Abstract
The neurotrophin (NT) hypothesis proposes that the obstruction of retrograde transport at the optic nerve head results in the deprivation of neurotrophic support to retinal ganglion cells (RGC) leading to apoptotic cell death in glaucoma. An important corollary to this concept is the implication that appropriate enhancement of neurotrophic support will prolong the survival of injured RGC indefinitely. This hypothesis is, perhaps, the most widely recognized theory to explain RGC loss resulting from exposure of the eye to elevated intraocular pressure (IOP). Recent studies of NT signaling using rat glaucoma models, have examined the endogenous responses of the retina to pressure exposure as well as studies designed to augment NT signaling in order to rescue RGC from apoptosis following pressure-induced injury. The examination of these studies in this review reveals a number of consistent observations and provides direction for further investigations of this hypothesis.
Keywords: glaucoma, intraocular pressure, neurotrophic factors, TRK receptors, p75 neurotrophin receptor
1. Introduction
The retinal pathology of glaucoma is characterized by selective RGC loss that is attributed to cell death by apoptosis (Quigley et al. 1995; Kerrigan et al. 1997; Quigley 1999). The specific downstream signals and effector molecules involved in RGC apoptosis have been the subject of intense investigation and scientific review (Nickells 1999; Farkas & Grosskreutz 2001; Huang et al. 2005; Libby et al. 2005; Nickells 2007), and will not be considered in detail here. However, the key upstream signals that trigger the apoptotic cascade in RGC in glaucoma are less clear and deciphering them is the main subject of this review.
Elevated IOP is the most recognized risk factor for primary open-angle glaucoma. Studies in primates demonstrate that experimentally elevated IOP results in axonal transport obstruction at the optic nerve head (Minckler et al. 1977; Minckler et al. 1978). When IOP is acutely elevated in rats, the retrograde transport of radiolabeled brain-derived neurotrophic factor (BDNF), a potent trophic factor for RGC, is obstructed (Quigley et al. 2000). Further, immunolocalization studies suggest that the BDNF receptor, TRKB, accumulates in the optic nerve head (Pease et al. 2000). These observations support the suggestion that transport obstruction inhibits the retrograde delivery of NT-TRK receptor complexes from the brain to the RGC soma, resulting in the deprivation of neurotrophic support that triggers apoptosis (Quigley 1995; Pease et al. 2000; Quigley et al. 2000; Vrabec & Levin 2007). An important implication of this concept is the supposition that the appropriate therapeutic manipulation of the NT signaling pathways will indefinitely prolong the survival of injured RGC. These closely related concepts are referred to as the NT hypothesis and will be examined in this review.
2. NT and RGC Survival
This hypothesis is attractive because NT are known to promote neuronal survival and regeneration in many experimental paradigms. When deprived of retrograde neurotrophic support from target neurons, developing sensory and sympathetic neurons die by apoptosis (Purves 1988; Oppenheim 1991). The application of exogenous BDNF to the superior colliculus reduces developmental cell RGC death (Raff et al. 1993; Frade et al. 1997; Ma et al. 1998). NT have important survival properties for adult neurons as well. The application of exogenous NT, especially BDNF, to isolated RGC prolongs their survival in culture (Johnson et al. 1986; Rodriguez-Tebar et al. 1989; Cohen-Cory & Fraser 1994; Meyer-Franke et al. 1995; Rohrer et al. 2001). Importantly, multiple studies indicate that the intravitreal injection of BDNF prolongs injured, adult RGC survival in vivo (Mey & Thanos 1993; Mansour-Robaey et al. 1994; Peinado-Ramon et al. 1996; Di Polo et al. 1998; Chen & Weber 2001).
BDNF is a member of the nerve growth factor family of NT, which also includes nerve growth factor (NGF), NT3, and NT4/5. These NT exert their effects by binding to either specific tyrosine kinase (TRK) receptors or the P75NT receptor (P75NTR), forming what are generally understood to be survival and death signaling complexes, respectively. The specific TRK/NT partners are: TRKA/NGF, TRKB/BDNF, TRKB/NT4/5 and TRKC/NT3. TRK receptor activation of downstream survival signaling pathways include both extracellular signal-regulated kinases (ERK) and AKT. All four NT bind to P75NTR and, while P75NTR signaling is complex, it generally includes the downstream activation of JUN N-terminal kinases (JNK) and is coupled to the mitochondrial apoptotic pathway (Dhanasekaran & Reddy 2008).
Importantly, NT are locally produced in the retina (Ugolini et al. 1995; Cui et al. 2002; Vecino et al. 2002; Spalding et al. 2004; Seki et al. 2005). Therefore, while increased IOP may prevent the retrograde transport of NT-receptor complexes, endogenous retinal sources could provide adequate compensation. The activity and physiological roles of NT from various sources for the RGC is still lacking and, additionally, NT can have different effects on somal and axonal compartments (Kimpinski et al. 1997; Toma et al. 1997; Kuruvilla et al. 2000).
While the pro-survival role of TRK receptor-mediated NT signaling is well-established and P75NTR can signal cell apoptosis (Miller & Kaplan 2001), studies have continued to reveal diverse, sometimes opposing, roles for both ligands and receptors, as well as to identify new signaling partners (Kalb 2005). Maximal activation and substrate specificity for NGF is achieved when TRKA is co-expressed with P75NTR; suggesting that a high specificity receptor complex of TRK, P75NTR and NT is formed (He & Garcia 2004; Nykjaer et al. 2005; Wehrman et al. 2007) or degradation of the activated receptor is inhibited (Makkerh et al. 2005). Paradoxically, P75NTR can promote neuronal survival via nuclear factor kappa B signaling (NFκB) (Hamanoue et al. 1999; Mamidipudi et al. 2002) and TRK receptor activation has been found to induce neuronal death under certain circumstances (Kalb 2005). Another layer of complexity is added by the discovery that proNT, the precursors of mature NT, can be secreted and serve as death signals in complex with P75NTR and sortilin (Lee et al. 2001; Teng et al. 2005).
While studies show that NT administration can slow the rate of RGC loss following injury, so far NT augmentation strategy has resulted in temporary, rather than permanent, cell survival (Clarke et al. 1998; Di Polo et al. 1998; Isenmann et al. 1998; Bahr 2000; Leaver et al. 2006), perhaps due to a reduced availability of NT receptors in injured RGC (Cheng et al. 2002; Chen & Weber 2004). Sustained RGC survival remains the goal of neuroprotective strategies based on the NT hypothesis. In addition, as appealing as the NT hypothesis may be, it does fail to take into account some potentially important observations. First, BDNF knock-out mice have been shown to have normal axon counts, illustrating that while exogenous BDNF may suppress developmental neuronal loss, an endogenous supply is not essential for RGC survival (Cellerino et al. 1997). Additionally, survival and axon extension of RGC in vitro has been shown to require multiple growth factors as well as the elevation of intracellular cyclic AMP and electrical activity (Meyer-Franke et al. 1995; Goldberg et al. 2002), implying that complex signaling relationships are necessary for cell survival and function in vivo. Finally, while the administration of NT can result prolonged RGC survival, there is also evidence that exogenous NT also can downregulate TRK receptor expression levels in the neural cells and tissues, including the retina (Frank et al. 1997; Knusel et al. 1997; Sommerfeld et al. 2000; Chen & Weber 2004; Gibbons & Bailey 2005).
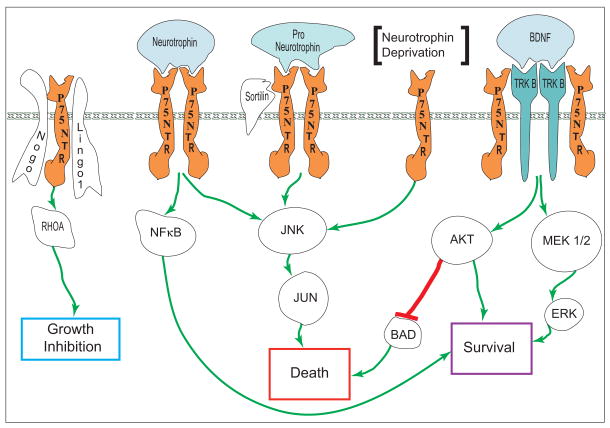
Figure 1 offers a simplified summary of the NT signaling pathways that are mentioned in this review.
