Abstract
Background
Islet transplantation is emerging as a treatment option for selected patients with type 1 diabetes. The limited human islet supply from cadavers and poor islet yield and quality remain substantial impediments to progress in the field. Use of porcine islets holds great promise for large-scale application of islet transplantation. Consistent isolation of porcine islets is dependent on advances in pancreas procurement and preservation, and islet isolation requiring detailed knowledge of the porcine pancreatic anatomy. The primary aim of this study was to describe the vascular and ductal anatomy of the porcine pancreas in order to guide and improve organ preservation and enzyme perfusion.
Methods
Pancreata were removed by en bloc viscerectomy from 65 female Landrace pigs.
Results
15% of organs exhibited inconsistent vascular branching from the celiac trunk. All organs had uniform patterns of branching at the superior mesenteric artery. The superior and inferior mesenteric veins (IMV) merged to become the portal vein in all but one case in which the IMV drained into the splenic vein. 97% of pancreata had three lobes: duodenal (DL), connecting (CL), and splenic (SL); 39% demonstrated ductal communication between the CL and the other two lobes; 50% had ductal communication only between the CL and DL; and 11% presented other types of ductal delineation.
Conclusions
Accounting for the variations in vascular and ductal anatomy, as detailed in this study, will facilitate development of protocols for preservation, optimal enzyme administration, and pancreas distention and digestion, and ultimately lead to substantial improvements in isolation outcomes.
Keywords: Pancreatic islet transplantation, Porcine pancreas anatomy, Surgical diabetes management
INTRODUCTION
Islet transplantation (ITx) is a promising treatment option for selected patients with type 1 diabetes1,2. Increasing evidence demonstrates the ability of human islet allografts to consistently restore normoglycemia and insulin independence in immunosuppressed recipients without the procedural risks associated with vascularized pancreas transplantation3–9. Technical and immunological challenges, however, remain prior to larger scale, cost-effective application of ITx. Technical challenges relate to the limited human islet supply from cadavers and to the low islet yield and quality associated with donor brain death and long cold-ischemia times during organ procurement, storage, and transportation10–13.
The use of islets isolated from pig pancreata, a source with an unlimited supply14, will have a marked impact as it will enable the application of ITx to a larger segment of the population in need. The recent achievement of long-term diabetes reversal after porcine islet xenotransplantation in non-human primates (NHP) demonstrated the potential of islet xenotransplantation in humans15,16.
In addition to addressing safety concerns, clinical use of porcine islets will also require the development and implementation of protocols that maximize the viable islet yield per donor pig pancreas17,18. Consistent isolation of large numbers of high-quality islets from porcine donors is dependent on advances in organ procurement, organ preservation, and islet isolation, all of which will benefit from detailed knowledge of the porcine pancreatic anatomy19–21. Even though studies of porcine pancreatic anatomy have been previously published22, the detailed information needed for optimizing pancreas preservation and ductal perfusion is unavailable. Since variations in porcine anatomy have been reported as limiting in these studies23,24, it is important to establish an understanding of the extent and implications of such anatomical variations of the pig pancreas in the context of pancreas procurement, pancreas preservation, and islet isolation.
The first goal of this investigation was to gain a detailed understanding of the porcine pancreas vascular anatomy so as to facilitate the development and implementation of improved procurement and perfusion-based preservation techniques. The second goal was to develop a better understanding of the ductal branching structure and gain critical knowledge so as to facilitate the development of improved techniques for ductal enzyme loading for optimal pancreas distention prior to islet isolation.
MATERIALS AND METHODS
A total of 65 female Landrace non-heart beating donor pigs were pancreatectomized in order to study the vascular and ductal anatomy of the pancreas. The age of the animals was between 6 and 24 months. The mean weight was 452 ± 99 lb, with a range between 248 and 680 lb. All experiments were conducted according to the rules and regulations of the Institutional Animal Care and Use Committee of the University of Minnesota. Animals were heparinized, sacrificed by sodium pentobarbital overdose, and then, following the cessation of heart rhythm completely bled out and eviscerated. All of the internal organs were placed onto a procurement table. The pancreas was then removed by en bloc viscerectomy to study the pancreatic lobes and general anatomy. The vasculature was studied in situ by dissecting and identifying all the vessels that supply the pancreas, allowing for the investigation of perfusion-based preservation techniques. The pancreatic vascular supply and ductal drainage systems were studied following organ procurement bythe infusion of colored 0.9% NaCl saline.
Surgical procedure
Once all the internal organs were out of the pig abdominal cavity, the greater omentum was incised.
The peritoneum that covers the surface of the viscera was opened and the tail of the pancreas (distal splenic lobe (SL)) was dissected from its posterior attachments starting laterally adjacent to the spleen. The upper and lower margins of the pancreatic tail were freed of their mesenteric attachments. The tail of the pancreas was isolated and the spleen was then mobilized.
The distal splenic artery and vein were divided to the left of the pancreas.
With the splenic artery as a landmark, the celiac trunk (CT) was located and dissected to completely expose the upper part of the abdominal aorta.
The tail and distal portions of the body (proximal SL) of the pancreas were mobilized to the junction of the splenic vein, superior mesenteric vein (SMV), and portal vein (PV).
The posterior attachments of the pancreas and all tissues between the splenic artery and the junction of the splenic vein and PV were divided.
The dissection between the left adrenal gland and the pancreas was later determined to provide more rapid access to the CT, the superior mesenteric artery (SMA), and the aorta, which was isolated by sectioning of the fibromuscular extensions of the diaphragmatic crura and the abdominal lymphatic duct.
