awareness of the external environment, i.e., during definitive sleep. Colrain et al. 12 and Gora et al. 11 indicated that the N350 in auditory evoked potentials (AEPs) and respiratory-related evoked potentials (RREPs) was difficult to observe in stage 1-alpha but was readily apparent in stage 1-theta. Stage 1-alpha was therefore similar to wakefulness while stage 1-theta was more similar to the actual sleep state
The N350 is prominent in averages of evoked vertex sharp waves 88; however, it also appears as a component in the averages of K-complexes during stage 2, 3 and 4 of sleep 88. Bastien and Campbell 89 hypothesized that the N350 might act as a trigger for the much larger amplitude N550 and several studies have reported that N350 is larger in averages of responses containing K-complexes than in averages of non-K-complex responses 8, 9, 90–92. The nature of the interrelationships between the vertex sharp wave-related N350 and the K-complex-related N550 remains to be fully determined, although it may be that the neural generator mechanism responsible for the vertex sharp wave at sleep onset, is able to trigger the K-complex generator once sleep has become more stable. Certainly, N350 replaces the P300 in the sleep onset period concurrent with the loss of alpha EEG activity (see Figure 3). This raises the possibility that it reflects an active inhibitory process designed to facilitate sleep onset.
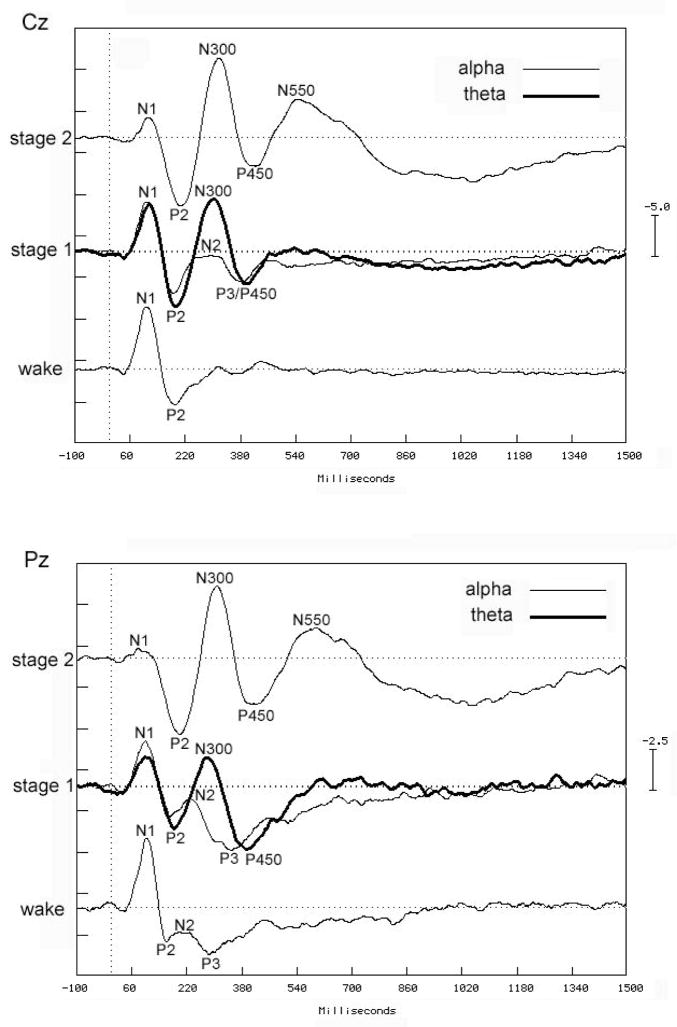
Figure 3.
Grand mean waveforms during wake, stage 1 alpha, stage 1 theta and stage 2 sleep recorded at Cz (upper panel) and Pz (lower panel). Voltage is plotted on the y-axis in microvolts. Negativity at the scalp relative to the reference (linked ears) is shown as an upward deflection. The data highlight the appearance of an N350 (labeled N300 in the figure), maximal at Cz, when stimuli presented within stage 1 sleep occur during a brief period of theta activity. The Pz panel highlights the presence of a P300 (labeled P3) to rare target tones during wakefulness, that persists into stage 1 alpha, but is replaced by the N350 with the onset of theta activity. Adapted from 12 with permission.
Kallai et al. 93 recently adopted an innovative strategy of combing traditional ERP and evoked “40 Hz oscillation” measures. An external stimulus can entrain the naturally occurring 40 Hz oscillation in the EEG forcing it to be in phase with stimulus presentation. There has been much recent interest in the 40 Hz response as a measure of conscious “binding”. It is thought to index stimulus recognition and possibly facilitate integration of sensory association areas. The 40 Hz oscillation has been studied in wakefulness but predictably and in REM sleep, but found to be absent in SWS, when studied with magnetoencephalography. The presentation of stimuli acted to reset the oscillation in wakefulness but not in REM 94. Kallai et al. 93 compared the stimulus evoked 40Hz response to N1 and the N350. N1 is readily apparent following presentation of auditory stimuli in the waking state and may reflect a general type of consciousness 64. The N350, as indicated above, can serve as a measure of the loss of consciousness during sleep onset. Stimuli consisted of single tones presented with a 3 s inter-stimulus interval. Consistent with the resetting data observed by Llinas and Ribary 94 nor evoked r40Hz response was seen in SWS or in REM. N1 decreased dramatically in light sleep, and consistent with the 40 Hz data, was absent in SWS. N350 was very large in both light sleep and SWS. As others have reported, N1 was apparent in stage REM but was much attenuated when a smaller N350 could also be recorded. The 40 Hz response was not apparent during stage REM. The combined results would indicate that there is little evidence of awareness of the external environment during SWS perhaps because of inhibition of input as indicated by the large N350. During REM sleep, there was however some evidence of the processing of external stimulus input (reduced N1), but perhaps not sufficiently enough to allow for its awareness or because of a failure of sensory integration (absence of 40 Hz response).
