Summary
Airway hypersensitivity is a common pathophysiological feature in various airway inflammatory diseases. Increasing evidence suggests that activation of the transient receptor potential vanilloid type 1 receptor (TRPV1) plays an important part in the manifestation of various symptoms of airway hypersensitivity. This mini-review focuses on recent studies that have revealed several potential contributing factors to the increase in TRPV1 sensitivity in pulmonary sensory neurons during airway inflammatory reaction. In addition, chronic allergic airway inflammation induces a pronounced over-expression of TRPV1 in neurofilament-positive pulmonary sensory neurons in nodose ganglia. A better understanding of the mechanisms underlying the increase in sensitivity and/or expression of TRPV1 during acute and chronic airway inflammation should generate the necessary information for developing effective therapeutic interventions to alleviate airway hypersensitivity.
Introduction
Transient receptor potential vanilloid type 1 receptor (TRPV1) is a polymodal transducer, and belongs to the superfamily of TRP ion channels [1]. TRPV1 is a tetrameric membrane protein with four identical subunits, and each subunit contains six transmembrane-spaning domains, which form a non-selective cation channel with a high permeability to Ca2+. Since it was cloned in 1997 [2], the TRPV1 expressed on the sensory nerve terminals has been recognized as “a molecular gateway” to nociceptive sensation in somatic and visceral tissues [3]. In the last several years, the expression of TRPV1 on the sensory nerves in the respiratory tract and its important role in the regulation of the airway function, especially in disease conditions, have been increasingly recognized [4–7].
The involvement of TRPV1 in the manifestation of various symptoms in airway diseases have been extensively discussed in several recent reviews [4,5,7]. This mini-review is intended to focus specifically on more recent findings of the involvements of TRPV1 in the development of airway hypersensitivity associated with inflammatory reactions in the respiratory tract. We will further discuss the mechanisms possibly underlying the up-regulation of TRPV1 sensitivity and expression under these pathophysiological conditions.
TRPV1-expressing sensory nerves in airways
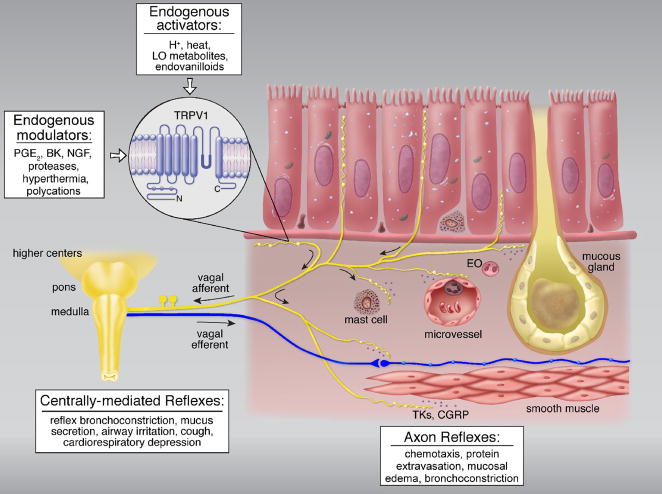
The afferent activities arising from sensory terminals in the lung and airways are conducted primarily by branches of vagus nerves, and project to the nucleus tractus solitarius in the medulla. Among these sensory nerves, TRPV1 is expressed predominantly in non-myelinated (C-fiber) afferents [8], which represent >75% of the afferent fibers in the pulmonary branch of the vagus nerve. One unique feature of these nerves is the extensive co-expression of TRPV1 with certain sensory neuropeptides, namely tachykinins and calcitonin gene-related peptide (CGRP) [9], that are synthesized in the cell bodies of these neurons located in the nodose and jugular ganglia [10]. Another prominent anatomical feature of these sensory nerves is the axonal arborization of their endings that either extend into the space between epithelial cells or form network-like plexus immediately beneath the basement membrane of epithelium [9,11] (Figure 1), suggesting a role of these afferents in regulating the airway responses to inhaled irritants [12,13]. When these TRPV1-expressing nerve endings are activated either by inhaled irritants or endogenous TRPV1 activators (see details in a later section), centrally-mediated reflex responses are elicited, which include reflex bronchoconstriction and mucus hypersecretion via the cholinergic pathway, accompanied by the sensation of airway irritation and urge to cough (Figure 1). In anesthetized animals, it also elicits the classical “pulmonary chemoreflexes”, characterized by the triad of apnea, bradycardia and hypotension [12]. Activation of TRPV1 also triggers Ca2+ influx and release of tachykinins and CGRP from the sensory terminals. These sensory neuropeptides can act on a number of effector cells in the respiratory tract (e.g., smooth muscles, cholinergic ganglia, mucous glands, immune cells), and elicit the local “axon reflexes” such as bronchoconstriction, protein extravasation and inflammatory cell chemotaxis [10] (Figure 1). These actions generated by tachykinins and CGRP have been well documented in rodents, but the degree of their relative importance in human airways remains to be fully established [10,13].
Figure 1.
