Abstract
Objective
Vascular endothelial growth factor receptor 2 (VEGFR2) is a receptor tyrosine kinase that regulates vascular physiology. However, mechanism(s) by which VEGFR2 signaling and trafficking is co-ordinated are not clear. Here, we have tested endocytic Rab GTPases for regulation of VEGFR2 trafficking and signaling linked to endothelial cell migration.
Methods and Results
Quiescent VEGFR2 displays endosomal localization and co-localization with the Rab5a GTPase, an early endosome fusion regulator. Expression of GTP or GDP-bound Rab5a mutants block activated VEGFR2 trafficking and degradation. Manipulation of Rab7a GTPase activity associated with late endosomes using overexpression of wild-type or mutant proteins blocks activated VEGFR2 trafficking and degradation. Depletion of Rab7a decreased VEGFR2 Y1175 phosphorylation but increased p42/44 (pERK1/2) MAPK phosphorylation. Endothelial cell migration was increased by Rab5a depletion but decreased by Rab7a depletion.
Conclusions
Rab5a and Rab7a regulate VEGFR2 trafficking towards early and late endosomes. Our data suggest that VEGFR2-mediated regulation of endothelial function is dependent on different but specific Rab-mediated GTP hydrolysis activity required for endosomal trafficking.
Keywords: VEGFR2, Rab, trafficking, signaling, migration
Introduction
Vascular Endothelial Growth Factor A (VEGF-A) is an essential regulator of vascular physiology.1 VEGF-A plays key roles in vasculogenesis and angiogenesis during development.2 Angiogenesis is needed for embryogenesis and wound healing but is also associated with cancer, eye diseases and other chronic disorders. The importance of VEGF-A is underlined by the fact that deletion of a single mouse VEGF-A allele causes embryonic lethality due to the impairment of vascular development.3, 4 The VEGF-A gene gives rise to at least 6-12 different variants, with VEGF-A165 (termed VEGF-A) being the most abundant and pro-angiogenic isoform.5, 6 VEGF-A can bind to two different but closely related endothelial receptor tyrosine kinases (RTKs): fms-like tyrosine kinase (VEGFR1 or Flt-1) and kinase insert domain receptor (VEGFR2 or KDR). A major pro-angiogenic axis is based on VEGF-A binding to VEGFR2, stimulating tyrosine kinase activity, autophosphorylation and signaling to downstream target proteins which regulate diverse processes including chemotaxis, migration, vascular permeability, cell survival and proliferation.7 Such physiological responses are regulated via multiple effectors including focal adhesion kinase (FAK), c-Akt/PKB, extracellular-signal related kinase (ERK) pathways and stimulation of nitric oxide and prostaglandin synthesis.8
Whereas VEGFR2 signaling pathways that regulate endothelial responses are well characterized, the spatial and temporal order underlying such events remains elusive. For example, VEGFR2 plasma membrane activation leads to trafficking to endosomes and degradation in lysosomes.9 Simultaneously, VEGFR2 activation leads to tyrosine phosphorylation and intracellular signaling.7, 8 How VEGFR2-mediated signaling events from different intracellular locations are co-ordinated is not known. Increasing evidence points to the requirement for co-ordination of RTK trafficking with signaling from different endocytic compartments.10, 11 RTK trafficking comprises of a series of complex highly regulated events with ligand binding leading to receptor activation and rapid internalization followed by degradation that is linked to signaling attenuation. When epidermal growth factor receptor (EGFR) clathrin-dependent endocytosis is inhibited by a mutant dynamin, EGF-stimulated signaling is enhanced.12 However, mitogen-activated protein kinase (MAPK) activation is suppressed, showing a need for EGFR trafficking. Endosome-associated EGFR can still autophosphorylate and recruit various effectors such as Grb2, a mediator of Ras and MAPK signaling.13 Another RTK, the nerve growth factor receptor TrkA, can also form endosomal signaling complexes in neurons.14 Receptor-ligand complexes could thus continue signaling until sequestration into the multi-vesicular body before recycling back to the plasma membrane or lysosomal degradation.15, 16
Membrane trafficking, targeting and degradation along the endocytic pathway is regulated by a wide array of regulatory molecules including GTPases, ubiquitin ligases, clathrin- and ubiquitin-binding effectors. Rabs are Ras-related small GTPases that program vesicle docking and fusion. Rab proteins can be used as tools to dissect the trafficking itinerary of different proteins associated with the exo- and endocytic pathways.17-20 We have used endocytic Rabs to test involvement in VEGFR2 trafficking and signaling in primary human endothelial cells. We show that VEGFR2 trafficking is dependent on Rab5a and Rab7a. Manipulation of Rab function indicates early or late endosome-specific modulation of VEGFR2 signaling and endothelial migration.