N550 as a measure of delta generation capability
Bastien and Campbell 89 were the first to separately sort and average responses to stimuli that did (KC+) and did not (KC−) elicit a K-complex. Unlike the N350, the later N550 is absent or greatly diminished in KC− averages of responses to both auditory and respiratory stimuli 95, 96. Bastien and Campbell 89 argued that the N550 in the KC+ average gives a very good estimate of the properties of the “pure” K-complex.
The view that K-complexes (and by extension the N550 in the KC+ average) are reflective of a brain state that is conducive to sleep, and represent the output of a delta frequency EEG generator mechanism has recently gained currency. The animal studies conducted by Steriade’s group 97–100 strongly support this view, by highlighting the similarity in underlying neurophysiology of K-complex and delta generation. More recently, human experimental studies have provided data that are also indicative of N550 reflecting delta generation. For example spontaneous K-complex densities are increased prior to transition to SWS compared to transitions to REM sleep 67. This increase prior to SWS was modeled by a linear regression and decreased with successive sleep cycles across the night following the established pattern of delta waves and slow wave activity originally proposed by Borbely 101. Evoked K-complexes are also more likely in periods preceding SWS than those preceding REM sleep 2, however the KC+ N550 did not differ. Further supportive data comes from looking at the rebound phenomenon following sleep fragmentation. N550 amplitude is significantly larger in recovery sleep following a night in which sleep was fragmented, when compared to baseline 102, 103, and paralleled an increase in slow wave sleep and in delta power 102.
The utility of having the evoked K-complex and its associated N550 component as measures of delta generation is clearly seen in studies of normal and pathological aging. Given the decrease in the amplitude of EEG associated with aging, it is likely that the use of a 75 μV criterion for the definition of delta activity dramatically underestimates the amount of SWS 104. The use of ERPs enables an estimate of the extent of delta generation independently of the rather arbitrary 75 μV criterion. This has enabled the determination that elderly subjects are still capable of generating delta frequency events in stage 2 sleep, although less frequently than younger subjects 105 with the N550 amplitude being on average −60 μV at Fz as compared to the −134 μV seen in younger subjects 105 (see Figure 4).
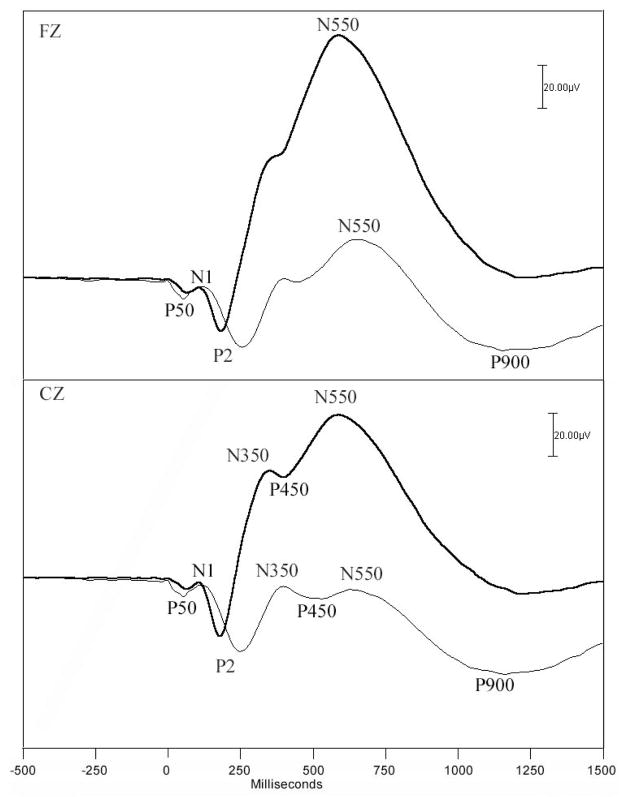
Figure 4.
Averaged auditory ERPs from stage 2 NREM sleep in young (dark lines) and elderly (light lines) healthy subjects, adapted from 105 with permission. Data are presented from Fz (upper panel) and Cz (lower panel) electrode sites, and are based on the averages of only those responses containing K-complexes (KC+). The waveforms display small P50 (middle latency) and N1 components, and a prominent P2, that is larger in the elderly. N350 is more prominent at Cz, and N550 more prominent at Fz.
Alcoholism is known to lead to a reduction in SWS 106. Determination of the impact of alcoholism on delta generation is thus problematic in older subjects. Again, ERPs can be used to provide further insight about this issue. The N550 in middle-aged alcoholics has been shown to be significantly reduced relative to age-matched controls 107. Finally, in another recent example, physiological delta generation capability as measured with ERPs, was shown to be reduced in Alzheimer’s disease (AD) patients compared to elderly controls 108, despite the increase in pathological delta frequency activity seen in wakefulness and sleep in AD patients.