Dissection continued along the superior margin of the pancreas. The left gastric artery (LGA) was divided at its origin from the splenic artery. The body of the pancreas wraps the PV with a large anterior and thin posterior ring, termed the “portal ring”.
After dissecting the hepatoduodenal ligament, the common bile duct (which enters proximally into the duodenum on the major duodenal papilla, about 2–5 cm from the pylorus) was cut off at its entrance into the duodenum, and the proper hepatic and gastroduodenal arteries were ligated.
The body of the pancreas and the connecting lobe (CL) were dissected free from the PV by ligating the vascular branches between them.
The head of the pancreas (duodenal lobe (DL)) was then dissected and mobilized to the right of the aorta. The head of the pancreas is in a C-shape with respect to the duodenum and is attached to the second, third, and fourth parts of the duodenum. The duodenum was pulled up and the pancreas was dissected free from the right portion of the PV and the infrahepatic vena cava.
The pancreas was then carefully separated from the pancreaticoduodenal vascular arcade so that the pancreatic branches of this vessel could be individually ligated.
The main pancreatic duct opens about 20 cm distally into the second (descending) portion of the duodenum. The peritoneum on the right side and behind the lower part of the duodenum was subsequently incised and the transverse colonic ligament was sharply dissected to expose the SMA and the SMV.
The right part of the CT, the SMA, and the aorta were then isolated.
The inferior pancreatic artery was also ligated, after the dissection of the SMA in the root of the aorta, about 2 cm caudal to the CT.
The pancreas was excised by dissection and individual ligation and division of the small vessels from the SMA to the pancreas.
The portal ring around the PV was dissected free from the surrounding structures.
The proximal and distal duodenum were ligated and transected.
The inferior and anterior aspect of the gland is attached to the mesocolon, which had to be transected to remove the organ completelyin finishing the total pancreatectomy.
The pancreas was ready to be removed after sectioning of the portal ring and after transection of the vasculature.
Pancreatic lobes
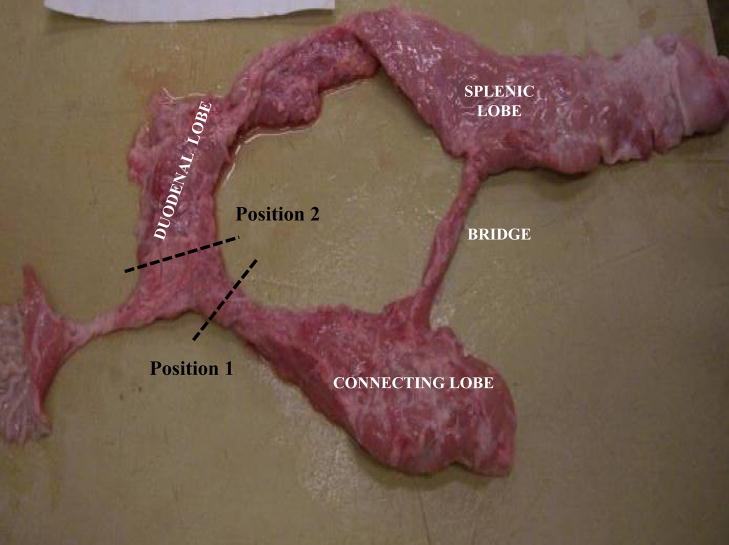
The pancreas is composed of three lobes featuring a nodular surface with irregular margins (Fig. 1). The “splenic” lobe (corresponding to the tail and body in the human pancreas) is situated posteriorly and is attached to the spleen and the stomach. The “duodenal” lobe (corresponding to the head of the pancreas) is located adjacent to the duodenum while the “connecting” lobe (corresponding to the uncinate process) is an extension of the pancreas which is attached to the anterior aspect of the portal vein. There is a “bridge” of pancreatic tissue serving as an anatomical connection between the splenic and connecting lobes.
Figure 1.
Photograph of an excised pig pancreas exhibiting normal anatomy with the duodenal, splenic, and connecting lobes, as well as the bridge.
Position 1: Dotted lines indicate the positioning of the clamp restricting flow to the connecting lobe.
Position 2: Dotted lines indicate the positioning of the clamp restricting flow to the duodenal lobe.