Figure 1.
A simplified summary of the NT signaling pathways discussed in this review. NT mediated RGC survival signals are principally mediated via AKT and ERK, while P75NTR is involved in multiple pathways culminating in apoptotic RGC death. However, P75NTR interaction with TRK receptors appears necessary for high affinity ligand binding and P75NTR signaling mediated via NFκB promotes cell survival. Further, P75NTR has plays a key role in RhoA-mediated axon outgrowth inhibition.
3. Modeling Glaucoma in Laboratory Rats
With these concepts in mind, experimental glaucoma models in rats have been used to explore aspects of the NT hypothesis. These models all rely on the production of elevated IOP and their characteristics and suitability have been reviewed (Danias et al. 2002; Levkovitch-Verbin 2004; Morrison et al. 2005; Pang & Clark 2007; Morrison et al. 2008a). While many variations in how the models are modified and implemented exist, there are basically three techniques commonly used to elevate IOP. These are referred to as the laser, hypertonic saline and cautery models, designations that will be used throughout this review.
In the laser model, the limbus and underlying trabecular meshwork are damaged by multiple laser burns (Ueda et al. 1998; WoldeMussie et al. 2001; Levkovitch-Verbin et al. 2002). In the hypertonic saline model, a method originated in our laboratory, the trabecular meshwork and Schlemm’s canal are sclerosed by the retroinjection of hypertonic saline into the isolated limbal plexus via a tiny episcleral vein (Morrison et al. 1997). In both models, damage is to the aqueous outflow pathways and results in outflow obstruction (Morrison et al. 1997; Levkovitch-Verbin et al. 2002; Nissirios et al. 2008). This is important because aqueous outflow obstruction is a principle characteristic of human primary open angle glaucoma (Tamm & Fuchshofer 2007).
In addition, both methods result in IOP elevation up to approximately twice normal levels, producing a roughly equivalent range of optic nerve axon and RGC loss (up to 90%) over a period of weeks (Morrison et al. 2005). In the hypertonic saline model, pressure elevation is generally delayed until several days following the injection and multiple injections may be necessary to produce stable IOP elevation. We find that the pattern and magnitude of pressure elevation varies among animals. In the laser model, pressure is rapidly elevated following treatment but tends to normalize after a period of a few weeks, requiring a second laser treatment if sustained elevation is desired.
In the other commonly used method, a number of larger veins located just posterior to the rectus muscle insertions are cauterized, apparently reducing venous outflow from the globe and producing ocular venous congestion (Grozdanic et al. 2003; Morrison et al. 2005). These veins have been identified as either episcleral or vortex veins (Shareef et al. 1995; Grozdanic et al. 2003). The method results in a similar degree of IOP elevation as the others. However, in the cautery model, the injury progresses at a slower, linear rate (Garcia-Valenzuela et al. 1995; Sawada & Neufeld 1999; Ko et al. 2001), reaching a maximum of about 40% in 10 to 28 weeks. Axonal degeneration in this model has not been well characterized, but also appears to be significantly less than in the laser or hypertonic saline models (Shareef et al. 1999; Grozdanic et al. 2003; Wang et al. 2004) and aqueous outflow may not be significantly obstructed (Nissirios et al. 2008).
In all methods, careful documentation of IOP is necessary to allow pressure dose comparisons between experimental groups and correlation of responses to IOP history. The equipment and techniques available for IOP measurement in rodents have been recently reviewed (Morrison et al. 2005; Pang et al. 2005). Two important factors that impact IOP are frequently ignored when pressure data is collected. The use of common anesthetics produces an unpredictable lowering of IOP in rats (Jia et al. 2000b). Additionally, there is a 10 mm Hg circadian range of IOP in rats, peaking during the dark phase. In glaucoma model eyes with normal light phase IOP measurements, this dark phase peak may be doubled and result in axonal degeneration (Jia et al. 2000a). IOP measurements in conventionally housed rats should be obtained during both light and dark phases, or alternatively, housing in constant, low-level light will stabilize the circadian variations without producing retinal injury (Jia et al. 2000a; Morrison et al. 2005). Ignoring the effects of circadian rhythms and anesthesia is likely to lead to a significant underestimation of the actual IOP experienced by the eye.
4. Endogenous NT Function In Rat Glaucoma Models
4.1 Endogenous NT And Receptor Levels
Using the hypertonic saline model and immunohistochemistry, we examined the sequence of changes in retinal and optic nerve head protein distribution that accompany exposure to elevated IOP (Johnson et al. 2000). At IOP levels approximately twice normal values for one week, immunostaining for BDNF and NT4/5 was reduced in the optic nerve head and the inner layers of the retina, changes that were accompanied by evidence of optic nerve axon degeneration. After longer exposures, retinas contained scattered individual RGC layer somas that were strongly labeled by NT antibodies, suggesting upregulation of NT expression in some injured RGC. RGC layer apoptosis was increased at all time points, even in retinas with no immunohistochemical evidence of altered NT distribution. Still, the relatively early loss of retinal NT immunostaining offers some support for the hypothesis that NT deprivation contributes to apoptotic RGC loss.
Rudzinski and colleagues, using the vein cautery model, evaluated the effect of elevated IOP on endogenous retinal NT and NT receptors at time points up to 28 days post surgery (Rudzinski et al. 2004). Retinal BDNF mRNA levels remained relatively unchanged until day 28, when they approximately doubled, NT4/5 was unchanged, NT3 declined slightly and NGF peaked at about two-fold at 14 days, then declined to control levels at 28 days. Receptor message level analysis indicated that TRKB remained constant during the experimental period, while TRKC mRNA levels were significantly increased to approximately 300%. This TRKC increase was due to the upregulation of truncated, inactive TRKC isoforms, while levels of the active, full-length form remained unchanged. TRKA levels increased slowly, reaching significance at about 2.3 fold at day 28 while increases in P75NTR levels were not significant. By western analysis, BDNF, NGF, NT3 and TRKA protein levels paralleled message levels, while levels of TRKB and TRKC were not determined. Fluorescent co-localization studies indicated that increases in TRKA occurred in RGC, while the increased, inactive TRKC was localized to RGC layer glia. From this study, the authors concluded that elevated IOP induces time-dependent changes in retinal NT and receptor levels that are related to the stress and consequent death of RGC. Their study also confirms the linear rate of RGC loss in this model and also suggests that the observed fluctuations have negligible impact on the rate of apoptosis. Further, levels of NT and their receptors are generally increased, providing little evidence that elevated IOP results in RGC NT deprivation. In contrast, these observations illustrate the potential importance of NT-receptor signaling other than that of BDNF-TRKB.
Also using the cautery model, Kim et al (Kim et al. 2007) found that elevated IOP resulted in elevated retinal BDNF message levels that peaked over four times control levels at 1 week, then declined to approximately twice control levels at 4 and 8 weeks. While the pattern of BDNF response was different from that found by Rudzinski et al., this study also found an overall increase in BDNF mRNA levels in glaucoma model retinas.
In a recent study, Coassin et al (Coassin et al. 2008), used the hypertonic saline model to produce eyes with at least 20% IOP elevation. They found a gradual increase in retinal NGF protein by ELISA, reaching significance at 35 days post-injection, coinciding with a significant increase in the number of apoptotic RGC. They also detected a gradual increase in NGF mRNA expression and, at 35 days, decreased ratios for TRKA relative to P75NTR. From these observations, they concluded that NGF is overexpressed in experimental glaucoma, but not to an extent sufficient to support RGC survival, perhaps due to the relatively greater upregulation of P75NTR. Again, this study illustrates the potential importance of multiple NT signaling pathways to RGC survival.
