Abstract
Frailty is characterized by vulnerability to acute stressors and is a consequence of decline in overall function and physiologic reserves. An estimated 7% of the US population older than 65 years and 30% of octogenarians are frail. The domains to define frailty include mobility, strength, balance, motor processing, cognition, nutrition, endurance, and physical activity. Pathophysiologic pathways leading to frailty involve a multisystem cascade that includes neuroendocrine dysfunction with lower insulinlike growth factor and dehydroepiandrosterone sulfate and an altered inflammatory milieu with increased levels of C-reactive protein, interleukins, tumor necrosis factor α, and abnormal coagulation. Frailty predicts death and heralds the transition to disability in general populations. As the population with coronary artery disease shifts toward older patients, physicians must consider the role of frailty in their patients. This review will enable clinicians to recognize frailty and consider its relevance in their daily practice. We also elaborate on reasons to consider frailty in older adults with cardiovascular disease and focus on its early identification, on referral to specialists, and on care after serious cardiac events.
During the past century, the average life expectancy has lengthened, resulting in a larger older population presenting with acute and chronic cardiovascular diseases. Variation in the health status of older patients is apparent, ranging from robust to frail. An estimated 7% of the US population older than 65 years and 30% of octogenarians are frail.1 Frailty, a construct well described and debated in the geriatric literature, is characterized by vulnerability to acute stressors and is a consequence of decline in overall function and physiologic reserves. Frail patients have a reduced ability to maintain homeostasis in the face of acute stress. Cardiovascular practice guidelines acknowledge the importance of global health status in older adults, stating that decisions on management in the elderly should reflect considerations of general health, comorbidities, cognitive status, and life expectancy.2-4As the population with coronary artery disease (CAD) ages, cardiologists must be ready to consider the role of frailty in their patients.5 In light of this situation, this review examines the definitions, incidence and prevalence, proposed pathophysiologic pathways, and outcomes associated with frailty. In so doing, it will enable clinicians to recognize frailty and consider its relevance in their daily practice.
English-language publications in PubMed and references from relevant articles published between 1970 and 2007 were reviewed. Main search terms were frailty, comorbidity, falls, endocrine or inflammatory markers of frailty, and sarcopenia. Articles were selected on the basis of quality, relevance to the central concept of frailty, importance in illustrating a proposed pathophysiology, or the level of attention that they had previously received in the field.
FRAILTY AS A CONCEPT
From observing people who have aged successfully, it is apparent that chronological age correlates loosely with biological age.6 This was highlighted in a population of older adults, in which adjustment for 27 biological risk factors including comorbidity, social status, lifestyle and disease factors, cognition, and frailty substantially reduced the association between chronological age and 5-year mortality (ages 80-84 years: unadjusted relative risk, 4.1; adjusted relative risk, 1.7).7 This suggests that factors beyond age explain much of the increased mortality associated with age. There is growing recognition that a biological state, referred to as frailty, affects older populations.
Although the definition of frailty continues to evolve, its hallmark is decreased resistance to stressors. All organisms start out with redundancy of structure and function, which serves them well during environmental stresses. A review of human bodily systems reveals that one third of organ capacity is adequate for normal organ function.8 The remaining reserves of organ function vary according to lifetime exposures to injury and illness. As the biological reserves are diminished, any challenge can exceed the capacity for recovery. Those without remaining buffers are prone to negative health outcomes, such as disability and death (Figure 1).9 The limited reserves could easily become apparent only when a stressful event (such as acute cardiac illness) unmasks them, as in myocardial infarction. Frailty is distinct from the related concepts of comorbidity, defined as the burden of coexisting medical illnesses, and disability, the limited ability for self-care.10 These distinctions are paramount and are elaborated further in this review.
FIGURE 1.