Schematic illustration of the function of TRPV1-expressing sensory nerves and their interaction with other cell types in airway mucosa. EO, eosinophil; LO, lipooxygenase; PGE2, prostaglandin E2; BK, bradykinin; NGF, nerve growth factor; MBP, major basic proteins derived from eosinophil degranulation; TKs, tachykinins; CGRP, calcitonin gene-related peptide. See text for details. (Adapted from reference 13)
Airway hypersensitivity: plasticity of TRPV1 in airway diseases
Airway hypersensitivity, characterized by exaggerated sensory (e.g., airway irritation, dyspnea, etc.) and reflexogenic responses (e.g., cough, bronchoconstriction, etc.) to inhaled irritants and certain endogenously released mediators, is a common pathophysiological feature in patients with airway inflammatory diseases such as asthma, bronchitis, viral infection, etc. Increasing and compelling evidence reported in recent studies suggests that the TRPV1 channel plays a pivotal role in the manifestation of various symptoms of airway hypersensitivity in these patients [4,5]. For example, cough sensitivity to TRPV1 activators, capsaicin or citric acid aerosol, was markedly elevated in patients with asthma or airway inflammation [14,15]. Endogenous TRPV1 activators such as H+ and lipooxygenase metabolites are consistently detected in the bronchoalveolar lavage fluid, sputum and/or exhaled breath condensate of patients with inflammatory airway diseases [5]. In addition, certain endogenous inflammatory mediators (e.g., prostaglandin E2, bradykinin, etc.), though not a TRPV1 activator themselves, can markedly enhance the sensitivity of TRPV1 and lower its threshold for activation [5]. Furthermore, cough sensitivity to acute inhalation challenge of ovalbumin aerosol was elevated in sensitized guinea pigs, and the enhanced response was significantly attenuated by TRPV1 antagonists, suggesting the involvement of TRPV1 in the airway hypersensitivity induced by chronic allergic inflammation [16]. Recent studies further revealed that the increased TRPV1-mediated responses is associated with increased expression of the TRPV1 channel in bronchopulmonary sensory nerves in certain chronic airway diseases [17,18]. These observations collectively suggest that plasticity of TRPV1 develops upon the action of various inflammatory mediators and cytokines during airway inflammatory reaction. Either an enhanced sensitivity or an increase in expression of TRPV1, a given level of stimulation is expected to evoke a greater afferent discharge of the TRPV1-expressing sensory nerves in the airways, and consequently more severe sensory and reflex responses, resulting in airway hypersensitivity.
Increased sensitivity of TRPV1
The sensitivity of TRPV1 can be elevated by the actions of a number of chemical, physical and biological factors, as discussed in details in several comprehensive reviews [1,3,4–6]. In this mini-review, we will focus our attention on three of these factors because recent investigations have revealed new information about their involvements in the development of airway hypersensitivity associated with airway inflammatory reaction.
Increase in airway temperature
Tissue inflammation is known to lead to local hyperemia and an increase in temperature in the inflamed area. A recent study reported that the end-expiratory temperature plateau was 2.7°C higher in mild allergic asthmatic children than in healthy children, and the difference was closely correlated with the exhaled nitric oxide concentration as well as the sputum eosinophil percentage [19]. An earlier study in adult patients also showed a faster rise of exhaled temperature in asthmatics than matching controls [20]. These findings seem to suggest that exhaled breath temperature is related to the degree of airway inflammation in asthma.
The four subtypes of TRPV channels, TRPV1–4, are generally considered as the primary sensors for warm and hot temperatures in mammalian species, and each is activated in a different temp range (> 43°C for TRPV1; > 52°C for TRPV2; > 34–38 °C for TRPV3; > 27–35 °C for TRPV4) [21]. A recent study has demonstrated the expression of both mRNA and receptor proteins of all these four subtypes of TRPVs in the cell bodies of sensory neurons innervating the lung structures in rats [22]. These neurons isolated in primary culture also exhibit distinct temperature sensitivity in whole-cell patch-clamp electrophysiological recording experiments [22]. When the temperature was raised from normal (~36°C) to hyperthermic (~40.6°C) level of the rat body temperature and held constant, the inward currents evoked by capsaicin and 2-aminoethoxydiphenyl borate (2-APB), a non-selective activator of TRPV1–3 receptors, were both significantly increased [23]. This potentiating effect was clearly present even at a moderate level of hyperthermia (~39°C). However, it was largely attenuated by selective TRPV1 antagonists, capsazepine or AMG 9810 [23], and completely absent in pulmonary nodose/jugular neurons isolated from TRPV1-null mice [24], suggesting the possible involvement of a positive interaction between hyperthermia and these chemical activators at the TRPV1 channel. Surprisingly, although hyperthermia also potentiated the TRPV1-mediated response to H+, it inhibited the responses mediated through the acid sensing ion channels [23]. The specific site(s) and mechanism underlying this interaction are not known, but an involvement of cytoplasmic COOH-terminal domain of the TRPV1 receptor in the conformational changes has been suggested [25]. Furthermore, in a recent study Voets et al. [26] have clearly demonstrated that increasing temperature to 42°C shifted the TRPV1 channel activation curve (open probability vs. voltage) from a non-physiological positive voltage range towards the negative potential (Figure 2). This large shift of voltage-dependent activation curve to a physiologically relevant voltage range with a relatively small gating charge may explain in part the hyperthermia-induced hypersensitivity in pulmonary sensory neurons expressing the TRPV1 [26,27]. This positive interaction between hyperthermia and chemical activators of TRPV1 is particularly relevant because an increase in tissue temperature during inflammatory reaction may occur concurrently with the release of several endogenous TRPV1 activators such as proton, polycations and certain lipoxygenase metabolites of arachidonic acid (e.g., 12S- and 15S-hydroperoxyeicosatetraenoic acid) in the airways [5].