Methods
Materials
Chemicals and reagents were obtained from Sigma-Aldrich (Poole, UK) or Invitrogen (Paisley, UK) unless stated otherwise. Primary antibodies include mouse anti-transferrin receptor, mouse anti-EEA1, mouse anti-Rab7a (Abcam, Cambridge, UK), rabbit anti-Cathepsin D (BD Transduction Labs, Erembodegem, Belgium) rabbit anti-pY1175 VEGFR2, rabbit anti-c-Akt, rabbit anti-p42/44 MAPK (Cell Signaling Technology, Danvers, USA), anti-VEGFR2 and anti-TfR antibodies.9, 21 HRP-conjugated secondary antibodies were from Jackson ImmunoResearch labs (Soham, UK) whilst AlexaFluor-conjugated secondary antibodies were from Invitrogen (Amsterdam, UK).
Cell culture
Human umbilical vein endothelial cells (HUVEC) were isolated from umbilical cords as previously described.22, 23 HUVECs were cultured in defined endothelial cell basal medium (Promocell, Heidelberg, Germany). Cells were seeded onto tissue culture plastic (Nunc, Roskilde, Denmark) pre-coated with 0.1% (w/v) porcine skin gelatin. Cells were generally passaged with a dilution of 1:3 to 1:4. HUVECs were grown for up to 4 passages and exhibited characteristic cobblestone morphology and routinely checked for endothelial character using the endothelial-specific marker Von Willebrand Factor that accumulates in Weibel-Palade bodies.22
Gene manipulation, transfection and RNAi
HUVECs were transiently transfected with plasmid DNA in OptiMEM (Invitrogen) supplemented with 10% fetal calf serum (FCS) using an EasyJECT electroporator (Flowgen, Nottingham, UK). The cell suspension was then plated out and allowed to recover in complete medium for 48 hrs prior to ligand stimulation, fixation and processing for microscopy or lysis for Western blotting. pEGFP-Rab7a constructs were from B. van Deurs (Copenhagen, Denmark). pEGFP-Rab5a constructs were from B. Knoll (University of Houston, Texas, USA).
HUVECs were transfected with RNAi duplexes using Lipofectamine 2000 (Invitrogen, Paisley, UK). Cells were treated and assayed 48 hrs following transfection. RNAi targeting using Rab7a siRNA duplexes were designed, synthesized and annealed by Eurogentec (Brussels, Belgium). RNAi sequences were designed using proprietary software (Eurogentec, Brussels, Belgium) and the of the various siRNA duplexes tested, the best were used. Rab5a siRNA duplex sense strand was 5′-GUCCGCUGUUGGCAAAUCA-3′ whereas Rab7a siRNA duplex had a sense strand was 5′-CUGCUGCGUUCUCCUAUUU-3′. Non-targeting siRNA duplex (Silencer Negative Control #1; Ambion, Warrington, UK) was also used as a negative control.
Immunofluorescence microscopy
Cells were fixed, processed for immunofluorescence and visualized using wide-field deconvolution microscopy as described previously.9, 22, 23 Images were subsequently converted to .tif files and imported into PowerPoint. Quantification of co-localized pixels was performed using IMARIS software (Bitplane AG, Switzerland) on imported deconvolved 3-D datasets. Quantification of the degree of overlap between VEGFR2 and other cell markers was performed using the Coloc module in IMARIS v4.0.6 with a threshold value taken as 10% of the maximum intensity. Images of triple labeled cells were acquired using an Inverted Zeiss LSM510 META Axiovert 200M laser scanning confocal microscope (Carl Zeiss, Oberkochen, Germany) with a 63x objective. Single slice sections were imaged, averaged and collected using Zeiss acquisition software.
Immunoblotting
SDS-PAGE analysis and immunoblotting was performed as described previously.9, 22, 23 A Fuji LAS-3000 system and software was used to capture chemiluminescence images and band intensities were determined by densitometry using Advanced Image Data Analyzer (AIDA) 2.11 software (FujiFilm, Tokyo, Japan).
Cell migration
Control or siRNA duplex-treated HUVECs were trypsinized and seeded at 5×104 cells per ml into a 24-well plate with 8 μm pore size Transwell inserts (Becton-Dickinson, Oxford, UK) within the upper chamber and 50 ng/ml VEGF-A in the lower chamber for migration to occur. After 16 hrs, filters were fixed, stained with hematoxylin-eosin and excised for microscopy. Random fields from each image was counted for calculation of % number of cells migrated onto filter underside vs. non-treated control.
