Abstract
We determined the persistence at various times (3, 7, 14, 21, 28, 42 and 56 days) of eight tobacco smoke carcinogen and toxicant biomarkers in the urine of 17 smokers who stopped smoking. The biomarkers were 1-hydroxy-2-(N-acetylcysteinyl)-3-butene (1) and 1-(N-acetylcysteinyl)-2-hydroxy-3-butene (2) [collectively called MHBMA for monohydroxybutyl mercapturic acid] and 1,2-dihydroxy-4-(N-acetylcysteinyl)butane (3) [DHBMA for dihydroxybutyl mercapturic acid], metabolites of 1,3-butadiene; 1-(N-acetylcysteinyl)-propan-3-ol (4, HPMA for 3-hydroxypropyl mercapturic acid), a metabolite of acrolein; 2-(N-acetylcysteinyl)butan-4-ol (5, HBMA for 4-hydroxybut-2-yl mercapturic acid), a metabolite of crotonaldehyde; (N-acetylcysteinyl)benzene (6, SPMA for S-phenyl mercapturic acid), a metabolite of benzene; (N-acetylcysteinyl)ethanol (7, HEMA for 2-hydroxyethyl mercapturic acid), a metabolite of ethylene oxide; 1-hydroxypyrene (8) and its glucuronides (1-HOP), metabolites of pyrene; and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (9) and its glucuronides (total NNAL), a biomarker of exposure to 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). These biomarkers represent some of the major carcinogens and toxicants in cigarette smoke: 1,3-butadiene, acrolein, crotonaldehyde, benzene, ethylene oxide, polycyclic aromatic hydrocarbons (PAH), and NNK. With the exception of DHBMA, levels of which did not change after cessation of smoking, all other biomarkers decreased significantly after 3 days of cessation (P<0.001). The decreases in MHBMA, HPMA, HBMA, SPMA, and HEMA were rapid, nearly reaching their ultimate levels (81 – 91% reduction) after 3 days. The decrease in total NNAL was gradual, reaching 92% after 42 days, while reduction in 1-HOP was variable among subjects to about 50% of baseline. Since DHBMA did not change upon smoking cessation, there appear to be sources of this metabolite other than 1,3-butadiene. The results of this study demonstrate that the tobacco smoke carcinogen/toxicant biomarkers MHBMA, HPMA, HBMA, SPMA, HEMA, 1-HOP, and NNAL are related to smoking and are good indicators of the impact of smoking on human exposure to 1,3-butadiene, acrolein, crotonaldehyde, benzene, ethylene oxide, PAH and NNK.
Keywords: NNAL, 1-hydroxypyrene, mercapturic acids, cigarette smoking
Introduction
While cigarette smoking causes 90% of lung cancer, resulting in over 3,000 deaths per day in the world, only about 15% of smokers will eventually get lung cancer, and we presently lack the ability to predict which smoker is susceptible (1). Some progress has been made in developing models for identifying a high risk profile for smokers, including terms such as family history of lung cancer and number of years of smoking (2). Tobacco carcinogen and toxicant biomarkers, quantitative measurements of exposure and fate of specific tobacco carcinogens and toxicants, could in principle become an important part of a risk algorithm to identify susceptible smokers, hopefully at a young age when intervention with appropriate preventive measures is still feasible. Tobacco carcinogen and toxicant biomarkers are also important for the evaluation and potentially the regulation of new and existing tobacco products (3). Our goal is to develop a panel of tobacco carcinogen and toxicant biomarkers applicable in these activities.
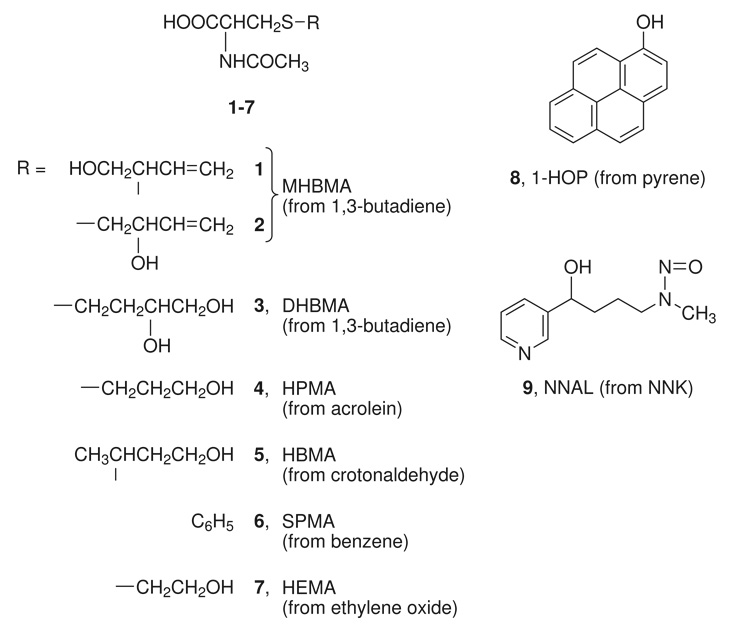
One validation criterion for a tobacco carcinogen or toxicant biomarker is its relationship to tobacco use. This can be determined by comparing levels of the biomarker in people who use tobacco products compared to those who do not, and by assessing the decrease in a biomarker level when people stop using the product. The latter approach is perhaps the most powerful because each subject serves as his or her own control. Therefore, in the study presented here, we evaluated the relationship to cigarette smoking of eight tobacco carcinogen and toxicant biomarkers (Chart 1): 1-hydroxy-2-(N-acetylcysteinyl)-3-butene (1) and 1-(N-acetylcysteinyl)-2-hydroxy-3-butene (2) [collectively called MHBMA for monohydroxybutyl mercapturic acid] and 1,2-dihydroxy-4-(N-acetylcysteinyl)butane (3) [DHBMA for dihydroxybutyl mercapturic acid], metabolites of 1,3-butadiene (4,5); 1-(N-acetylcysteinyl)-propan-3-ol (4, HPMA for 3-hydroxypropyl mercapturic acid), a metabolite of acrolein (6); 2-(N-acetylcysteinyl)butan-4-ol (5, HBMA for 4-hydroxybut-2-yl mercapturic acid), a metabolite of crotonaldehyde (7); (N-acetylcysteinyl)benzene (6, SPMA for S-phenyl mercapturic acid), a metabolite of benzene (8); (N-cetylcysteinyl)ethanol (7, HEMA for 2-hydroxyethyl mercapturic acid), a metabolite of ethylene oxide (9); 1-hydroxypyrene (8) and its glucuronides (1-HOP), metabolites of pyrene and a widely accepted biomarker of exposure to carcinogenic polycyclic aromatic hydrocarbons (PAH) (10); and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (9) and its glucuronides (total NNAL), an established biomarker of exposure to the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) (10).
Chart 1.