Figure 2.
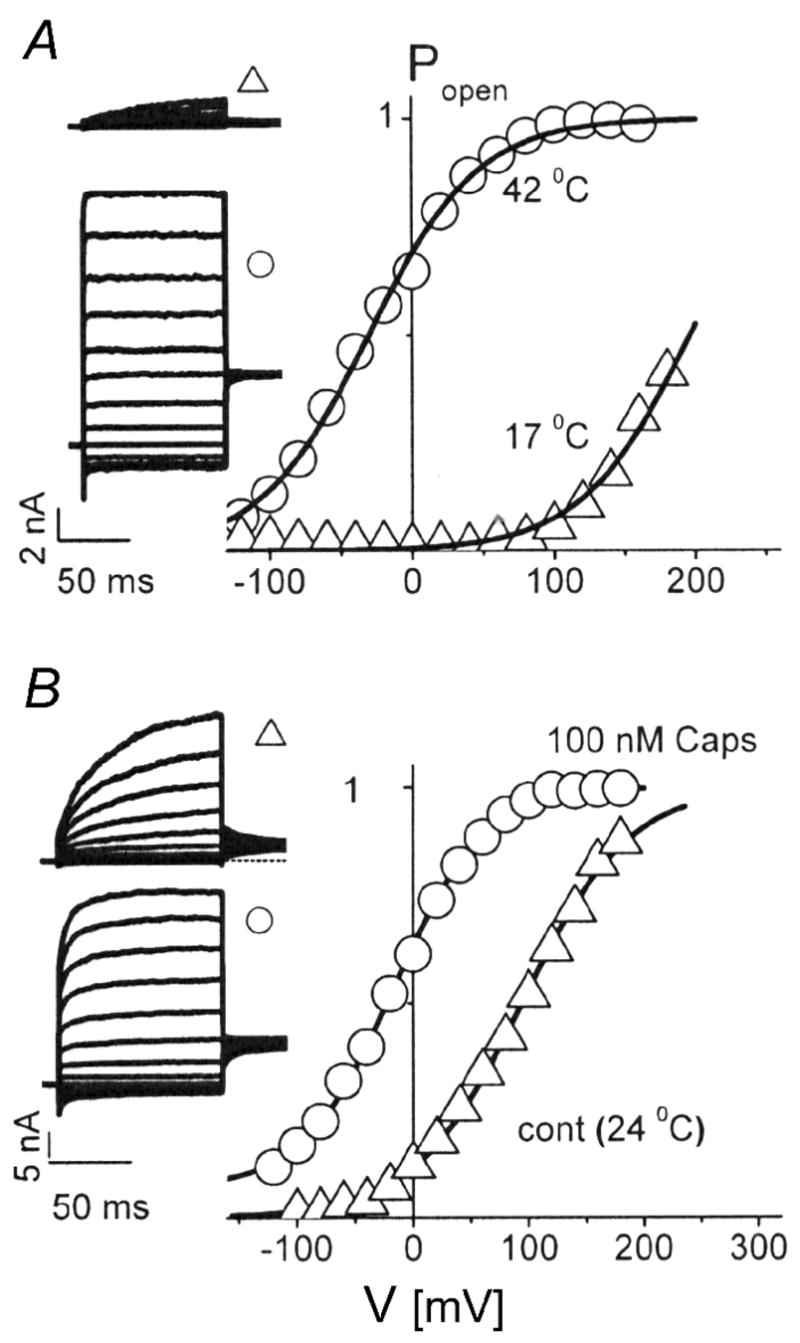
Voltage shifts of TRPV1 activation curves by temperature and capsaicin. A, voltage dependence of the open probability of TRPV1 channels at 17°C (triangles) and 42°C (circles). The inset shows the respective current families obtained from a voltage-step protocols (holding potential 0 mV, voltage steps of 100-ms duration from −120 to +160 mV in 40-mV increments). Note the leftward shift of the activation curve towards negative potentials by increasing the temperature. B, the same voltage protocol as in A, and the temperature was held at 24°C. Activation of TRPV1 by capsaicin (100 nM; circles) also caused a pronounced leftward shift of the activation curve. (Adapted from reference 27)
Protease-activated receptors-2 (PAR2)
PAR2 belongs to a family of G-protein-coupled, seven-transmembrane-domain receptors named PARs that are uniquely activated by proteolysis [28]. PAR2 activation occurs after cleavage of its extracellular N-terminal domain by specific proteases, revealing a new N-terminus that acts as a tethered ligand binding to and activating the receptor (Figure 3). Expression of PAR2 has been demonstrated in a variety of cells in the lung and airways, including TRPV1-positive sensory neurons [29]. Mast cell tryptase, trypsin and trypsin-like proteases, and coagulation factors VIIa and Xa are considered as the endogenous agonists of PAR2 [29,30]. PAR2 can also be activated by certain airborne allergens such as house dust mite Der P1, P3 and P9 [31]. In addition, tissue kallikreins, a large family of secreted serine proteases with tryptic or chymotryptic activity, are recently proposed as physiological regulators of PAR2 [32].