Results
VEGFR2 early endosome trafficking is regulated by Rab5a
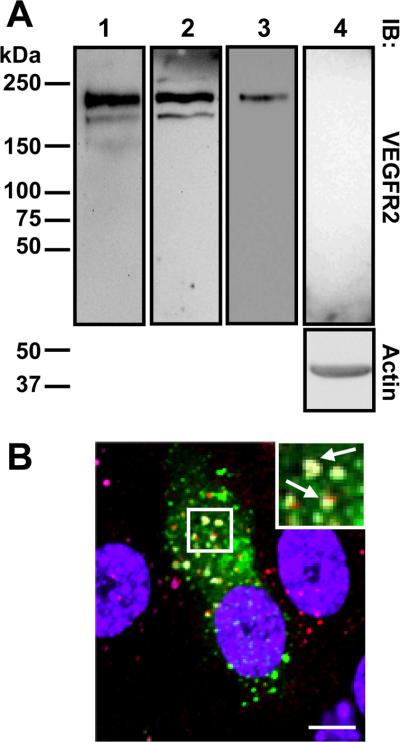
Our previous work showed evidence for activated VEGFR2 trafficking through the endocytic pathway before lysosomal degradation.9 One likelihood is that such trafficking is dependent on the Rab5a GTPase that regulates early endosome fusion.24 VEGFR2 is evident as both immature (~200 kDa) and mature glycosylated (~230 kDa) polypeptides detected in primary human endothelial cells using VEGFR2-specific antibodies to either extracellular or cytoplasmic domains (Figure 1A). Microscopy on non-permeabilized or permeabilized endothelial cells showed VEGFR2 distribution at the plasma membrane and punctate endosomes (Figure I please see www.ahajournals.org). Expression of wild-type GFP-tagged Rab5a showed co-localization with VEGFR2 in a punctate intracellular compartment (Figure 1B). VEGFR2 and GFP-Rab5a showed ~60% co-distribution in transfected cells. VEGFR2 and Rab5a also showed co-localization with a key marker, early endosomal antigen 1 (Figure 1B).
Figure 1.
VEGFR2 expression and localization. A, Endothelial cell lysates probed with anti-VEGFR2 extracellular domain (lanes 1 and 3), anti-VEGFR2 cytoplasmic domain (lane 2) or anti-VEGFR2 extracellular domain plus competing recombinant VEGFR2 (lane 4). B, Cells labeled for GFP-Rab5a (green), EEA1 (cyan), VEGFR2 (red) and nuclei (blue). Bar, 10 μm.
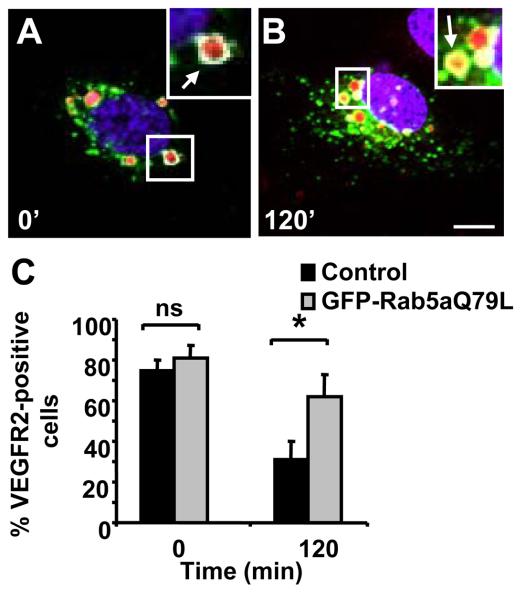
A GTPase-deficient Rab5a mutant (Q79L) programs a membrane-associated state which allows plasma membrane endocytosis but stimulates endosome fusion.25 However, Rab5aQ79L membrane association prevents endosome-derived vesicle fission resulting in enlarged endosomes.26, 27 Rab5aQ79L expression was used to investigate VEGFR2 trafficking through endosomes. Overexpression of GFP-Rab5aQ79L in endothelial cells caused enlarged endosomes which contained both EEA1 and VEGFR2 (Figure 2A). VEGF-A stimulation for 120 min had no effect on VEGFR2 localization (Figure 2B), indicating that VEGFR2 is trapped within these endosomes in the presence of Rab5aQ79L. Quantification of cells containing both VEGFR2 and Rab5aQ79L showed a 2-fold increase in VEGFR2 labeling in VEGF-A-stimulated cells compared to non-stimulated controls (Figure 2C). Rab5a could be depleted using RNAi and signaling events such as VEGFR2 tyrosine phosphorylation (~1.3 fold) and MAPK p42/44 phosphorylation (~1.5-fold) were both elevated and prolonged (Figure II please see www.ahajournals.org). However, 15 min after VEGF-A stimulation, VEGFR2 phosphorylation is enhanced by Rab5a depletion (Figure II please see www.ahajournals.org). Thus, Rab5a GTPase activity may regulate both VEGFR2 trafficking and phosphorylation status in early endosomes.
Figure 2.
Rab5aQ76L causes VEGFR2 accumulation within enlarged endosomes. Endothelial cells expressing GFP-Rab5aQ79L stimulated with VEGF-A for 0 (A) or 120 (B) min labeled for EEA1 (cyan), VEGFR2 (red) and nuclei (blue). Bar, 10 μm. C, Quantification of VEGFR2-labeled cells overexpressing GFP-Rab5aQ79L after VEGF-A stimulation (*, p<0.05).
