Abstract
This study describes the synthesis, characterization, and in vitro evaluation of N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer–gadolinium (Gd)–doxorubicin (Dox) conjugates. Copolymers of HPMA were derivatized to incorporate side chains for Gd chelation and Dox conjugation. The conjugates were characterized by their side chain contents, T1 relaxivity (r1), stability, and in vitro cytotoxicity. High stability and relaxivity of these conjugates coupled with low toxicity show their potential for monitoring the in vivo fate of HPMA-based drug delivery systems by magnetic resonance imaging techniques.
Keywords: contrast agents, drug delivery, HPMA copolymers, magnetic resonance imaging, relaxivity, water-soluble polymer
Introduction
Detection and prediction of the fate of drug delivery systems within the tumor is of critical importance in cancer therapy. Prediction of the fate of drug delivery systems derived from standard pharmacokinetic models is frequently inadequate because of the complex nature of 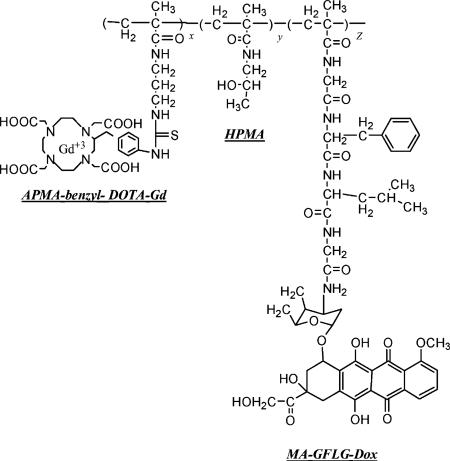 tumor blood flow and microenvironment. Although tissue drug concentrations within the tumor and non-target organs can be sampled with microdialysis[1] or biopsy, non-invasive alternatives for evaluating the distribution of polymeric drug delivery systems are yet to be developed.
tumor blood flow and microenvironment. Although tissue drug concentrations within the tumor and non-target organs can be sampled with microdialysis[1] or biopsy, non-invasive alternatives for evaluating the distribution of polymeric drug delivery systems are yet to be developed.
To date, the most commonly used non-invasive imaging modalities are nuclear,[2,3] magnetic resonance (MR),[4,5] and optical techniques.[6-8] Among these approaches, MR imaging (MRI) combines the benefits of high spatial resolution[9] with unique capability to simultaneously elicit both anatomic and physiological information.
The use of MR contrast agents to enhance the contrast of images in medical imaging is critical. Low molecular weight gadolinium (Gd) complexes, are currently used as extracellular MR contrast agents in a large fraction of clinical examinations. Several macromolecular contrast agents are in preclinical and clinical trials.[10] These are of interest because they have a prolonged blood pool retention time and can leak out only in compromised endothelium. Due to hyperpermeability of neoplastic blood vessels in tumor tissues,[11,12] macromolecular contrast agents show potential for imaging and characterization of tumors. Accumulation of macromolecules at the tumor site via the “enhanced permeability and retention” (EPR) effect[12] allow targeting of anticancer drugs to solid tumors. Consequently, by attaching both the contrast agent and the chemotherapeutic agent to the polymeric side chains, one can follow the fate of polymeric drug conjugates and subsequent correlation with treatment.
N-(2-hydroxypropyl)methacrylamide (HPMA) copolymers are non-toxic, non-immunogenic water soluble polymeric carriers that are in various stages of clinical trials for cancer therapy.[13] Previously the potential of HPMA copolymers for passive and active delivery of contrast agents was reported.[14-17] In this study we report the synthesis, characterization, in vitro stability, and cytotoxicity of a macromolecular drug delivery system based on HPMA copolymer containing both a chemotherapeutic drug and an MRI contrast agent.
Chemicals and Reagents
2,2′-azoisobutyronitrile (AIBN) and Gd (III) chloride hexahydrate (GdCl3 · 6H2O) were obtained from Aldrich (Milwaukee, WI, USA). N-3-aminopropylmethacrylamide (APMA) was obtained from Polysciences, Inc. (Warrington, PA, USA). p-Isothiocyanatobenzyl-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid ( p-SCN-Bz-DOTA) was obtained from Macrocyclics (Dallas, TX, USA). N,N,N′,N′-ethylenediaminetetraacetic acid (EDTA) disodium salt dihydrate was obtained from USB Corporation (Cleveland, OH, USA), fetal bovine serum (FBS) from QBI (Gaithersburg, MD, USA) and Calf bovine serum from ATCC (Manassas, VA, USA). All other chemicals were purchased from Sigma (St. Louis, MO, USA) and were of reagent grade.
Cell Culture
Human breast cancer cell line MDA-MB-435 (ATCC HTB-129; ATCC) was cultured in DMEM (ATCC 30−2002) supplemented with 10% heat inactivated FBS and 1% penicillin. Mouse fibroblast cell line NIH/3T3 (ATCC CRL-1658; ATCC) was cultured in DMEM supplemented with 10% heat inactivated calf bovine serum. Both cell lines were grown at 37 °C in a humidified atmosphere of 5% CO2.