Over the last few years, we have also investigated in vivo alterations in retinal NT and receptor mRNA and protein levels using our hypertonic saline glaucoma model, with preliminary findings published in abstract form (Jia et al. 2004; Cepurna et al. 2007). In contrast to some previous findings, our completed studies [manuscript currently under review at EER] indicate that while retinal BDNF mRNA and mature protein levels are not significantly altered, there are moderate alterations in receptor expression. Message levels for TRKB decrease and P75NTR increase linearly, reaching values in the most injured retinas that are 67% (p<0.01) and 210% (p<0.001) of controls, respectively. However, regardless of these message changes, the activated, pro-survival form of TRKB protein actually demonstrates a slight, but significant, linear increase with increasing nerve injury (p<0.03), while P75NTR protein levels are not significantly altered.
In summary, these studies offer no more than scant support for the NT hypothesis. Rather, they suggest that multiple NT signaling pathways are activated in pressure-induced RGC injury, implying complex cellular responses.
4.2 Alterations In Endogenous NT Survival And Apoptosis Signaling Pathway Intermediates
Neuronal injury is linked to induction of the JUN transcription factor and its prolonged phosphorylation by JNK is linked to apoptosis (Oppenheim et al. 1990; Koistinaho et al. 1993; Estus et al. 1994; Tournier et al. 2000; Lei et al. 2002). Therefore, using the laser model, Levkovitch-Verbin and colleagues examined the time course of retinal immunostaining for JUN and activated pJUN following IOP elevation (Levkovitch-Verbin et al. 2005). They found immunolabeling for both peaked at 1 week following IOP elevation and simultaneously retinal levels of pJUN protein were increased by approximately 60%. While JUN activation is associated with RGC death by apoptosis, it also is implicated in regenerative and survival responses (Hull & Bahr 1994; Robinson 1994; Schaden et al. 1994; Lu et al. 2003), supporting multiple roles for the transcription factor following retinal injury.
Using the laser model, Kwong and Caprioli also examined the correlation between RGC layer apoptosis labeling and activated JNK labeling of RGC in eyes with IOP levels at about 150% (Kwong & Caprioli 2006). By immunolabeling, they found that the number of activated JNK-labeled RGC layer cells doubled at 5-weeks in glaucoma model retinas. Retrograde fluorescent labeling identified some of these cells as RGC and some apoptotic cells were positive for activated JNK. These observations support the association of JNK activation with the process of RGC apoptosis.
Kim and Park used the cautery model to examine activation of NT survival pathway signaling at time points up to 6 weeks (Kim & Park 2005). They also confirmed the gradual, steady loss of RGC in this model, with a 7% loss at two weeks and 30% at 6 weeks. Using immunohistochemistry and western analysis, they examined TRK-activated signaling via AKT that results in the phosphorylation of BAD (pBAD), inhibiting its pro-apoptotic activity. They found that levels of activated AKT and pBAD peaked at 1 week post-surgery, before falling to levels below control values by 6 weeks, suggesting that this pro-survival pathway response is short lived.
They also found evidence of activation of the extrinsic (caspase 8) signaling pathway, involving FAS ligand and FAS Associated Death Domain (FADD). Generally, this pathway is associated with immune and cell mediated toxicity, but it also may be activated via the intrinsic (mitochondrial) caspase pathway (Weishaupt et al. 2003). Kim and Park found elevated levels of pro-apoptotic FAS ligand, FADD, and activated caspase 8 at all time points following cautery. By fluorescent retrograde labeling and immunohistochemistry, they showed co-localization of these proteins to RGC. In a follow-up study, they found that FAS ligand colocalized to microglia and FADD to both Muller cells and RGC, suggesting potential glial participation in the extrinsic caspase activation process (Ju et al. 2006). Because pro-survival events tended to peak earlier than some of the pro-apoptotic ones, Kim and Park concluded that the former delayed RGC death, although they did find apoptotic RGC even during peak pro-survival signaling.
Levkovitch-Verbin et al followed up their previous study by an examination of both apoptotic and survival pathway activation in the laser model (Levkovitch-Verbin et al. 2007). Prior to peak levels of apoptotic caspase 3 activation at day 15, they found peak expression of activated pro-apoptotic p38 as well as pro-survival ERK1 and AKT. While IOP levels returned to normal values at about 3 weeks, active caspase 3 remained at very high levels up to 30 days and JNK levels peaked at the same time, suggesting prolonged retinal pro-apoptotic responses following IOP normalization.
The upregulation of caspase pathways in experimental glaucoma was further studied by Huang et al using the hypertonic saline model (Huang et al. 2005). In eyes with 10 days of elevated IOP, they used laser capture microdissection to select retrograde-labeled RGC and quantitative RT-PCR to determine message levels. They demonstrated upregulated levels of both caspase 8 and 9 mRNA, initiators of the extrinsic and intrinsic (mitochondrial) caspase cascades, respectively. Simultaneously, the number of retrograde labeled RGC had fallen by 34%. Western analysis showed increased levels of activated caspases 8 and 9 proteins in glaucoma model retinas. The activation of caspase 8 adds to the evidence that death signaling pathways other than those regulated by P75NTR play a role in RGC loss in glaucoma.
5. Neuroprotection Strategies Designed to Rescue Injured RGC
While our knowledge of the early responses of RGC to pressure-induced injury is still very incomplete, glaucoma models have been implemented in a number of studies designed to support RGC survival during and after pressure-induced injury.
5.1 Neuroprotection by Application of Exogenous NT
Using the cautery model, Ko and colleagues examined the effect of multiple intravitreal BDNF injections on RGC survival (Ko et al. 2000). RGC were retrograde labeled and IOP elevated to sustained values of about twice baseline. Without BDNF treatment, RGC loss after 37 days was 27%. Four sequential injections of BDNF significantly reduced this loss to 19% and augmentation of BDNF with a systemic free-radical scavenger limited the cell loss to 10%. As a follow up to this study, a time course of RGC survival with BDNF treatment alone was determined (Ko et al. 2001). At 33 and 47 days following IOP elevation, vehicle treatment produced 16% and 27% RGC loss, while BDNF significantly decreased this loss to 9% and 17% at the same respective time points.
Martin et al implemented a gene therapy strategy to sustain BDNF availability to injured RGC (Martin et al. 2003). Two weeks prior to IOP elevation by laser treatment, they intravitreally injected a modified viral vector transgene that increased retinal BDNF levels by four fold. Then, at four weeks following IOP elevation, they evaluated RGC survival by counting morphologically intact axons in the optic nerve. They found that axon loss in the BDNF-treated group was 32%, significantly less than the 52% loss found in untreated glaucoma model nerves.
Both these studies lend support to previous observations indicating that application of exogenous BDNF can prolong the survival of injured RGC.
5.2 Neuroprotection by Alteration of NT Receptor Activation
Recently, Shi et al., hypothesized that the pharmacologic activation of TRKA or inhibition of P75NTR might augment the protective effect of therapeutic IOP reduction in glaucoma model eyes (Shi et al. 2007). Using the cautery model, they produced a 1.7 fold IOP elevation over 4 months. RGC loss in untreated cauterized eyes was 16% at 3 weeks and 35% at 6 weeks. Daily betaxolol following two weeks of IOP elevation effectively normalized IOP, similar to previous observations (Morrison et al. 1998; Rudzinski et al. 2004). In betaxolol-treated glaucoma model eyes, RGC loss was 7% at 3 weeks and 20% at 6 weeks, demonstrating that IOP normalization reduced, but did not eliminate, RGC loss.
To determine if residual cell losses could be prevented by TRKA activation, the authors used dual sequential intravitreal injections of a TRKA agonist, with and without betaxolol pressure normalization. With the TRKA agonist alone, the RGC loss was reduced, but with betaxolol and the TRKA agonist, the cell loss was reduced to only 11% at 6 weeks, a value is not significantly different from the loss after initial two weeks of pressure elevation. This suggested that the combination of IOP normalization and TRKA activation successfully prevented the continuing phase of RGC loss following the initial IOP insult. Administration of NGF alone did not provide this protection. Shi and colleagues also used a similar set of experiments to determine if a P75NTR antagonist might be neuroprotective, but no alteration in response was found. Together, these findings suggest that pharmacological activation of TRKA may promote adult RGC survival in eyes with previous pressure-induced injury and provide further evidence for multiple trophic factor signaling pathways in RGC survival.