Trafficking of VEGFR2 through late endosomes is regulated by Rab7a
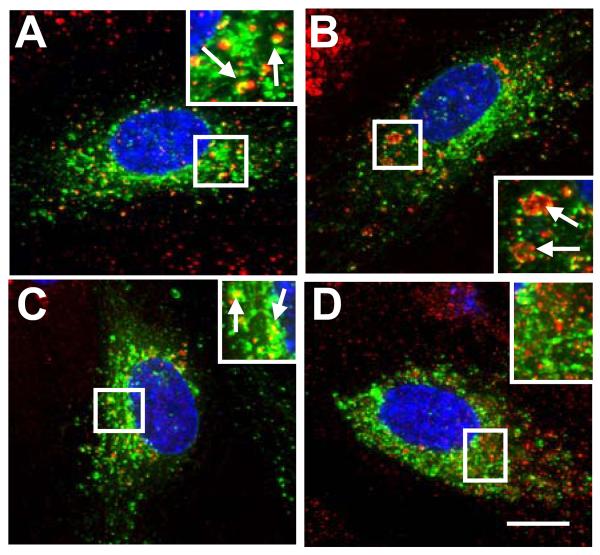
Are other GTPases are involved in VEGFR2 endosomal trafficking? The Rab7a GTPase regulates early to late endosome trafficking including receptor tyrosine kinases such as epidermal growth factor receptor.28 We tested a role for this GTPase using transfection of a GFP-tagged Rab7a in primary endothelial cells. We found that Rab7a in endothelial cells showed co-localization with a late endosome marker, CD63 (Figure 3B), and a lysosomal enzyme, cathepsin D (Figure 3C). However, Rab7a showed no significant co-localization with early endosome markers such as EEA1 (Figure 3A) or the transferrin receptor, TfR (Figure 3D). Thus Rab7a is a marker for a endocytic compartment that is distinct from Rab5a.
Figure 3.
Rab7a localization in endothelial cells. HUVECs expressing GFP-Rab7a (A-D, green) were labeled for EEA1 (A, red), CD63 (B, red), cathepsin D (C, red) or transferrin receptor (D, red) and nuclei (blue). Insets show 2-fold magnification and arrows denote proximity or co-distribution. Bar, 10 μm.
We used GFP-tagged Rab7a expression in transfected endothelial cells to assess effects on endogenous VEGFR2 trafficking (Figure 4). Cells were stimulated for 30 min or 120 min with VEGF-A and assessed for VEGFR2 localization in cells expressing GFP-Rab7a compared to non-stimulated controls. Cells that overexpressed wild-type Rab7a consistently displayed increased VEGFR2 staining in punctate structures resembling endosomes, 30 min after ligand stimulation (Figure 4B). Quantification shows an ~4-fold increase in VEGFR2 labeling of cells overexpressing Rab7a, 30 min after VEGF-A stimulation (Figure 4D). Further stimulation with VEGF-A for 120 min showed a 94% decrease in VEGFR2 labeled cells during Rab7a expression (H. Jopling and S. Ponnambalam, unpublished data). This is corroborated by the decreased VEGFR2 and Rab7a co-localization after treatment with VEGF-A for 120 min (Figure 4D).
Figure 4.
Rab7a and VEGFR2 trafficking. A-C, Endothelial cells expressing GFP-Rab7a (green) were stimulated with VEGF-A for 0 (A), 30 (B) or 120 (C) min and labeled for VEGFR2 (red) and nuclei (blue). Bar, 10 μm. D, Quantification of VEGFR2-labeled cells overexpressing GFP-Rab7a after VEGF-A stimulation (*, p<0.05).
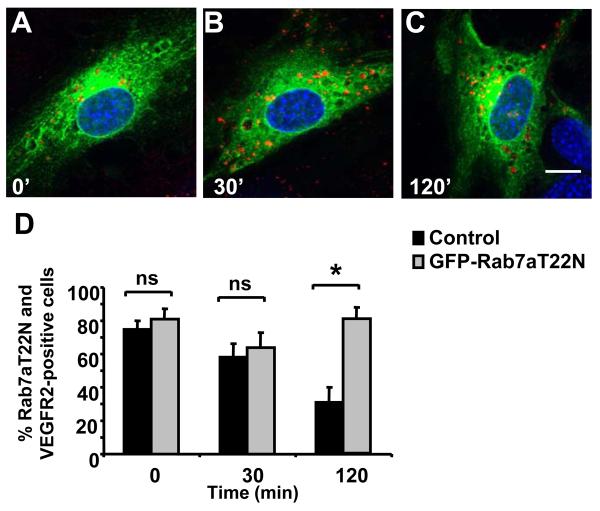
We expressed a GDP-bound Rab7aT22N mutant in endothelial cells to further check for effects on VEGFR2 trafficking and degradation (Figure 5). GFP-Rab7aT22N showed a widely dispersed homogenous staining consistent with a non-membrane or cytosolic localization (Figure 5A-5C). Again, VEGFR2 labeling was evident in control (Figure 5A) and VEGF-A-stimulated cells (Figure 5B-5C) in punctate structures resembling late endosomes. Interestingly, VEGFR2 labeling persisted 120 min after VEGF-A stimulation (Figure 5C), different to that observed upon expression of wild-type Rab7a (Figure 4). Expression of the GTP-bound Rab7aQ67L mutant also caused VEGFR2 accumulation in punctate endosomes after prolonged VEGF-A stimulation (Figure S3A). A fraction of endosomes showed co-labeling of Rab7aT22N, VEGFR2 and EEA1 after VEGF-A stimulation for 120 min (Figure III please see www.ahajournals.org), indicating that endocytic trafficking of VEGFR2 was perturbed. Quantification showed ~2.5-fold increase in VEGFR2 labeling in cells expressing Rab7aT22N that had been stimulated with VEGF-A for 120 min in comparison to non-transfected endothelial cells (Figure 5D).