Synthesis and Characterization of Polymer–Drug–Contrast Agent Conjugates
Monomer Synthesis
Methacryloylglycylphenylalanylleucylglycyl doxorubicin (MA-GFLG-Dox)[18] and HPMA[19] were synthesized by previously described methods. Comonomer APMA-benzyl-DOTA was synthesized by reacting APMA with p-SCN-Bz-DOTA in dry dimethylsulfoxide (DMSO). The p-SCN-Bz-DOTA was reacted at 1.2 molar excess to APMA. The comonomer was characterized by UV spectrometry (λmax=274 nm) and mass spectrometry (Mol. Wt. 693).
Polymer Synthesis
HPMA copolymer precursors with Dox [p-(DOTA–Gd)–Dox] and without Dox [p-(DOTA–Gd)] (Table 1) were synthesized by free radical precipitation copolymerization. First the polymer backbones were synthesized using monomers of HPMA, APMA-benzyl- DOTA, and MA-GFLG-Dox in predetermined molar compositions (Table 1). All polymerizations were carried out in acetone/DMSO using AIBN as the initiator. 3-Mercaptopropionic acid (0.01 mol-%) was used as a chain transfer agent to control the molecular weight of HPMA copolymer–DOTA conjugate. The ratio of monomers/initiator/solvent in the feed was kept constant at 12.5:0.6:86.9 (wt.-%), respectively. The comonomer mixture was purged with nitrogen for 5 min. The ampoule was sealed at 50 °C for 24 h. The polymers were isolated by precipitation of the resulting solution into ether. The contents of side chains terminating in Dox were determined by UV spectrophotometry (ε=11 500 L · mol−1 · cm−1, λmax=482 nm, water).
Table 1.
Physicochemical characteristics of HPMA copolymer–contrast agent conjugates.
| Samplea) | Feed comonomer composition |
Polymer characteristics (mmol · g−1 polymer) |
b) |
nc) |
Relaxivity |
|||
|---|---|---|---|---|---|---|---|---|
| HPMA |
APMA–DOTA |
MA–GFLG–Dox |
Dox content | Gd content | g · mol−1 | s−1 · mM−1 Gd | ||
| mol-% | ||||||||
| p-(DOTA–Gd) | 90 | 10 | 0 | – | 0.41 ± 0.014 | 35 000 | 1.6 | 19.6 |
| p-(DOTA–Gd)–Dox | 85 | 10 | 5 | 0.26 ± 0.05 | 0.19 ± 0.021 | 34 000 | 1.4 | 32.5 |
For structures of polymer-contrast agent conjugates see Figure 1
Weight average molecular weight of polymer precursor
Polydispersity index.
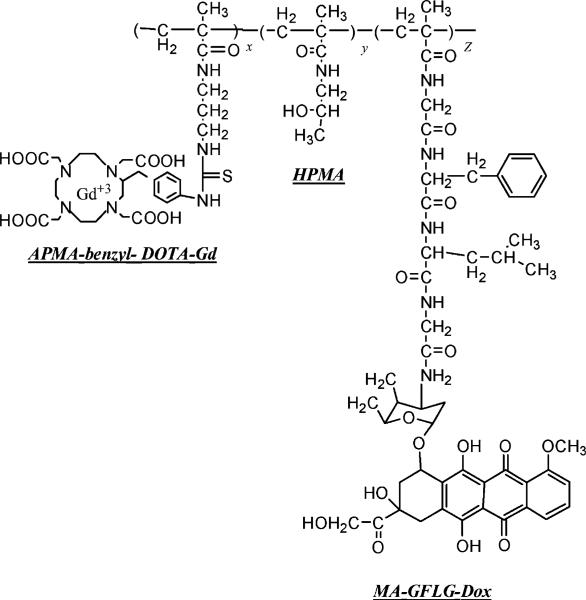
In the second step, polymer conjugates and Gd (III) chloride hexahydrate (GdCl3 · 6H2O) (1.5:1 molar equivalents relative to the DOTA content of the feed) were dissolved in deionized water. The pH of the solution was maintained at 5−5.5 overnight by gradual addition of NaOH,[15] EDTA disodium salt dehydrate (EDTA/Gd, 1:1) was added into the solutions to chelate the excess and non-specifically bound Gd. After stirring for 30 min, the solution was purified over a PD10 size exclusion column (GE Healthcare, NJ, USA), to remove the EDTA-chelated Gd and other unreacted low molecular weight monomers from the polymeric conjugates. The polymer conjugates were dialyzed and lyophilized. The chemical structure of a typical polymeric construct is shown in Figure 1.
Figure 1.
General structure of HPMA copolymer–(DOTA–Gd)–Dox conjugates. (HPMA; APMA-benzyl-DOTA; Gd; MA-GFLG-Dox).