Caution needs to be exercised however, when interpreting the impact of pathology on sleep ERPs. Gora et al. 42 reported decreased N550 amplitudes to inspiratory occlusion stimuli in OSAS patients and interpreted this finding as evidence of a sleep-specific blunting of afferent transfer to the cortex. Subsequently Afifi et al. 40 replicated this finding for inspiratory occlusions, but found no difference between OSAS patients and controls for the auditory N550. Thus it is likely that the respiratory N550 effect is due to poor transduction of specific pressure changes to afferent neural signals due to altered upper airway mechanics, rather than a generalized altering of CNS processing.
Practice points
ERPs provide an exquisitely sensitive index of the extent of sensory and cognitive processing during wakefulness, independent of motor function, and under conditions in which the subject’s attention need not be maintained on the task at hand.
Daytime ERPs are not reliably impaired in insomnia.
P300 (P3b) shows variable effects in OSAS; however P3a may be a more sensitive measure.
P300 shows consistent alterations in daytime studies of Narcolepsy.
P300 is sensitive to onset of sleep. It is much reduced during stage 1 when the subject no longer is capable of overtly detecting the target stimulus or when theta activity dominates the EEG.
The amplitude of P300 is reduced following total sleep deprivation
Sleep fragmentation has less of an impact on P300 but leads to decreases in N1 amplitude.
ERPs that are maximum over fronto-central areas of the scalp such as the novel P3 (possibly P3a) and the error related negativity (ERN) show marked attenuation following sleep deprivation.
P300 cannot be recorded in NREM sleep (with the possible exception of “own name” processing).
An N1 is visible and P300 may be visible in REM sleep (but with a different topographic distribution), presumably reflecting the altered extent of cognitive processing relative to wakefulness.
In NREM sleep, N350 is a useful measure of the onset of drowsiness and may well indicate the engagement of a sleep-specific inhibitory mechanism that also produces vertex sharp waves and possibly triggers K-complexes as spontaneous and evoked EEG phenomena.
N550 can be viewed as a marker of EEG delta generation capability and thus as a functional measure of CNS structural integrity.
Research Agenda
Daytime assessment of the impact of sleep disruption in both experimental and pathological contexts should focus more on ERP measures designed to index frontal cortical processing. Such frontal functions may include the automatic switching of attention to highly relevant stimulus input, the detection of novelty, the inhibition of inappropriate response and error detection.
More research needs to focus on N350 as a marker of the loss of conscious processing of stimuli during drowsiness. It would appear to have particular potential for use in the study of the sleep onset period in insomniacs, and possibly in applied settings such as drowsy driving.
More attention should be focussed on the K-complex reflecting underlying neural mechanisms that must be operational to produce a single delta frequency synchronized waveform. When viewed in this context, the N550 becomes a useful measure of delta generation capability.
Acknowledgments
Dr. Colrain is supported by the United States’ National Institutes of Health (NIH) grants AA14211, AA05965, AA12388, HL58585 and DA16427. Dr. Campbell is supported from research grants from the National Scientific and Engineering Research Council (NSERC) of Canada.
Footnotes
P300 is also called “P3b” to distinguish from an earlier latency “P3a”. While the P3s are similar in that they are elicited by rare stimulus events, a number of features distinguish them, as will be explained later in this article.
Some authors also distinguish between the novel P3 and P3a. The novel P3 is elicited by a highly novel stimulus while the P3a can be elicited by any obtrusive stimulus or high perceptible stimulus change, even if they are not “novel”. The novel stimulus does however represent a highly perceptible stimulus change. Both the novel P3 and P3a share a common fronto-central scalp topography and can be elicited in the absence of attention.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Regan DM. Human Brain Electrophysiology. New York: Elsevier; 1995. [Google Scholar]
- 2.Nicholas CL, Trinder J, Crowley KE, et al. The impact of slow wave sleep proximity on evoked K-complex generation. Neurosci Lett. 2006;404(1–2):127–31. doi: 10.1016/j.neulet.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 3.Dinges DF, Kribbs NB. Performing while sleepy: effects of experimentally induced sleepiness. In: Monk TH, editor. Sleep, Sleepiness and Performance. Chichester, UK: John Wiley and Sons; 1991. pp. 98–128. [Google Scholar]