In an alternative approach to inhibiting P75NTR signaling, Fu and colleagues, hypothesized that blocking the function of a P75NTR signaling partner, Lingo1, would be neuroprotective to injured RGC (Fu et al. 2008). Lingo1-P75NTR signaling was originally associated with the inhibition of axonal regeneration (Mi et al. 2004), but it may be important to note that Lingo-1 has other signaling partners in addition to P75NTR (Zhao et al. 2008). Dual laser treatments were used to produce sustained IOP elevation to approximately twice normal levels. Without additional treatment, Lingo1 protein levels increased approximately 1.6 fold and RGC loss was approximately 13% at 2 weeks and 20% at 4 weeks. When Lingo1 function was blocked by intravitreal injection of either soluble Lingo1 or anti-Lingo1 antibody, this loss was successfully eliminated. Further, blocking Lingo1 was found to reduce pro-apoptotic RHOA GTPase, reduce JNK activation and sustain pro-survival AKT signaling in glaucoma model retinas (Dubreuil et al. 2003). Together these findings suggest that Lingo1-related signaling plays a more important role in RGC death than previously appreciated.
5.3 Neuroprotection by Enhancement Of Downstream NT Survival Signaling Pathways
ERK1/2, a common downstream kinase activated by many trophic factors, plays a key role in RGC survival (Cheng et al. 2002). Therefore, Zhou et al hypothesized that stable activation of ERK1/2 would protect RGC in a glaucoma model (Zhou et al. 2005). To test this, they used the hypertonic saline model and gene transfer of the immediate upstream kinase (MEK1) responsible for ERK1/2 activation. Initially, they demonstrated that transfection with constitutively active MEK1 (CA-MEK1) resulted in a significant increase in levels of activated ERK1/2. Then, they labeled RGC with a retrograde tracer and generated CA-MEK1, wild-type MEK1 (WT) and other control groups. Next, they used the hypertonic saline method to produce groups of rats with consistent IOP histories within and between groups. Finally, they examined RGC survival following 5 and 7 weeks of ocular hypertension. At both durations, they found significant RGC protection in the CA-MEK1 group, with approximately 50% greater RGC survival than in the WT group. They also found approximately 50% greater axon survival in the CA-MEK1 group. This data supported their conclusion that prolonged Erk1/2 pathway activation is neuroprotective to injured RGC.
6. Roles for Additional Trophic Factors in RGC Survival
In addition to members of the NGF family, other trophic factors may contribute to RGC survival by signaling via AKT and/or ERK kinases. Using the cautery model to initially double IOP levels, Kanamori and colleagues found a slow decline to baseline IOP at about three months and a 35% RGC loss at six months (Kanamori et al. 2004). They examined retinal immunostaining patterns for total and activated forms of AKT in flat-mounted retinas during that time course. Labeling for activated AKT was absent in the normal retina, but peaked at three days in glaucoma model retinas and was localized, in part, to RGC and their processes. The authors had anticipated a lower level of active AKT due to inhibited retrograde transport of NT-TRK complexes. This unexpected observation led them to hypothesize that signaling via the separate insulin-like growth factor (IGF)/IGF receptor signaling pathway might lead to the activation of the AKT pro-survival pathway in the glaucomatous retinas. By western analysis, they found significantly increased activated forms of both IGF receptor and AKT at three days following IOP elevation, supporting their conclusion that endogenous retinal signaling via IGF may provide an early, although ultimately inadequate, neuroprotective response to RGC injury.
A potential survival enhancing role for glial cell line-derived neurotrophic factor (GDNF) was investigated by Jiang et al recently using the hypertonic saline model (Jiang et al. 2007). GDNF also signals via pathways distinct from TRK or P75NTR. After showing that biodegradable microspheres persist in the vitreous for at least 6 weeks, they injected GDNF and control microspheres at one week after an initial hypertonic saline injection. At eight weeks following a second hypertonic saline injection, retinas and optic nerves were collected and analyzed by immunohistochemistry and histology. In this study, pressure levels rose gradually, stabilizing at 3 weeks at about twice normal values in all experimental groups. Immunolabeling for GDNF and its receptors showed that these proteins were localized, in part, to RGC. In retinas treated with GDNF spheres, the authors reported decreased nerve head cupping, increased nerve fiber layer thickness and significantly increased inner plexiform layer thickness. RGC layer neuronal and axonal survival was significantly increased by about 50%, while glial activation appeared less. These observations led the authors to conclude that sustained GDNF delivery offers significant neuroprotection to injured RGC.
In studies using the laser model, Ji and colleagues examined neuroprotection following intravitreal ciliary neurotrophic factor (CNTF) injection (Ji et al. 2004). In control glaucoma model eyes, RGC loss was 13% at two weeks and 22% at four weeks. In these eyes, immunostaining for CNTF remained constant in the RGC layer, but increased in the inner plexiform layer. Activation of the JAK/STAT signaling pathway was indicated by an increase in immunolabeled pSTAT3 in both layers during the first days of IOP elevation. RGC loss was significantly less in CNTF treated eyes, -7% at 2 weeks and 5% at 4 weeks. Western analysis demonstrated that CNTF injections prolonged the retinal activation of pSTAT, although the localization of this increase was not reported. The authors concluded that the pSTAT increase was correlated with the protective effect of CNTF on injured RGC.
7. Conclusions
The NT hypothesis is, perhaps, the most widely recognized theory proposed to explain RGC loss due to elevated IOP and, importantly, it incorporates axonal injury at the optic nerve head as a key component. The currently available rat glaucoma models offer the opportunity to examine this hypothesis in a paradigm in which only the risk factor of IOP is altered. As summarized in this review, recent studies of NT signaling using these in vivo models have encompassed both the endogenous responses of the retina to pressure exposure as well as experimental strategies to rescue RGC from apoptosis following pressure-induced injury.
The use of multiple models, variations in their application, small sample sizes, different qualitative and quantitative analysis techniques, as well as the limited scope and duration of many studies undoubtedly impact the conclusions that can be drawn and, perhaps, result in apparent contradictions. Another important limitation is that, in most studies, RGC responses were not isolated from those of other potentially affected retinal cells. However, there are some general observations that have a degree of consistency. First, experimental support for the assumption that RGC are deprived of neurotrophic support before they become committed to apoptosis is quite limited (Johnson et al. 2000; Quigley et al. 2000), particularly considering the ample evidence that NT and other trophic factors are produced endogenously in the retina (Ugolini et al. 1995; Cui et al. 2002; Vecino et al. 2002; Rudzinski et al. 2004; Spalding et al. 2004; Seki et al. 2005; Coassin et al. 2008). Secondly, while multiple NT and other trophic factor signaling pathways contribute to RGC well being, elevated IOP exposure appears to trigger multiple pro-apoptotic signaling pathways as well (Kim & Park 2005; Levkovitch-Verbin et al. 2007; Fu et al. 2008). Thirdly, pro-survival and pro-apoptotic NT signaling events demonstrate dynamic inter-relationships (Rudzinski et al. 2004; Kim & Park 2005; Levkovitch-Verbin et al. 2007; Coassin et al. 2008). While our knowledge and understanding of the process initiated by elevated IOP exposure is fragmented and incomplete, efforts to inhibit pro-apoptotic signaling have been limited and deserve further attention. It is important to note that the only effective long-term survival strategy for RGC soma in an experimental glaucoma model so far is the knockout of the pro-apoptotic BAX gene in DBA/2J mice (Libby et al. 2005). Additionally, multiple studies suggest that while initial pro-survival signals are short-lived and inadequate, prolonging and intensifying these signals can delay RGC loss (Ko et al. 2000; Cheng et al. 2002; Kanamori et al. 2004; Jiang et al. 2007).
Finally, although experimental support for the NT hypothesis is limited, the theory still provides the best explanation of how axonal injury at the optic nerve head results in RGC death in glaucoma. Therefore, further examination of the theory and its implications is essential, particularly investigations utilizing experimental animal glaucoma models and evolving microanalysis techniques, such as laser capture microdissection and microproteomics, to more precisely determine in vivo RGC responses to glaucomatous insults (Huang et al. 2004; Huang et al. 2006; Zhang et al. 2006; Guo et al. 2008; Gutstein et al. 2008; Morrison et al. 2008b).