Physicochemical Characterization
Copolymer Gd contents were determined using inductively coupled plasma optical emission spectroscopy (ICP-OES) (Galbraith, Knoxville, TN). Dox content of the p-(DOTA–Gd)–Dox conjugate was assessed by UV spectrophotometry at 482 nm. The weight average molecular weight and molecular weight distribution of the polymeric conjugates were estimated by size exclusion chromatography (SEC) on a Superose 12 HR 10/30 column (GE Healthcare, Piscataway, NJ, USA) using a fast protein liquid chromatography (FPLC) system (GE Healthcare) and HPMA homopolymer fractions of known molecular weight as calibration standards.
Relaxivity Measurements
The T1 relaxation times for HPMA copolymer–(DOTA–Gd) chelates of four concentrations (from 0.1 to 0.015 mm) and water were determined on a 1.5 T MR system (Eclipse, Philips Medical System, Cleveland, OH). T1 was measured by an inversion recovery fast spin echo imaging sequence using inversion times (TI) of 50, 100, 200, 400, 700, 1 400, 2 000, and 2 800 ms, an echo time (TE) of 12 ms, and an echo train length of 8 at a repeat time TR of 6 000 ms. All images were obtained from a single axial slice with a 20×15 cm field of view (FOV), 3 mm slice thickness, 256×192 matrix, and one excitation. T1 for each solution and deionized water were calculated using MATLAB (The Mathworks, Inc., Natick, MA, USA). The r1 value was calculated from the slope of the plot of (1/T1, solution−1/T1, water) versus concentration of contrast agent (mm), where T1, solution is the T1 of each dilution of the contrast agent and T1, water is the T1 of water without contrast agent.
Stability of p-(DOTA–Gd) Complex
The stability of the HPMA copolymer–contrast agent complex was evaluated across a range of pH (Table 2). Aliquots of 2 mg · mL−1 HPMA–contrast agent complex were incubated in phosphate buffer at pH 3, 5, and 7 for 1, 3, and 5 d at room temperature. At each time point samples were eluted on a PD10 size exclusion column (GE Healthcare), to remove decomplexed Gd and other low molecular weight impurities. The samples were lyophilized and Gd contents of polymeric conjugates were measured by ICP-OES. Results were reported as% Gd bound compared to p-(DOTA–Gd) (Table 1). The free Gd content of the polymers was determined using Arsenaso III assay.[20]
Table 2.
Stability of HPMA copolymer–(DOTA–Gd) complex as a function of pH.
| Days | pH = 3 |
pH = 5 |
pH = 7 |
|---|---|---|---|
| % Gd bounda) | |||
| 1 | 95.9 | 97.5 | 99.1 |
| 3 | 89.9 | 95.9 | 98.9 |
| 5 | 85.8 | 95.9 | 98.5 |
The data represent the means of duplicate points.
The stability of Gd–DOTA complex was also evaluated in the presence of a range of excess concentrations of a competitive chelator namely EDTA (Table 3). Briefly polymer–DOTA conjugates and GdCl3 · 6H2O (1.5:1 molar equivalents relative to the DOTA content of the feed) were dissolved in deionized water. The pH of the solution was maintained at 5.0−5.5 overnight by gradual addition of 1 n NaOH solution. The solution was divided into four equal volumes and EDTA disodium salt dihydrate was added at 1, 5, 25, and 125 times of Gd concentration. Polymeric solution with (1:1 EDTA/Gd) concentration was treated as a control. After stirring for 30 min, the solutions were eluted in a PD10 size exclusion column to remove the EDTA-chelated Gd and other unreacted low molecular weight monomers from the polymeric conjugate. The samples were lyophilized and Gd contents of polymeric complexes were measured by ICP-OES. Results were reported as% Gd bound compared to p-(DOTA–Gd) (Table 1). The free Gd content of the polymers was determined using Arsenaso III assay.[20]
Table 3.
Stability of HPMA copolymer–(DOTA–Gd) complex in the presence of EDTA.
| No. | EDTA/Gd | Stability (% Gd bound)a) |
|---|---|---|
| 1 | 1:1 | 100 |
| 2 | 5:1 | 88.2 |
| 3 | 25:1 | 84.2 |
| 4 | 125:1 | 57.9 |
The data represent the means of duplicate points.
Cytotoxicity of Polymeric Complexes
The toxicity of polymeric conjugates was assessed using model breast cancer (MDA-MB-435) and non-cancerous fibroblast (NIH/3T3) cell lines. Cells were seeded on 96-well culture plates at a concentration of 3 000 cells per well and allowed to attach for 24 h at 37 °C and in humidified atmosphere of 5% CO2. Subsequently, the medium was removed and 100 μL of HPMA copolymer–(DOTA–Gd) conjugate in DMEM (10% serum) was added to obtain final concentrations of 1−1 000 μm Gd equivalent. MTT assay was performed at 24, 48, and 72 h to determine time dependent effects on toxicity. The same experiment was performed with HPMA copolymer–DOTA–Dox conjugates (with and without Gd) at concentrations between 1 and 10 000 nm Dox equivalent. Cells were assayed at 560 nm on a microplate reader (SPECTRAmax plus, Molecular Devices, Sunnyvale, CA, USA). The toxicity of the conjugates in all experiments was expressed as% of viable cells. Statistical significance of differences in toxicity between different samples was analyzed using two-tailed unpaired student's t-test.