- 4.Koslowsky M, Babkoff H. Meta-analysis of the relationship between total sleep deprivation and performance. Chronobiol Int. 1992;9(2):132–6. doi: 10.3109/07420529209064524. [DOI] [PubMed] [Google Scholar]
- 5.Pilcher JJ, Huffcutt AI. Effects of sleep deprivation on performance: a meta-analysis. Sleep. 1996;19(4):318–26. doi: 10.1093/sleep/19.4.318. [DOI] [PubMed] [Google Scholar]
- 6.Donchin E, Coles MG. Is the P300 component a manifestation of context. updating? Behavior and Brain Sciences. 1988;11:357–427. [Google Scholar]
- 7*.Ogilvie RD, Simons IA, Kuderian RH, et al. Behavioral, event-related potential, and EEG/FFT changes at sleep onset. Psychophysiology. 1991;28(1):54–64. doi: 10.1111/j.1469-8986.1991.tb03386.x. [DOI] [PubMed] [Google Scholar]
- 8.Harsh J, Voss U, Hull J, et al. ERP and behavioral changes during the wake/sleep transition. Psychophysiology. 1994;31(3):244–52. doi: 10.1111/j.1469-8986.1994.tb02213.x. [DOI] [PubMed] [Google Scholar]
- 9.Niiyama Y, Fujiwara R, Satoh N, et al. Endogenous components of event-related potential appearing during NREM stage 1 and REM sleep in man. Int J Psychophysiol. 1994;17(2):165–74. doi: 10.1016/0167-8760(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 10.Cote KA, De Lugt DR, Campbell KB. Changes in the scalp topography of event-related potentials and behavioral responses during the sleep onset period. Psychophysiology. 2002;39(1):29–37. doi: 10.1017/S0048577202992188. [DOI] [PubMed] [Google Scholar]
- 11.Gora J, Colrain IM, Trinder J. Respiratory-related evoked potentials during the transition from alpha to theta EEG activity in stage 1 NREM sleep. J Sleep Res. 1999;8(2):123–34. doi: 10.1046/j.1365-2869.1999.00144.x. [DOI] [PubMed] [Google Scholar]
- 12.Colrain IM, Di Parsia P, Gora J. The impact of prestimulus EEG frequency on auditory evoked potentials during sleep onset. Can J Exp Psychol. 2000;54(4):243–54. doi: 10.1037/h0087344. [DOI] [PubMed] [Google Scholar]
- 13.Bastuji H, Garcia-Larrea L, Franc C, et al. Brain processing of stimulus deviance during slow-wave and paradoxical sleep: a study of human auditory evoked responses using the oddball paradigm. J Clin Neurophysiol. 1995;12(2):155–67. doi: 10.1097/00004691-199503000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Lee HJ, Kim L, Kim YK, et al. Auditory event-related potentials and psychological changes during sleep deprivation. Neuropsychobiology. 2004;50(1):1–5. doi: 10.1159/000077933. [DOI] [PubMed] [Google Scholar]
- 15.Lee HJ, Kim L, Suh KY. Cognitive deterioration and changes of P300 during total sleep deprivation. Psychiatry Clin Neurosci. 2003;57(5):490–6. doi: 10.1046/j.1440-1819.2003.01153.x. [DOI] [PubMed] [Google Scholar]
- 16.Morris AM, So Y, Lee KA, et al. The P300 event-related potential. The effects of sleep deprivation. J Occup Med. 1992;34(12):1143–52. [PubMed] [Google Scholar]
- 17*.Cote KA, Milner CE, Osip SL, et al. Waking quantitative electroencephalogram and auditory event-related potentials following experimentally induced sleep fragmentation. Sleep. 2003;26(6):687–94. doi: 10.1093/sleep/26.6.687. [DOI] [PubMed] [Google Scholar]
- 18.Kingshott RN, Cosway RJ, Deary IJ, et al. The effect of sleep fragmentation on cognitive processing using computerized topographic brain mapping. J Sleep Res. 2000;9(4):353–7. doi: 10.1046/j.1365-2869.2000.00223.x. [DOI] [PubMed] [Google Scholar]
- 19*.Gosselin A, De Koninck J, Campbell KB. Total sleep deprivation and novelty processing: implications for frontal lobe functioning. Clin Neurophysiol. 2005;116(1):211–22. doi: 10.1016/j.clinph.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 20.Squires NK, Squires KC, Hillyard SA. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalogr Clin Neurophysiol. 1975;38(4):387–401. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- 21.Knight RT. Decreased response to novel stimuli after prefrontal lesions in man. Electroencephalogr Clin Neurophysiol. 1984;59(1):9–20. doi: 10.1016/0168-5597(84)90016-9. [DOI] [PubMed] [Google Scholar]
- 22.Muller-Gass A, Gonthier I, Desrochers A, et al. Multiple P3 evidence of a two-stage process in word gender decision. Neuroreport. 2000;11(16):3527–31. doi: 10.1097/00001756-200011090-00025. [DOI] [PubMed] [Google Scholar]
- 23.Cycowicz YM, Friedman D. The old switcheroo: when target environmental sounds elicit a novelty P3. Clin Neurophysiol. 2004;115(6):1359–67. doi: 10.1016/j.clinph.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Salmi J, Huotilainen M, Pakarinen S, et al. Does sleep quality affect involuntary attention switching system? Neurosci Lett. 2005;390(3):150–5. doi: 10.1016/j.neulet.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 25*.Atienza M, Cantero JL, Dominguez-Marin E. Mismatch negativity (MMN): an objective measure of sensory memory and long-lasting memories during sleep. Int J Psychophysiol. 2002;46(3):215–25. doi: 10.1016/s0167-8760(02)00113-7. [DOI] [PubMed] [Google Scholar]