Arterial anatomy
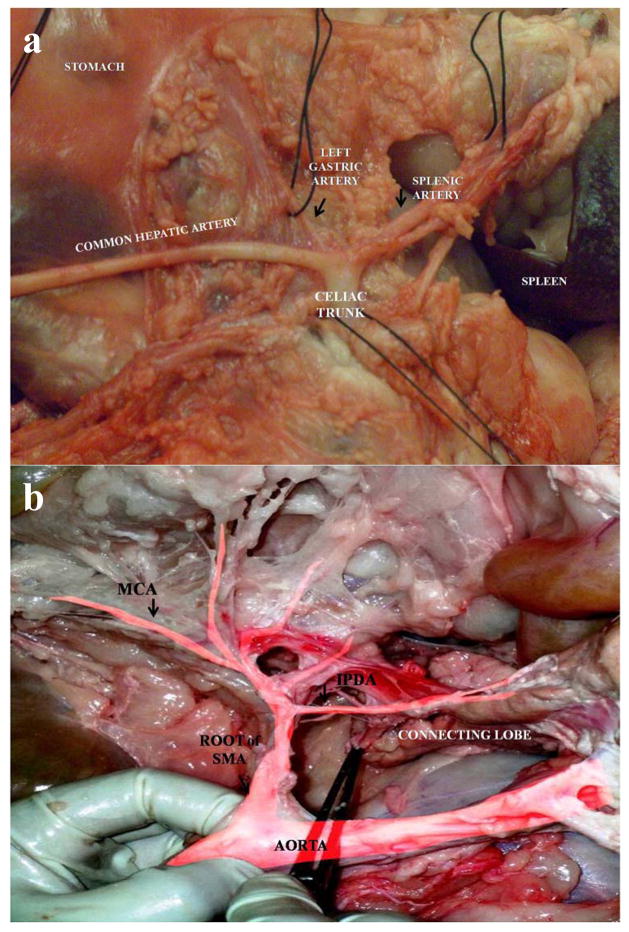
The abdominal aorta is retroperitoneal. The viscera had to be moved aside and the parietal peritoneum had to be removed to locate the aorta. The aorta was immediately dissected from the rest of the tissue. The CT was the first branch off the abdominal aorta infra-diaphragmatically and branched into the splenic artery, the LGA, and the common hepatic artery, as is common in the human (Fig. 2a). These arteries were immediately identified and transected distally to the pancreas. The splenic artery was the first branch identified, dissected, and tied. The posterior or dorsal pancreatic artery (PPA) is a small pancreatic branch originating from the splenic or hepatic artery and was next identified along the upper border of the pancreas. In some cases the PPA can have its origin more proximal off of the splenic artery, but distal to the celiac trifurcation. In some animals, more than one PPA were identified with large variability in size. The other two main branches from the CT were then identified. The first was the LGA, which runs parallel to the left gastric vein to supply the stomach, and the other was the common hepatic artery which supplies the liver with arterial blood. The proper hepatic artery and common bile duct were ligated and divided. The gastroduodenal artery arises from the hepatic artery before its bifurcation into the right and left hepatic artery. The pyloric region of the gastroduodenal artery exhibits two branches that supply the duodenal lobe of the pancreas: (1) the superior pancreaticoduodenal artery and (2) the right gastroepiploic (or gastro-omental) artery. The superior pancreaticoduodenal artery supplies the descending duodenum in addition to the DL. This artery anastomoses with the inferior pancreaticoduodenal artery that originates from the SMA. The pancreaticoduodenal vascular arcade runs between the pancreas and the duodenum, and also extends branches to both organs.
Figure 2.
a: Celiac trunk and the main branches (splenic artery, hepatic artery, and left gastric artery).
b: Arterial system (superior mesenteric artery with distal branches). The branch of SMA to pancreas (IPDA) is distributed mainly in the connecting lobe.
SMA, superior mesenteric artery; IPDA, inferior pancreaticoduodenal artery; MCA, middle colic artery.
Caudal to the CT was the SMA and the renal arteries (found by following the abdominal aorta to the level of the kidneys). The SMA and the SMV were isolated at the lower edge of the pancreas on the left side of the PV. The SMA gives branches (jejunal arteries, right colic artery, middle colic artery, and ileocolic artery) that supply the distal part of the descending duodenum to the proximal part of the ascending colon. The CL, the bridge, and the inferior aspect of the SL are vascularized by an arterial branch, the inferior pancreatic artery, which emerges from the inferior pancreaticoduodenal arterial arcade (Fig. 2b).
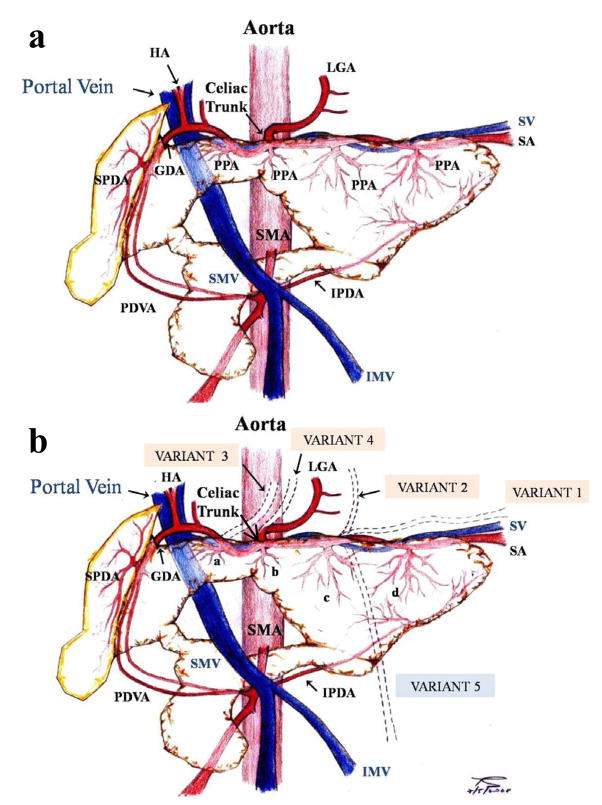
In order to study which lobes are supplied by which main arteries, the aorta was cut open longitudinally and then the CT and the SMA were cannulated separately. Then, colored saline was infused through them to highlight (by introducing visible contrast) the distribution of the arterial blood supply. Once the infusion was completed, the vascular anatomy was mapped by recording the regions supplied by the CT and SMA, respectively, and by dissecting to determine the borders of these regions. The differently-colored saline infusions were surprisingly visible, even to the naked eye. The normal pig pancreatic anatomy along with the major variants are presented in Fig. 3a and 3b respectively.
Figure 3.
Diagram of (a) normal vascular pancreatic anatomy and (b) the major variants of vascular pancreatic anatomy.
a: GDA: Gastroduodenal artery; HA: Hepatic artery; IMV: Inferior mesenteric vein; IPDA: Inferior pancreaticoduodenal artery; LGA: Left gastric artery; PDVA: Pancreaticoduodenal vascular arcade; PPA: Posterior pancreatic artery; SA: Splenic artery; SMA: Superior mesenteric artery; SMV: Superior mesenteric vein; SPDA: Superior pancreaticoduodenal artery; SV: Splenic vein.
b: a: Posterior pancreatic artery from the hepatic artery; b: Posterior pancreatic artery from the celiac trunk; c: Posterior pancreatic artery from the proximal splenic artery; d: Posterior pancreatic artery from the distal splenic artery.