Structures of urinary biomarkers (8 and 9 also occur as glucuronides)
These biomarkers represent some of the most important carcinogens and toxicants in cigarette smoke. 1,3-Butadiene, acrolein, crotonaldehyde, benzene, and ethylene oxide are found mainly in the gas phase of cigarette smoke while PAH and NNK are particulate phase constituents (1). 1,3-Butadiene (13 – 51 µg per cigarette mainstream smoke, FTC/ISO conditions, (11)), a potent multi-organ carcinogen in mice, with weaker activity in rats, is classified by the International Agency for Research on Cancer (IARC) as “carcinogenic to humans”, Group 1 (12). Acrolein (54 – 155 µg per cigarette (11)) is highly cilia toxic and induces mutations in the p53 gene similar to those caused by PAH diol epoxides and commonly found in lung tumors from smokers (13,14). Crotonaldehyde (11 – 17 µg per cigarette, FTC/ISO conditions (15)) is mutagenic in various systems and causes liver tumors in rats (16). Benzene (15 – 59 µg per cigarette (11)) is a known human leukemogen while ethylene oxide (7 µg per cigarette, (1)) is associated with lymphatic and hematopoietic cancers in humans and causes tumors at various sites in laboratory animals: both are IARC group 1 carcinogens (17,18). PAH [6 – 70 ng per cigarette (total of 7 carcinogenic PAH)](11) and NNK (29 – 270 ng per cigarette (11)) are generally accepted as causes of lung cancer in smokers: benzo[a]pyrene, a representative carcinogenic PAH, and NNK are both IARC group 1 carcinogens (19,20).
In this study, the 8 biomarkers discussed above were quantified in the urine of 17 smokers, at baseline, and after 3, 7, 14, 21, 28, 42, and 56 days of smoking cessation.
Experimental Procedures
Chemicals
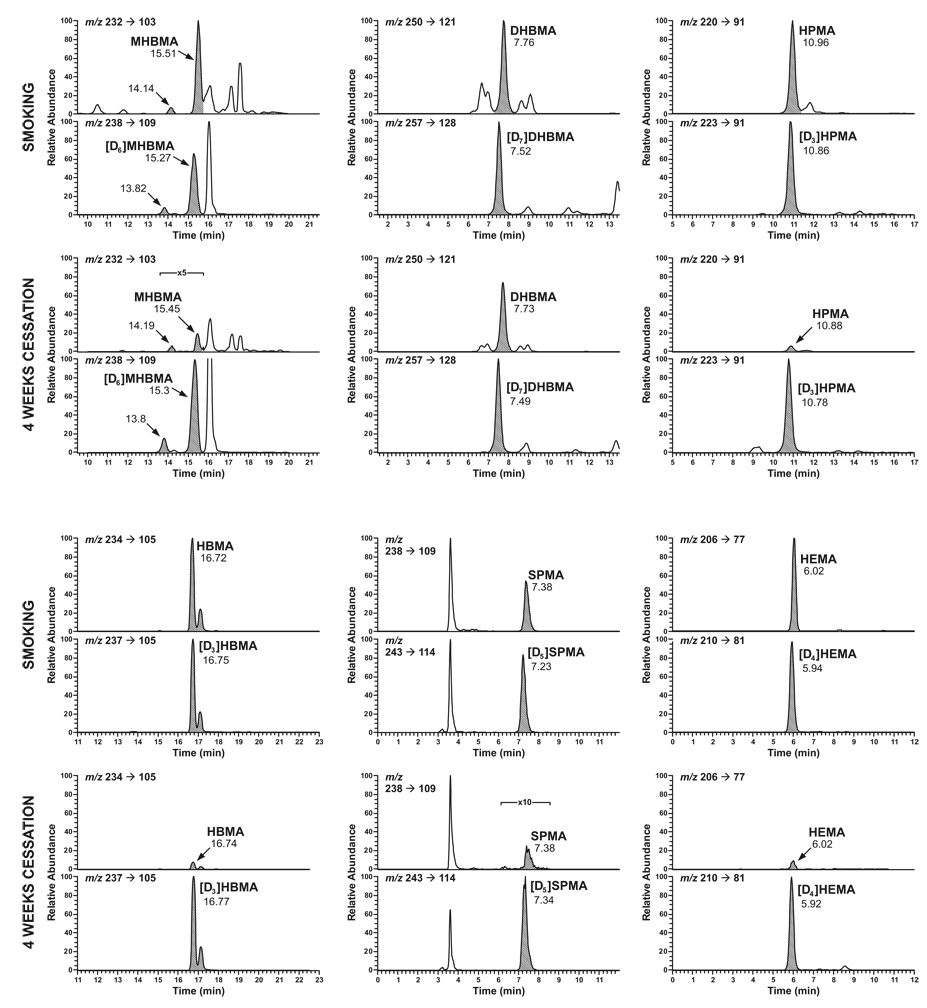
Mercapturic acid standards 1 – 7, NNAL, and internal standards were obtained from Toronto Research Chemicals, Toronto, Ontario. The internal standards were: [1,1,2,3,4,4-hexadeutero]1-hydroxy-2-(N-acetylcysteinyl)-3-butene and [1,1,2,3,4,4-hexadeutero]1-(N-acetylcysteinyl)-2-hydroxy-3-butene ([D6]MHBMA), [1,1,2,3,3,4,4-heptadeutero]1,2-dihydroxy-4-(N-acetylcysteinyl)butane ([D7]DHBMA), [CD3]1-(N-acetycysteinyl)propan-3-ol ([D3]HPMA), [cysteinyl-CD3]2-(N-acetylcysteinyl)butan-4-ol [D3]HBMA, [benzene-D5]N-acetylcysteinyl)benzene ([D5]SPMA), and [1,1,2,2-tetradeutero](N-acetylcysteinyl)ethanol [D4]HEMA. LC-ESI-MS/MS analysis of [D6]MHBMA showed that it had an impurity which eluted at 16.1 min (see Figure 1). The impurity was tentatively identified as [D6]1-(N-acetylcysteinyl)-4-hydroxy-2-butene by HPLC analysis as previously reported (21). Oasis MAX mixed mode reverse phase anion exchange solid phase extraction cartridges (500 mg) were obtained from Waters Corp. (Milford, MA).
Figure 1.
Chromatograms obtained upon LC-ESI-MS/MS-SRM analysis of MHBMA, DHBMA, HPMA, HBMA, SPMA, and HEMA in the urine of a smoker at baseline (denoted by “smoking” and in that subject’s urine after 4 weeks of cessation. Each pair of chromatograms was obtained by SRM for the analyte (upper) and internal standard (lower), represented by the shaded peaks. For MHBMA, the peak eluting at 14.14 min in the top chromatogram is one diastereomer of 1 while the peak eluting at 15.51 min is the other diastereomer of 1 and both diastereomers of 2, as determined by HPLC analysis (21). The same distribution applies to the other MHBMA chromatograms. The two peaks in the HBMA chromatograms are presumed to be diastereomers.
Analysis of Mercapturic Acids in Urine
All solutions and buffers were freshly prepared on the same day that the assay was to be performed. To 2 mL of urine in a 10 mL centrifuge tube was added the internal standards: [D6]MHBMA (1250 ng), [D7]DHBMA (2000 ng), [D3]HPMA (100 ng), [D3]HBMA (2000 ng), [D5]SPMA (50 ng), and [D4]HEMA (100 ng). An Oasis MAX cartridge was preconditioned with 6 mL of MeOH and 6 mL of 2% aq NH4OH. The sample was applied and the cartridge was washed with 6 mL of MeOH and 6 mL of 2% aq NH4OH. The cartridge was dried by applying a vacuum for 2 min, then blown dry with N2, and washed with 6 mL of 2% formic acid. For collection of the fraction containing MHBMA, DHBMA, HPMA, HBMA, and HEMA, 5 mL of 30% MeOH in 2% aq formic acid was added to the cartridge and a 5 mL fraction was collected in a 10 mL glass centrifuge tube. The cartridge was then washed with 5 mL of 50% MeOH in 2% aq formic acid. The fraction containing SPMA was then collected in a 10 mL glass centrifuge tube by addition of 5 mL of 90% MeOH in 2% aq formic acid. The two fractions containing the mercapturic acids were concentrated to dryness with a centrifugal evaporator, operated overnight without heating. The residues were transferred to 200 µL plastic autosampler vials with two aliquots of 80/20:CH3CN/MeOH. The solvents were removed on a Speedvac and the residues stored at −20 °C until analysis. For LC-APCI-MS/MS analysis, the residues were redissolved in 50 µL of 95% 15 mM NH4OAc/5% MeOH with sonication.