Figure 5.
Rab7aT22N blocks VEGFR2 trafficking. A-C, Endothelial cells expressing GFP-Rab7aT22N (green) were stimulated with VEGF-A for 0 (A), 30 (B) or 120 (C) min and labeled for VEGFR2 (red) and nuclei (blue). Bar, 10 μm. D, Quantification of VEGFR2-labeled cells overexpressing GFP-Rab7aT22N after VEGF-A stimulation (*, p<0.05).
VEGFR2 intracellular signaling is Rab7a-dependent
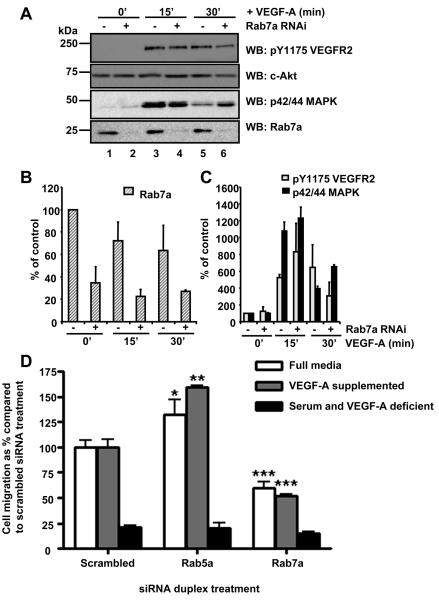
Increasing evidence indicates that functional RTK signaling complexes such as those involving EGFR29 or c-Met30 are present on endosomes. To test whether this is true for VEGFR2, we used RNAi to deplete Rab7a levels and probe for VEGFR2 signaling (Figure 6). Using a siRNA duplex specific for Rab7a, depletion of ~65% was achieved in primary human endothelial cells compared to control mock-transfected cells (Figure 6A, 6B). Neither control nor Rab7a-depleted cells showed tyrosine phosphorylation of residue Y1175 in VEGFR2 (pY1175) without VEGF-A stimulation (Figure 6A, lanes 1, 2). However, after VEGF-A stimulation for 15 min, tyrosine phosphorylation of VEGFR2 (pY1175) was clearly evident (Figure 6A, lanes 3, 4). However, the VEGFR2 pY1175 epitope was decreased in Rab7a-depleted cells in comparison to controls (Figure 6A, lanes 5, 6). Quantification showed ~2-fold decrease in VEGFR phosphorylation (pY1175) in VEGF-A-stimulated and Rab7a-depleted cells 30 min after VEGF-A stimulation (Figure 3C).
Figure 6.
Rab7a regulates VEGFR2 signaling and cell migration. A, VEGF-A stimulation and immunoblotting of control (lanes 1, 3, 5) or Rab7a-depleted cells (lanes 2, 4, 6). B, Rab7a quantification (n=3, ±SEM). C, VEGFR2 pY1175 and p42/44 MAPK quantification (n=3, ±SEM). D, Control or Rab-depleted cells assayed for cell migration.
As an indicator of downstream VEGFR2 signaling, we further examined p42/44 MAPK phosphorylation in control and Rab7a-depleted cells. In resting endothelial cells, phosphorylated p42/44 MAPK was not evident (Figure 6A, lanes 1, 2). However, following VEGF-A stimulation for 15 min, levels of the phosphorylated p42/44 MAPK epitope exhibited ~11-fold increase in Rab7a-depleted cells (Figure 6A, lane 4). In addition, there was a dramatic increase in intracellular staining for p42/44 MAPK after VEGF-A stimulation at both 10 and 30 min time points after VEGF-A addition (Figure IV please see www.ahajournals.org). Importantly, at 30 min after VEGF-A stimulation although p42/44 MAPK phosphorylation showed a 4-fold increase in controls (Figure 6A, lanes 5), this was increased ~7-fold in Rab7a-depleted cells (Figure 6C, lane 6). Thus Rab7a depletion caused ~1.8-2-fold changes in VEGF-A-stimulated VEGFR2 and p42/44 MAPK phosphorylation levels. Analysis of p42/44 MAPK localization in Rab7a-depleted cells using microscopy showed no morphological difference to control scrambled siRNA-transfected cells (Figure IV please see www.ahajournals.org).