Acknowledgments
Supported by NIH grants EY010145, EY016866 and an unrestricted grant from Research to Prevent Blindness, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bahr M. Live or let die - retinal ganglion cell death and survival during development and in the lesioned adult CNS. Trends Neurosci. 2000;23(10):483–90. doi: 10.1016/s0166-2236(00)01637-4. [DOI] [PubMed] [Google Scholar]
- Cellerino A, Carroll P, Thoenen H, Barde YA. Reduced Size of Retinal Ganglion Cell Axons and Hypomyelination in Mice Lacking Brain-Derived Neurotrophic Factor. Mol Cell Neurosci. 1997;5:397–408. doi: 10.1006/mcne.1997.0641. [DOI] [PubMed] [Google Scholar]
- Cepurna WO, Johnson EC, Jia L, Morrison JC. Retinal Ganglion Cell Loss in a Glaucoma Model: Does Neurotrophin Deprivation Play a Critical Role? Invest. Ophthalmol Vis Sci. 2007;48(5):3276. [Google Scholar]
- Chen H, Weber AJ. BDNF enhances retinal ganglion cell survival in cats with optic nerve damage. Invest Ophthalmol Vis Sci. 2001;42(5):966–74. [PubMed] [Google Scholar]
- Chen H, Weber AJ. Brain-derived neurotrophic factor reduces TrkB protein and mRNA in the normal retina and following optic nerve crush in adult rats. Brain Res. 2004;1011(1):99–106. doi: 10.1016/j.brainres.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Cheng L, Sapieha P, Kittlerova P, Hauswirth WW, Di Polo A. TrkB gene transfer protects retinal ganglion cells from axotomy-induced death in vivo. J Neurosci. 2002;22(10):3977–86. doi: 10.1523/JNEUROSCI.22-10-03977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke DB, Bray GM, Aguayo AJ. Prolonged administration of NT-4/5 fails to rescue most axotomized retinal ganglion cells in adult rats. Vision Res. 1998;38(10):1517–24. doi: 10.1016/s0042-6989(97)00341-6. [DOI] [PubMed] [Google Scholar]
- Coassin M, Lambiase A, Sposato V, Micera A, Bonini S, Aloe L. Retinal p75 and bax overexpression is associated with retinal ganglion cells apoptosis in a rat model of glaucoma. Graefes Arch Clin Exp Ophthalmol. 2008;246(12):1743–9. doi: 10.1007/s00417-008-0913-5. [DOI] [PubMed] [Google Scholar]
- Cohen-Cory S, Fraser SE. BDNF in the development of the visual system of Xenopus. Neuron. 1994;12(4):747–61. doi: 10.1016/0896-6273(94)90328-x. [DOI] [PubMed] [Google Scholar]
- Cui Q, Tang LS, Hu B, So KF, Yip HK. Expression of trkA, trkB, and trkC in injured and regenerating retinal ganglion cells of adult rats. Invest Ophthalmol Vis Sci. 2002;43(6):1954–64. [PubMed] [Google Scholar]
- Danias J, Shen F, Goldblum D, Chen B, Ramos-Esteban J, Podos SM, Mittag T. Cytoarchitecture of the retinal ganglion cells in the rat. Invest Ophthalmol Vis Sci. 2002;43(3):587–94. [PubMed] [Google Scholar]
- Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27(48):6245–51. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Polo A, Aigner LJ, Dunn RJ, Bray GM, Aguayo AJ. Prolonged delivery of brain-derived neurotrophic factor by adenovirus- infected Muller cells temporarily rescues injured retinal ganglion cells. Proc Natl Acad Sci U S A. 1998;95(7):3978–83. doi: 10.1073/pnas.95.7.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil CI, Winton MJ, McKerracher L. Rho activation patterns after spinal cord injury and the role of activated Rho in apoptosis in the central nervous system. J Cell Biol. 2003;162(2):233–43. doi: 10.1083/jcb.200301080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estus S, Zaks WJ, Freeman RS, Gruda M, Bravo R, Johnson EM., Jr Altered gene expression in neurons during programmed cell death: identification of c-jun as necessary for neuronal apoptosis. J Cell Biol. 1994;127(6 Pt 1):1717–27. doi: 10.1083/jcb.127.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas RH, Grosskreutz CL. Apoptosis, neuroprotection, and retinal ganglion cell death: an overview. Int Ophthalmol Clin. 2001;41(1):111–30. doi: 10.1097/00004397-200101000-00011. [DOI] [PubMed] [Google Scholar]
- Frade JM, Bovolenta P, Martinez-Morales JR, Arribas A, Barbas JA, Rodriguez-Tebar A. Control of early cell death by BDNF in the chick retina. Development. 1997;124(17):3313–20. doi: 10.1242/dev.124.17.3313. [DOI] [PubMed] [Google Scholar]
- Frank L, Wiegand SJ, Siuciak JA, Lindsay RM, Rudge JS. Effects of BDNF infusion on the regulation of TrkB protein and message in adult rat brain. Exp Neurol. 1997;145(1):62–70. doi: 10.1006/exnr.1997.6440. [DOI] [PubMed] [Google Scholar]
- Fu QL, Hu B, Wu W, Pepinsky RB, Mi S, So KF. Blocking LINGO-1 function promotes retinal ganglion cell survival following ocular hypertension and optic nerve transection. Invest Ophthalmol Vis Sci. 2008;49(3):975–85. doi: 10.1167/iovs.07-1199. [DOI] [PubMed] [Google Scholar]
- Garcia-Valenzuela E, Shareef S, Walsh J, Sharma SC. Programmed cell death of retinal ganglion cells during experimental glaucoma. Exp Eye Res. 1995;61(1):33–44. doi: 10.1016/s0014-4835(95)80056-5. [DOI] [PubMed] [Google Scholar]
- Gibbons AS, Bailey KA. BDNF and NT-3 regulation of trkB and trkC mRNA levels in the developing chick spinal cord. Neurosci Lett. 2005;385(1):41–5. doi: 10.1016/j.neulet.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Goldberg JL, Espinosa JS, Xu Y, Davidson N, Kovacs GT, Barres BA. Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron. 2002;33(5):689–702. doi: 10.1016/s0896-6273(02)00602-5. [DOI] [PubMed] [Google Scholar]
- Grozdanic SD, Betts DM, Sakaguchi DS, Kwon YH, Kardon RH, Sonea IM. Temporary elevation of the intraocular pressure by cauterization of vortex and episcleral veins in rats causes functional deficits in the retina and optic nerve. Exp Eye Res. 2003;77(1):27–33. doi: 10.1016/s0014-4835(03)00089-7. [DOI] [PubMed] [Google Scholar]
- Guo Y, Johnson EC, Dyck JA, Cepurna WO, Doser T, Morrison JC. Retinal Ganglion Cell Layer Gene Responses to Elevated Pressure and Progressive Axon Loss in Experimental Glaucoma. Invest Ophthalmol Vis Sci. 2008;49(5):3697. [Google Scholar]
- Gutstein HB, Morris JS, Annangudi SP, Sweedler JV. Microproteomics: analysis of protein diversity in small samples. Mass Spectrom Rev. 2008;27(4):316–30. doi: 10.1002/mas.20161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanoue M, Middleton G, Wyatt S, Jaffray E, Hay RT, Davies AM. p75-mediated NF-kappaB activation enhances the survival response of developing sensory neurons to nerve growth factor. Mol Cell Neurosci. 1999;14(1):28–40. doi: 10.1006/mcne.1999.0770. [DOI] [PubMed] [Google Scholar]
- He XL, Garcia KC. Structure of nerve growth factor complexed with the shared neurotrophin receptor p75. Science. 2004;304(5672):870–5. doi: 10.1126/science.1095190. [DOI] [PubMed] [Google Scholar]
- Huang W, Fileta J, Guo Y, Grosskreutz CL. Downregulation of Thy1 in retinal ganglion cells in experimental glaucoma. Curr Eye Res. 2006;31(3):265–71. doi: 10.1080/02713680500545671. [DOI] [PubMed] [Google Scholar]
- Huang W, Dobberfuhl A, Ingelsson M, Filippopoulos T, Grosskreutz CL. Detection of gene expression changes of caspase 8 and Thy1 in rat retinal ganglion cells after optic nerve crush using laser capture microdissection (LCM) with real-time PCR. Investigative Ophthalmology & Visual Science. 2004;45:U787–U787. [Google Scholar]