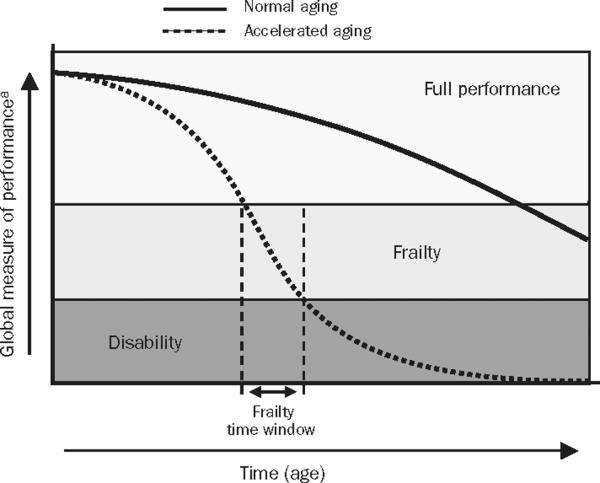
Trajectories of health and functioning. Full performance = high functional reserve that allows patients to face environmental perturbations with ease; frailty = patients are at high risk of homeostasis disruption and consequent negative health outcomes, including disability and death, probably from exhaustion of functional reserve. From J Endocrinol Invest,9 with permission from the Italian Society of Endocrinology.
a Can be physical, cognitive, social, or quality of life.
FRAILTY DEFINITIONS
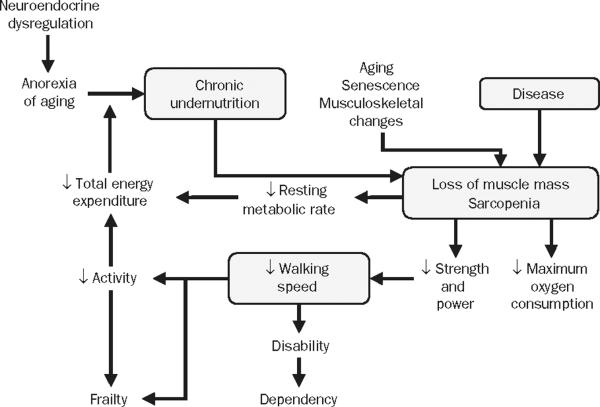
There is no single best definition of frailty, as this construct is a constellation of clinical attributes. Frailty does not fit easily with the typical organ-specific model of disease. The frailty phenotype represents the complex relationship among sarcopenia, physical activity, nutritional intake, and energy expenditure. Sarcopenia leads to poor muscle strength, which limits mobility and physical activity, thereby reducing energy expenditure and nutritional intake. This leads in turn to weight loss and worsening sarcopenia. In 2001, investigators from Johns Hopkins developed a conceptual framework of frailty combining attributes of body composition, nutrition, and mobility into an explanatory pathophysiologic phenotype (Figure 2).1 Table 1 lists some other definitions as well.1,11-17
FIGURE 2.
Cycle of frailty combines elements of body composition, nutrition, and mobility into a pathophysiologic pathway. In this pathway, sarcopenia and poor muscle strength, by limiting mobility and physical activity, reduce total energy expenditure and nutritional intake, thereby causing weight loss and further sarcopenia. In defining frailty, involvement of musculoskeletal system is central and paramount. Loss of movement capacity frequently accelerates decline in other organ systems. From J Gerontol A Biol Sci Med Sci,1 with permission of the Gerontological Society of America. Copyight © 2001.
TABLE 1.