A key question is whether Rab activity is required for physiological responses by the endothelium. A key endothelial function is cell migration required for vasculogenesis, angiogenesis and wound healing.31 To test this idea, we depleted Rab5 or Rab7 levels using RNAi and monitored endothelial cell migration in different types of cell growth media containing or lacking VEGF-A (Figure 6D). In control cells transfected with a scrambled siRNA duplex, there was substantial migration in media containing excess VEGF-A. However, in serum-free media lacking VEGF-A, there was only ~20% cell migration compared to that observed in the presence of VEGF-A. Depletion of Rab7a showed pronounced inhibition of VEGF-A-stimulated migration in any of the three media conditions, with ~50-60% cell migration compared to controls (Figure 6D). Surprisingly, depletion of Rab5a levels using RNAi showed the opposite effect with either a ~25% increase (full growth media) or a ~60% increase (VEGF-A-supplemented media) in cell migration compared to control cells (Figure 6D).
Discussion
Herein, we provide evidence for a Rab-dependent mechanism for regulating VEGFR2 trafficking and signaling from endosomes in primary endothelial cells. Manipulation of GTPase activity through overexpression of GFP-tagged wild-type or mutant Rab7a proteins perturbed endothelial VEGFR2 trafficking, degradation and signaling. Intriguingly, Rab7a depletion decreased VEGFR2 tyrosine autophosphorylation on residue Y1175, a characteristic signature in VEGFR2-mediated activation and signaling.32 This correlated with ~50% decrease in VEGF-A-stimulated endothelial cell migration after Rab7a depletion, suggesting a requirement for Rab7a in VEGFR2-mediated signaling. However, in Rab7a-depleted cells, there was increased p42/44 MAPK levels 30 min after VEGF-A addition, suggesting increased signaling through this arm of the pathway. A likely explanation is that VEGFR2-regulated signaling to p42/p44 MAPK occurs at the early endosome and is prolonged in Rab7a-depleted cells as VEGFR2 cannot progress towards lysosomal degradation. A different study has also argued that VEGFR2-regulated p42/44 MAPK activation can occur via endosomes33 rather than solely at the plasma membrane. RTKs such as EGFR28, 29, PDGFR34, c-Met35 and TrkA14, 36, 37 have also been found to be capable of signaling from early endosomes. These lines of evidence suggest existence of at least two distinct VEGF-A and VEGFR2-regulated signaling pathways, one of which regulates cell migration and this is distinct from the MAPK signaling pathway.
Our findings also support requirement for the Rab5a GTPase in early endosome sorting of both quiescent and activated VEGFR2. Both Rab5a and VEGFR2 showed substantial co-localization with a marker for early endosomes, EEA1. VEGF-A stimulation caused activated VEGFR2 accumulation in early endosomes with a block in subsequent lysosomal degradation of VEGFR2. Intriguingly, Rab5a depletion caused ~25-60% increase in VEGF-A-stimulated endothelial cell migration, suggesting that VEGFR2-mediated signaling in prolonged under such conditions. Rab5a expression in immortalized epithelial cells also causes quiescent EGFR accumulation in endosomes but it is unclear whether this is due to increased endocytosis or inhibition of EGFR recycling.38 Rab5a is also implicated in clathrin-coated pit formation39 suggesting that this GTPase may act at multiple steps from the plasma membrane to early endosomes. However, we cannot exclude the possibility that Rab5aQ79L inhibits activation of VEGFR2, leading to abrogation of activated VEGFR2 trafficking. In addition, we observed enhanced VEGFR2 phosphorylation upon knockdown of Rab5a.
Downstream of early endosomes, Rab7a was implicated in activated VEGFR2 egress out of late endosomes towards degradation in lysosomes. The dominant-negative GDP-bound Rab7aT22N mutant perturbs late endosome trafficking40, 41 and renders late endosomes inaccessible to activated RTKs. In transformed epithelial cells, Rab7a perturbation decreased both EGF and EGFR degradation and modulation of EGFR signaling.28 Similarly, Rab7a-linked effects causes endosomal accumulation of TrkA 37 and viral spike glycoproteins.42, 43
Identification of Rab-mediated steps in the VEGFR2 endocytic itinerary argues for a network of interactions that impact on vascular features such as angiogenesis and blood pressure. Indeed, Rab GTPase activity can be modulated by RTK activation to provide a positive feedback mechanism.19, 44 VEGFR2 trafficking through the endocytic pathway argues for signaling from plasma membrane and later compartments with p42/44 MAPK activation occurring on endosomes. Intriguingly, a recent report suggests that the strength of a c-Met-derived signal is dependent on plasma membrane or endosome location with major consequences for nuclear gene expression.45 VEGF-A stimulation of Rab5a or Rab7a depleted cells suggest lesser effects on endothelial cell proliferation (A. F. Odell & S. Ponnambalam, unpublished data) but this requires further study. The evidence from our study leads us to conclude that different effectors are recruited to VEGFR2 to impose a spatiotemporal framework for trafficking and signaling leading to changes in vascular physiology in response to VEGF-A ligand. Investigation of the link between Rab GTPase activity and VEGF-A receptor activation may provide routes to modulate vascular physiology in human health and disease.