Results
Characterization of Conjugates
Characteristics of HPMA–(DOTA–Gd) complexes with and without Dox are summarized in Table 1. The content of MA–GFLG–Dox in the copolymers was 0.26 mmol · g−1 corresponding to 92% of the feed comonomer content. Subsequent chelation of Gd to the DOTA side chains of the conjugates resulted in Gd incorporation efficiency of 51 and 87% of the DOTA molecules per polymer backbone with and without Dox, respectively. Both HPMA-linked Gd conjugates exhibited relaxation (r1) values higher than the commercially available Gd–DOTA contrast agent (Dotarem®).[21] Polymeric-Gd complex with Dox exhibited 1.6 times higher relaxivity than polymeric complex without Dox. The estimated weight average molecular weight of the polymers was 35 and 34 kDa with polydispersity index of 1.6 and 1.4 (Table 1), typical of similar polymeric conjugates reported in the literature.[14-16]
Chelate Stability as a Function of pH
Stability studies were performed in physiological and acidic pH conditions. Results showed that at pH 7 and 5 less than 5% of Gd was decomplexed in 5 d suggesting the high kinetic stability of the HPMA copolymer–(DOTA–Gd) complex. At these pH values, decomplexation was not observed beyond 3 d. At pH = 3, 85.8% of Gd remained chelated after 5 d (Table 2). Arsenazo III assay showed less than 2% free Gd in each sample suggesting more than 98% of Gd was chelated in each final sample.
Competitive Chelate Challenge Study
Gd-labeling in the presence of increase in concentrations of a competitive chelator EDTA, resulted in lower Gd content of polymeric complexes (Table 3). The amount of Gd decomplexed over 30 min increased linearly with respect to the concentration of added EDTA compared to control. Arsenazo III assay showed less than 2% free Gd in each sample after 30 min incubation with EDTA and purification, suggesting more than 98% of Gd was chelated in final samples.
Cytotoxicity of Polymeric Complexes
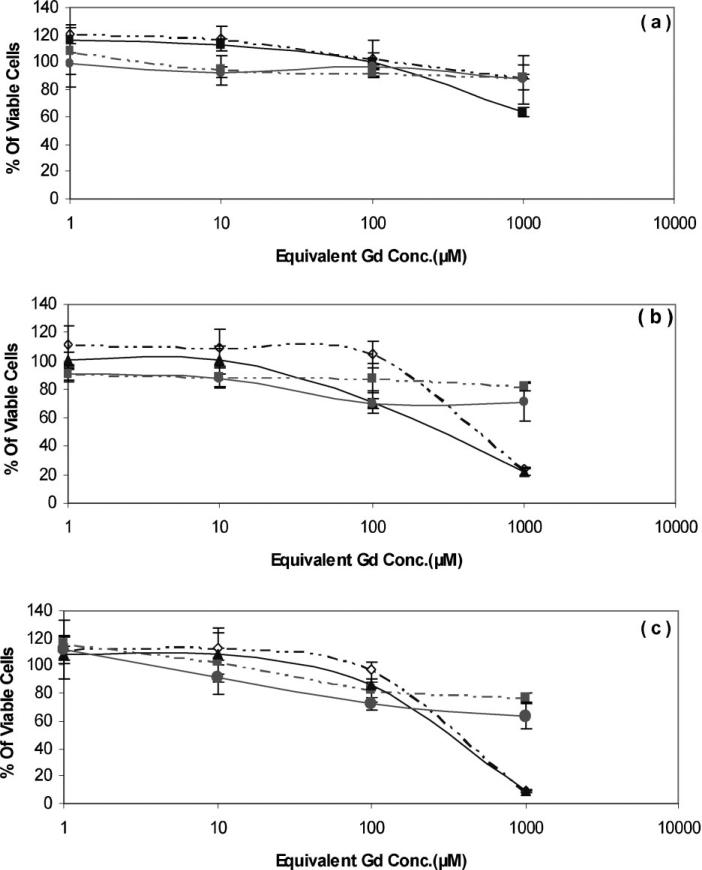
The results of time- and concentration-dependent cytotoxicity of HPMA–(DOTA–Gd) complex (without Dox) on MDA-MB-435 are presented in Figure 2. Toxicity was represented as percentage of viable cells following treatment with the polymeric system and was compared to Magnevist, a commercially available Gd chelate contrast agent, at incremental polymer concentrations (1, 10, 100, and 1 000 μm Gd equivalent). At concentrations between 1 and 100 μm, polymer–Gd chelate showed significantly lower toxicity compared to Magnevist (Gd–DTPA) after 72 h (p<0.019). No significant differences between these compounds after 24 and 48 h at concentrations between 1 and 100 μm equivalent of Gd were observed. At 1 000 μm Gd equivalent concentration,polymer–Gdcomplex showed significantly higher toxicity than Magnevist after 48 and 72 h (p<0.025). No significant difference between these compounds was observed after 24 h at this concentration. The same experiment was performed on NIH/3T3 cell line. Although after 72 h, polymer–Gd complex showed a higher trend in percentage of viable cells on healthy fibroblast cell line, at each concentration there was no significant difference between the cytotoxicity of the same conjugates (Figure 2).