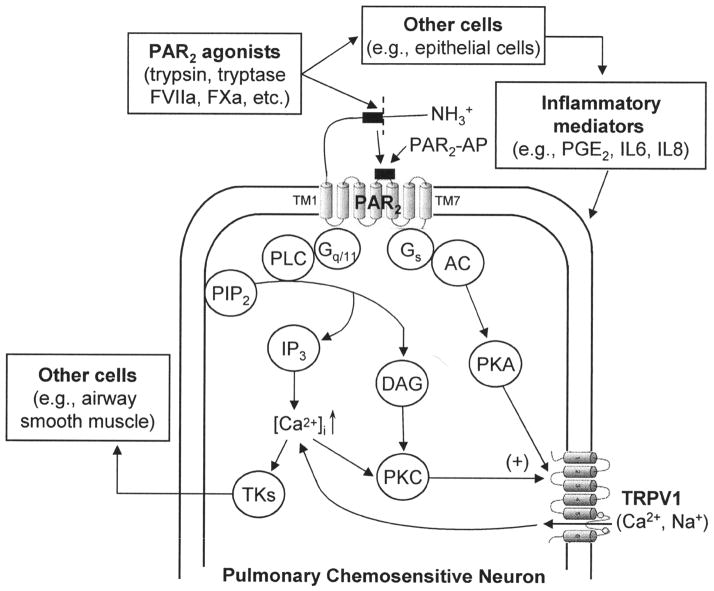
Figure 3.
Schematic illustration of the hypothesized interaction between PAR2 and TRPV1 in pulmonary sensory neurons. PAR2, protease-activated receptor-2; FVIIa, coagulation factor VIIa; FXa, coagulation factor Xa; PAR2-AP, synthetic PAR2 activating peptide; TM, transmembrane domain; Gq/11 and Gs, G proteins; PLC, phospholipase C; PIP2, phosphatidylinositol 4,5-bisphosphate; IP3, inositol 1,4,5-triphosphate; DAG, diacylglycerol; PKC, protein kinase C; [Ca2+]i, intracellular Ca2+ concentration; TKs, tachykinins; AC, adenylate cyclase; PKA, protein kinase A; PGE2, prostaglandin E2, IL, interleukin. See text for details.
Compelling evidence indicates that PAR2 plays a critical role in the pathogenesis of airway inflammation and airway hyperresponsiveness. The elevated levels of both the endogenous agonists and the expression of PAR2 have been reported from patients and animals under airway inflammatory conditions [30,33]. Activation of PAR2 in the lung induces airway constriction, lung inflammation, and protein-rich pulmonary edema [34–36]. These effects are inhibited by either perineural capsaicin treatment of both vagi or the combination of neurokinin-1 (NK1), NK2 and CGRP receptor antagonists, indicating the involvement of neuropeptides released from TRPV1-containing C-fiber afferents. Indeed, a recent study showed that PAR2 activation up-regulates the excitability of rat pulmonary chemosensitive neurons and potentiate the capsaicin-induced, TRPV1-mediated pulmonary chemoreflex responses [37]. PAR2 activation also exaggerates the TRPV1-dependent tussive response in guinea pigs [38]. Furthermore, PAR2 activation is often associated with release of various proinflammatory mediators including prostanoids such as prostaglandin E2, and cytokines such as interleukin (IL)-6 and IL-8 [29,39,40]. These mediators are known to have potential regulatory effects on the sensitivity of TRPV1 [5].
The signaling mechanisms of PAR2 are not fully understood. In a number of cell systems, PAR2has been reported to be coupled to Gq/11 protein to activate phospholipase C, resulting in generation of second messengers, inositol 1,4,5-triphosphate and diacylglycerol, which further trigger mobilization of Ca2+ and activation of protein kinase C [28]. Involvements of PKC, PKA and possibly PKD have recently been proposed in PAR2-induced sensitization of TRPV1 as well as other TRP channels in sensory neurons [37,41–43] (Figure 3).
Polycations
Recent studies have reported that extracellular cations such as Mg2+ and Ca2+ can sensitize and gate TRPV1 [44]. A similar effect can also be generated by polyamines (such as spermine, spermidine and putrescine), the organic polycations that are known to modulate inflammation and nociception [45]. More importantly, it has been reported that the blood levels of these polyamines were significantly elevated in patients during asthmatic attack [46]. The study by Ahern et al. further suggested that the extracellular acidic residues ASP-646 and Glu-648, which are located near the pore-forming region of TRPV1, play an important role in polyamine regulation. Their hypothesis was supported by the finding that spermine failed to increase the whole-cell current evoked by protons in TRPV1-expressing oocytes [45].