- 26.Falkenstein M, Hohnsbein J, Hoormann J, et al. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol. 1991;78(6):447–55. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- 27.Kerns JG, Cohen JD, MacDonald AW, 3rd, et al. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303(5660):1023–6. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 28.Tsai LL, Young HY, Hsieh S, et al. Impairment of error monitoring following sleep deprivation. Sleep. 2005;28(6):707–13. doi: 10.1093/sleep/28.6.707. [DOI] [PubMed] [Google Scholar]
- 29.Scheffers MK, Humphrey DG, Stanny RR, et al. Error-related processing during a period of extended wakefulness. Psychophysiology. 1999;36(2):149–57. [PubMed] [Google Scholar]
- 30.Murphy TI, Richard M, Masaki H, et al. The effect of sleepiness on performance monitoring: I know what I am doing, but do I care? J Sleep Res. 2006;15(1):15–21. doi: 10.1111/j.1365-2869.2006.00503.x. [DOI] [PubMed] [Google Scholar]
- 31.Yeung N, Cohen JD, Botvinick MM. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev. 2004;111(4):931–59. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
- 32.Gehring WJ, Goss B, Coles MGH, et al. A neural system for the error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- 33.Dorrian J, Lamond N, Dawson D. The ability to self-monitor performance when fatigued. J Sleep Res. 2000;9(2):137–44. doi: 10.1046/j.1365-2869.2000.00195.x. [DOI] [PubMed] [Google Scholar]
- 34.Sangal RB, Sangal JM. Obstructive sleep apnea and abnormal P300 latency topography. Clin Electroencephalogr. 1997;28(1):16–25. doi: 10.1177/155005949702800104. [DOI] [PubMed] [Google Scholar]
- 35.Sangal R, Sangal J. Abnormal visual P300 latency in obstructive sleep apnea does not change acutely upon treatment with CPAP. Sleep. 1997;20(9):702–704. doi: 10.1093/sleep/20.9.702. [DOI] [PubMed] [Google Scholar]
- 36.Sangal RB, Sangal JM. P300 latency: abnormal in sleep apnea with somnolence and idiopathic hypersomnia, but normal in narcolepsy. Clin Electroencephalogr. 1995;26(3):146–153. doi: 10.1177/155005949502600305. [DOI] [PubMed] [Google Scholar]
- 37.Kotterba S, Rasche K, Widdig W, et al. Neuropsychological investigations and event-related potentials in obstructive sleep apnea syndrome before and during CPAP-therapy. J Neurol Sci. 1998;159(1):45–50. doi: 10.1016/s0022-510x(98)00131-2. [DOI] [PubMed] [Google Scholar]
- 38.Rumbach L, Krieger J, Kurtz D. Auditory event-related potentials in obstructive sleep apnea: effects of treatment with nasal continuous positive airway pressure. Electroencephalography and Clinical Neurophysiology. 1991;8(5):454–457. doi: 10.1016/0168-5597(91)90094-e. [DOI] [PubMed] [Google Scholar]
- 39.Walsleben J, Squires NK, Rothenberger VL. Auditory event-related potentials and brain dysfunction in sleep apnea. Electroencephalogr Clin Neurophysiol. 1989;74(4):297–311. doi: 10.1016/0168-5597(89)90060-9. [DOI] [PubMed] [Google Scholar]
- 40*.Afifi L, Guilleminault C, Colrain IM. Sleep and respiratory stimulus specific dampening of cortical responsiveness in OSAS. Respir Physiol Neurobiol. 2003;136(2–3):221–34. doi: 10.1016/s1569-9048(03)00084-3. [DOI] [PubMed] [Google Scholar]
- 41.Sangal RB, Sangal JM. Measurement of P300 and sleep characteristics in patients with hypersomnia: do P300 latencies, P300 amplitudes, and multiple sleep latency and maintenance of wakefulness tests measure different factors? Clin. Electroencephalogr. 1997;28(3):179–184. doi: 10.1177/155005949702800311. [DOI] [PubMed] [Google Scholar]
- 42.Gora J, Trinder J, Pierce R, et al. Evidence of a sleep-specific blunted cortical response to inspiratory occlusions in mild obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2002;166(9):1225–34. doi: 10.1164/rccm.2106005. [DOI] [PubMed] [Google Scholar]
- 43.Gosselin N, Mathieu A, Mazza S, et al. Deficits in involuntary attention switching in obstructive sleep apnea syndrome. Neurosci Lett. 2006;408(1):73–78. doi: 10.1016/j.neulet.2006.08.046. [DOI] [PubMed] [Google Scholar]
- 44.Bruder GE, Towey JP, Stewart JW, et al. Event-related potentials in depression: influence of task, stimulus hemifield and clinical features on P3 latency. Biol Psychiatry. 1991;30(3):233–46. doi: 10.1016/0006-3223(91)90108-x. [DOI] [PubMed] [Google Scholar]
- 45.Anderer P, Semlitsch HV, Saletu B, et al. Effects of hormone replacement therapy on perceptual and cognitive event-related potentials in menopausal insomnia. Psychoneuroendocrinology. 2003;28(3):419–45. doi: 10.1016/s0306-4530(02)00032-x. [DOI] [PubMed] [Google Scholar]