- Huang W, Dobberfuhl A, Filippopoulos T, Ingelsson M, Fileta JB, Poulin NR, Grosskreutz CL. Transcriptional up-regulation and activation of initiating caspases in experimental glaucoma. Am J Pathol. 2005;167(3):673–81. doi: 10.1016/S0002-9440(10)62042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull M, Bahr M. Regulation of immediate-early gene expression in rat retinal ganglion cells after axotomy and during regeneration through a peripheral nerve graft. J Neurobiol. 1994;25(1):92–105. doi: 10.1002/neu.480250109. [DOI] [PubMed] [Google Scholar]
- Isenmann S, Klocker N, Gravel C, Bahr M. Short communication: protection of axotomized retinal ganglion cells by adenovirally delivered BDNF in vivo. Eur J Neurosci. 1998;10(8):2751–6. doi: 10.1046/j.1460-9568.1998.00325.x. [DOI] [PubMed] [Google Scholar]
- Ji JZ, Elyaman W, Yip HK, Lee VW, Yick LW, Hugon J, So KF. CNTF promotes survival of retinal ganglion cells after induction of ocular hypertension in rats: the possible involvement of STAT3 pathway. Eur J Neurosci. 2004;19(2):265–72. doi: 10.1111/j.0953-816x.2003.03107.x. [DOI] [PubMed] [Google Scholar]
- Jia L, Cepurna WO, Johnson EC, Morrison JC. Patterns of intraocular pressure elevation after aqueous humor outflow obstruction in rats. Invest Ophthalmol Vis Sci. 2000a;41(6):1380–5. [PubMed] [Google Scholar]
- Jia L, Cepurna WO, Johnson EC, Morrison JC. Effect of general anesthetics on IOP in rats with experimental aqueous outflow obstruction. Invest Ophthalmol Vis Sci. 2000b;41(11):3415–9. [PubMed] [Google Scholar]
- Jia L, Cepurna WO, Barber SL, Morrison JC, Johnson EC. Retinal neurotrophin and Trk receptor mRNA expression following elevated intraocular pressure or optic nerve transection. Investigative Ophthalmology & Visual Science. 2004;45:U787–U787. [Google Scholar]
- Jiang C, Moore MJ, Zhang X, Klassen H, Langer R, Young M. Intravitreal injections of GDNF-loaded biodegradable microspheres are neuroprotective in a rat model of glaucoma. Mol Vis. 2007;13:1783–92. [PubMed] [Google Scholar]
- Johnson EC, Deppmeier LM, Wentzien SK, Hsu I, Morrison JC. Chronology of optic nerve head and retinal responses to elevated intraocular pressure. Invest Ophthalmol Vis Sci. 2000;41(2):431–42. [PubMed] [Google Scholar]
- Johnson JE, Barde YA, Schwab M, Thoenen H. Brain-derived neurotrophic factor supports the survival of cultured rat retinal ganglion cells. J Neurosci. 1986;6(10):3031–8. doi: 10.1523/JNEUROSCI.06-10-03031.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju KR, Kim HS, Kim JH, Lee NY, Park CK. Retinal glial cell responses and Fas/FasL activation in rats with chronic ocular hypertension. Brain Res. 2006;1122(1):209–21. doi: 10.1016/j.brainres.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Kalb R. The protean actions of neurotrophins and their receptors on the life and death of neurons. Trends Neurosci. 2005;28(1):5–11. doi: 10.1016/j.tins.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Kanamori A, Nakamura M, Nakanishi Y, Nagai A, Mukuno H, Yamada Y, Negi A. Akt is activated via insulin/IGF-1 receptor in rat retina with episcleral vein cauterization. Brain Res. 2004;1022(1–2):195–204. doi: 10.1016/j.brainres.2004.06.077. [DOI] [PubMed] [Google Scholar]
- Kerrigan LA, Zack DJ, Quigley HA, Smith SD, Pease ME. TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch Ophthalmol. 1997;115(8):1031–5. doi: 10.1001/archopht.1997.01100160201010. [DOI] [PubMed] [Google Scholar]
- Kim HS, Park CK. Retinal ganglion cell death is delayed by activation of retinal intrinsic cell survival program. Brain Res. 2005;1057(1–2):17–28. doi: 10.1016/j.brainres.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Kim HS, Chang YI, Kim JH, Park CK. Alteration of retinal intrinsic survival signal and effect of alpha2-adrenergic receptor agonist in the retina of the chronic ocular hypertension rat. Vis Neurosci. 2007;24(2):127–39. doi: 10.1017/S0952523807070150. [DOI] [PubMed] [Google Scholar]
- Kimpinski K, Campenot RB, Mearow K. Effects of the neurotrophins nerve growth factor, neurotrophin-3, and brain-derived neurotrophic factor (BDNF) on neurite growth from adult sensory neurons in compartmented cultures. J Neurobiol. 1997;33(4):395–410. doi: 10.1002/(sici)1097-4695(199710)33:4<395::aid-neu5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Knusel B, Gao H, Okazaki T, Yoshida T, Mori N, Hefti F, Kaplan DR. Ligand-induced down-regulation of Trk messenger RNA, protein and tyrosine phosphorylation in rat cortical neurons. Neuroscience. 1997;78(3):851–62. doi: 10.1016/s0306-4522(96)00616-1. [DOI] [PubMed] [Google Scholar]
- Ko ML, Hu DN, Ritch R, Sharma SC. The combined effect of brain-derived neurotrophic factor and a free radical scavenger in experimental glaucoma. Invest Ophthalmol Vis Sci. 2000;41(10):2967–71. [PubMed] [Google Scholar]
- Ko ML, Hu DN, Ritch R, Sharma SC, Chen CF. Patterns of retinal ganglion cell survival after brain-derived neurotrophic factor administration in hypertensive eyes of rats. Neurosci Lett. 2001;305(2):139–42. doi: 10.1016/s0304-3940(01)01830-4. [DOI] [PubMed] [Google Scholar]
- Koistinaho J, Hicks KJ, Sagar SM. Long-term induction of c-jun mRNA and Jun protein in rabbit retinal ganglion cells following axotomy or colchicine treatment. J Neurosci Res. 1993;34(2):250–5. doi: 10.1002/jnr.490340213. [DOI] [PubMed] [Google Scholar]
- Kuruvilla R, Ye H, Ginty DD. Spatially and functionally distinct roles of the PI3-K effector pathway during NGF signaling in sympathetic neurons. Neuron. 2000;27(3):499–512. doi: 10.1016/s0896-6273(00)00061-1. [DOI] [PubMed] [Google Scholar]
- Kwong JM, Caprioli J. Expression of phosphorylated c-Jun N-terminal protein kinase (JNK) in experimental glaucoma in rats. Exp Eye Res. 2006;82(4):576–82. doi: 10.1016/j.exer.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Leaver SG, Cui Q, Plant GW, Arulpragasam A, Hisheh S, Verhaagen J, Harvey AR. AAV-mediated expression of CNTF promotes long-term survival and regeneration of adult rat retinal ganglion cells. Gene Ther. 2006;13(18):1328–41. doi: 10.1038/sj.gt.3302791. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294(5548):1945–8. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Lei K, Nimnual A, Zong WX, Kennedy NJ, Flavell RA, Thompson CB, Bar-Sagi D, Davis RJ. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH(2)-terminal kinase. Mol Cell Biol. 2002;22(13):4929–42. doi: 10.1128/MCB.22.13.4929-4942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkovitch-Verbin H. Animal models of optic nerve diseases. Eye. 2004;18(11):1066–74. doi: 10.1038/sj.eye.6701576. [DOI] [PubMed] [Google Scholar]
- Levkovitch-Verbin H, Quigley HA, Martin KR, Valenta D, Baumrind LA, Pease ME. Translimbal laser photocoagulation to the trabecular meshwork as a model of glaucoma in rats. Invest Ophthalmol Vis Sci. 2002;43(2):402–10. [PubMed] [Google Scholar]