Airway infiltration of eosinophils and the release of their granule-derived, low molecular weight and highly cationic proteins (i.e., major basic protein, eosinophil cationic protein, eosinophil peroxidase and eosinophil-derived neurotoxin) occur in a variety of airway inflammatory diseases including asthma [47]. Furthermore, the increases in both the number of eosinophils and levels of eosinophil-derived cationic proteins in the bronchoalveolar lavage fluid of asthmatic patients are correlated with the severity of the disease [48]. Recent studies have demonstrated that these eosinophil-derived cationic proteins can directly sensitize the capsaicin-evoked, TRPV1-mediated whole-cell responses in isolated rat pulmonary sensory neurons; the effect of these proteins was completely abolished when their cationic charges were neutralized by mixing with a polyanion [49]. Whether these eosinophil granule proteins increase the sensitivity of TRPV1 via a similar mechanism as polyamines remains to be determined.
Over-expression of TRPV1 in chronic airway inflammation
In addition to increased excitability of the channel, an increase in the TRPV1 receptor protein expression can also contribute to the airway hypersensitivity, particularly in chronic airway diseases. For example, the TRPV-1 immunoreactive nerve profile was five-fold higher in the biopsies of bronchial tissue from patients with chronic cough than healthy individuals [17]. Furthermore, there was a significant correlation between the cough sensitivity to capsaicin inhalation challenge and the density of TRPV1-expressing nerves in the mucosa of patients with chronic cough [17,50]. These observations have provided strong evidence to suggest an increase in expression of TRPV1 in the sensory endings of airway mucosa may be involved in the development of chronic cough. A recent study showed that, after chronic airway inflammation was induced by allergen sensitization, capsaicin evoked a pronounced stimulatory effect on vagal bronchopulmonary myelinated afferents, which normally exhibit no or very little sensitivity to capsaicin [18]. Immunohistochemical experiments further indicated a pronounced increase in the proportion of TRPV1-expressing bronchopulmonary neurons in nodose ganglia of sensitized rats, and the increased TRPV1 expression was found mainly in neurofilament-positive neurons (myelinated neurons) [18]. This observation is in general agreement with another recent finding of an increased number of TRPV1- immunoreactive axons within the tracheal epithelium and around smooth muscles of ovalbumin-sensitized guinea pigs by Watanabe et al. [51]. The mechanism underlying the phenotypic change in TRPV1 expression in pulmonary myelinated neurons was not known. However, a possible involvement of neurotrophins such as brain-derived neurotrophic factor and nerve growth factor should be considered. These neurotrophins are known to up-regulate the expression of TRPV1 in sensory neurons [52,53,54,55], or promote translocation of the TRPV1 to cell membrane [56], and their synthesis and release have been shown to increase in allergic airways [57,58].
Conclusion
Airway hypersensitivity is a common and debilitating problem for patients with various airway inflammatory diseases. Cumulative evidence indicates that TRPV1 plays a key part in the manifestation of various symptoms of airway hypersensitivity, suggesting that TRPV1 is an important target for pharmacological interventions. To obtain the necessary information for developing new effective therapeutic strategy, further investigations on the mechanisms involved in the increase in sensitivity and/or expression of TRPV1 during both acute and chronic airway disease conditions are required. In addition, it is evident that the interaction between TRPV1 and other ion channels and regulatory receptor proteins that are also expressed on these airway sensory nerves is important in modulating the function of airway sensory neurons. Thus, TRPV1 functions not only as a transducer, but also an integrator of multiple endogenous activators and modulatory molecules. A better understanding of the overall role of TRPV1 in regulating the excitability of these neurons during airway inflammatory reaction is probably one of the most important steps in uncovering the underlying mechanism of airway hypersensitivity.
Acknowledgments
The work was supported in part by USPHS grants from the National Institutes of Health (HL58686 to L.-Y.L.; AI76714 to Q.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 2.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 3.Caterina MJ, Julius D. The vanilloid receptor a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 4.•.Geppetti P, Materazzi S, Nicoletti P. The transient receptor potential vanilloid 1. role in airway inflammation and disease. Eur J Pharmacol. 2006;533:207–214. doi: 10.1016/j.ejphar.2005.12.063. This is a comprehensive review of the possible involvement of TRPV1 in various airway diseases and the putative endogenous TRPV1 activators in the airways. [DOI] [PubMed] [Google Scholar]