Acknowledgments
Sources of funding
This work was supported by the British Heart Foundation (S.P., J.H.W., I.C.Z., N.M.H.), The Wellcome Trust (J.H.W., S.P.) and a BBSRC DTA PhD studentship (H.M.J.). We thank B. Knoll (Houston, USA) and B. van Deurs (Copenhagen, Denmark) for reagents. We thank G. Howell for bioimaging assistance and A. Bruns for comments on the manuscript.
Footnotes
Disclosures
None.
References
- 1.Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res. 2001;49:568–581. doi: 10.1016/s0008-6363(00)00268-6. [DOI] [PubMed] [Google Scholar]
- 2.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2004;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 5.Cross MJ, Dixelius J, Matsumoto T, Claesson-Welsh L. VEGF-receptor signal transduction. Trends Biochem Sci. 2003;28:488–494. doi: 10.1016/S0968-0004(03)00193-2. [DOI] [PubMed] [Google Scholar]
- 6.Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer. 2008;8:880–887. doi: 10.1038/nrc2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang S, Toy K, Ingle G, Zlot C, Williams PM, Fuh G, Li B, de Vos A, Gerritsen ME. Vascular endothelial growth factor-induced genes in human umbilical vein endothelial cells: relative roles of KDR and Flt-1 receptors. Arterioscler Thromb Vasc Biol. 2002;22:1797–1803. doi: 10.1161/01.atv.0000038995.31179.24. [DOI] [PubMed] [Google Scholar]
- 8.Zachary I. VEGF signalling: integration and multi-tasking in endothelial cell biology. Biochem Soc Trans. 2003;31:1171–1177. doi: 10.1042/bst0311171. [DOI] [PubMed] [Google Scholar]
- 9.Ewan LC, Jopling HM, Jia H, Mittar S, Bagherzadeh A, Howell GJ, Walker JH, Zachary IC, Ponnambalam S. Intrinsic tyrosine kinase activity is required for vascular endothelial growth factor receptor 2 ubiquitination, sorting and degradation in endothelial cells. Traffic. 2006;7:1270–1282. doi: 10.1111/j.1600-0854.2006.00462.x. [DOI] [PubMed] [Google Scholar]
- 10.Sorkin A, Von Zastrow M. Signal transduction and endocytosis: close encounters of many kinds. Nat Rev Mol Cell Biol. 2002;3:600–614. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- 11.Kermorgant S, Parker PJ. c-Met signalling: spatio-temporal decisions. Cell Cycle. 2005;4:352–355. doi: 10.4161/cc.4.3.1519. [DOI] [PubMed] [Google Scholar]
- 12.Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- 13.Miaczynska M, Pelkmans L, Zerial M. Not just a sink: endosomes in control of signal transduction. Curr Opin Cell Biol. 2004;16:400–406. doi: 10.1016/j.ceb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Howe CL, Valletta JS, Rusnak AS, Mobley WC. NGF signaling from clathrin-coated vesicles: evidence that signaling endosomes serve as a platform for the Ras-MAPK pathway. Neuron. 2001;32:801–814. doi: 10.1016/s0896-6273(01)00526-8. [DOI] [PubMed] [Google Scholar]
- 15.Urbe S. Ubiquitin and endocytic protein sorting. Essays Biochem. 2005;41:81–98. doi: 10.1042/EB0410081. [DOI] [PubMed] [Google Scholar]
- 16.Urbe S, McCullough J, Row P, Prior IA, Welchman R, Clague MJ. Control of growth factor receptor dynamics by reversible ubiquitination. Biochem Soc Trans. 2006;34:754–756. doi: 10.1042/BST0340754. [DOI] [PubMed] [Google Scholar]
- 17.Mohrmann K, van der Sluijs P. Regulation of membrane transport through the endocytic pathway by rabGTPases. Mol Membr Biol. 1999;16:81–87. doi: 10.1080/096876899294797. [DOI] [PubMed] [Google Scholar]
- 18.Jordens I, Marsman M, Kuijl C, Neefjes J. Rab proteins, connecting transport and vesicle fusion. Traffic. 2005;6:1070–1077. doi: 10.1111/j.1600-0854.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 19.Bucci C, Chiariello M. Signal transduction gRABs attention. Cell Signal. 2006;18:1–8. doi: 10.1016/j.cellsig.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Towler MC, Prescott AR, James J, Lucocq JM, Ponnambalam S. The manganese cation disrupts membrane dynamics along the secretory pathway. Exp Cell Res. 2000;259:167–179. doi: 10.1006/excr.2000.4958. [DOI] [PubMed] [Google Scholar]
- 22.Howell GJ, Herbert SP, Smith JM, Mittar S, Ewan LC, Mohammed M, Hunter AR, Simpson N, Turner AJ, Zachary I, Walker JH, Ponnambalam S. Endothelial cell confluence regulates Weibel-Palade body formation. Mol Membr Biol. 2004;21:413–421. doi: 10.1080/09687860400011571. [DOI] [PubMed] [Google Scholar]