Figure 2.
Comparison of cytotoxicity of varying concentrations of HPMA copolymer–Gd chelate using MTT assay on MDA-MB-435 and NIH/3T3 cell lines after: (a) 24 h; (b) 48 h; and (c) 72 h. p-Gd (NIH/3T3) (◇); Magnevist (NIH/3T3) (■); p-Gd (MDA-MB-435) (▲); Magnevist (MDA-MB-435) (•); Data represent the means of triplicate ± standard error. For structures and characteristics of the samples see Figure 1 and Table 1.
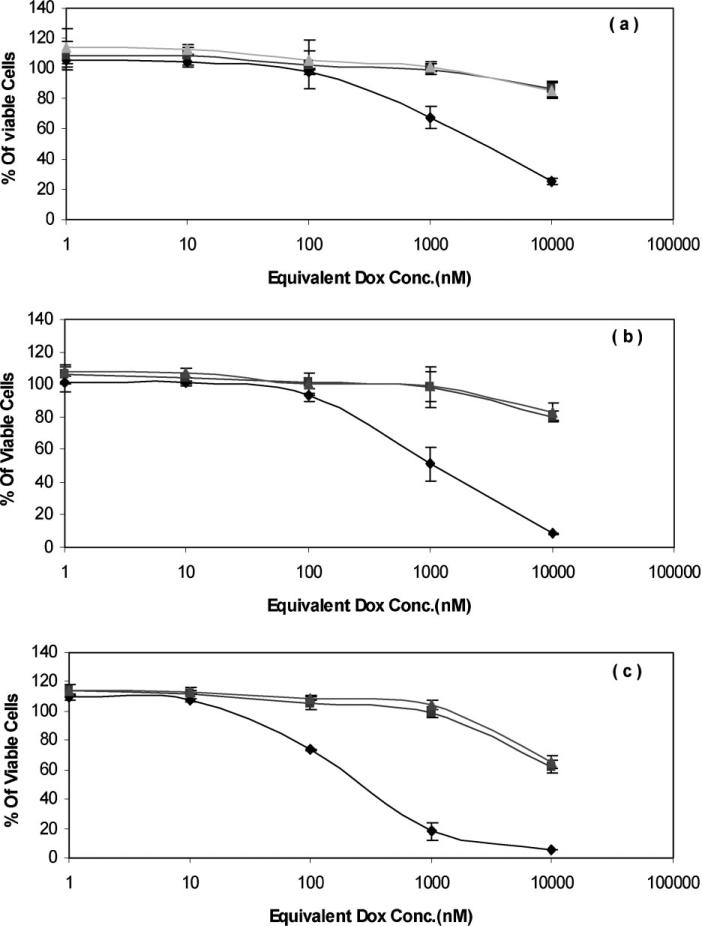
Toxicity of HPMA–DOTA–Gd conjugates with and without Dox on MDA-MB-435 cell line was compared to each other at 24, 48, and 72 h (Figure 3). No significant difference in toxicity of polymer– drug conjugates with and without Gd was observed, suggesting Gd does not interfere with the effect of Dox. Toxicity of polymeric Dox conjugate is significantly less than free Dox suggesting a slower endocytic mechanism of uptake for the conjugates compared to rapid diffusion of free Dox.
Figure 3.
Effect of Gd on cytotoxicity of HPMA copolymer–Dox conjugates at 37 °C after: (a) 24 h; (b) 48 h; and (c) 72 h. Dox (◇); p-Dox–DOTA (■); p-Dox–DOTA–Gd (▲) using MTT assay. Data represent the means of triplicate ± standard error. For structures and characteristics of the samples see Figure 1 and Table 1.
Discussion
The use of polymeric conjugates to selectively deliver cytotoxic anticancer drugs to tumor tissues is well established.[22] Water-soluble polymer anticancer drug conjugates have demonstrated good aqueous solubility, increased half-life in the body, high antitumor effects, and lower toxicity. These systems have the advantages of passive as well as active targeting of the tumor tissues.[23] Several HPMA copolymer based anti-cancer agents are currently in Phase I/II clinical trials.[22] Non-invasive imaging methods of quantifying in vivo pharmacokinetics of these copolymers were developed using scintigraphic imaging of γ-emitting isotopes and SPECT.[24-30] Despite the use of radiolabeled HPMA copolymers a number of shortcomings of isotopes limit their clinical utility.[31] These limitations include radiation, low tissue penetration, and high cost. Therefore, non-invasive methods for imaging of such drug delivery systems for correlation of localization with therapy need to be developed.