LC-APCI-MS/MS-SRM analysis was carried out on a TSQ Quantum Discovery Max instrument (Thermoelectron, San Jose, CA). The HPLC was equipped with a 250 × 4.6 mm Synergi C12 4µ, Max-RP, 80 angstrom pore size column (Phenomenex, Torrance, CA). For analysis of the fraction containing MHBMA, DHBMA, HPMA, HBMA, and HEMA, elution was carried out with 15 mM NH4OAc, pH 6.8 (solvent A) and MeOH (solvent B) at a flow rate of 0.8 mL/min, using the following gradient (time, % of solvent B): 0–5 min, 5; 5 – 10 min, 5 to 10; 10 – 15 min, 10 to 45; 15 – 20 min, 45; 20 – 22 min, 45 to 95; 22 to 25 min, 95; 25 to 27 min, 95 to 5, then reinject. For the fraction containing SPMA, elution was isocratic for 20 min with 55% solvent A. The APCI ion source was operated in the negative ion mode with the following settings: corona discharge needle, 1.8µA (1.5kV); vaporizer temperature, 450 °C; N2 sheath gas pressure, 30 psi; and N2 auxilliary gas pressure, 5psi. The capillary temperature was 200 °C with a voltage of −35 V. Other MS parameters were: collision energy, 13V; Ar collision gas pressure, 1mTorr; peak width, Q1 (full width at half maximum), 0.70; Q3 (full width at half maximum), 0.70; scan width (m/z), 0.40; scan time (sec), 0.1. Transitions for SRM are summarized in Table 1.
Table 1.
Some characteristics of the mercapturic acid analysis by LC-APCI-MS/MS-SRM
| Analyte | Transition monitored (m/z)a | Expected valueb | Observed valuec | R2 | Precision (CV, %) | Assay detection limit (pmol/mL)d |
|---|---|---|---|---|---|---|
| MHBMA | 232 → 103 | 4.04 ± 0.64 | 2.37 | 0.99 | 16 | 3.2 |
| [D6]MHBMA | 238 → 109 | |||||
| DHBMA | 250 → 121 | 300 ± 26.0 | 326 | 0.98 | 8.7 | 12 |
| [D7]DHBMA | 257 → 128 | |||||
| HPMA | 220 → 91 | 1440 ± 135 | 1440 | 0.90 | 9.4 | 2.3 |
| [D3]HPMA | 223 → 91 | |||||
| HBMA | 234 → 105 | 2800 ± 170 | 3190 | 1.0 | 6.1 | 0.21 |
| [D3]HBMA | 237 → 105 | |||||
| SPMA | 238 → 109 | 1.58 ± 0.12 | 1.52 | 0.99 | 7.6 | 0.013 |
| [D5]SPMA | 243 → 114 | |||||
| HEMA | 206 → 77 | 6.41 ± 0.77 | 5.99 | 0.94 | 12.1 | 0.24 |
| [D4]HEMA | 210 → 81 |
Results from cleavage of S-CH2 bond with charge retention on S-containing fragment (see Chart 1)
Based on replicate (N=6) analysis of pooled smokers' urine; values are ng/ml ± S.D.
y intercept from known addition experiment with pooled smokers' urine; values are ng/ml
Starting with 2 mL urine
Accuracy was determined by spiking 2 mL of a pooled smokers’ urine sample with five levels of the 6 mercapturic acids as follows (ng): MHBMA 4, 8, 16, 32, 64; DHBMA 500, 1000, 2000, 3000, 4000; HPMA 500, 1000, 2000, 3000, 4000; HBMA 4000, 8000, 16000, 32000, 64000; SPMA 1, 2, 4, 8, 16; HEMA 5, 10, 20, 30, 40 and carrying out the analyses. Precision was determined by replicate analyses (intra-day) of 6–8 aliquots of pooled smokers’ urine.
Analysis of 1-HOP and Total NNAL in Urine
Study Design and Urine Samples
Cigarette smokers wanting to quit smoking were recruited from the local metropolitan area using posted brochures in doctors’ offices, advertisements in campus and metropolitan newspapers, on radio, cable TV, and the internet, and by word of mouth. Subjects were included in the study if they were 18 – 70 years old, smoked at least 10 cigarettes per day for at least one year and were generally in good physical and mental health. They were excluded if they experienced contraindications for nicotine replacement use, used any other nicotine containing products, used medications that might interact with biomarkers, or were pregnant or nursing.
Subjects who passed screening were asked to continue to use their own brand of cigarettes for 2 weeks. They were required to attend baseline clinic visits once during Week 1 (Day -14) and once during Week 2 (Day -7) of the study, and on these days collected a 24 h urine sample. The urine collection started with the second void on the morning of their visit and continued through the first void of the next day. Urine collections were brought to the clinic the day after their clinic visit, or were frozen in their personal freezer until the following visit. Subjects quit smoking after this baseline period and were required to refrain from smoking. They attended clinic on Days 3, 7, 14, 21, 28, 42, and 56 after quitting, and collected 24h urine samples on these days. At every clinic visit, subjects completed questionnaires on health, withdrawal symptoms, tobacco use and alcohol use. Vital signs, weight and expired carbon monoxide were obtained. Subjects were asked to complete daily diaries of withdrawl symptoms, cigarettes smoked, study product used and number of alcoholic drinks per day.
Subjects received nicotine patch or nicotine gum or lozenge as a smoking cessation aid, and chose their preferred product. Those who had difficulty achieving abstinence on a single nicotine replacement product were offered combined therapies. Subjects were paid in increasing amounts contingent on their ability to stay abstinent. They also received behavioral counseling at each clinic visit.
Statistical Analysis
Due to a distribution skewed to high values, all biomarkers were analyzed on the natural log scale. The paired t-test compared the initial change from baseline to day 3 for each biomarker and the repeated measures analysis of variance evaluated the rate of change during follow-up, starting from day 3 to day 56. A p-value < 0.05 was considered statistically significant. No p-value adjustments were made for multiple comparisons.
Results
For the analysis of mercapturic acids, a new combined method was developed involving solid phase extraction on a mixed mode anion exchange/ reverse phase cartridge followed by LC-APCI-MS/MS-SRM analysis. Two fractions were collected from the solid phase extraction cartridge. The first more polar fraction contained MHBMA, DHBMA, HPMA, HBMA, and HEMA and the second contained the more hydrophobic SPMA. These two fractions were each analyzed for the mercapturic acids using the transitions summarized in Table 1. In each case, the transition monitored resulted from cleavage of the S-CH2 bond with negative charge retention on the fragment containing sulfur. Typical chromatograms are illustrated in Figure 1. Accuracy, precision, and detection limits were generally acceptable for the purpose of this study (Table 1), although the MHBMA analysis was not optimal, probably due to a trailing peak which co-eluted partially with the analyte (Figure 1). Recoveries ranged from 20 –100%.
Seventeen subjects (11 female) completed the study. Their mean age (± S.D.) was 43.9 ± 11.0 years (range 23 – 58), 16 were Caucasian, and 1 was African-American. They had been smoking an average of 17.3 ± 12.3 years and smoked 21.8 ± 6.7 cigarettes per day.