- 5.•.Jia Y, Lee LY. Role of TRPV receptors in respiratory diseases. Biochim Biophys Acta. 2007;1772:915–927. doi: 10.1016/j.bbadis.2007.01.013. A comprehensive review of the expression and activators/modulators of TRPV1 and TRPV4 in the lung, and the potential role of these channels in the pathogenesis of various acute and chronic airway diseases. [DOI] [PubMed] [Google Scholar]
- 6.Gunthorpe MJ, Szallasi A. Peripheral TRPV1 receptors as targets for drug development new molecules and mechanisms. Curr Pharm Des. 2008;14:32–41. doi: 10.2174/138161208783330754. [DOI] [PubMed] [Google Scholar]
- 7.Takemura M, Quarcoo D, Niimi A, Dinh QT, Geppetti P, Fischer A, Chung KF, Groneberg DA. Is TRPV1 a useful target in respiratory diseases? Pulm Pharmacol Ther. 2008;21:833–839. doi: 10.1016/j.pupt.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Ho CY, Gu Q, Lin YS, Lee LY. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol. 2001;127:113–124. doi: 10.1016/s0034-5687(01)00241-9. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe N, Horie S, Michael GJ, Keir S, Spina D, Page CP, Priestley JV. Immunohistochemical co-localization of transient receptor potential vanilloid (TRPV)1 and sensory neuropeptides in the guinea-pig respiratory system. Neuroscience. 2006;141:1533–1543. doi: 10.1016/j.neuroscience.2006.04.073. [DOI] [PubMed] [Google Scholar]
- 10.De Swert KO, Joos GF. Extending the understanding of sensory neuropeptides. Eur J Pharmacol. 2006;533:171–181. doi: 10.1016/j.ejphar.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 11.Adriaensen D, Timmermans JP, Brouns I, Berthoud HR, Neuhuber WL, Scheuermann DW. Pulmonary intraepithelial vagal nodose afferent nerve terminals are confined to neuroepithelial bodies an anterograde tracing and confocal microscopy study in adult rats. Cell Tissue Res. 1998;293:395–405. doi: 10.1007/s004410051131. [DOI] [PubMed] [Google Scholar]
- 12.Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol. 2001;125:47–65. doi: 10.1016/s0034-5687(00)00204-8. [DOI] [PubMed] [Google Scholar]
- 13.Lee LY, Undem BJ. Brochopulmonary vagal sensory nerves. Chapter 11. In: Undem BJ, Weinreich D, editors. Advances in Vagal Afferent Neurobiology. CRC Press; 2005. pp. 279–313. [Google Scholar]
- 14.O’Connell F, Thomas VE, Studham JM, Pride NB, Fuller RW. Capsaicin cough sensitivity increases during upper respiratory infection. Respir Med. 1996;90:279–286. doi: 10.1016/s0954-6111(96)90099-2. [DOI] [PubMed] [Google Scholar]
- 15.Doherty MJ, Mister R, Pearson MG, Calverley PM. Capsaicin responsiveness and cough in asthma and chronic obstructive pulmonary disease. Thorax. 2000;55:643–649. doi: 10.1136/thorax.55.8.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLeod RL, Fernandez X, Correll CC, Phelps TP, Jia Y, Wang X, Hey JA. TRPV1 antagonists attenuate antigen-provoked cough in ovalbumin sensitized guinea pigs. Cough. 2006;2:10. doi: 10.1186/1745-9974-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groneberg DA, Niimi A, Dinh QT, Cosio B, Hew M, Fischer A, Chung KF. Increased expression of transient receptor potential vanilloid-1 in airway nerves of chronic cough. Am J Respir Crit Care Med. 2004;170:1276–1280. doi: 10.1164/rccm.200402-174OC. [DOI] [PubMed] [Google Scholar]
- 18.••.Zhang G, Lin RL, Wiggers ME, Snow DM, Lee LY. Altered expression of TRPV1 and sensitivity to capsaicin in pulmonary myelinated afferents following chronic airway inflammation in the rat. J Physiol. 2008;586:5771–5786. doi: 10.1113/jphysiol.2008.161042. Chronic airway inflammation induced by ovalbumin sensitization generated a pronounced over-expression of TRPV1 in neurofilament-positive pulmonary sensory neurons in nodose ganglia and upregulated the capsaicin sensitivity in myelinated bronchopulmonary afferents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.•.Piacentini GL, Peroni D, Crestani E, Zardini F, Bodini A, Costella S, Boner AL. Exhaled air temperature in asthma. methods and relationship with markers of disease. Clin Exp Allergy. 2007;37:415–419. doi: 10.1111/j.1365-2222.2007.02663.x. This is the first direct evidence indicating a distinctly higher airway temperature (average 2.7 °C) in asthmatic patients than that in healthy individuals. [DOI] [PubMed] [Google Scholar]
- 20.Paredi P, Kharitonov SA, Barnes PJ. Faster rise of exhaled breath temperature in asthma. a novel marker of airway inflammation? Am J Respir Crit Care Med. 2002;165:181–184. doi: 10.1164/ajrccm.165.2.2103053. [DOI] [PubMed] [Google Scholar]
- 21.Dhaka A, Viswanath V, Patapoutian A. TRP Ion Channels and Temperature Sensation. Annu Rev Neurosci. 2006;29:135–161. doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- 22.•.Ni D, Gu Q, Hu HZ, Gao N, Zhu MX, Lee LY. Thermal sensitivity of isolated vagal pulmonary sensory neurons. role of transient receptor potential vanilloid receptors. Am J Physiol Regul Integr Comp Physiol. 2006;291:R541–550. doi: 10.1152/ajpregu.00016.2006. This study characterized the thermal-sensitive properties of pulmonary sensory neurons, and demonstrated that increasing temperature to >34.4 °C exerted a stimulatory effect on these neurons via activation of TRPVs. [DOI] [PubMed] [Google Scholar]