- 23.Herbert SP, Ponnambalam S, Walker JH. Cytosolic Phospholipase A2-{alpha} Mediates Endothelial Cell Proliferation and Is Inactivated by Association with the Golgi Apparatus. Mol Biol Cell. 2005;16:3800–3809. doi: 10.1091/mbc.E05-02-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- 25.Hunker CM, Kruk I, Hall J, Giambini H, Veisaga ML, Barbieri MA. Role of Rab5 in insulin receptor-mediated endocytosis and signaling. Arch Biochem Biophys. 2006;449:130–142. doi: 10.1016/j.abb.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 26.Barbieri MA, Li G, Mayorga LS, Stahl PD. Characterization of Rab5:Q79L-stimulated endosome fusion. Arch Biochem Biophys. 1996;326:64–72. doi: 10.1006/abbi.1996.0047. [DOI] [PubMed] [Google Scholar]
- 27.Dinneen JL, Ceresa BP. Expression of dominant negative rab5 in HeLa cells regulates endocytic trafficking distal from the plasma membrane. Exp Cell Res. 2004;294:509–522. doi: 10.1016/j.yexcr.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Taub N, Teis D, Ebner HL, Hess MW, Huber LA. Late endosomal traffic of the epidermal growth factor receptor ensures spatial and temporal fidelity of mitogen-activated protein kinase signaling. Mol Biol Cell. 2007;18:4698–4710. doi: 10.1091/mbc.E07-02-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Pennock S, Chen X, Wang Z. Endosomal signaling of epidermal growth factor receptor stimulates signal transduction pathways leading to cell survival. Mol Cell Biol. 2002;22:7279–7290. doi: 10.1128/MCB.22.20.7279-7290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kermorgant S, Zicha D, Parker PJ. PKC controls HGF-dependent c-Met traffic, signalling and cell migration. EMBO J. 2004;23:3721–3734. doi: 10.1038/sj.emboj.7600396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J. 2001;20:2768–2778. doi: 10.1093/emboj/20.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J Cell Biol. 2006;174:593–604. doi: 10.1083/jcb.200602080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Pennock SD, Chen X, Kazlauskas A, Wang Z. Platelet-derived growth factor receptor-mediated signal transduction from endosomes. J Biol Chem. 2004;279:8038–8046. doi: 10.1074/jbc.M311494200. [DOI] [PubMed] [Google Scholar]
- 35.Kermorgant S, Zicha D, Parker PJ. Protein kinase C controls microtubule-based traffic but not proteasomal degradation of c-Met. J Biol Chem. 2003;278:28921–28929. doi: 10.1074/jbc.M302116200. [DOI] [PubMed] [Google Scholar]
- 36.Beattie EC, Howe CL, Wilde A, Brodsky FM, Mobley WC. NGF signals through TrkA to increase clathrin at the plasma membrane and enhance clathrin-mediated membrane trafficking. J Neurosci. 2000;20:7325–7333. doi: 10.1523/JNEUROSCI.20-19-07325.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saxena S, Bucci C, Weis J, Kruttgen A. The small GTPase Rab7 controls the endosomal trafficking and neuritogenic signaling of the nerve growth factor receptor TrkA. J Neurosci. 2005;25:10930–10940. doi: 10.1523/JNEUROSCI.2029-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dinneen JL, Ceresa BP. Continual expression of Rab5(Q79L) causes a ligand-independent EGFR internalization and diminishes EGFR activity. Traffic. 2004;5:606–615. doi: 10.1111/j.1398-9219.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 39.McLauchlan H, Newell J, Morrice N, Osborne A, West M, Smythe E. A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Curr Biol. 1998;8:34–45. doi: 10.1016/s0960-9822(98)70018-1. [DOI] [PubMed] [Google Scholar]
- 40.Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: a key to lysosome biogenesis. Mol Biol Cell. 2000;11:467–480. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ceresa BP, Bahr SJ. rab7 activity affects epidermal growth factor:epidermal growth factor receptor degradation by regulating endocytic trafficking from the late endosome. J Biol Chem. 2006;281:1099–1106. doi: 10.1074/jbc.M504175200. [DOI] [PubMed] [Google Scholar]
- 42.Feng Y, Press B, Wandinger-Ness A. Rab 7: an important regulator of late endocytic membrane traffic. J Cell Biol. 1995;131:1435–1452. doi: 10.1083/jcb.131.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vonderheit A, Helenius A. Rab7 associates with early endosomes to mediate sorting and transport of Semliki forest virus to late endosomes. PLoS Biol. 2005;3:e233. doi: 10.1371/journal.pbio.0030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barbieri MA, Roberts RL, Gumusboga A, Highfield H, Alvarez-Dominguez C, Wells A, Stahl PD. Epidermal growth factor and membrane trafficking. EGF receptor activation of endocytosis requires Rab5a. J Cell Biol. 2000;151:539–550. doi: 10.1083/jcb.151.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kermorgant S, Parker PJ. Receptor trafficking controls weak signal delivery: a strategy used by c-Met for STAT3 nuclear accumulation. J Cell Biol. 2008;182:855–863. doi: 10.1083/jcb.200806076. [DOI] [PMC free article] [PubMed] [Google Scholar]