MRI is a powerful non-invasive diagnostic modality that can provide high quality anatomic images and physiological data. The most significant advantage of MRI compared to scintigraphy is its high spatial resolution. Correlation of a detailed map of the delivery system deposition within the tissue with the local pathologic features may help optimize the structure of the polymeric drug conjugates for personalized medicine. Therefore, components of the delivery system, such as molecular weight, charge, targeting moiety, drug content, drug releasing mechanism, etc. may be adjusted to achieve superior therapeutic effect in individual patients. Although MRI is less quantitative than some other imaging modalities such as positron emission tomography (PET) and computed tomography (CT), the limitation concerning ionizing radiation does not apply to MRI.
Advances in applications of MRI for cancer imaging have depended predominantly on the use of contrast agents to enhance the appearance of the lesions. The most widely used contrast agents are chelates of Gd that have a strong magnetic field. However, the pharmacokinetic properties of these low molecular weight agents limit their application in many cases including cancer imaging. Attachment of Gd to macromolecular carriers improves blood retention time and accumulation at tumor site because of hyperpermeability of neoplastic blood vessels.[11,12] Macromolecular contrast agents have potential for improved blood pool pharmacokinetics and MR contrast enhancement when compared to low molecular weight contrast agents.[32,33]
In this study, we evaluated a multifunctional macro-molecular delivery system based on water soluble HPMA copolymers, consisting of a contrast agent Gd and a chemotherapeutic agent Dox. Dox was conjugated to the polymeric backbone via a lysosomally degradable peptide spacer (GFLG). The idea is that by attaching both a chemotherapeutic and an imaging agent to the same polymeric carrier it is possible to develop compounds with a relaxivity suitable for MR imaging of the fate of the drug delivery system at the tumor site. This will allow us to correlate the time and extent of tumor localization on the one hand and efficacy and toxicity on the other. The insight gained with this information can further be used to evaluate the effectiveness of therapy in individual patients based on tumor conditions (size, vascularization) and cancer stage.
HPMA copolymers are advantageous as macromolecular carriers because of the ability to tailor make the polymer backbone and control the content of side chains by facile chemical manipulations.[13] Previously it was shown that Gd chelated to HPMA copolymers can be used to monitor diseases such as rheumatoid arthritis and breast cancer.[15,17] The long circulation and local accumulation of these contrast agents allowed the performance of more detailed imaging procedures. Here, we designed a polymeric contrast agent containing Dox as a model chemotherapy agent to evaluate the relaxivity, stability, cytotoxicity, and physicochemical properties of drug containing HPMA copolymer–Gd complex.
The average molecular size of the conjugates (≈34.5 kDa) was lower than the glomerular filtration threshold of 45 kDa for HPMA copolymers.[34] This size is considered optimal for effective clearance of the polymer from the body over time. Observed relaxivities for HPMA copolymer contrast agent conjugates were improved over commercially available contrast agent Gd–DOTA (Table 1). Conjugation of Gd–DOTA to larger macromolecules is known to increase relaxivity by reducing rotational correlation time.[35] This has been observed for many Gd-based complexes[36-40] as well as HPMA-based contrast agents.
Relaxivity of HPMA copolymer–(DOTA–Gd)–Dox conjugate (Table 1) was higher than HPMA copolymer–Gd conjugate probably due to hydrophobic interactions between Dox molecules that may lead to inter- and intra-molecular interactions with overall slower local motions and global rotation. Hence, it may be possible to monitor the sustained incremental accumulation of HPMA copolymer–Dox conjugates at the tumor site with relatively higher contrast. The high kinetic stability of polymeric Gd–DOTA conjugate at physiological and acidic pH values (to simulate lysosomal conditions) is in agreement with the stability values for small molecular weight Gd–DOTA.
Results of cytotoxicity test showed lower toxicity (p<0.019) for polymeric conjugates at 72 h suggesting that gradual accumulation of polymeric contrast agent does not cause toxicity on breast cancer cell line. At 1 000 μm equivalent of Gd, polymer–Gd conjugate showed significantly higher toxicity compared to Magnevist at 48 h (p<0.025) and 72 h (p<0.010) suggesting time dependent endocytosis of the polymeric complex at high concentrations might be toxic. The same experiment was performed on NIH/3T3 cell line and showed lower trend of cytotoxicity on a healthy fibroblast cell line compared to a cancerous cell line at each concentration, probably due to higher uptake rate of the cancer cell line. The toxicity of polymeric drug conjugates with and without Gd on MDA-MB-435 (Figure 3) suggests that there is no interference between Gd and Dox effect.
Conclusion
In summary, HPMA copolymer–(DOTA–Gd)–Dox conjugates were synthesized and characterized. The conjugates were stable and showed higher relaxivity values than commercially available Gd–DOTA contrast agent (Dotarem). The polymeric conjugate with Dox exhibited 1.6 times higher relaxivity than the polymeric conjugate without Dox. High relaxivity and stability of these conjugates coupled with low toxicity show the potential of these systems for monitoring the in vivo fate of HPMA copolymer–drug conjugates and further correlation of localization with efficacy in cancer treatment.
Acknowledgements
This study received financial support from a pre-doctoral DOD fellowship (W81XWH0410341) and a grant from the National Institutes of Health (R01 EB007171).