Levels of the eight biomarkers at baseline and times after smoking cessation are summarized in Table 2. Baseline values of the biomarkers ranged from 1–3 nmol/24h for NNAL, 1-HOP, and SPMA; 60 – 100 nmol/24h for HEMA and MHBMA; and 1,000 – 10,000 nmol/24h for DHBMA, HPMA, and HBMA.
Table 2.
Levels of eight urinary biomarkers in smokers' urine at baseline and at times after cessation of smoking
| Mean ± S.D. (N=17) Amount (nmol/24h) at Day | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Biomarker | Baseline | 3 | 7 | 14 | 21 | 28 | 42 | 56 | |
| MHBMA | 66.1± 69.4 | 5.42 ± 4.35 | 6.12 ± 5.64 | 6.07 ± 5.10 | 4.67 ± 2.75 | 7.49 ± 13.81 | 5.08 ± 3.68 | 3.66 ± 2.41 | |
| DHBMA | 1038 ± 514 | 875 ± 635 | 886± 558 | 622 ± 340 | 769 ± 316 | 791 ± 382 | 781 ± 269 | 662 ± 248 | |
| HPMA | 10020 ± 5150 | 1336 ± 923 | 1362 ± 622 | 1626 ± 1587 | 1381 ± 653 | 1440 ± 741 | 1847 ± 1083 | 1500 ± 1005 | |
| HBMA | 1965 ± 1001 | 265 ± 113 | 269 ± 95 | 270 ± 130 | 242 ± 83 | 331 ± 148 | 269 ± 118 | 273 ± 153 | |
| SPMA | 3.20 ± 3.80 | 0.396 ± 0.345 | 0.276 ± 0.234 | 0.165 ± 0.136 | 0.203 ± 0.163 | 0.357 ± 0.249 | 0.254 ± 0.263 | 0.214 ± 0.214 | |
| HEMA | 102 ± 47.1 | 24.0 ± 16.8 | 20.5 ± 11.3 | 21.2 ± 16.3 | 19.9 ± 15.0 | 38.8 ± 29.6 | 19.2 ± 18.1 | 19.2 ± 13.6 | |
| 1-HOP | 1.36 ± 0.776 | 0.826 ± 1.07 | 0.750 ± 0.545 | 1.06 ± 1.87 | 1.12 ± 1.81 | 0.783 ± 1.09 | 0.542 ± 0.224 | 1.09 ± 1.97 | |
| Total NNAL | 2.70 ± 2.03 | 0.935 ± 0.496 | 0.761 ± 0.491 | 0.433 ± 0.321 | 0.343 ± 0.223 | 0.261 ± 0.175 | 0.199 ± 0.162 | 0.132 ± 0.113 | |
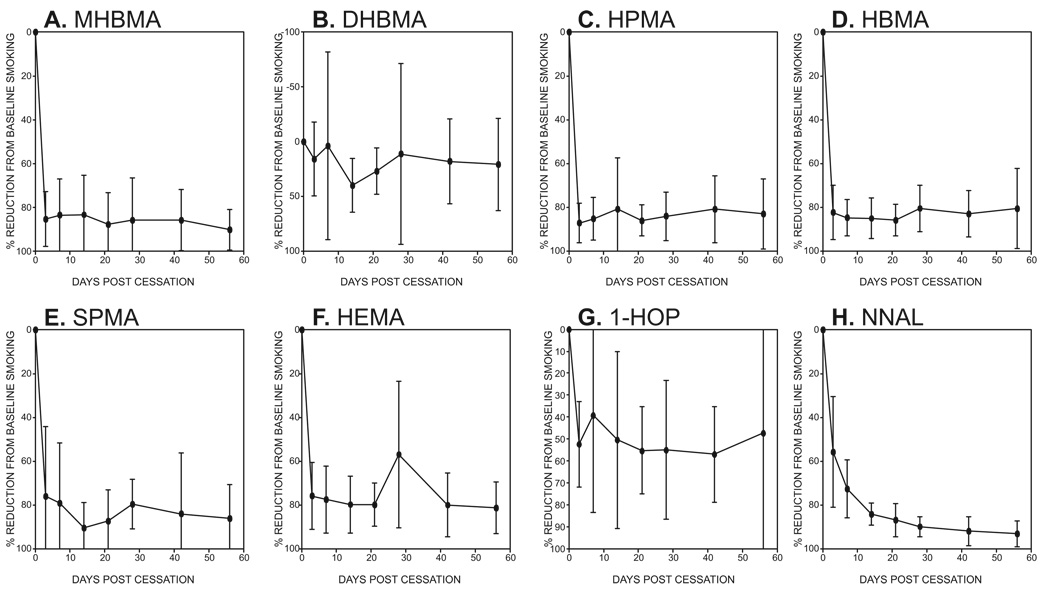
Relative changes in levels of the eight biomarkers after cessation are illustrated in Figure 2A–H. Levels of all the mercapturic acids except DHBMA decreased rapidly after smoking cessation (Figure 2A, C, D, E, F). In each case, the mean reduction from baseline was approximately 80%. Levels of DHBMA did not change significantly over the course of the study (Figure 2B). Levels of 1-HOP decreased to about 50% of the baseline values in most subjects, but two subjects had highly variable levels after cessation, presumably due to environmental exposures, and these were excluded from Figure 2G. Amounts of NNAL decreased gradually, with reductions of 57%, 71%, and 86% after 3, 7, and 21 days, respectively (Figure 2H). With the exception of DHBMA, all decreases in biomarker levels from baseline to day 3 were significant (P<0.001), and this was true for 1-HOP when all subjects were included. After the initial reduction from baseline to day 3, the rate of decline from day 3 to day 56 was only significant for NNAL (P<0.001) and MHBMA (P = 0.04). The percent reduction in biomarker levels correlated with cigarettes smoked per day at baseline for HPMA (P = 0.012), HBMA (P = 0.023), SPMA (P< 0.001), and HEMA (P = 0.017)
Figure 2.
Percent reduction from baseline of eight tobacco carcinogen and toxicant biomarkers at various intervals after cessation. Values are means ± S.D. (N = 17), except for 1-HOP (N = 15) for which 2 subjects with highly variable data were omitted.
Discussion
The results of this study demonstrate that 7 of the 8 urinary biomarkers investigated here are definitely related to smoking. Levels of these urinary biomarkers – 5 mercapturic acids, 1-HOP, and total NNAL – decreased significantly after smoking cessation. Only one biomarker – DHBMA – was unaffected by smoking cessation. All of the subjects included in this analysis were confirmed abstainers from smoking after baseline based on their total NNAL values. These results demonstrate that smoking is a major source of exposure to 1,3-butadiene, acrolein, crotonaldehyde, benzene, ethylene oxide, PAH and NNK. In this respect, it is noteworthy that 1,3-butadiene, benzene, ethylene oxide, benzo[a]pyrene (a representative PAH), and NNK are considered carcinogenic to humans (12,17–20).