Prevalence, Incidence, and Various Definitions of Frailtya
| Reference | Prevalence (%) | Incidence (%) | Data source | Measure of frailty |
|---|---|---|---|---|
| Fried etal,1 2001 | 6.9 | 4-y, 7.2 | CHS | Gait speed, weight loss, physical activity, exhaustion, grip strength |
| Newman etal,11 2001 | 11.0 | NA | CHS | Same as above |
| Jumadilova et al,12 2005 | 30.0 | NA | Nursing home residents | Urinary incontinence |
| Holroyd-Leduc et al,13 2004 | 14.8 | NA | AHEAD study | Urinary incontinence |
| Studenski etal,14 2004 | NA | NA | CGIC-PF instrument | Six intrinsic and 7 consequence domains |
| Jones et al,15 2004 | 17.0, mild | NA | MGAT trial | FI-CGA, comprising 10 standard domains |
| 58.0, moderate | ||||
| 25.0, severe | ||||
| Puts et al,16 2005 | 19.0 | 14.1 | LASA | Nine frailty markers: body weight, peak expiratory flow, cognition, vision and hearing problems, incontinence, sense of mastery, depressive symptoms, and physical activity |
| Woods etal,17 2005 | 16.3 | 14.8 | WHI observational study | Criteria for frailty same as proposed by Fried et al1 |
AHEAD = Asset and Health Dynamics Among the Oldest Old; CGIC-PF = Clinical Global Impression of Change in Physical Frailty; CHS = Cardiovascular Health Study; FI-CGA = Frailty Index-Complete Geriatric Assessment; LASA = Longitudinal Aging Study Amsterdam; MGAT = Mobile Geriatric Assessment Team; NA = not available; WHI = Women's Health Initiative.
Previously, health care professionals relied on subjective assessments to identify frailty, but a recent consensus report lists 8 domains in this phenotype: mobility, strength, balance, motor processing, cognition, nutrition, endurance, and physical activity.4 Performance measurements of muscle strength, such as grip strength and gait speed, are included with clinical history and functional status measures. Performance assessment identifies variations in strength at or below the level of daily function. This is important because sarcopenia, defined as age-related decline in lean muscle mass, is a key component of the frailty phenotype. During a person's lifetime, muscle strength and muscle mass are highest in the teen years and begin to decrease after age 30 years. After age 50 years, muscle strength decreases at a rate of 10% to 15% per decade of life, paralleled by a decline in the number of fast-twitch fibers.18-20
INCIDENCE AND PREVALENCE
Multiple definitions for frailty have resulted in varying estimates of its prevalence. The prevalence of frailty in the recent Longitudinal Aging Study Amsterdam (LASA) involving 1720 community-dwelling adults older than 65 years was 19%16; however, it was 6.9% in the older Cardiovascular Health Study (CHS) that used different criteria (Table 2).1 Differences in the populations (communitydwelling residents vs nursing home residents) and age ranges (>65 years to centenarians) also limit estimates. There is a paucity of data on frailty in cardiovascular populations. In contrast to the overall CHS group, subgroups with cardiovascular disease had higher prevalence of frailty.11 In 2 small prospective studies, frailty, as defined by the CHS criteria, was observed in 20% of older patients (≥65 years) undergoing percutaneous coronary interventions21 and 27% of older patients (≥70 years) with serious CAD at cardiac catheterization.22
TABLE 2.
Definition of Frailty as Proposed by the Cardiovascular Health Study
| Unintentional weight loss | 4.5 kg in the past year |
| Exhaustion | CES-D |
| Physical activity | Minnesota Leisure Time Activity questionnaire |
| Walk time | Cutoff for time to walk 4.6 m criteria |
| Men, stratified by height | |
| ≤173 cm | ≥7 s |
| ≤173 cm | ≤6 s |
| Women | |
| ≤159cm | ≥7 s |
| >159cm | ≤6 s |
| Grip strength | Cutoff for grip strength (kg) |
| Men, stratified by BMI | criterion for frailty |
| ≤24.0 | ≤29.0 |
| 24.1-26.0 | ≤30.0 |
| 26.1-28.0 | ≤30.0 |
| >28.0 | ≤32.0 |
| Women, stratified by BMI | |
| ≤23.0 | ≤17.0 |
| 23.1-26.0 | 17.3 |
| 26.1-29.0 | 18.0 |
| >29.0 | 21.0 |
| Frailty | 3 or more core elements |
| Intermediate frailty (prefrail) | 1 or 2 core elements |
BMI = body mass index; CES-D = Center for Epidemiologic Studies Depression Scale.