Footnotes
Current Address: Departments of Pharmaceutics and Pharmaceutical Chemistry and Bioengineering, University of Utah, Salt Lake City, Utah 84108, USA
Contributor Information
Bahar Zarabi, Department of Pharmaceutical Sciences, University of Maryland, Baltimore, Maryland 21201, USA; Center for Nanomedicine and Cellular Delivery, University of Maryland, Baltimore, Maryland 21201, USA.
Jiachen Zhuo, Department of Radiology, University of Maryland, Baltimore, Baltimore, Maryland 21201, USA.
Rao Gullapalli, Center for Nanomedicine and Cellular Delivery, University of Maryland, Baltimore, Maryland 21201, USA; Department of Radiology, University of Maryland, Baltimore, Baltimore, Maryland 21201, USA.
Hamidreza Ghandehari, Department of Pharmaceutical Sciences, University of Maryland, Baltimore, Maryland 21201, USA; Center for Nanomedicine and Cellular Delivery, University of Maryland, Baltimore, Maryland 21201, USA.
References
- 1.Nakashima M, Shibata S, Tokunaga Y, Fujita H, Anda T, Arizono K, Tomiyama N, Sasaki H, Ichikawa M. J. Pharm. Pharmacol. 1997;49:777. doi: 10.1111/j.2042-7158.1997.tb06111.x. [DOI] [PubMed] [Google Scholar]
- 2.Bogdanov AA, Lewin M, Weissleder R. Adv. Drug Deliv. Rev. 1999;37:279. doi: 10.1016/s0169-409x(98)00098-2. [DOI] [PubMed] [Google Scholar]
- 3.Tjuvajev JG, Avril N, Oku T, Sasajima T, Miyagawa T, Joshi R, Safer M, Beattie B, DiResta G, Daghighian F, Augensen F, Koutcher J, Zweit J, Humm J, Larson SM, Finn R, Blasberg R. Cancer Res. 1998;58:4333. [PubMed] [Google Scholar]
- 4.Weissleder R, Reimer P, Lee AS, Wittenberg J, Brady TJ. Am. J. Roentgenol. 1990;155:1161. doi: 10.2214/ajr.155.6.2122660. [DOI] [PubMed] [Google Scholar]
- 5.Weissleder R, Simonova M, Bogdanova A, Bredow S, Enochs WS, Bogdanov A., Jr. Radiology. 1997;204:425. doi: 10.1148/radiology.204.2.9240530. [DOI] [PubMed] [Google Scholar]
- 6.Weissleder R, Tung CH, Mahmood U, Bogdanov A., Jr. Nat. Biotechnol. 1999;17:375. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 7.Benaron DA, Stevenson DK. Science. 1993;259:1463. doi: 10.1126/science.8451643. [DOI] [PubMed] [Google Scholar]
- 8.Contag PR, Olomu IN, Stevenson DK, Contag CH. Nat. Med. 1998;4:245. doi: 10.1038/nm0298-245. [DOI] [PubMed] [Google Scholar]
- 9.Smith BR, Johnson GA, Groman EV, Linney E. Proc. Natl. Acad. Sci. USA. 1994;91:3530. doi: 10.1073/pnas.91.9.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padhani AR. Br. J. Radiol. 2003;76:60. doi: 10.1259/bjr/24373060. [DOI] [PubMed] [Google Scholar]
- 11.Gerlowski LE, Jain RK. Microvasc. Res. 1986;31:288. doi: 10.1016/0026-2862(86)90018-x. [DOI] [PubMed] [Google Scholar]
- 12.Maeda H, Seymour LW, Miyamoto Y. Bioconjug. Chem. 1992;3:351. doi: 10.1021/bc00017a001. [DOI] [PubMed] [Google Scholar]
- 13.Kopecek J, Kopeckova P, Minko T, Lu ZR. Eur. J. Pharm. Biopharm. 2000;50:61. doi: 10.1016/s0939-6411(00)00075-8. [DOI] [PubMed] [Google Scholar]
- 14.Huang Y, Nan A, Rosen GM, Winalski CA, Schneider E, Tsai P, Ghandehari H. Macromol. Biosci. 2003;3:647. [Google Scholar]
- 15.Wang D, Miller SC, Sima M, Parker D, Buswell H, Goodrich CH, Kopeckova P, Kopecek J. (Pharm. Res.).2004;21:1741. doi: 10.1023/b:pham.0000045232.18134.e9. [DOI] [PubMed] [Google Scholar]
- 16.Zarabi B, Nan A, Zhou J, Gullapalli R, Ghandehari H. Mol. Pharm. 2006;3:550. doi: 10.1021/mp060072i. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Ye F, Jeong E, Sun Y, Parker DL, Lu ZR. Pharm. Res. 2007;6:1208. doi: 10.1007/s11095-007-9252-1. [DOI] [PubMed] [Google Scholar]