GDA: Gastroduodenal artery; HA: Hepatic artery; IMV: Inferior mesenteric vein; IPDA: Inferior pancreaticoduodenal artery; LGA: Left gastric artery; PDVA: Pancreaticoduodenal vascular arcade; SA: Splenic artery; SMA: Superior mesenteric artery; SMV: Superior mesenteric vein; SPDA: Superior pancreaticoduodenal artery; SV: Splenic vein.
VARIANT 1: “Accessory” splenic artery travelling along with the splenic artery; VARIANT 2: LGA from the splenic artery; VARIANT 3: LGA from the common hepatic artery; VARIANT 4: LGA from the CT; VARIANT 5: Inferior mesenteric vein draining into the splenic vein.
Venous blood outflow (drainage)
The PV collects the blood from stomach, pancreas, intestine, and spleen. The PV, on its way from the root of the mesentery to the liver, penetrates the pancreas at an acute angle so that it lies caudally on the ventral surface and rostrally on the dorsal surface of the pancreas. The PV has two branches which drain into it, the splenic vein and the SMV. The splenic vein drains the body and tail of the pancreas and it is partly surrounded by pancreatic tissue. Veins draining blood from the stomach (left gastric vein, left gastroepiploic vein) also come out into the splenic vein.
The SMV passes through the portal ring receiving the inferior pancreaticoduodenal vein. The inferior mesenteric vein (IMV) usually flows into the SMV, which would be considered an unusual variation in the human anatomy. Small branches drain the CL into the SMV. The gastroduodenal vein empties into the SMV immediately before its junction with the splenic vein. The gastroduodenal vein receives small veins from the DL. Peripherally, the gastroduodenal vein receives the superior pancreaticoduodenal vein which anastomoses with the inferior pancreaticoduodenal vein.
Pancreatic duct
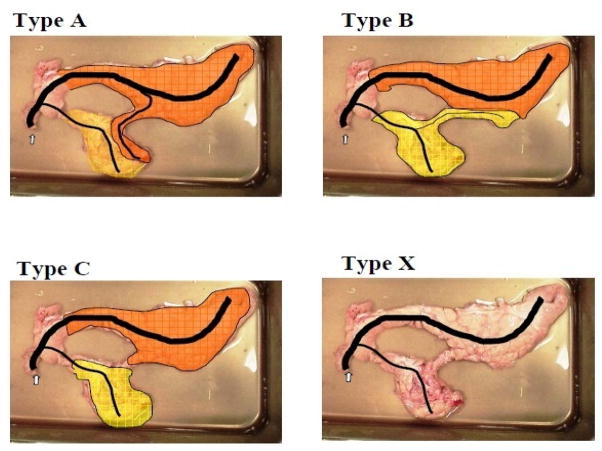
Following dissection, the pancreas was removed from the viscera utilizing an en bloc style procurement technique and placed onto a cooled dissection tray for trimming. The next step was the cannulation of the pancreatic papilla. In some cases, the pancreatic duct was cannulated in situ during the dissection of the viscera. Ductal anatomy was investigated by infusing colored saline into the main pancreatic duct while clamping access to the CL (position 1 in Fig. 1) and tracing the color change. Next, the CL was infused with a differently colored saline solution while access to the DL was clamped off (position 2 in Fig. 1). The pancreas was then further dissected to determine the extent of ductal communication between the lobes. A classification scheme for the different types of pancreatic ductal networks was designed by observing the ductal anatomy (Fig. 4).
Figure 4.
Classification of the different duct types.
STATISTICAL ANALYSIS
We performed a descriptive analysis of the number of cases reporting median and extreme, average, and standard deviation (SD). For statistical analyses, we used the Statistical Package for Social Sciences program (SPSS Inc, Chicago, USA).
RESULTS
The pig pancreas is a retroperitoneal organ, with comparable anatomical orientation and localization to the human. However, there are significant differences between the pig and human pancreas with respect to the number and distribution of the pancreatic lobes. In our series, 63 of the 65 porcine pancreata examined (97%), exhibited the three lobes (duodenal, splenic, and connecting) that we considered the normal representation of the pancreas in the porcine model (Fig. 1). One pancreas presented no “bridging tissue” between the SL and the CL, and another case exhibited a “ring-shaped” pancreas (the portal vein ran parallel to the duodenum and both were covered by the DL). A third pancreas that had the three expected lobes also contained an additional piece or elongation of pancreas tissue extending from the SL. After the pig pancreas was removed and trimmed each organ was weighed. The mean weight of the whole trimmed gland was 347±103 g, with a range from 190 to 698 g. The mean weight of the different lobes was: duodenal, 75±9 g (68–85); splenic, 246±50 g (188–308); connecting, 84 ±17 g (71–114).
The arterial and venous vasculature was studied in 61 animals. It was observed that 51 pigs (84%) exhibited normal branching from the CT. We found variations in CT anatomy in 10 cases (Table 1, Fig. 3b). All pigs exhibited traditional branching at the SMA, sending branches to the CL of the pancreas (inferior pancreaticoduodenal artery). However, one displayed a branch below the SMA that supplied the bowel and another presented additional branches from the SMA towards the colon without variation in the pancreatic supply. Another pig had a branch to the bowel that came off the SMA before the pancreatic branches. Anatomical findings related to the PPA were very diverse and are shown in Table 2 (Fig. 3b). It was found that the PPA most commonly branches from the hepatic artery (54%).