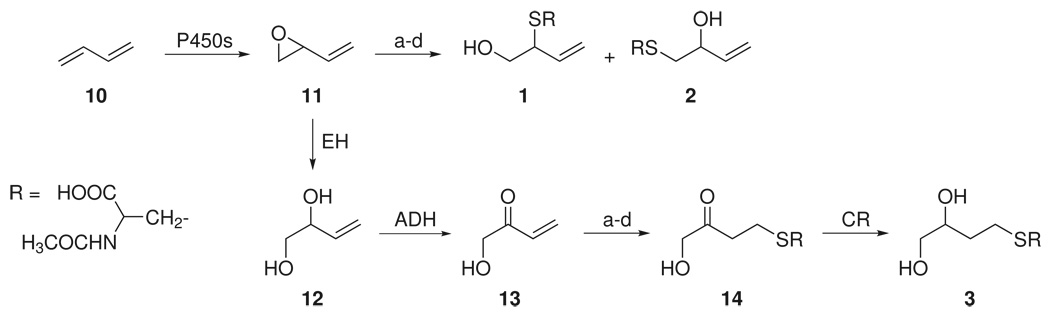
Our data clearly demonstrate different fates of metabolites of 1,3-butadiene (10, Scheme 1) after smoking cessation: MHBMA decreased significantly but DHBMA did not. These urinary metabolites of 1,3-butadiene have been thoroughly characterized in rats and mice treated with the labeled compound (25–27). MHBMA is formed by glutathione conjugation of 1,3-butadiene monoxide (11, Scheme 1), followed by normal metabolic degradation of the glutathione conjugates to mercapturic acids 1 and 2. Since attack can occur at either the 1- or 2-position of 11, a mixture of two regioisomers (1 and 2) is produced and each regioisomer is a mixture of two diastereomers (21). The route of formation of DHBMA has been studied in some detail (Scheme 1). Epoxide 11 undergoes hydration catalyzed by epoxide hydrolase, producing diol 12. This is followed by alcohol dehydrogenase catalyzed oxidation of the secondary hydroxyl group to hydroxymethyl vinyl ketone (13, Scheme 1). Michael addition of glutathione to 13 is then followed by reduction of the carbonyl and normal metabolic processing to give DHBMA (3). This pathway has been confirmed by administration of the relevant precursors to rats and mice (28).
Scheme 1.
Metabolism of 1,3-butadiene (10) to MHBMA (1 and 2) and DHBMA (3)
a. GSH, GSTs; b. γ-glutamyltranspeptidase; c. cysteinylglycine dipeptidase; d. cysteine S-conjugate N-acetyltransferase; EH, epoxide hydrolase; ADH, alcohol dehydrogenase; CR, carbonyl reductase
GC-MS (29,30), GC-MS/MS (31,32), and LC-MS/MS (4,5,33–35) methods have been developed and applied for the quantitation of MHBMA and DHBMA in human urine after occupational exposure to 1,3-butadiene (5,29–32,34–38) and in smokers and non-smokers (4,30,33,39). Consistent with our results, most of these studies have shown that MHBMA is related to 1,3-butadiene exposure while DHBMA, which is present in far higher concentrations, is not related to exposure. Apparently there are significant sources of exposure to ketone 13 or other precursors to DHBMA other than metabolism of 1,3-butadiene. In one study of the effects of changes in smoking on 1,3-butadiene metabolites in urine, MHBMA levels decreased by 18% when smokers switched from cellulose acetate to charcoal filtered cigarettes (4), while a second study demonstrated a decrease of 50–80%, and also reported decreases of 90 – 95% when smokers stopped (39).
HPMA was characterized as a major urinary metabolite of acrolein in rats (40). It is believed to arise by initial Michael addition of glutathione, followed by normal metabolic processing to the mercapturic acid, and reduction. HBMA is similarly formed in the metabolism of crotonaldehyde (41).
Analyses of HPMA and HBMA by LC-MS/MS have been reported (6,7,42). Our results are consistent with a previous study in which we showed that HPMA levels decreased significantly by 78% in smokers who abstained for 4 weeks (6). In other studies, levels of HPMA were significantly higher in smokers than non-smokers in an investigation of 274 smokers and 100 non-smokers in Germany (8). Levels of HPMA and HBMA decreased by 8% and 17%, respectively, when smokers switched from cellulose acetate to charcoal filter cigarettes (4), but in a second study HPMA decreased by 50 – 75% (39). Small decreases in HPMA were observed in a study in which smokers switched from full-flavor to light or ultra-light cigarettes (43). A significant 35% decrease in urinary HPMA was noted in smokers who switched to a second-generation electrically heated cigarette smoking system compared to those who continued smoking conventional cigarettes for 12 months (44).
Benzene is metabolized to benzene oxide which undergoes glutathione conjugation followed by dehydration to yield S-glutathionyl benzene. This is converted metabolically to SPMA. The capture of benzene oxide by glutathione at pH 7 is inefficient compared to its rearrangement to phenol, accounting for the fact that SPMA is a minor metabolite of benzene (45). Nevertheless, SPMA has proven to be a useful and specific biomarker of benzene exposure (10,46–50), with LC-MS/MS being used extensively for quantitation (4,51–56). Levels of SPMA are consistently higher in smokers than in non-smokers (8,10,55,56). The reported levels in smokers are somewhat higher than observed here. We did not use acid hydrolysis, which converts a pre-mercapturic acid to SPMA, and this could account in part for the difference (57). Significant decreases in urinary SPMA were observed when smokers switched from conventional cellulose acetate to charcoal filter cigarettes (4,39). No or modest decreases in SPMA were observed in a study in which smokers switched from full-flavor to light or ultra-light cigarettes (43).
HEMA is formed by direct conjugation of ethylene oxide with glutathione, followed by normal metabolic processing (58,59). Variable amounts of this metabolite were detected in workers exposed to ethylene oxide (60). LC-MS/MS has been used to quantify HEMA in urine (9,61), with higher levels found in smokers than in non-smokers.
Many studies have investigated levels of 1-HOP in the urine of cigarette smokers and in general levels are about twice as high as those in non-smokers although some studies report greater differences (10,62,63). A recent study of smokers and non-smokers in Germany reported similar data (mean 0.90 nmol/24 h in smokers and 0.46 nmol/24 h in non-smokers) to those summarized previously and reported here (8). When smokers switched from their own brand to a second-generation electrically heated cigarette smoking system with lower levels of combustion products, their 1-HOP levels decreased by 53% (44) but there was little decrease upon switching from full-flavored to light or ultra-light cigarettes (43) which is consistent with our previous data (64), or to cigarettes with charcoal filters (39). In an earlier study, we observed about a 50% decrease in 1-HOP levels among smokers who abstained for 2 or 4 weeks (65). All of these results are consistent with those observed here, in which the overall decrease in urinary 1-HOP levels was about 40–50% depending on whether we included all 17 subjects or excluded two who clearly had other exposures.
As shown in Figure 2G, levels of total NNAL decreased only gradually after cessation of smoking. This finding replicates that of our previous study in which a very similar gradual decrease of total NNAL was observed (66). This gradual decrease contrasts markedly to the curves for the mercapturic acids (except DHBMA) which clearly demonstrate a rapid drop in urinary concentrations after 3 days of cessation. The slow release of NNAL also contrasts with data for nicotine plus nicotine glucuronide and cotinine plus cotinine glucuronide, compounds of similar molecular weight and polarity, for which curves were similar to those of the mercapturic acids (66). These data support our hypothesis that there is a receptor or protein binding site for NNAL in the body (67).
In summary, the results of this study validate the biomarkers MHBMA, HPMA, HBMA, SPMA, HEMA, 1-HOP, and NNAL with respect to cigarette smoking, while DHBMA is clearly not related to smoking, nor does it appear to be related to 1,3-butadiene exposure. The results also demonstrate and confirm that cigarette smoking is a major source of exposure to 1,3-butadiene, acrolein, crotonaldehyde, benzene, ethylene oxide, PAH, and NNK. The validated biomarkers will continue to be useful in studies of carcinogen and toxicant uptake in smokers.