- 18.Ulbrich K, Subr V, Strohalm J, Plocova D, Jelinkova M, Rihova B. J. Controlled Release. 2000;64:63. doi: 10.1016/s0168-3659(99)00141-8. [DOI] [PubMed] [Google Scholar]
- 19.Strohalm J, Kopecek J. Angew. Makromol. Chem. 1978;70:109. [Google Scholar]
- 20.Gouin S, Winnik FM. Bioconjug. Chem. 2001;12:372. doi: 10.1021/bc000109w. [DOI] [PubMed] [Google Scholar]
- 21.Bousquet JC, Saini S, Stark DD, Hahn PF, Nigam M, Wittenberg J, Ferrucci JT., Jr. Radiology. 1988;166:693. doi: 10.1148/radiology.166.3.3340763. [DOI] [PubMed] [Google Scholar]
- 22.Duncan R. Nat. Rev. Drug Discov. 2003;2:347. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 23.Mitra A, Nan A, Line BR, Ghandehari H. Polymeric Conjugates for Angiogenesis Targeted Tumor Imaging and Therapy. In: Amiji M, editor. Nanotechnology for Cancer Therapeutics. CRC Press; Boca Raton, Florida: 2007. p. 159. [Google Scholar]
- 24.Kissel M, Peschke P, Subr V, Ulbrich K, Schuhmacher J, Debus J, Friedrich E. PDA J. Pharm. Sci. Technol. 2001;55:191. [PubMed] [Google Scholar]
- 25.Lammers T, Kuhnlein R, Kissel M, Subr V, Etrych T, Pola R, Pechar M, Ulbrich K, Storm G, Huber P, Peschke P. J. Controlled Release. 2005;110:103. doi: 10.1016/j.jconrel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Vasey PA, Kaye SB, Morrison R, Twelves C, Wilson P, Duncan R, Thomson AH, Murray LS, Hilditch TE, Murray T, Burtles S, Fraier D, Frigerio E, Cassidy J. Clin. Cancer Res. 1999;5:83. [PubMed] [Google Scholar]
- 27.Pimm MV, Perkins AC, Strohalm J, Ulbrich K, Duncan R. J. Drug Target. 1996;3:385. doi: 10.3109/10611869608996829. [DOI] [PubMed] [Google Scholar]
- 28.Pimm MV, Perkins AC, Strohalm J, Ulbrich K, Duncan R. J. Drug Target. 1996;3:375. doi: 10.3109/10611869608996828. [DOI] [PubMed] [Google Scholar]
- 29.Mitra A, Nan A, Ghandehari H, McNeill E, Mulholland J, Line BR. Pharm. Res. 2004;21:1153. doi: 10.1023/b:pham.0000033001.49737.b7. [DOI] [PubMed] [Google Scholar]
- 30.Julyan PJ, Seymour LW, Ferry DR, Daryani S, Boivin CM, Doran J, Avid M, Anderson D, Christodoulou C, Young AM, Hesslewood S, Kerr DJ. J. Controlled Release. 1999;57:281. doi: 10.1016/s0168-3659(98)00124-2. [DOI] [PubMed] [Google Scholar]
- 31.Fischman AJ, Babich JW, Strauss HW. J. Nucl. Med. 1993;34:2253. [PubMed] [Google Scholar]
- 32.Brasch RC. Magn. Reson. Med. 1991;22:282. doi: 10.1002/mrm.1910220225. [DOI] [PubMed] [Google Scholar]
- 33.Desser TS, Rubin DL, Fan Q, Muller HH, Young SW, Kellar KE, Wellons JA, Ladd DL, Toner JT, Snow RA. Invest. Radiol. 1994;29:S65. doi: 10.1097/00004424-199406001-00022. [DOI] [PubMed] [Google Scholar]
- 34.Seymour LW, Duncan R, Strohalm J, Kopecek J. J. Biomed. Mater. Res. 1987;21:1341. doi: 10.1002/jbm.820211106. [DOI] [PubMed] [Google Scholar]
- 35.Meyer D, Schaefer M, Bonnemain B. Invest. Radiol. 1988;23:S232. doi: 10.1097/00004424-198809001-00048. [DOI] [PubMed] [Google Scholar]
- 36.Caravan P, Greenfield M, Li Z, Sherry A. Inorg. Chem. 2001;40:6580. doi: 10.1021/ic0102900. [DOI] [PubMed] [Google Scholar]
- 37.Caravan P, Ellison J, Mcmurry T, Lauffer R. Chem. Rev. 1999;99:2293. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 38.Nivorozhkin A, Kolodziej A, Caravan P, Greenfield M, Lauffer R, Mcmurry T. Angew. Chem. Int. Ed. 2001;40:2903. [PubMed] [Google Scholar]
- 39.Kiessling F, Heilmann M, Lammers T, Ulbrich K, Subr V, Peschke P, Waengler B, Mier W, Schrenk H, Bock M, Schad L, Semmler W. Bioconjug. Chem. 2006;17:42. doi: 10.1021/bc0501909. [DOI] [PubMed] [Google Scholar]
- 40.Mohs AM, Zong Y, Guo J, Parker DL, Lu ZR. Biomacro-molecules. 2005;6:2305. doi: 10.1021/bm050194g. [DOI] [PubMed] [Google Scholar]