Table 1.
Variations of the celiac trunk anatomy
| VARIATIONS CELIAC TRUNK ANATOMY | n =10 |
|---|---|
| “Accesory” splenic artery traveling along with the splenic artery | 1/10 |
| LGA from the splenic artery | 3/10 |
| LGA from the common hepatic artery | 2/10 |
| Two LGA, one from the CT and another from splenic artery | 2/10 |
| Two LGA from the root of the CT | 1/10 |
| No CT. Hepatic and splenic arteries from aorta and LGA from splenic artery | 1/10 |
LGA, left gastric artery; CT, celiac trunk
Table 2.
Anatomical origin of the posterior pancreatic artery
| POSTERIOR PANCREATIC ARTERY | N | % |
|---|---|---|
| Splenic artery | 6 | 21.43 |
| Hepatic artery | 15 | 53.57 |
| Splenic and Hepatic artery | 6 | 21.43 |
| Root CT | 1 | 3.57 |
| Total | 28 | 100 |
CT, celiac trunk
After inserting the cannulas into the root of the CT and the SMA, and infusing each one with different colors, it became apparent that the CT supplied the entire DL and the majority of the SL. The SMA supplied the CL, the bridge, and the inferior aspect of the SL.
The venous outflow occurred through the PV as a result of the merging of the splenic vein and SMV. The splenic vein typically traveled around the superior and posterior aspect of the pancreas and drained the body and the tail (SL). The rest of the pancreas was drained by branches coming from the SMV and the PV. In the cases we examined, the SMV and IMV merged to become the PV in all but one pig; the exception was the IMV draining into the splenic vein, as is the traditional case in humans (Fig. 3b). The left gastric vein drained into the splenic vein in all studied cases.
The pancreatic ductal network was traced by differential colored saline infusions in 36 pancreata. Fourteen of the 36 pigs (39%) of the pigs exhibited ductal communication between the CL and the other two lobes. Eleven pigs (31%) exhibited ductal communication only from the SL to the CL with the clamp in position 1 (Fig. 4, type A). In one case (2.8%) the communication only existed from the CL to the SL when the clamp was in position 2 (Fig. 4, type B). In two cases (5.6%), the colored infusion illustrated that the whole organ could be infused through from both communications, with the clamp in either position (Type A + B). Eighteen pigs (50%) of the pigs exhibited no ductal communication between the SL and CL, but featured communications solely from the DL (Type C). We classified a type X (neither type A, B, or C) ductal anatomy in four cases (11.1%). In this category, one pig exhibited no anatomical connection between the SL and CL (bridge atresia); another pig exhibited two separate ducts (one for the DL and SL, and the other for the CL); and in two pigs, the pancreata exhibited minimal ductal communication between the CL and distal SL after the initial infusion and clamping off of the connection between the DL and SL. After clamping off the connection between the DL and CL, the infused colored saline flowed into the DL and the proximal SL.
DISCUSSION
There is a lack of literature describing the detailed anatomy of the pig pancreas. Comprehensive knowledge of the pig pancreatic anatomy is essential for improvements in pancreas procurement, preservation, and islet isolation protocols25,26. It has been reported that the pancreatic anatomy may vary from donor to donor27 and several studies28,29 have demonstrated the importance of pig strain on islet isolation outcome. Although the use of different breeds was considered, it was decided to focus on Landrace pigs, as this breed is considered to be the most suitable donor for islet isolation30–32. Recent literature33–35 suggests that this breed is desirable for use in xenotransplantation since isolations yield large numbers of islets and pancreata contain a high islet volume density (3.4%) when compared to other breeds (1–2%). For this reason the investigation was focused on understanding the anatomy of the Landrace breed in detail. Krickhahn et al36 demonstrated that pancreatic islet size may be an important parameter influencing islet yield after isolation and this may be dependent on the strain of the pig.
The present study provides more detailed information about the distribution of the lobes in the pancreas and demonstrates that almost 97% of pancreata exhibited three lobes. One pancreas presented no bridge and another case exhibited a “ring” shaped pancreas. It is important to recognize these kinds of variations if the porcine pancreas is to be used as a source of islets for clinical xenotransplantation. It is critical to understand the functional relationship between pancreatic ducts and drained pancreatic tissue for proper infusion of digestive enzymes and the subsequent isolation of islets. A lack of anatomical knowledge can result in poor or incomplete distension and digestion of the gland. In the 1970s, Calne et al37 had described two lobes attached near the duodenum to the body of the pancreas. Other authors38 divided the pig pancreas into three parts, but named these parts differently as compared to our study: body of the pancreas (corpus pancreatis), right lobe of the pancreas (lobus pancreatis dexter), left lobe of the pancreas (lobus pancreatis sinister). Kumagai et al39 described the pancreas as consisting of the tail, body, head, and the bridge lobes. Gänger et al40, described the pig donor operation to obtain duodenopancreaticosplenic specimen for experimental transplantation. They located the orifice of pancreatic duct in the distal tip of the uncinate process, which corresponds to a duodenal lobe according to our nomenclature. Our data provides a reliable system to classify the pancreatic lobes and the variants in the anatomy eliminating inconsistency in the naming and distribution of the lobes described in the literature; this is an important point with major implications in the field of pig islet isolation and xenotransplantation.