- Levkovitch-Verbin H, Harizman N, Dardik R, Nisgav Y, Vander S, Melamed S. Regulation of cell death and survival pathways in experimental glaucoma. Exp Eye Res. 2007;85(2):250–8. doi: 10.1016/j.exer.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Levkovitch-Verbin H, Quigley HA, Martin KR, Harizman N, Valenta DF, Pease ME, Melamed S. The transcription factor c-jun is activated in retinal ganglion cells in experimental rat glaucoma. Exp Eye Res. 2005;80(5):663–70. doi: 10.1016/j.exer.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Libby RT, Li Y, Savinova OV, Barter J, Smith RS, Nickells RW, John SW. Susceptibility to neurodegeneration in a glaucoma is modified by Bax gene dosage. PLoS Genet. 2005;1(1):17–26. doi: 10.1371/journal.pgen.0010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Cui Q, Yip HK, So KF. c-Jun expression in surviving and regenerating retinal ganglion cells: effects of intravitreal neurotrophic supply. Invest Ophthalmol Vis Sci. 2003;44(12):5342–8. doi: 10.1167/iovs.03-0444. [DOI] [PubMed] [Google Scholar]
- Ma YT, Hsieh T, Forbes ME, Johnson JE, Frost DO. BDNF injected into the superior colliculus reduces developmental retinal ganglion cell death. J Neurosci. 1998;18(6):2097–107. doi: 10.1523/JNEUROSCI.18-06-02097.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkerh JP, Ceni C, Auld DS, Vaillancourt F, Dorval G, Barker PA. p75 neurotrophin receptor reduces ligand-induced Trk receptor ubiquitination and delays Trk receptor internalization and degradation. EMBO Rep. 2005;6(10):936–41. doi: 10.1038/sj.embor.7400503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamidipudi V, Li X, Wooten MW. Identification of interleukin 1 receptor-associated kinase as a conserved component in the p75-neurotrophin receptor activation of nuclear factor-kappa B. J Biol Chem. 2002;277(31):28010–8. doi: 10.1074/jbc.M109730200. [DOI] [PubMed] [Google Scholar]
- Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci U S A. 1994;91(5):1632–6. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KR, Quigley HA, Zack DJ, Levkovitch-Verbin H, Kielczewski J, Valenta D, Baumrind L, Pease ME, Klein RL, Hauswirth WW. Gene therapy with brain-derived neurotrophic factor as a protection: retinal ganglion cells in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2003;44(10):4357–65. doi: 10.1167/iovs.02-1332. [DOI] [PubMed] [Google Scholar]
- Mey J, Thanos S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993;602(2):304–17. doi: 10.1016/0006-8993(93)90695-j. [DOI] [PubMed] [Google Scholar]
- Meyer-Franke A, Kaplan MR, Pfrieger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15(4):805–19. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- Mi S, Lee X, Shao Z, Thill G, Ji B, Relton J, Levesque M, Allaire N, Perrin S, Sands B, et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci. 2004;7(3):221–8. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- Miller FD, Kaplan DR. Neurotrophin signalling pathways regulating neuronal apoptosis. Cell Mol Life Sci. 2001;58(8):1045–53. doi: 10.1007/PL00000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minckler DS, Bunt AH, Johanson GW. Orthograde and retrograde axoplasmic transport during acute ocular hypertension in the monkey. Invest Ophthalmol Vis Sci. 1977;16(5):426–41. [PubMed] [Google Scholar]
- Minckler DS, Bunt AH, Klock IB. Radioautographic and cytochemical ultrastructural studies of axoplasmic transport in the monkey optic nerve head. Invest Ophthalmol Vis Sci. 1978;17(1):33–50. [PubMed] [Google Scholar]
- Morrison JC, Johnson E, Cepurna WO. Rat models for glaucoma research. Prog Brain Res. 2008a;173:285–301. doi: 10.1016/S0079-6123(08)01121-7. [DOI] [PubMed] [Google Scholar]
- Morrison JC, Johnson EC, Cepurna W, Jia L. Understanding mechanisms of pressure-induced optic nerve damage. Prog Retin Eye Res. 2005;24(2):217–240. doi: 10.1016/j.preteyeres.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Morrison JC, Nylander KB, Lauer AK, Cepurna WO, Johnson E. Glaucoma drops control intraocular pressure and protect optic nerves in a rat model of glaucoma. Invest Ophthalmol Vis Sci. 1998;39(3):526–31. [PubMed] [Google Scholar]
- Morrison JC, Moore CG, Deppmeier LM, Gold BG, Meshul CK, Johnson EC. A rat model of chronic pressure-induced optic nerve damage. Exp Eye Res. 1997;64(1):85–96. doi: 10.1006/exer.1996.0184. [DOI] [PubMed] [Google Scholar]
- Morrison JC, Guo Y, Dyck JA, Cepurna WO, Doser T, Johnson EC. Increasing the Sensitivity of Detecting Cell Responses to Elevated Intraocular Pressure: A Comparsion of Whole Retina Tt Retinal Ganglion Cell Layer. Invest Ophthalmol Vis Sci. 2008b;49(5):3698. doi: 10.1167/iovs.09-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickells RW. Apoptosis of retinal ganglion cells in glaucoma: an update of the molecular pathways involved in cell death. Surv Ophthalmol. 1999;43(Suppl 1):S151–61. doi: 10.1016/s0039-6257(99)00029-6. [DOI] [PubMed] [Google Scholar]
- Nickells RW. From ocular hypertension to ganglion cell death: a theoretical sequence of events leading to glaucoma. Can J Ophthalmol. 2007;42(2):278–87. [PubMed] [Google Scholar]
- Nissirios N, Chanis R, Johnson E, Morrison J, Cepurna WO, Jia L, Mittag T, Danias J. Comparison of anterior segment structures in two rat glaucoma models: an ultrasound biomicroscopic study. Invest Ophthalmol Vis Sci. 2008;49(6):2478–82. doi: 10.1167/iovs.07-0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykjaer A, Willnow TE, Petersen CM. p75NTR--live or let die. Curr Opin Neurobiol. 2005;15(1):49–57. doi: 10.1016/j.conb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW, Prevette D, Tytell M, Homma S. Naturally occurring and induced neuronal death in the chick embryo in vivo requires protein and RNA synthesis: evidence for the role of cell death genes. Dev Biol. 1990;138(1):104–13. doi: 10.1016/0012-1606(90)90180-q. [DOI] [PubMed] [Google Scholar]
- Pang IH, Clark AF. Rodent models for glaucoma retinopathy and optic neuropathy. J Glaucoma. 2007;16(5):483–505. doi: 10.1097/IJG.0b013e3181405d4f. [DOI] [PubMed] [Google Scholar]
- Pang IH, Wang WH, Millar JC, Clark AF. Measurement of mouse intraocular pressure with the Tono-Pen. Exp Eye Res. 2005;81(3):359–60. doi: 10.1016/j.exer.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Pease ME, McKinnon SJ, Quigley HA, Kerrigan-Baumrind LA, Zack DJ. Obstructed axonal transport of BDNF and its receptor TrkB in experimental glaucoma. Invest Ophthalmol Vis Sci. 2000;41(3):764–74. [PubMed] [Google Scholar]
- Peinado-Ramon P, Salvador M, Villegas-Perez MP, Vidal-Sanz M. Effects of axotomy and intraocular administration of NT-4, NT-3, and brain-derived neurotrophic factor on the survival of adult rat retinal ganglion cells. A quantitative in vivo study. Invest Ophthalmol Vis Sci. 1996;37(4):489–500. [PubMed] [Google Scholar]
- Purves D. Body and brain: a trophic theory of neural connections. Cambridge, Mass: Harvard University Press; 1988. p. 231. [DOI] [PubMed] [Google Scholar]