Data from J Gerontol A Biol Sci Med Sci.1
FRAILTY AND COMORBIDITY
The distinction between frailty and comorbidity is paramount despite overlap between constructs of comorbid conditions, frailty, and disability as demonstrated in the CHS population.10 Of 2576 patients with comorbidity, 249 were frail. Frail patients were more likely to have a history of cardiovascular disease (31% vs 15%), chronic heart failure (14% vs 1%), diabetes (32% vs 19%), and hypertension (49% vs 37%) than their counterparts who were not frail.23 In addition to higher prevalence of cardiovascular disease, frail elderly patients had more subclinical cardiovascular disease identified by carotid ultrasonography, electrocardiography, and echocardiography and had more infarct-like lesions visible on brain magnetic resonance imaging.11 In a separate study, the presence of CAD was associated with a greater likelihood of subsequent decline in gait speed over time, a major factor in the frailty phenotype.24 Weakness and exhaustion, characteristics of frailty, are symptoms of heart disease as well.25 Additionally, exacerbation of heart disease can unmask frailty.
BIOLOGICAL UNDERPINNINGS OF FRAILTY
The “cycle of frailty” (Figure 2) describes a multisystem cascade that includes neuroendocrine dysfunction with lower insulinlike growth factor (IGF) and dehydroepiandrosterone sulfate and an altered inflammatory milieu with increased levels of C-reactive protein (CRP), interleukins, tumor necrosis factor α, and abnormal coagulation.1,26 There are several reasons to suspect inflammatory and endocrine links with frailty. First, inflammatory markers (such as interleukin-6 and CRP) are elevated with normal aging, doubling between ages 40 and 65 years.9 The exact stimuli for higher inflammatory markers with aging are not well known and could reflect a rising burden of tissue damage, acute or chronic infection, or general deterioration in homeostatic mechanisms for repair.27-30 Second, high levels of such markers are also associated with cardiovascular events and mortality.31 Several studies have linked elevated levels of CRP with future incident adverse cardiovascular events.32 A common denominator for frailty and cardiovascular disease could be higher levels of inflammatory markers (eg, CRP). Therefore, studies of how frailty, inflammation, and CAD are related are needed. Third, high levels of cytokines can induce skeletal muscle loss and neuroendocrine dysregulation.33 Despite interesting theoretical links, the data on biomarkers in frailty are cross-sectional and preliminary (Table 3).16,34-40
TABLE 3.
Biomarkers and Frailtya
| Reference | Biomarkers studied | Data source | Observations in frailty |
|---|---|---|---|
| Semba et al,34 2005 | CD4+andCD8+ T cells | Women's Health and Aging Studies | CD8+ and CD28- higher |
| Leng et al,35 2004 | IGF-1, DHEA-S, IL-6 | Community-dwelling adults | IGF-1 and DHEA-S lower |
| Leng et al,36 2004 | IL-6, TNF-α, andIL-10 | Community-dwelling adults | Lower LPS-induced PBMC proliferation ratio and higher IL-6 |
| Cohen et al,37 2003 | IL-6 and D-dimer measurement | Duke Established Populations for Epidemiologic Studies of the Elderly | Highest levels of IL-6 and D-dimer had increased mortality |
| Bruunsgaard et al,38 2003 | TNF-α and IL-6 measurement | Danish Centenarian Study | TNF-α: an independent prognostic marker for mortality |
| Walston et al,39 2002 | CRP, fibrinogen, factors VII and VIII | Cardiovascular Health Study | Higher CRP, fibrinogen, factor VIII, and D-dimer in frail patients |
| van den Biggelaar et al,40 2004 | LPS-induced IL-1β, IL-6, TNF-α, IL-1RA, IL-10 | Leiden 85-plus study | Low levels of LPS-induced markers associated with higher mortality |
| Puts et al,16 2005 | 25(OH)D, CRP, IL-6, and IGF-1 | LASA | Low 25(OH)D associated with prevalent/incident frailty; elevated CRP with incident frailty |
CRP = C-reactive protein; DHEA-S = dehydroandrosterone sulfate; IGF = insulinlike growth factor; IL = interleukin; LASA = Longitudinal Aging Study Amsterdam; LPS = lipopolysaccharide; 25(OH)D = 25-hydroxyvitamin D; PBMC = peripheral blood mononuclear cells; TNF = tumor necrosis factor.