The present study has demonstrated 10 variations related to the CT anatomy and normal branching coming from the SMA. The distribution and disposition of the PPA were unpredictable (Table 2). The most common origin of the PPA in the series of Morel et al21 was from the splenic artery along the upper border of the pancreas. They found that only 2 pigs exhibited a major pancreatic artery coming from the hepatic artery, but a small arterial branch from the splenic artery was also observed. Shokouh-Amiri MH et al20 had classified the variations in arterial blood supply of the porcine pancreas with three types as they related to the major pancreatic artery (PPA in our study). Nonetheless, they found a PPA arising from the root of the CT. They concluded that segmental pancreatic autotransplantation was technically possible in all animals regardless of the type of arterial supply if the anatomy of the vasculature is known adequately. In contrast, Traverso and McFarlene23, based on the description of variations in the arterial blood supply to the body and tail of the pancreas, concluded that the pig is unsuitable for pancreatic autotransplantation studies.
Some authors41 used the tail of the pancreas for various experiments, to minimize complexities associated with variations in the anatomy of the head of the pancreas. To develop a pig model either for whole or segmental pancreas transplantation, the arterial anatomy of the pancreas must be precisely mapped out. The blood supply of the pancreas is provided by the celiac and superior mesenteric arteries. The DL of the pancreas receives its blood supply from the superior pancreaticoduodenal artery, which is in all cases a branch of the gastroduodenal artery. In contrast with this finding, Schröder et al42, described the origin of the superior pancreaticoduodenal artery from the splenic artery. The SL is supplied by the splenic artery and the inferior pancreaticoduodenal artery which branches off from the SMA and also supplies the CL and the bridge as well.
After the abdominal aorta was opened through the anterior wall and cannulas were placed into the CT and SMA, the organ was infused with colored saline. It was observed that the color was distributed homogenously throughout the whole gland. The finding demonstrated that CT and SMA cannulation is a suitable technique to sufficiently perfuse the pancreas and could be used in other experiments related to pancreas preservation. In fact, Zhang et al43 used the pig model for pancreaticoduodenal transplantation and the donors were perfused via the abdominal aorta without clamping the portal venous outflow. Gäbel et al44, used the entire pancreas with intact vascular supply to get a graft from a pig donor to transplant in a pig recipient. The organ was perfused via the CT only with no mention of the SMA. These authors described early graft failure caused by concomitant acute pancreatic necrosis, probably followed by vascular complications. To avoid these kinds of complications, it could be very useful to dissect the CT and the SMA selectively to ensure good perfusion of the entire pancreas, even if you use it for islet transplantation or for a vascularized graft for pancreas transplantation.
In our study, as well as in others20,21, few variations were found in the venous drainage of the pancreas. In only one pig the IMV drained into the splenic vein instead of the SMV.
The impetus to study the pancreatic ductal system was to better understand the ductal branching structure for the purpose of applying the knowledge to the development of enzyme perfusion and pancreatic distention techniques within the context of the pig model. All but one pancreas exhibited only one main pancreatic duct. Only in one case we found two separate ducts, one for the DL and SL and the other for the CL. There are some discrepancies in the literature. König-Liebich38 described the dual origin of the pancreas as arising from dorsal and ventral primordial buds, stating that some species have two pancreatic ducts, and the pig is one of them. The accessory pancreatic duct enters the duodenum at the minor duodenal papilla located distal to the major duodenal papilla22. On the other hand, Swindle45 asserted that the pancreas is related to the proximal duodenum with a single pancreatic duct entering the duodenal lumen distal to the common bile duct. Morel et al21 in a series of 49 Yorkshire pigs, found one pancreatic duct emerging from the lower right part of the pancreatic head, draining into the second part of the duodenum. Only in one instance of this study was an accessory duct found draining the connecting lobe into the third part of the duodenum. Pitkaranta et al46, developed an experimental model of chronic pancreatitis in the pig. They observed a papilla obstruction failure rate of 30%, suggesting the presence of an accessory duct. However, our data do not support these findings.
Even though the pancreatic ductal system and its variability remain the Achilles’ heel in islet isolation, the detailed anatomy described here helps to overcome the difficulties associated with complete distention of the gland. Understanding the variable ductal distribution facilitates simple adaptation of technical distention protocols to make the process more efficient and effective.
In conclusion, the vascular anatomy of the pig pancreas makes it suitable for perfusion via the suprarenal aorta, as is the case in humans. The knowledge of the variations in the anatomy in the vascular supply could help to develop successful new models of whole or segmental pig pancreas preservation and transplantation and islet isolation and transplantation25,26. However, the ductal anatomy can be highly variable, with branching from the connecting lobe being extremely inconsistent. If cannulation is not carefully performed, one portion of the organ may not be properly distended with proteolytic enzymes, resulting in a substantial loss of the islets present in this portion. Careful consideration of all these anatomical findings is likely to facilitate less variable and more economical pig islet isolation for research studies and therapeutic purposes.
Acknowledgments
The authors thank Efstathios Avgoustiniatos, Louis Kidder, Kristen Maynard, Phillip Rozak, and Kate Mueller for assistance in manuscript preparation and review; A.N. Balamurugan and Melanie Graham for helpful discussions; and Shuichiro Matsumoto, Takayuki Anazawa, Thomas Gilmore, Brian Perrault, and Lucas Mutch for surgical assistance.
Research funding provided by grants from the National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases (R01DK068717 and R43 DK070400), the Iacocca Foundation, the Schott Foundation, and the Carol Olson Memorial Diabetes Research Fund.