Acknowledgements
This study was supported by NIH grant DA-13333 and contract N01-PC-64402. We thank Peter Villalta for assistance with mass spectrometry, carried out in the Analytical Biochemistry Shared Resource, and Bruce Lindgren for statistical analyses, performed in the Biostatistics and Informatics Shared Resource of the Masonic Cancer Center. These shared resources are supported in part by NIH grant CA-77598.
References
- 1.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. vol. 83. Lyon, FR: IARC; 2004. Tobacco Smoke and Involuntary Smoking; pp. 33–1187. [PMC free article] [PubMed] [Google Scholar]
- 2.Spitz MR, Etzel CJ, Dong Q, Amos CI, Wei Q, Wu X, Hong WK. An expanded risk prediction model for lung cancer. Cancer Prev. Res. 2008;1:250–254. doi: 10.1158/1940-6207.CAPR-08-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hatsukami DK, Benowitz NL, Rennard SI, Oncken C, Hecht SS. Biomarkers to assess the utility of potential reduced exposure tobacco products. Nicotine and Tob. Res. 2006;8:600–622. doi: 10.1080/14622200600858166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scherer G, Urban M, Engl J, Hagedorn HW, Riedel K. Influence of smoking charcoal filter tipped cigarettes on various biomarkers of exposure. Inhal. Toxicol. 2006;18:821–829. doi: 10.1080/08958370600747945. [DOI] [PubMed] [Google Scholar]
- 5.Sapkota A, Halden RU, Dominici F, Groopman JD, Buckley TJ. Urinary biomarkers of 1,3-butadiene in environmental settings using liquid chromatography isotope dilution tandem mass spectrometry. Chem Biol Interact. 2006;160:70–79. doi: 10.1016/j.cbi.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Carmella SG, Chen M, Zhang Y, Zhang S, Hatsukami DK, Hecht SS. Quantitation of acrolein-derived 3-hydroxypropylmercapturic acid in human urine by liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry: effects of cigarette smoking. Chem. Res. Toxicol. 2007;20:986–990. doi: 10.1021/tx700075y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scherer G, Urban M, Hagedorn HW, Feng S, Kinser RD, Sarkar M, Liang Q, Roethig HJ. Determination of two mercapturic acids related to crotonaldehyde in human urine: influence of smoking. Hum Exp Toxicol. 2007;26:37–47. doi: 10.1177/0960327107073829. [DOI] [PubMed] [Google Scholar]
- 8.Scherer G, Engl J, Urban M, Gilch G, Janket D, Riedel K. Relationship between machine-derived smoke yields and biomarkers in cigarette smokers in Germany. Regul. Toxicol. Pharmacol. 2007;47:171–183. doi: 10.1016/j.yrtph.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Calafat AM, Barr DB, Pirkle JL, Ashley DL. Reference range concentrations of N-acetyl-S-(2-hydroxyethyl)-L-cysteine, a common metabolite of several volatile organic compounds, in the urine of adults in the United States. J Expo. Anal. Environ. Epidemiol. 1999;9:336–342. doi: 10.1038/sj.jea.7500032. [DOI] [PubMed] [Google Scholar]
- 10.Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23:907–922. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]
- 11.Roemer E, Stabbert R, Rustemeier K, Veltel DJ, Meisgen TJ, Reininghaus W, Carchman RA, Gaworski CL, Podraza KF. Chemical composition, cytotoxicity and mutagenicity of smoke from US commercial and reference cigarettes smoked under two sets of machine smoking conditions. Toxicology. 2004;195:31–52. doi: 10.1016/j.tox.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 12.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. v. 97. Lyon, FR: IARC; 2008. in press. [PMC free article] [PubMed] [Google Scholar]
- 13.Kensler CJ, Battista SP. Components of cigarette smoke with ciliary-depressant activity. Their selective removal by filters containing activated charcoal granules. N. Engl. J Med. 1963;269:1161–1166. doi: 10.1056/NEJM196311282692202. [DOI] [PubMed] [Google Scholar]
- 14.Feng Z, Hu W, Hu Y, Tang MS. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc. Natl. Acad. Sci. U. S. A. 2006;103:15404–15409. doi: 10.1073/pnas.0607031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Counts ME, Hsu FS, Laffoon SW, Dwyer RW, Cox RH. Mainstream smoke constituent yields and predicting relationships from a worldwide market sample of cigarette brands: ISO smoking conditions. Regul. Toxicol. Pharmacol. 2004;39:111–134. doi: 10.1016/j.yrtph.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 16.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. vol. 63. Lyon, France: IARC; 1995. Dry cleaning, some chlorinated solvents and other industrial chemicals; pp. 373–391. [PMC free article] [PubMed] [Google Scholar]
- 17.International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. vol. 60. Lyon, FR: IARC; 1994. Some Industrial Chemicals; pp. 73–159. [Google Scholar]
- 18.International Agency for Research on Cancer. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. vol. 29. Lyon, FR: IARC; 1982. Some Industrial Chemicals and Dyestuffs; pp. 93–148. [PubMed] [Google Scholar]
- 19.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. v. 89. Lyon, FR: IARC; 2007. Smokeless tobacco and tobacco-specific nitrosamines; pp. 548–553. [PMC free article] [PubMed] [Google Scholar]
- 20.Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Cogliano V. Carcinogenicity of polycyclic aromatic hydrocarbons. Lancet. Oncol. 2005;6:931–932. doi: 10.1016/s1470-2045(05)70458-7. [DOI] [PubMed] [Google Scholar]
- 21.Elfarra AA, Sharer JE, Duescher RJ. Synthesis and characterization of N-acetyl-L-cysteine S-conjugates of butadiene monoxide and their detection and quantitation in urine of rats and mice given butadiene monoxide. Chem. Res. Toxicol. 1995;8:68–76. doi: 10.1021/tx00043a009. [DOI] [PubMed] [Google Scholar]
- 22.Carmella SG, Han S, Fristad A, Yang Y, Hecht SS. Analysis of total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human urine. Cancer Epidemiol. Biomarkers & Prev. 2003;12:1257–1261. [PubMed] [Google Scholar]
- 23.Carmella SG, Le K, Hecht SS. Improved method for determination of 1-hydroxypyrene in human urine. Cancer Epidemiol. Biomarkers & Prev. 2004;13:1261–1264. [PubMed] [Google Scholar]
- 24.Carmella SG, Chen M, Han S, Briggs A, Jensen J, Hatsukami DK, Hecht SS. Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chem. Res. Toxicol. 2008 doi: 10.1021/tx800479s. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabourin PJ, Burka LT, Bechtold WE, Dahl AR, Hoover MD, Chang IY, Henderson RF. Species differences in urinary butadiene metabolites; identification of 1,2-dihydroxy-4-(N-acetylcysteinyl)butane, a novel metabolite of butadiene. Carcinogenesis. 1992;13:1633–1638. doi: 10.1093/carcin/13.9.1633. [DOI] [PubMed] [Google Scholar]