The role of hormones in frailty has also been studied. In studies of longitudinal aging, free testosterone index, IGF, and physical activity were predictors of sarcopenia.41,42 In the Women's Health and Aging Study, low IGF-1 levels were associated with poor knee extensor muscle strength, slow movement, and difficulty with mobility.43 Despite low hormone levels in frail individuals, replacement hormones have not been shown to be useful.44 In a 2-year placebo-controlled, randomized trial involving 144 elderly men and women, neither dehydroepiandrosterone sulfate nor low-dose testosterone replacement had any physiologically relevant beneficial effects on body composition, physical performance, insulin sensitivity, or quality of life.44 More recently, meta-analyses suggested that testosterone/dihydrotestosterone therapy produced a moderate increase in muscle strength in men participating in 11 randomized trials.45 Testosterone supplementation during 6 months in older men with a low normal testosterone concentration did not affect functional status or cognition but increased lean body mass and had mixed metabolic effects.
Alterations in the biological milieu have associations with frailty, but these links remain theoretical; research is needed to elucidate the biological mechanisms contributing to the development and worsening of frailty.
EFFECT OF FRAILTY ON OUTCOMES
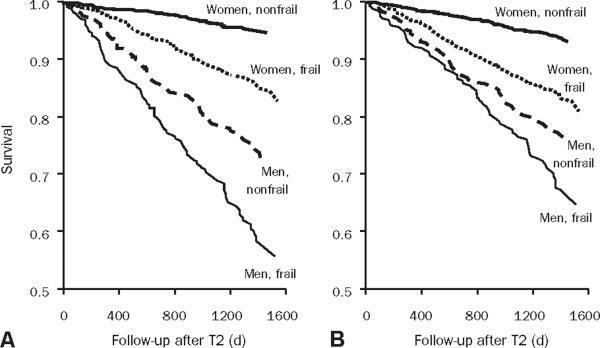
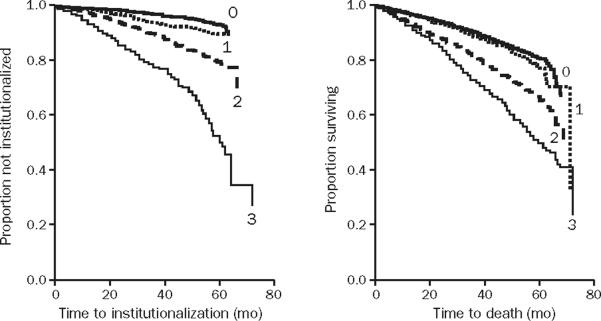
Patient-reported functional status and comorbid conditions are prognostic determinants of death in cardiovascular populations.23,46,47 Frailty predicts death and heralds the transition to disability in general populations.48 In the Women's Health Initiative, frail patients were almost twice as likely to die as patients not determined to be frail.17 Among 1720 respondents in LASA, 5-year mortality in men with frailty was 50% as compared with 15% in nonfrail men; findings were similar in women (Figure 3).16,49 Frailty also predicts falls, poor function for activities of daily living (ADL), and hospitalization. In the Canadian comprehensive sampling of 9008 community residents stratified by age, a dose-response association between frailty and subsequent institutionalization and death was observed (Figure 4).50,51 Frailty predicts adverse outcomes incrementally and independently from coexisting medical conditions. In a prospective cohort study of 5886 older adults, objective measures of subclinical disease and disease severity were independent and joint predictors of 5-year mortality (12%), along with male sex, relative poverty, physical activity, smoking, indicators of frailty, and disability.7 The Health and Retirement Study used the 1998 wave of 11,701 persons older than 50 years and incorporated both comorbid conditions and functional measures to develop and validate a prognostic index for 4-year mortality.52 Data are sparse on the prognostic relevance of frailty on CAD events. Two studies from the Cooperative Cardiovascular Project found frailty (defined as urinary incontinence, inability to walk, and low body mass index) to be a predictor of 1-year mortality and stroke after acute myocardial infarction.53,54
FIGURE 3.