Abbreviations
- CL
connecting lobe
- CT
celiac trunk
- DL
duodenal lobe
- IMV
inferior mesenteric vein
- ITx
islet transplantation
- LGA
left gastric artery
- NHP
non-human primate
- PPA
posterior or dorsal pancreatic artery
- PV
portal vein
- SL
splenic lobe
- SMA
superior mesenteric artery
- SMV
superior mesenteric vein
References
- 1.Fiorina P, Secchi A. Pancreatic islet cell transplant for treatment of diabetes. Endocrinol Metab Clin North Am. 2007;36(4):999–1013. doi: 10.1016/j.ecl.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Hogan A, Pileggi A, Ricordi C. Transplantation: current developments and future directions; the future of clinical islet transplantation as a cure for diabetes. Front Biosci. 2008;13:1192–205. doi: 10.2741/2755. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–8. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 4.Hering BJ, Kandaswamy R, Ansite JD, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293(7):830–5. doi: 10.1001/jama.293.7.830. [DOI] [PubMed] [Google Scholar]
- 5.Frank A, Deng S, Huang X, et al. Transplantation for type I diabetes: comparison of vascularized whole-organ pancreas with isolated pancreatic islets. Ann Surg. 2004;240(4):631–40. doi: 10.1097/01.sla.0000140754.26575.2a. discussion 640–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318–30. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 7.Froud T, Ricordi C, Baidal DA, et al. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am J Transplant. 2005;5(8):2037–46. doi: 10.1111/j.1600-6143.2005.00957.x. [DOI] [PubMed] [Google Scholar]
- 8.Keymeulen B, Gillard P, Mathieu C, et al. Correlation between beta cell mass and glycemic control in type 1 diabetic recipients of islet cell graft. Proc Natl Acad Sci U S A. 2006;103(46):17444–9. doi: 10.1073/pnas.0608141103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badet L, Benhamou PY, Wojtusciszyn A, et al. Expectations and strategies regarding islet transplantation: metabolic data from the GRAGIL 2 trial. Transplantation. 2007;84(1):89–96. doi: 10.1097/01.tp.0000268511.64428.d8. [DOI] [PubMed] [Google Scholar]
- 10.Kin T, Murdoch TB, Shapiro AM, Lakey JR. Estimation of pancreas weight from donor variables. Cell Transplant. 2006;15(2):181–5. doi: 10.3727/000000006783982133. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto S, Zhang G, Qualley S, et al. Analysis of donor factors affecting human islet isolation with current isolation protocol. Transplant Proc. 2004;36(4):1034–6. doi: 10.1016/j.transproceed.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Ponte GM, Pileggi A, Messinger S, et al. Toward maximizing the success rates of human islet isolation: influence of donor and isolation factors. Cell Transplant. 2007;16(6):595–607. doi: 10.3727/000000007783465082. [DOI] [PubMed] [Google Scholar]
- 13.Sakuma Y, Ricordi C, Miki A, et al. Factors that affect human islet isolation. Transplant Proc. 2008;40(2):343–5. doi: 10.1016/j.transproceed.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rood PP, Buhler LH, Bottino R, Trucco M, Cooper DK. Pig-to-nonhuman primate islet xenotransplantation: a review of current problems. Cell Transplant. 2006;15(2):89–104. doi: 10.3727/000000006783982052. [DOI] [PubMed] [Google Scholar]
- 15.Hering BJ, Wijkstrom M, Graham ML, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12(3):301–3. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 16.Cardona K, Korbutt GS, Milas Z, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12(3):304–6. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 17.O’Neil JJ, Stegemann JP, Nicholson DT, Gagnon KA, Solomon BA, Mullon CJ. The isolation and function of porcine islets from market weight pigs. Cell Transplant. 2001;10(3):235–46. doi: 10.3727/000000001783986792. [DOI] [PubMed] [Google Scholar]
- 18.Mellert J, Hering BJ, Liu X, et al. Critical islet mass for successful porcine islet autotransplantation. J Mol Med. 1999;77(1):126–9. doi: 10.1007/s001090050319. [DOI] [PubMed] [Google Scholar]
- 19.Jensen SL, Kühl C, Nielsen OV, Holst JJ. Isolation and perfusion of the porcine pancreas. Scand J Gastroenterol Suppl. 1976;37:57–61. [PubMed] [Google Scholar]
- 20.Shokouh-Amiri MH, Rahimi-Saber S, Andersen HO. Segmental pancreatic autotransplantation in the pig. Transplantation. 1989;47(1):42–4. doi: 10.1097/00007890-198901000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Morel P, Kaufmann DB, Matas A, et al. Total pancreatectomy in the pig for islet transplantation. Technical alternatives. Transplantation. 1991;52(1):11–5. doi: 10.1097/00007890-199107000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Skjennald A. Anatomy of the liver and pancreas in the domestic swine, with special reference to vascular structures. Scand J Gastroenterol. 1982;17:16–31. [Google Scholar]
- 23.Traverso LW, MacFarlane S. Pancreas autotransplantation: unsuitability of the swine as a model. Transplantation. 1987;44(3):450–1. doi: 10.1097/00007890-198709000-00026. [DOI] [PubMed] [Google Scholar]