- Quigley HA. Ganglion cell death in glaucoma: pathology recapitulates ontogeny. Aust N Z J Ophthalmol. 1995;23(2):85–91. doi: 10.1111/j.1442-9071.1995.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999;18(1):39–57. doi: 10.1016/s1350-9462(98)00014-7. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36(5):774–86. [PubMed] [Google Scholar]
- Quigley HA, McKinnon SJ, Zack DJ, Pease ME, Kerrigan-Baumrind LA, Kerrigan DF, Mitchell RS. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest Ophthalmol Vis Sci. 2000;41(11):3460–6. [PubMed] [Google Scholar]
- Raff MC, Barres BA, Burne JF, Coles HS, Ishizaki Y, Jacobson MD. Programmed cell death and the control of cell survival: lessons from the nervous system. Science. 1993;262(5134):695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- Robinson GA. Immediate early gene expression in axotomized and regenerating retinal ganglion cells of the adult rat. Brain Res Mol Brain Res. 1994;24(1–4):43–54. doi: 10.1016/0169-328x(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Tebar A, Jeffrey PL, Thoenen H, Barde YA. The survival of chick retinal ganglion cells in response to brain-derived neurotrophic factor depends on their embryonic age. Dev Biol. 1989;136(2):296–303. doi: 10.1016/0012-1606(89)90256-x. [DOI] [PubMed] [Google Scholar]
- Rohrer B, LaVail MM, Jones KR, Reichardt LF. Neurotrophin receptor TrkB activation is not required for the postnatal survival of retinal ganglion cells in vivo. Exp Neurol. 2001;172(1):81–91. doi: 10.1006/exnr.2001.7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudzinski M, Wong TP, Saragovi HU. Changes in retinal expression of neurotrophins and neurotrophin receptors induced by ocular hypertension. J Neurobiol. 2004;58(3):341–54. doi: 10.1002/neu.10293. [DOI] [PubMed] [Google Scholar]
- Sawada A, Neufeld AH. Confirmation of the rat model of chronic, moderately elevated intraocular pressure. Exp Eye Res. 1999;69(5):525–31. doi: 10.1006/exer.1999.0732. [DOI] [PubMed] [Google Scholar]
- Schaden H, Stuermer CA, Bahr M. GAP-43 immunoreactivity and axon regeneration in retinal ganglion cells of the rat. J Neurobiol. 1994;25(12):1570–8. doi: 10.1002/neu.480251209. [DOI] [PubMed] [Google Scholar]
- Seki M, Tanaka T, Sakai Y, Fukuchi T, Abe H, Nawa H, Takei N. Muller Cells as a source of brain-derived neurotrophic factor in the retina: noradrenaline upregulates brain-derived neurotrophic factor levels in cultured rat Muller cells. Neurochem Res. 2005;30(9):1163–70. doi: 10.1007/s11064-005-7936-7. [DOI] [PubMed] [Google Scholar]
- Shareef S, Sawada A, Neufeld AH. Isoforms of nitric oxide synthase in the optic nerves of rat eyes with chronic moderately elevated intraocular pressure. Invest Ophthalmol Vis Sci. 1999;40(12):2884–91. [PubMed] [Google Scholar]
- Shareef SR, Garcia-Valenzuela E, Salierno A, Walsh J, Sharma SC. Chronic ocular hypertension following episcleral venous occlusion in rats [letter] Exp Eye Res. 1995;61(3):379–82. doi: 10.1016/s0014-4835(05)80131-9. [DOI] [PubMed] [Google Scholar]
- Shi Z, Birman E, Saragovi HU. Neurotrophic rationale in glaucoma: a TrkA agonist, but not NGF or a p75 antagonist, protects retinal ganglion cells in vivo. Dev Neurobiol. 2007;67(7):884–94. doi: 10.1002/dneu.20360. [DOI] [PubMed] [Google Scholar]
- Sommerfeld MT, Schweigreiter R, Barde YA, Hoppe E. Down-regulation of the neurotrophin receptor TrkB following ligand binding. Evidence for an involvement of the proteasome and differential regulation of TrkA and TrkB. J Biol Chem. 2000;275(12):8982–90. doi: 10.1074/jbc.275.12.8982. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Rush RA, Harvey AR. Target-derived and locally derived neurotrophins support retinal ganglion cell survival in the neonatal rat retina. J Neurobiol. 2004;60(3):319–27. doi: 10.1002/neu.20028. [DOI] [PubMed] [Google Scholar]
- Tamm ER, Fuchshofer R. What increases outflow resistance in primary open-angle glaucoma? Surv Ophthalmol. 2007;52(Suppl 2):S101–4. doi: 10.1016/j.survophthal.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25(22):5455–63. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma JG, Rogers D, Senger DL, Campenot RB, Miller FD. Spatial regulation of neuronal gene expression in response to nerve growth factor. Dev Biol. 1997;184(1):1–9. doi: 10.1006/dbio.1997.8515. [DOI] [PubMed] [Google Scholar]
- Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288(5467):870–4. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- Ueda J, Sawaguchi S, Hanyu T, Yaoeda K, Fukuchi T, Abe H, Ozawa H. Experimental glaucoma model in the rat induced by laser trabecular photocoagulation after an intracameral injection of India ink. Jpn J Ophthalmol. 1998;42(5):337–44. doi: 10.1016/s0021-5155(98)00026-4. [DOI] [PubMed] [Google Scholar]
- Ugolini G, Cremisi F, Maffei L. TrkA, TrkB and p75 mRNA expression is developmentally regulated in the rat retina. Brain Res. 1995;704(1):121–4. doi: 10.1016/0006-8993(95)01191-9. [DOI] [PubMed] [Google Scholar]
- Vecino E, Garcia-Grespo D, Garcia M, Martinez-Millan L, Sharma SC, Carrascal E. Rat retinal ganglion cells co-express brain derived neurotrophic factor (BDNF) and its receptor TrkB. Vision Res. 2002;42(2):151–7. doi: 10.1016/s0042-6989(01)00251-6. [DOI] [PubMed] [Google Scholar]
- Vrabec JP, Levin LA. The neurobiology of cell death in glaucoma. Eye. 2007;21(Suppl 1):S11–4. doi: 10.1038/sj.eye.6702880. [DOI] [PubMed] [Google Scholar]
- Wang J, Ge J, Sadun AA, Lam TT. Characteristics of optic nerve damage induced by chronic intraocular hypertension in rat. Yan Ke Xue Bao. 2004;20(1):25–9. [PubMed] [Google Scholar]
- Wehrman T, He X, Raab B, Dukipatti A, Blau H, Garcia KC. Structural and mechanistic insights into nerve growth factor interactions with the TrkA and p75 receptors. Neuron. 2007;53(1):25–38. doi: 10.1016/j.neuron.2006.09.034. [DOI] [PubMed] [Google Scholar]
- Weishaupt JH, Diem R, Kermer P, Krajewski S, Reed JC, Bahr M. Contribution of caspase-8 to apoptosis of axotomized rat retinal ganglion cells in vivo. Neurobiol Dis. 2003;13(2):124–35. doi: 10.1016/s0969-9961(03)00032-9. [DOI] [PubMed] [Google Scholar]
- WoldeMussie E, Ruiz G, Wijono M, Wheeler LA. Neuroprotection of retinal ganglion cells by brimonidine in rats with laser-induced chronic ocular hypertension. Invest Ophthalmol Vis Sci. 2001;42(12):2849–55. [PubMed] [Google Scholar]
- Zhang M, Budak MT, Lu W, Khurana TS, Zhang X, Laties AM, Mitchell CH. Identification of the A3 adenosine receptor in rat retinal ganglion cells. Mol Vis. 2006;12:937–48. [PubMed] [Google Scholar]
- Zhao XH, Jin WL, Wu J, Mi S, Ju G. Inactivation of glycogen synthase kinase-3beta and up-regulation of LINGO-1 are involved in LINGO-1 antagonist regulated survival of cerebellar granular neurons. Cell Mol Neurobiol. 2008;28(5):727–35. doi: 10.1007/s10571-007-9258-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Pernet V, Hauswirth WW, Di Polo A. Activation of the extracellular signal-regulated kinase 1/2 pathway by AAV gene transfer protects retinal ganglion cells in glaucoma. Mol Ther. 2005;12(3):402–12. doi: 10.1016/j.ymthe.2005.04.004. [DOI] [PubMed] [Google Scholar]