- 46.Anderer P, Saletu B, Saletu-Zyhlarz G, et al. Brain regions activated during an auditory discrimination task in insomniac postmenopausal patients before and after hormone replacement therapy: low-resolution brain electromagnetic tomography applied to event-related potentials. Neuropsychobiology. 2004;49(3):134–53. doi: 10.1159/000076722. [DOI] [PubMed] [Google Scholar]
- 47.Regestein QR, Dambrosia J, Hallett M, et al. Daytime alertness in patients with primary insomnia. Am J Psychiatry. 1993;150(10):1529–34. doi: 10.1176/ajp.150.10.1529. [DOI] [PubMed] [Google Scholar]
- 48.Szelenberger W, Niemcewicz S. Event-related current density in primary insomnia. Acta Neurobiol Exp (Wars) 2001;61(4):299–308. doi: 10.55782/ane-2001-1405. [DOI] [PubMed] [Google Scholar]
- 49.Devoto A, Violani C, Lucidi F, et al. P300 amplitude in subjects with primary insomnia is modulated by their sleep quality. J Psychosom Res. 2003;54(1):3–10. doi: 10.1016/s0022-3999(02)00579-2. [DOI] [PubMed] [Google Scholar]
- 50.Devoto A, Manganelli S, Lucidi F, et al. Quality of sleep and P300 amplitude in primary insomnia: a preliminary study. Sleep. 2005;28(7):859–63. doi: 10.1093/sleep/28.7.859. [DOI] [PubMed] [Google Scholar]
- 51*.Sforza E, Haba-Rubio J. Event-related potentials in patients with insomnia and sleep-related breathing disorders: evening-to-morning changes. Sleep. 2006;29(6):805–13. doi: 10.1093/sleep/29.6.805. [DOI] [PubMed] [Google Scholar]
- 52.de Lugt DR, Loewy DH, Campbell KB. The effect of sleep onset on event related potentials with rapid rates of stimulus presentation. Electroencephalogr Clin Neurophysiol. 1996;98(6):484–92. doi: 10.1016/0013-4694(96)94726-4. [DOI] [PubMed] [Google Scholar]
- 53.Noldy NE, Stelmack RM, Campbell KB. Event-related potentials and recognition memory for pictures and words: the effects of intentional and incidental learning. Psychophysiology. 1990;27(4):417–28. doi: 10.1111/j.1469-8986.1990.tb02337.x. [DOI] [PubMed] [Google Scholar]
- 54*.Webster KE, Colrain IM. Multichannel EEG analysis of respiratory evoked-potential components during wakefulness and NREM sleep. J Appl Physiol. 1998;85(5):1727–1735. doi: 10.1152/jappl.1998.85.5.1727. [DOI] [PubMed] [Google Scholar]
- 55*.Cote KA. Probing awareness during sleep with the auditory odd-ball paradigm. Int J Psychophysiol. 2002;46(3):227–41. doi: 10.1016/s0167-8760(02)00114-9. [DOI] [PubMed] [Google Scholar]
- 56.Cote KA, Etienne L, Campbell KB. Neurophysiological evidence for the detection of external stimuli during sleep. Sleep. 2001;24(7):791–803. [PubMed] [Google Scholar]
- 57.Broughton R. Performance and evoked potential measures of various states of daytime sleepiness. Sleep. 1982;5 (Suppl 2):S135–46. doi: 10.1093/sleep/5.s2.s135. [DOI] [PubMed] [Google Scholar]
- 58.Aguirre M, Broughton RJ. Complex event-related potentials (P300 and CNV) and MSLT in the assessment of excessive daytime sleepiness in narcolepsy-cataplexy. Electroencephalogr Clin Neurophysiol. 1987;67(4):298–316. doi: 10.1016/0013-4694(87)90116-7. [DOI] [PubMed] [Google Scholar]
- 59.Broughton R, Aguirre M, Dunham W. A comparison of multiple and single sleep latency and cerebral evoked potential (P300) measures in the assessment of excessive daytime sleepiness in narcolepsy-cataplexy. Sleep. 1988;11(6):537–45. doi: 10.1093/sleep/11.6.537. [DOI] [PubMed] [Google Scholar]
- 60.Sangal RB, Sangal JM, Belisle C. Visual P300 latency predicts treatment response to modafinil in patients with narcolepsy. Clin Neurophysiol. 1999;110(6):1041–7. doi: 10.1016/s1388-2457(99)00035-8. [DOI] [PubMed] [Google Scholar]
- 61.Naumann A, Bierbrauer J, Przuntek H, et al. Attentive and preattentive processing in narcolepsy as revealed by event-related potentials (ERPs) Neuroreport. 2001;12(13):2807–11. doi: 10.1097/00001756-200109170-00011. [DOI] [PubMed] [Google Scholar]
- 62.Halasz P. K-complex, a reactive EEG graphoelement of NREM sleep: an old chap in a new garment. Sleep Med Rev. 2005;9(5):391–412. doi: 10.1016/j.smrv.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 63.Bastuji H, Perrin F, Garcia-Larrea L. Semantic analysis of auditory input during sleep: studies with event related potentials. Int J Psychophysiol. 2002;46(3):243–55. doi: 10.1016/s0167-8760(02)00116-2. [DOI] [PubMed] [Google Scholar]
- 64.Campbell KB, Colrain IM. Event-related potential measures of the inhibition of information processing: II. The sleep onset period Int J Psychophysiol. 2002;46(3):197–214. doi: 10.1016/s0167-8760(02)00112-5. [DOI] [PubMed] [Google Scholar]
- 65.Colrain IM. The K-complex: a 7-decade history. Sleep. 2005;28(2):255–73. doi: 10.1093/sleep/28.2.255. [DOI] [PubMed] [Google Scholar]