- 24.Turégano-Fuentes F, Garcia-Menéndez C, Larrad-Jiménez A, Domínguez-Comesaña E, Sanz-Sánchez M, Pérez-Gallardo A. The feasibility of porcine pancreas autotransplantation--a case for controversy. Transplantation. 1990;49(5):1028–9. doi: 10.1097/00007890-199005000-00049. [DOI] [PubMed] [Google Scholar]
- 25.Scott WE, 3rd, Matsumoto S, Tanaka T, et al. Real-time noninvasive assessment of pancreatic ATP levels during cold preservation. Transplant Proc. 2008;40(2):403–6. doi: 10.1016/j.transproceed.2008.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor MJ, Baicu S, Leman B, Greene E, Vazquez A, Brassil J. Twenty-four hour hypothermic machine perfusion preservation of porcine pancreas facilitates processing for islet isolation. Transplant Proc. 2008;40(2):480–2. doi: 10.1016/j.transproceed.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabat M, Godlewska E, Kinasiewicz J, Urbanowicz A, Orłowski T. Assessment of some porcine strains as donors of islets of Langerhans. Transplant Proc. 2003;35(6):2343–4. doi: 10.1016/s0041-1345(03)00793-0. [DOI] [PubMed] [Google Scholar]
- 28.Dufrane D, D’hoore W, Goebbels RM, Saliez A, Guiot Y, Gianello Parameters favouring successful adult pig islet isolations for xenotransplantation in pig-to-primate models. Xenotransplantation. 2006;13(3):204–14. doi: 10.1111/j.1399-3089.2006.00275.x. [DOI] [PubMed] [Google Scholar]
- 29.Toso C, Brandhorst D, Oberholzer J, Triponez F, Bühler L, Morel P. Isolation of adult porcine islets of Langerhans. Cell Transplant. 2000;9(3):297–305. doi: 10.1177/096368970000900301. [DOI] [PubMed] [Google Scholar]
- 30.Ricordi C, Socci C, Davalli AM, et al. Isolation of the elusive pig islet. Surgery. 1990;107(6):688–94. [PubMed] [Google Scholar]
- 31.Socci C, Ricordi C, Davalli AM, et al. Selection of donors significantly improves pig islet isolation yield. Horm Metab Res Suppl. 1990;25:32–4. [PubMed] [Google Scholar]
- 32.Kim JH, Kim HI, Lee KW, et al. Influence of strain and age differences on the yields of porcine islet isolation: extremely high islet yields from SPF CMS miniature pigs. Xenotransplantation. 2007;14(1):60–6. doi: 10.1111/j.1399-3089.2006.00364.x. [DOI] [PubMed] [Google Scholar]
- 33.Prabhakaran S, Hering BJ. What strain of pig should be used? Xenotransplantation. 2008;15(2):83–6. doi: 10.1111/j.1399-3089.2008.00456.x. [DOI] [PubMed] [Google Scholar]
- 34.Kirchhof N, Hering BJ, Geiss V, Federlin K, Bretzel RG. Evidence for breed-dependent differences in porcine islets of Langerhans. Transplant Proc. 1994;26(2):616–7. [PubMed] [Google Scholar]
- 35.Ulrichs K, Bosse M, Heiser A, et al. Histomorphological characteristics of the porcine pancreas as a basis for the isolation of islets of Langerhans. Xenotransplantation. 1995;2:176–187. [Google Scholar]
- 36.Krickhahn M, Bühler C, Meyer T, Thiede A, Ulrichs K. The morphology of islets within the porcine donor pancreas determines the isolation result: successful isolation of pancreatic islets can now be achieved from young market pigs. Cell Transplant. 2002;11(8):827–38. [PubMed] [Google Scholar]
- 37.Calne RY, Sells RA, Marshall VC, et al. Multiple organ grafts in the pig. Techniques and results of pancreatic, hepatic, cardiac, and renal allografts. Br J Surg. 1972;59(12):969–77. doi: 10.1002/bjs.1800591210. [DOI] [PubMed] [Google Scholar]
- 38.Veterinary anatomy of domestic mammals. Texbook and colour atlas. 3 König-Liebich; [Google Scholar]
- 39.Kumagai N, O’Neil JJ, Barth RN, et al. Vascularized islet-cell transplantation in miniature swine. I. Preparation of vascularized islet kidneys. Transplantation. 2002;74(9):1223–30. doi: 10.1097/00007890-200211150-00005. [DOI] [PubMed] [Google Scholar]
- 40.Gänger KH, Mettler D, Höflin F, et al. Experimental pancreaticosplenic composite transplantation in the pig. Operative technique and assessment of graft function. Eur Surg Res. 1987;19(5):323–8. doi: 10.1159/000128717. [DOI] [PubMed] [Google Scholar]
- 41.Shokouh-Amiri MH, Rahimi-Saber S, Andersen HO, Jensen SL. Pancreas autotransplantation in pig with systemic or portal venous drainage. Effect on the endocrine pancreatic function after transplantation. Transplantation. 1996;61(7):1004–9. doi: 10.1097/00007890-199604150-00003. [DOI] [PubMed] [Google Scholar]
- 42.Schröder T, Rämö OJ, Joffe SN. Laser pancreatectomy. A comparison between dog and pig. Res Exp Med (Berl) 1988;188(3):227–33. doi: 10.1007/BF01852324. [DOI] [PubMed] [Google Scholar]
- 43.Zhang ZD, Han FH, Meng LX. Establishment of a pig model with enteric and portal venous drainage of pancreatoduodenal transplantation. World J Gastroenterol. 2005;11(35):5475–9. doi: 10.3748/wjg.v11.i35.5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gäbel H, Brynger H, Heding L, Säve-Söderbergh J, Wedel N, Lundholm K. Pancreas transplantation in streptozotocin-diabetic juvenile pigs. Evaluation of function among duct-ligated, duct-occluded, and nonligated allografts. Transplantation. 1983;36(6):609–14. [PubMed] [Google Scholar]
- 45.Swindle M. Technical Bulletin. Comparative anatomy of the pig. www.sinclairresearch.com.
- 46.Pitkäranta P, Kivisaari L, Nordling S, Saari A, Schröder T. Experimental chronic pancreatitis in the pig. Scand J Gastroenterol. 1989;24(8):987–92. doi: 10.3109/00365528909089245. [DOI] [PubMed] [Google Scholar]