- 26.Nauhaus SK, Fennell TR, Asgharian B, Bond JA, Sumner SC. Characterization of urinary metabolites from Sprague-Dawley rats and B6C3F1 mice exposed to [1,2,3,4-13C]butadiene. Chem Res Toxicol. 1996;9:764–773. doi: 10.1021/tx950196u. [DOI] [PubMed] [Google Scholar]
- 27.Richardson KA, Peters MM, Wong BA, Megens RH, van Elburg PA, Booth ED, Boogaard PJ, Bond JA, Medinsky MA, Watson WP, van Sittert NJ. Quantitative and qualitative differences in the metabolism of 14C-1,3-butadiene in rats and mice: relevance to cancer susceptibility. Toxicol. Sci. 1999;49:186–201. doi: 10.1093/toxsci/49.2.186. [DOI] [PubMed] [Google Scholar]
- 28.Sprague CL, Elfarra AA. Mercapturic acid urinary metabolites of 3-butene-1,2-diol as in vivo evidence for the formation of hydroxymethylvinyl ketone in mice and rats. Chem Res Toxicol. 2004;17:819–826. doi: 10.1021/tx049949f. [DOI] [PubMed] [Google Scholar]
- 29.Bechtold WE, Strunk MR, Chang IY, Ward JB, Jr, Henderson RF. Species differences in urinary butadiene metabolites: comparisons of metabolite ratios between mice, rats, and humans. Toxicol. Appl. Pharmacol. 1994;127:44–49. doi: 10.1006/taap.1994.1137. [DOI] [PubMed] [Google Scholar]
- 30.Fustinoni S, Soleo L, Warholm M, Begemann P, Rannug A, Neumann HG, Swenberg JA, Vimercati L, Colombi A. Influence of metabolic genotypes on biomarkers of exposure to 1,3-butadiene in humans. Cancer Epidemiol. Biomarkers Prev. 2002;11:1082–1090. [PubMed] [Google Scholar]
- 31.van Sittert NJ, Megens HJ, Watson WP, Boogaard PJ. Biomarkers of exposure to 1,3-butadiene as a basis for cancer risk assessment. Toxicol. Sci. 2000;56:189–202. doi: 10.1093/toxsci/56.1.189. [DOI] [PubMed] [Google Scholar]
- 32.Boogaard PJ, van Sittert NJ, Megens HJ. Urinary metabolites and haemoglobin adducts as biomarkers of exposure to 1,3-butadiene: a basis for 1,3-butadiene cancer risk assessment. Chem. Biol. Interact. 2001;135–136:695–701. doi: 10.1016/s0009-2797(01)00205-8. [DOI] [PubMed] [Google Scholar]
- 33.Urban M, Gilch G, Schepers G, van Miert E, Scherer G. Determination of the major mercapturic acids of 1,3-butadiene in human and rat urine using liquid chromatography with tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003;796:131–140. doi: 10.1016/j.jchromb.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Fustinoni S, Perbellini L, Soleo L, Manno M, Foa V. Biological monitoring in occupational exposure to low levels of 1,3-butadiene. Toxicol. Lett. 2004;149:353–360. doi: 10.1016/j.toxlet.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 35.McDonald JD, Bechtold WE, Krone JR, Blackwell WB, Kracko DA, Henderson RF. Analysis of butadiene urinary metabolites by liquid chromatography-triple quadrupole mass spectrometry. J Anal. Toxicol. 2004;28:168–173. doi: 10.1093/jat/28.3.168. [DOI] [PubMed] [Google Scholar]
- 36.Albertini RJ, Sram RJ, Vacek PM, Lynch J, Wright M, Nicklas JA, Boogaard PJ, Henderson RF, Swenberg JA, Tates AD, Ward JB., Jr Biomarkers for assessing occupational exposures to 1,3-butadiene. Chem Biol Interact. 2001;135–136:429–453. doi: 10.1016/s0009-2797(01)00181-8. [DOI] [PubMed] [Google Scholar]
- 37.Hayes RB, Zhang L, Yin S, Swenberg JA, Xi L, Wiencke J, Bechtold WE, Yao M, Rothman N, Haas R, O'Neill JP, Zhang D, Wiemels J, Dosemeci M, Li G, Smith MT. Genotoxic markers among butadiene polymer workers in China. Carcinogenesis. 2000;21:55–62. doi: 10.1093/carcin/21.1.55. [DOI] [PubMed] [Google Scholar]
- 38.Albertini RJ, Sram RJ, Vacek PM, Lynch J, Nicklas JA, van Sittert NJ, Boogaard PJ, Henderson RF, Swenberg JA, Tates AD, Ward JB, Jr, Wright M, Ammenheuser MM, Binkova B, Blackwell W, de Zwart FA, Krako D, Krone J, Megens H, Musilova P, Rajska G, Ranasinghe A, Rosenblatt JI, Rossner P, Rubes J, Sullivan L, Upton P, Zwinderman AH. Biomarkers in Czech workers exposed to 1,3-butadiene: a transitional epidemiologic study. Res Rep. Health Eff. Inst. 2003:1–141. [PubMed] [Google Scholar]
- 39.Sarkar M, Kapur S, Frost-Pineda K, Feng S, Wang J, Liang Q, Roethig H. Evaluation of biomarkers of exposure to selected cigarette smoke constituents in adult smokers switched to carbon-filtered cigarettes in short-term and long-term clinical studies. Nicotine Tob Res. 2008;10:1761–1772. doi: 10.1080/14622200802443718. [DOI] [PubMed] [Google Scholar]
- 40.Parent RA, Paust DE, Schrimpf MK, Talaat RE, Doane RA, Caravello HE, Lee SJ, Sharp DE. Metabolism and distribution of [2,3-14C]acrolein in Sprague-Dawley rats. II. Identification of urinary and fecal metabolites. Toxicol. Sci. 1998;43:110–120. doi: 10.1006/toxs.1998.2462. [DOI] [PubMed] [Google Scholar]
- 41.Gray JM, Barnsley EA. The metabolism of crotyl phosphate, crotyl alcohol and crotonaldehyde. Xenobiotica. 1971;1:55–67. doi: 10.3109/00498257109044379. [DOI] [PubMed] [Google Scholar]
- 42.Mascher DG, Mascher HJ, Scherer G, Schmid ER. High-performance liquid chromatographic-tandem mass spectrometric determination of 3-hydroxypropylmercapturic acid in human urine. J. Chromatogr. B Biomed. Sci. Appl. 2001;750:163–169. doi: 10.1016/s0378-4347(00)00385-6. [DOI] [PubMed] [Google Scholar]
- 43.Mendes P, Kapur S, Wang J, Feng S, Roethig H. A randomized, controlled exposure study in adult smokers of full flavor Marlboro cigarettes switching to Marlboro Lights or Marlboro Ultra Lights cigarettes. Regul. Toxicol. Pharmacol. 2008;51:295–305. doi: 10.1016/j.yrtph.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 44.Roethig HJ, Feng S, Liang Q, Liu J, Rees WA, Zedler BK. A 12-month, randomized, controlled study to evaluate exposure and cardiovascular risk factors in adult smokers switching from conventional cigarettes to a second-generation electrically heated cigarette smoking system. J Clin Pharmacol. 2008;48:580–591. doi: 10.1177/0091270008315316. [DOI] [PubMed] [Google Scholar]
- 45.Henderson AP, Barnes ML, Bleasdale C, Cameron R, Clegg W, Heath SL, Lindstrom AB, Rappaport SM, Waidyanatha S, Watson WP, Golding BT. Reactions of benzene oxide with thiols including glutathione. Chem. Res. Toxicol. 2005;18:265–270. doi: 10.1021/tx049781y. [DOI] [PubMed] [Google Scholar]