- 66.Bastien CH, Crowley KE, Colrain IM. Evoked potential components unique to non-REM sleep: relationship to evoked K-complexes and vertex sharp waves. Int J Psychophysiol. 2002;46(3):257–74. doi: 10.1016/s0167-8760(02)00117-4. [DOI] [PubMed] [Google Scholar]
- 67.De Gennaro L, Ferrara M, Bertini M. The spontaneous K-complex during stage 2 sleep: is it the ‘forerunner’ of delta waves? Neurosci Lett. 2000;291(1):41–3. doi: 10.1016/s0304-3940(00)01366-5. [DOI] [PubMed] [Google Scholar]
- 68.Crowley KE, Colrain IM. A review of the evidence for P2 being an independent component process: age, sleep and modality. Clin Neurophysiol. 2004;115(4):732–44. doi: 10.1016/j.clinph.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 69.Perrin F, Bastuji H, Garcia-Larrea L. Detection of verbal discordances during sleep. Neuroreport. 2002;13(10):1345–9. doi: 10.1097/00001756-200207190-00026. [DOI] [PubMed] [Google Scholar]
- 70.Brualla J, Romero MF, Serrano M, et al. Auditory event-related potentials to semantic priming during sleep. Electroencephalogr Clin Neurophysiol. 1998;108(3):283–90. doi: 10.1016/s0168-5597(97)00102-0. [DOI] [PubMed] [Google Scholar]
- 71.Sabri M, Campbell KB. Is the failure to detect stimulus deviance during sleep due to a rapid fading of sensory memory or a degradation of stimulus encoding? J Sleep Res. 2005;14(2):113–22. doi: 10.1111/j.1365-2869.2005.00446.x. [DOI] [PubMed] [Google Scholar]
- 72.Atienza M, Cantero JL, Quian Quiroga R. Precise timing accounts for posttraining sleep-dependent enhancements of the auditory mismatch negativity. Neuroimage. 2005;26(2):628–34. doi: 10.1016/j.neuroimage.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 73.Sabri M, Labelle S, Gosselin A, et al. Effects of sleep onset on the mismatch negativity (MMN) to frequency deviants using a rapid rate of presentation. Brain Res Cogn Brain Res. 2003;17(1):164–76. doi: 10.1016/s0926-6410(03)00090-9. [DOI] [PubMed] [Google Scholar]
- 74*.Muller-Gass A, Campbell K. Event-related potential measures of the inhibition of information processing: I. Selective attention in the waking state. Int J Psychophysiol. 2002;46(3):177–95. doi: 10.1016/s0167-8760(02)00111-3. [DOI] [PubMed] [Google Scholar]
- 75.Sabri M, Campbell KB. The effects of digital filtering on mismatch negativity in wakefulness and slow-wave sleep. J Sleep Res. 2002;11(2):123–7. doi: 10.1046/j.1365-2869.2002.00292.x. [DOI] [PubMed] [Google Scholar]
- 76.Perrin F, Garcia-Larrea L, Mauguiere F, et al. A differential brain response to the subject’s own name persists during sleep. Clin Neurophysiol. 1999;110(12):2153–64. doi: 10.1016/s1388-2457(99)00177-7. [DOI] [PubMed] [Google Scholar]
- 77.Perrin F, Bastuji H, Mauguiere F, et al. Functional dissociation of the early and late portions of human K-complexes. Neuroreport. 2000;11(8):1637–40. doi: 10.1097/00001756-200006050-00008. [DOI] [PubMed] [Google Scholar]
- 78.Pratt H, Berlad I, Lavie P. ‘Oddball’ event-related potentials and information processing during REM and non-REM sleep. Clin Neurophysiol. 1999;110(1):53–61. doi: 10.1016/s0168-5597(98)00044-6. [DOI] [PubMed] [Google Scholar]
- 79.Webster KE, Adey SA, Colrain IM. The effect of stimulus probability on P3 in the respiratory-related evoked potential. Psychophysiology. 2002;39(1):9–15. doi: 10.1017/S0048577202001282. [DOI] [PubMed] [Google Scholar]
- 80.Takahara M, Nittono H, Hori T. Effect of voluntary attention on auditory processing during REM sleep. Sleep. 2006;29(7):975–82. doi: 10.1093/sleep/29.7.975. [DOI] [PubMed] [Google Scholar]
- 81.Sallinen M, Kaartinen J, Lyytinen H. Processing of auditory stimuli during tonic and phasic periods of REM sleep as revealed by event-related brain potentials. J Sleep Res. 1996;5(4):220–8. doi: 10.1111/j.1365-2869.1996.00220.x. [DOI] [PubMed] [Google Scholar]
- 82.Cote KA, Campbell KB. The effects of varying stimulus intensity on P300 during REM sleep. Neuroreport. 1999;10(11):2313–8. doi: 10.1097/00001756-199908020-00017. [DOI] [PubMed] [Google Scholar]
- 83.Linden DE. The p300: where in the brain is it produced and what does it tell us? Neuroscientist. 2005;11(6):563–76. doi: 10.1177/1073858405280524. [DOI] [PubMed] [Google Scholar]
- 84.Atienza M, Cantero JL. Complex sound processing during human REM sleep by recovering information from long-term memory as revealed by the mismatch negativity (MMN) Brain Res. 2001;901(1–2):151–60. doi: 10.1016/s0006-8993(01)02340-x. [DOI] [PubMed] [Google Scholar]
- 85.Loewy DH, Campbell KB, Bastien C. The mismatch negativity to frequency deviant stimuli during natural sleep. Electroencephalogr Clin Neurophysiol. 1996;98(6):493–501. doi: 10.1016/0013-4694(96)95553-4. [DOI] [PubMed] [Google Scholar]