- 23.•.Ni D, Lee LY. Effect of increasing temperature on TRPV1-mediated responses in isolated rat pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol. 2008;294:L563–571. doi: 10.1152/ajplung.00336.2007. Increasing temperature within the physiological range generated a potentiating effect on the response to TRPV1 activators in pulmonary sensory neurons. [DOI] [PubMed] [Google Scholar]
- 24.Ni D, Lee LY. A lack of potentiating effect of increasing temperature on the responses to chemical activators in vagal sensory neurons isolated from TRPV1-null mice. Am J Physiol Lung Cell Mol Physiol. 2008;295:L897–904. doi: 10.1152/ajplung.90385.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.•.Vlachova V, Teisinger J, Susankova K, Lyfenko A, Ettrich R, Vyklicky L. Functional role of C-terminal cytoplasmic tail of rat vanilloid receptor 1. J Neurosci. 2003;23:1340–1350. doi: 10.1523/JNEUROSCI.23-04-01340.2003. This study provided convincing evidence that the structural basis of the thermal sensitivity of the TRPV1 resides in the distal half of the COOH-terminal of the receptor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.••.Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. This study clearly demonstrated that an increase in temperature shifted the TRPV1 activation curve towards physiological membrane potential, and suggested that the small gating charge of the TRPV1 channel is a crucial factor for the voltage shift. [DOI] [PubMed] [Google Scholar]
- 27.Nilius B, Talavera K, Owsianik G, Prenen J, Droogmans G, Voets T. Gating of TRP channels. a voltage connection? J Physiol. 2005;567:35–44. doi: 10.1113/jphysiol.2005.088377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ossovskaya VS, Bunnett NW. Protease-activated receptors contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 29.Reed CE, Kita H. The role of protease activation of inflammation in allergic respiratory diseases. J Allergy Clin Immunol. 2004;114:997–1008. doi: 10.1016/j.jaci.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 30.Sokolova E, Reiser G. A novel therapeutic target in various lung diseases airway proteases and protease-activated receptors. Pharmacol Ther. 2007;115:70–83. doi: 10.1016/j.pharmthera.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Ramachandran R, Hollenberg MD. Proteinases and signalling pathophysiological and therapeutic implications via PARs and more . Br J Pharmacol. 2008;153 (Suppl 1):S263–282. doi: 10.1038/sj.bjp.0707507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oikonomopoulou K, Hansen KK, Saifeddine M, Tea I, Blaber M, Blaber SI, Scarisbrick I, Andrade-Gordon P, Cottrell GS, Bunnett NW, et al. Proteinase-activated receptors, targets for kallikrein signaling. J Biol Chem. 2006;281:32095–32112. doi: 10.1074/jbc.M513138200. [DOI] [PubMed] [Google Scholar]
- 33.Knight DA, Lim S, Scaffidi AK, Roche N, Chung KF, Stewart GA, Thompson PJ. Protease-activated receptors in human airways upregulation of PAR-2 in respiratory epithelium from patients with asthma. J Allergy Clin Immunol. 2001;108:797–803. doi: 10.1067/mai.2001.119025. [DOI] [PubMed] [Google Scholar]
- 34.Ricciardolo FL, Steinhoff M, Amadesi S, Guerrini R, Tognetto M, Trevisani M, Creminon C, Bertrand C, Bunnett NW, Fabbri LM, et al. Presence and bronchomotor activity of protease-activated receptor-2 in guinea pig airways. Am J Respir Crit Care Med. 2000;161:1672–1680. doi: 10.1164/ajrccm.161.5.9907133. [DOI] [PubMed] [Google Scholar]
- 35.Barrios VE, Jarosinski MA, Wright CD. Proteinase-activated receptor-2 mediates hyperresponsiveness in isolated guinea pig bronchi. Biochem Pharmacol. 2003;66:519–525. doi: 10.1016/s0006-2952(03)00292-2. [DOI] [PubMed] [Google Scholar]
- 36.Su X, Camerer E, Hamilton JR, Coughlin SR, Matthay MA. Protease-activated receptor-2 activation induces acute lung inflammation by neuropeptide-dependent mechanisms. J Immunol. 2005;175:2598–2605. doi: 10.4049/jimmunol.175.4.2598. [DOI] [PubMed] [Google Scholar]
- 37.Gu Q, Lee LY. Hypersensitivity of pulmonary chemosensitive neurons induced by activation of protease-activated receptor-2 in rats. J Physiol. 2006;574:867–876. doi: 10.1113/jphysiol.2006.110312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gatti R, Andre E, Amadesi S, Dinh TQ, Fischer A, Bunnett NW, Harrison S, Geppetti P, Trevisani M. Protease-activated receptor-2 activation exaggerates TRPV1-mediated cough in guinea pigs. J Appl Physiol. 2006;101:506–511. doi: 10.1152/japplphysiol.01558.2005. [DOI] [PubMed] [Google Scholar]
- 39.Kauffman HF, Tomee JF, van de Riet MA, Timmerman AJ, Borger P. Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. J Allergy Clin Immunol. 2000;105:1185–1193. doi: 10.1067/mai.2000.106210. [DOI] [PubMed] [Google Scholar]