The Longitudinal Aging Study Amsterdam demonstrated poor survival rates among both men and women who were frail according to 9 frailty markers defined as static or dynamic and who consisted of 2257 respondents participating in 2 cycles: T1 in 1992-1993 and T2 in 1995-1996. A, Survival according to frailty status at T2. B, Survival according to frailty status at T1-T2. Fom J Am Geriatr Soc,49 with permission from Wiley-Blackwell Publishing.
FIGURE 4.
Canadian comprehensive sampling of 9008 community residents. Adjustments have been made for age and sex. Frailty scale in this study is based on geriatric status scale and demonstrates dose-response relationship between grades of frailty and subsequent institutionalization and death. 0 = those who walk without help, perform activities of daily living (ADL), are continent, and are not cognitively impaired; 1 = those with bladder incontinence only; 2 = those with 1 (2 if incontinent) or more of the following: need for assistance with mobility or ADL, cognitive impairment with dementia, or bowel or bladder incontinence; 3 = those with 2 (3 if incontinent) or more of totally dependent for transfers or 1 or more ADL, incontinence of bowel and bladder, and diagnosis of dementia. From The Lancet,50 with permission from Elsevier Limited.
RELEVANCE OF FRAILTY TO CARDIOVASCULAR CARE
Reasons to consider frailty in older adults with cardiovascular disease include its early identification, consideration of referral to specialists, and anticipation of care after major cardiac events. Although frailty definitions are useful in research, clinicians still rely on impressions to determine the vigor of individual patients. Yet frailty, and vulnerability that accompanies it, can be present before functional limitations or disability are apparent. Early recognition is hampered by the overlap with comorbidity and disability. Unintended weight loss, disability in ADLs or instrumental ADLs, and presence of multiple comorbid conditions in a complex cardiac patient should alert physicians to the possibility of associated frailty. Clinicians could screen older adults by performing simple tests, such as grip strength, gait speed, or quadriceps strength. Early recognition of advanced biological age would enable referral to a geriatric specialist, skilled in understanding the contributors to age-related declines, who could offer multidisciplinary interventions to slow or reverse the functional decline. Comprehensive geriatric assessments are geared to improving physical performance and quality of life. Many age-associated impairments are dynamic. In a community setting, up to 25% of elderly people who develop a new disability subsequently demonstrate full functional recovery. Assessment of psychosocial support after acute cardiac events can reduce admission to long-term care facilities.
Clinicians can also consider frailty in decision making. Accurate risk prediction has particular relevance to older adults because of unique risks, heterogeneity, and the value placed on preservation of independence. Assessing frailty can improve risk prediction in vulnerable patients who are elderly. Whereas treatment of frailty itself is limited, assessment can facilitate a multidisciplinary approach to care and direct greater attention to comorbid illnesses.