- 86.Atienza M, Cantero JL, Gomez CM. The initial orienting response during human REM sleep as revealed by the N1 component of auditory event-related potentials. Int J Psychophysiol. 2001;41(2):131–41. doi: 10.1016/s0167-8760(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 87.Kisley MA, Olincy A, Robbins E, et al. Sensory gating impairment associated with schizophrenia persists into REM sleep. Psychophysiology. 2003;40(1):29–38. doi: 10.1111/1469-8986.00004. [DOI] [PubMed] [Google Scholar]
- 88.Colrain IM, Webster KE, Hirst G, et al. The roles of vertex sharp waves and K-complexes in the generation of N300 in auditory and respiratory-related evoked potentials during early stage 2 NREM sleep. Sleep. 2000;23(1):97–106. [PubMed] [Google Scholar]
- 89*.Bastien C, Campbell K. The evoked K-complex: all-or-none phenomenon? Sleep. 1992;15(3):236–45. doi: 10.1093/sleep/15.3.236. [DOI] [PubMed] [Google Scholar]
- 90.Bastien C, Campbell K. Effects of rate of tone-pip stimulation on the evoked K-Complex. J Sleep Res. 1994;3(2):65–72. doi: 10.1111/j.1365-2869.1994.tb00109.x. [DOI] [PubMed] [Google Scholar]
- 91.Niiyama Y, Fushimi M, Sekine A, et al. K-complex evoked in NREM sleep is accompanied by a slow negative potential related to cognitive process. Electroencephalogr Clin Neurophysiol. 1995;95(1):27–33. doi: 10.1016/0013-4694(95)00021-p. [DOI] [PubMed] [Google Scholar]
- 92.Sallinen M, Kaartinen J, Lyytinen H. Is the appearance of mismatch negativity during stage 2 sleep related to the elicitation of K-complex? Electroencephalogr Clin Neurophysiol. 1994;91(2):140–8. doi: 10.1016/0013-4694(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 93.Kallai I, Harsh J, Voss U. Attention to external stimuli during wakefulness and sleep: evoked 40-Hz response and N350. Psychophysiology. 2003;40(6):955–66. doi: 10.1111/1469-8986.00114. [DOI] [PubMed] [Google Scholar]
- 94.Llinas R, Ribary U. Coherent 40-Hz oscillation characterizes dream state in humans. Proc Natl Acad Sci U S A. 1993;90(5):2078–81. doi: 10.1073/pnas.90.5.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Colrain IM, Webster KE, Hirst G. The N550 component of the evoked K-complex: a modality non-specific response? J. Sleep Res. 1999;8(4):273–280. doi: 10.1046/j.1365-2869.1999.00163.x. [DOI] [PubMed] [Google Scholar]
- 96.Cote KA, de Lugt DR, Langley SD, et al. Scalp topography of the auditory evoked K-complex in stage 2 and slow wave sleep. J Sleep Res. 1999;8(4):263–72. doi: 10.1046/j.1365-2869.1999.00164.x. [DOI] [PubMed] [Google Scholar]
- 97.Steriade M, Amzica F. Slow sleep oscillation, rhythmic K-complexes, and their paroxysmal developments. J Sleep Res. 1998;7(Suppl 1):30–5. doi: 10.1046/j.1365-2869.7.s1.4.x. [DOI] [PubMed] [Google Scholar]
- 98.Amzica F, Steriade M. The K-complex: its slow (<1-Hz) rhythmicity and relation to delta waves. Neurology. 1997;49(4):952–9. doi: 10.1212/wnl.49.4.952. [DOI] [PubMed] [Google Scholar]
- 99.Amzica F, Steriade M. Electrophysiological correlates of sleep delta waves. Electroencephalogr Clin Neurophysiol. 1998;107(2):69–83. doi: 10.1016/s0013-4694(98)00051-0. [DOI] [PubMed] [Google Scholar]
- 100.Amzica F, Steriade M. Cellular substrates and laminar profile of sleep K-complex. Neuroscience. 1998;82(3):671–86. doi: 10.1016/s0306-4522(97)00319-9. [DOI] [PubMed] [Google Scholar]
- 101.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1(3):195–204. [PubMed] [Google Scholar]
- 102.Nicholas CL, Trinder J, Colrain IM. Increased production of evoked and spontaneous K-complexes following a night of fragmented sleep. Sleep. 2002;25(8):882–7. [PubMed] [Google Scholar]
- 103.Peszka J, Harsh J. Effect of sleep deprivation on NREM sleep ERPs and related activity at sleep onset. Int J Psychophysiol. 2002;46(3):275–86. doi: 10.1016/s0167-8760(02)00115-0. [DOI] [PubMed] [Google Scholar]
- 104.Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16(1):40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- 105.Crowley K, Trinder J, Colrain IM. An examination of evoked K-complex amplitude and frequency of occurrence in the elderly. J Sleep Res. 2002;11(2):129–40. doi: 10.1046/j.1365-2869.2002.00293.x. [DOI] [PubMed] [Google Scholar]
- 106.Benca RM, Obermeyer WH, Thisted RA, et al. Sleep and psychiatric disorders. A meta-analysis Arch Gen Psychiatry. 1992;49(8):651–68. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- 107.Nicholas CL, Sullivan EV, Pfefferbaum A, et al. The effects of alcoholism on auditory evoked potentials during sleep. J Sleep Res. 2002;11(3):247–53. doi: 10.1046/j.1365-2869.2002.00298.x. [DOI] [PubMed] [Google Scholar]
- 108.Crowley K, Sullivan EV, Adalsteinsson E, et al. Differentiating pathologic delta from healthy physiologic delta in patients with Alzheimer disease. Sleep. 2005;28(7):865–70. doi: 10.1093/sleep/28.7.865. [DOI] [PubMed] [Google Scholar]