- 40.Lan RS, Knight DA, Stewart GA, Henry PJ. Role of PGE(2) in protease-activated receptor-1, -2 and -4 mediated relaxation in the mouse isolated trachea. Br J Pharmacol. 2001;132:93–100. doi: 10.1038/sj.bjp.0703776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.•.Amadesi S, Cottrell GS, Divino L, Chapman K, Grady EF, Bautista F, Karanjia R, Barajas-Lopez C, Vanner S, Vergnolle N, et al. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and A-dependent mechanisms in rats and mice. J Physiol. 2006;575:555–571. doi: 10.1113/jphysiol.2006.111534. The authors used a combination of experimental approaches, including analysis of localization and subcellular distribution of kinases, measurement of Ca2+ signaling and currents, and behavioral studies in conscious animals, to reveal a major role of PKC and PKA in PAR2 signaling and PAR2-induced sensitization of TRPV1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, Kobayashi K, Obata K, Yamanaka H, Noguchi K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest. 2007;117:1979–1987. doi: 10.1172/JCI30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, Materazzi S, Altier C, Cenac N, Zamponi GW, Bautista-Cruz F, et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol. 2007;578:715–733. doi: 10.1113/jphysiol.2006.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahern GP, Brooks IM, Miyares RL, Wang XB. Extracellular cations sensitize and gate capsaicin receptor TRPV1 modulating pain signaling. J Neurosci. 2005;25:5109–5116. doi: 10.1523/JNEUROSCI.0237-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.•.Ahern GP, Wang X, Miyares RL. Polyamines are potent ligands for the capsaicin receptor TRPV1. J Biol Chem. 2006;281:8991–8995. doi: 10.1074/jbc.M513429200. These authors show that polyamines directly activate TRPV1 in a charge-dependent manner and sensitize this receptor at a low concentration. Their experiments with TRPV1 mutants identified extracellular acidic residues (D646N and E648A) critical for polyamine regulation. [DOI] [PubMed] [Google Scholar]
- 46.Kurosawa M, Shimizu Y, Tsukagoshi H, Ueki M. Elevated levels of peripheral-blood, naturally occurring aliphatic polyamines in bronchial asthmatic patients with active symptoms. Allergy. 1992;47:638–643. doi: 10.1111/j.1398-9995.1992.tb02388.x. [DOI] [PubMed] [Google Scholar]
- 47.Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol. 2000;105:651–663. doi: 10.1067/mai.2000.105712. [DOI] [PubMed] [Google Scholar]
- 48.Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323:1033–1039. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- 49.Gu Q, Wiggers ME, Gleich GJ, Lee LY. Sensitization of isolated rat vagal pulmonary sensory neurons by eosinophil-derived cationic proteins. Am J Physiol Lung Cell Mol Physiol. 2008;294:L544–552. doi: 10.1152/ajplung.00271.2007. [DOI] [PubMed] [Google Scholar]
- 50.Mitchell JE, Campbell AP, New NE, Sadofsky LR, Kastelik JA, Mulrennan SA, Compton SJ, Morice AH. Expression and characterization of the intracellular vanilloid receptor (TRPV1) in bronchi from patients with chronic cough. Exp Lung Res. 2005;31:295–306. doi: 10.1080/01902140590918803. [DOI] [PubMed] [Google Scholar]
- 51.•.Watanabe N, Horie S, Spina D, Michael GJ, Page CP, Priestley JV. Immunohistochemical localization of transient receptor potential vanilloid subtype 1 in the trachea of ovalbumin-sensitized Guinea pigs. Int Arch Allergy Immunol. 2008;146(Suppl 1):28–32. doi: 10.1159/000126057. This study demonstrated that TRPV1-immunoreactive axons were more densely stained, and increased in number in the tracheal epithelium of ovalbumin-sensitized guinea pigs. [DOI] [PubMed] [Google Scholar]
- 52.Winter J. Brain derived neurotrophic factor, but not nerve growth factor, regulates capsaicin sensitivity of rat vagal ganglion neurones. Neurosci Lett. 1998;241:21–24. doi: 10.1016/s0304-3940(97)00978-6. [DOI] [PubMed] [Google Scholar]
- 53.Winston J, Toma H, Shenoy M, Pasricha PJ. Nerve growth factor regulates VR-1 mRNA levels in cultures of adult dorsal root ganglion neurons. Pain. 2001;89:181–186. doi: 10.1016/s0304-3959(00)00370-5. [DOI] [PubMed] [Google Scholar]
- 54.Anand U, Otto WR, Casula MA, Day NC, Davis JB, Bountra C, Birch R, Anand P. The effect of neurotrophic factors on morphology, TRPV1 expression and capsaicin responses of cultured human DRG sensory neurons. Neurosci Lett. 2006;399:51–56. doi: 10.1016/j.neulet.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 55.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 57.Bonini S, Lambiase A, Bonini S, Angelucci F, Magrini L, Manni L, Aloe L. Circulating nerve growth factor levels are increased in humans with allergic diseases and asthma. Proc Natl Acad Sci USA. 1996;93:10955–10960. doi: 10.1073/pnas.93.20.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lommatzsch M, Schloetcke K, Klotz J, Schuhbaeck K, Zingler D, Zingler C, Schulte-Herbruggen O, Gill H, Schuff-Werner P, Virchow JC. Brain-derived neurotrophic factor in platelets and airflow limitation in asthma. Am J Respir Crit Care Med. 2005;171:115–120. doi: 10.1164/rccm.200406-758OC. [DOI] [PubMed] [Google Scholar]