Early recognition of frailty is paramount, as it can enable identification of some treatable disorders associated with frailty, such as malignancy or major depression, and can allow physicians to discuss and introduce geriatric or palliative care approaches with patients and their families (Table 4). Timely recognition of early frailty can provide opportunities to ensure tailored, symptom-driven care geared toward improving the quality of life of elderly patients. Frailty can be arbitrarily divided into 3 stages— early, referring to the time of frailty recognition; middle, referring to the onset of functional decline; and late, referring to increasing functional decline, life-threatening illness, and imminent death.55 Several therapeutic approaches are being studied in patients diagnosed as being frail. They include various forms of physical and strengthening exercises and nutritional or hormonal supplementation (eg, testosterone), especially in hormone-deficient elderly men.56-60 The recognition, pathophysiology, and management of patients diagnosed as being frail is still evolving. With further insights into mechanisms and management, more concrete evidence-based guidelines will be available to aid in decision making about how to treat cardiac patients who meet criteria for frailty.
TABLE 4.
Key Points About Frailty
| Frailty is an evolving concept without disease-based treatment or diagnosis It is characterized by a reduced ability to maintain or regain homeostasis in the face of perturbations and is distinct from comorbidities and disability |
| Frailty is associated with higher mortality, disability, and hospitalization in the cardiac population |
| Early recognition of frailty may enable referral to a geriatric specialist, anticipation of care, and the initiation of interventions to delay its progression |
| Decision making should consider the multidimensional health status of older adults, including the contribution of frailty |
CONCLUSION
The aging of the population, with its high burden of vascular disease, will increase the number of older adults with cardiovascular disease and frailty. Some of the most troubling gaps in applying evidence-based treatments stem from a disconnect between biological and chronological age. Identifying impairment caused by aging in cardiovascular populations and its contribution to risk is thus of tremendous clinical importance. Safe, effective, equitable, and patient-centered health care can be achieved for this demographic group only by observing relevant outcomes within the framework of the multidimensional health issues of older adults.
Glossary
- ADL
activities of daily living
- CAD
coronary artery disease
- CHS
Cardiovascular Health Study
- CRP
C-reactive protein
- IGF
insulinlike growth factor
- LASA
Longitudinal Aging Study Amsterdam
Footnotes
Individual reprints of this article are not available.
REFERENCES
- 1.Fried LP, Tangen CM, Walston J, et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Alexander KP, Newby LK, Cannon CP, et al. Acute coronary care in the elderly, part I: non-ST-segment-elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115(19):2549–2569. doi: 10.1161/CIRCULATIONAHA.107.182615. [DOI] [PubMed] [Google Scholar]
- 3.Alexander KP, Newby LK, Armstrong PW, et al. Acute coronary care in the elderly, part II: ST-segment-elevation myocardial infarction: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115(19):2570–2589. doi: 10.1161/CIRCULATIONAHA.107.182616. [DOI] [PubMed] [Google Scholar]
- 4.Ferrucci L, Guralnik JM, Studenski S, Fried LP, Cutler GB, Jr, Walston JD. Interventions on Frailty Working Group. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc. 2004;52(4):625–634. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 5.Roger VL, Jacobsen SJ, Weston SA, Bailey KR, Kottke TE, Frye RL. Trends in heart disease deaths in Olmsted County, Minnesota, 1979-1994. Mayo Clin Proc. 1999;74(7):651–657. doi: 10.4065/74.7.651. [DOI] [PubMed] [Google Scholar]