- 46.Qu Q, Melikian AA, Li G, Shore R, Chen L, Cohen B, Yin S, Kagan MR, Li H, Meng M, Jin X, Winnik W, Li Y, Mu R, Li K. Validation of biomarkers in humans exposed to benzene: urine metabolites. Am. J. Ind. Med. 2000;37:522–531. doi: 10.1002/(sici)1097-0274(200005)37:5<522::aid-ajim8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 47.Feng S, Roethig HJ, Liang Q, Kinser R, Jin Y, Scherer G, Urban M, Engl J, Riedel K. Evaluation of urinary 1-hydroxypyrene, S-phenylmercapturic acid, trans,trans-muconic acid, 3-methyladenine, 3-ethyladenine, 8-hydroxy-2'-deoxyguanosine and thioethers as biomarkers of exposure to cigarette smoke. Biomarkers. 2006;11:28–52. doi: 10.1080/13547500500399730. [DOI] [PubMed] [Google Scholar]
- 48.Kim S, Vermeulen R, Waidyanatha S, Johnson BA, Lan Q, Rothman N, Smith MT, Zhang L, Li G, Shen M, Yin S, Rappaport SM. Using urinary biomarkers to elucidate dose-related patterns of human benzene metabolism. Carcinogenesis. 2006;27:772–781. doi: 10.1093/carcin/bgi297. [DOI] [PubMed] [Google Scholar]
- 49.Qu Q, Cohen BS, Shore R, Chen LC, Li G, Jin X, Melikian AA, Yin S, Yan H, Xu B, Li Y, Mu R, Zhang X, Li K. Benzene exposure measurement in shoe and glue manufacturing: a study to validate biomarkers. Appl. Occup. Environ. Hyg. 2003;18:988–998. doi: 10.1080/714044188. [DOI] [PubMed] [Google Scholar]
- 50.van Sittert NJ, Boogaard PJ, Beulink GD. Application of the urinary S-phenylmercapturic acid test as a biomarker for low levels of exposure to benzene in industry. Br. J. Ind. Med. 1993;50:460–469. doi: 10.1136/oem.50.5.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barbieri A, Sabatini L, Accorsi A, Roda A, Violante FS. Simultaneous determination of t,t-muconic, S-phenylmercapturic and S-benzylmercapturic acids in urine by a rapid and sensitive liquid chromatography/electrospray tandem mass spectrometry method. Rapid Commun. Mass Spectrom. 2004;18:1983–1988. doi: 10.1002/rcm.1580. [DOI] [PubMed] [Google Scholar]
- 52.Maestri L, Negri S, Ferrari M, Ghittori S, Imbriani M. Determination of urinary S-phenylmercapturic acid, a specific metabolite of benzene, by liquid chromatography/single quadrupole mass spectrometry. Rapid Commun. Mass Spectrom. 2005;19:1139–1144. doi: 10.1002/rcm.1904. [DOI] [PubMed] [Google Scholar]
- 53.Lin LC, Shih JF, Shih TS, Li YJ, Liao PC. An electrospray ionization tandem mass spectrometry based system with an online dual-loop cleanup device for simultaneous quantitation of urinary benzene exposure biomarkers trans,trans-muconic acid and S-phenylmercapturic acid. Rapid Commun. Mass Spectrom. 2004;18:2743–2752. doi: 10.1002/rcm.1687. [DOI] [PubMed] [Google Scholar]
- 54.Lin LC, Tyan YC, Shih TS, Chang YC, Liao PC. Development and validation of an isotope-dilution electrospray ionization tandem mass spectrometry method with an on-line sample clean-up device for the quantitative analysis of the benzene exposure biomarker S-phenylmercapturic acid in huma urine. Rapid Commun. Mass Spectrom. 2004;18:1310–1316. doi: 10.1002/rcm.1488. [DOI] [PubMed] [Google Scholar]
- 55.Schettgen T, Musiol A, Alt A, Kraus T. Fast determination of urinary S-phenylmercapturic acid (S-PMA) and S-benzylmercapturic acid (S-BMA) by column-switching liquid chromatography-tandem mass spectrometry. J Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008;863:283–292. doi: 10.1016/j.jchromb.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 56.Sabatini L, Barbieri A, Indiveri P, Mattioli S, Violante FS. Validation of an HPLC-MS/MS method for the simultaneous determination of phenylmercapturic acid, benzylmercapturic acid and o-methylbenzyl mercapturic acid in urine as biomarkers of exposure to benzene, toluene and xylenes. J Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008;863:115–122. doi: 10.1016/j.jchromb.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 57.Paci E, Pigini D, Cialdella AM, Faranda P, Tranfo G. Determination of free and total S-phenylmercapturic acid by HPLC/MS/MS in the biological monitoring of benzene exposure. Biomarkers. 2007;12:111–122. doi: 10.1080/13547500601007943. [DOI] [PubMed] [Google Scholar]
- 58.Gerin M, Tardif R. Urinary N-acetyl-S-2-hydroxyethyl-L-cysteine in rats as biological indicator of ethylene oxide exposure. Fundam. Appl. Toxicol. 1986;7:419–423. [PubMed] [Google Scholar]
- 59.Tardif R, Goyal R, Brodeur J, Gerin M. Species differences in the urinary disposition of some metabolites of ethylene oxide. Fundam. Appl. Toxicol. 1987;9:448–453. doi: 10.1016/0272-0590(87)90027-3. [DOI] [PubMed] [Google Scholar]
- 60.Popp W, Vahrenholz C, Przygoda H, Brauksiepe A, Goch S, Muller G, Schell C, Norpoth K. DNA-protein cross-links and sister chromatid exchange frequencies in lymphocytes and hydroxyethyl mercapturic acid in urine of ethylene oxide-exposed hospital workers. Int Arch Occup. Environ. Health. 1994;66:325–332. doi: 10.1007/BF00378365. [DOI] [PubMed] [Google Scholar]
- 61.Barr DB, Ashley DL. A rapid, sensitive method for the quantitation of N-acetyl-S-(2-hydroxyethyl)-L-cysteine in human urine using isotope-dilution HPLC-MS-MS. J Anal. Toxicol. 1998;22:96–104. doi: 10.1093/jat/22.2.96. [DOI] [PubMed] [Google Scholar]
- 62.Jacob J, Seidel A. Biomonitoring of polycyclic aromatic hydrocarbons in human urine. J Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;778:31–47. doi: 10.1016/s0378-4347(01)00467-4. [DOI] [PubMed] [Google Scholar]
- 63.Hansen AM, Mathiesen L, Pedersen M, Knudsen LE. Urinary 1-hydroxypyrene (1-HP) in environmental and occupational studies- a review. Int J Hyg. Environ. Health. 2008;211:471–503. doi: 10.1016/j.ijheh.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 64.Hecht SS, Murphy SE, Carmella SG, Li S, Jensen J, Le C, Joseph AM, Hatsukami DK. Similar uptake of lung carcinogens by smokers of regular, light, and ultra-light cigarettes. Cancer Epidemiol. Biomarkers & Prev. 2005;14:693–698. doi: 10.1158/1055-9965.EPI-04-0542. [DOI] [PubMed] [Google Scholar]
- 65.Hatsukami DK, Lemmonds C, Zhang Y, Murphy SE, Le C, Carmella SG, Hecht SS. Evaluation of carcinogen exposure in people who used "reduced exposure" tobacco products. J. Natl. Cancer Inst. 2004;96:844–852. doi: 10.1093/jnci/djh163. [DOI] [PubMed] [Google Scholar]
- 66.Hecht SS, Carmella SG, Chen M, Koch JFD, Miller AT, Murphy SE, Jensen JA, Zimmerman CL, Hatsukami DK. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Res. 1999;59:590–596. [PubMed] [Google Scholar]
- 67.Hecht SS. Progress and challenges in selected areas of tobacco carcinogenesis. Chem. Res. Toxicol. 2008;21:160–171. doi: 10.1021/tx7002068. [DOI] [PMC free article] [PubMed] [Google Scholar]