- 6.Mitnitski AB, Graham JE, Mogilner AJ, Rockwood K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. 2002;2:1. doi: 10.1186/1471-2318-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried LP, Kronmal RA, Newman AB, et al. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279(8):585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 8.Bortz WM., II A conceptual framework of frailty: a review. J Gerontol A Biol Sci Med Sci. 2002;57(5):M283–M288. doi: 10.1093/gerona/57.5.m283. [DOI] [PubMed] [Google Scholar]
- 9.Ferrucci L, Cavazzini C, Corsi A, et al. Biomarkers of frailty in older persons. J Endocrinol Invest. 2002;25(10):10–15. (suppl) [PubMed] [Google Scholar]
- 10.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 11.Newman AB, Gottdiener JS, McBurnie MA, et al. Cardiovascular Health Study Research Group. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56(3):M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 12.Jumadilova Z, Zyczynski T, Paul B, Narayanan S. Urinary incontinence in the nursing home: resident characteristics and prevalence of drug treatment. Am J Manag Care. 2005;11(4 suppl):S112–S120. [PubMed] [Google Scholar]
- 13.Holroyd-Leduc JM, Mehta KM, Covinsky KE. Urinary incontinence and its association with death, nursing home admission, and functional decline. J Am Geriatr Soc. 2004;52(5):712–718. doi: 10.1111/j.1532-5415.2004.52207.x. [DOI] [PubMed] [Google Scholar]
- 14.Studenski S, Hayes RP, Leibowitz RQ, et al. Clinical global impression of change in physical frailty: development of a measure based on clinical judgment. J Am Geriatr Soc. 2004;52(9):1560–1566. doi: 10.1111/j.1532-5415.2004.52423.x. [DOI] [PubMed] [Google Scholar]
- 15.Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc. 2004;52(11):1929–1933. doi: 10.1111/j.1532-5415.2004.52521.x. [DOI] [PubMed] [Google Scholar]
- 16.Puts MT, Visser M, Twisk JW, Deeg DJ, Lips P. Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol (Oxf) 2005;63(4):403–411. doi: 10.1111/j.1365-2265.2005.02355.x. [DOI] [PubMed] [Google Scholar]
- 17.Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc. 2005;53(8):1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 18.Melton LJ, III, Khosla S, Crowson CS, O'Connor MK, O'Fallon WM, Riggs BL. Epidemiology of sarcopenia. J Am Geriatr Soc. 2000;48(6):625–630. [PubMed] [Google Scholar]
- 19.Reuben DB, Frank JC, Hirsch SH, McGuigan KA, Maly RC. A randomized clinical trial of outpatient comprehensive geriatric assessment coupled with an intervention to increase adherence to recommendations. J Am Geriatr Soc. 1999;47(3):269–276. doi: 10.1111/j.1532-5415.1999.tb02988.x. [DOI] [PubMed] [Google Scholar]
- 20.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–763. doi: 10.1093/oxfordjournals.aje.a009520. published correction appears in Am J Epidemiol. 1999;149(12):1161] [DOI] [PubMed] [Google Scholar]
- 21.Singh MRV, Rihal C, Lennon R, et al. Correlates of frailty in patients with coronary heart disease undergoing percutaneous coronary interventions. Circulation. 2007;115(21):E556. [Google Scholar]
- 22.Purser JL, Kuchibhatla MN, Fillenbaum GG, Harding T, Peterson ED, Alexander KP. Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriatr Soc. 2006;54(11):1674–1681. doi: 10.1111/j.1532-5415.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 23.Rumsfeld JS, MaWhinney S, McCarthy M, Jr, et al. Participants of the Department of Veterans Affairs Cooperative Study Group on Processes, Structures, and Outcomes of Care in Cardiac Surgery. Health-related quality of life as a predictor of mortality following coronary artery bypass graft surgery. JAMA. 1999;281(14):1298–1303. doi: 10.1001/jama.281.14.1298. [DOI] [PubMed] [Google Scholar]
- 24.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 25.Brieger D, Eagle KA, Goodman SG, et al. GRACE Investigators. Acute coronary syndromes without chest pain, an underdiagnosed and undertreated high-risk group: insights from the Global Registry of Acute Coronary Events. Chest. 2004;126(2):461–469. doi: 10.1378/chest.126.2.461. [DOI] [PubMed] [Google Scholar]
- 26.Walston J, Fried LP. Frailty and the older man. Med Clin North Am. 1999;83(5):1173–1194. doi: 10.1016/s0025-7125(05)70157-7. [DOI] [PubMed] [Google Scholar]
- 27.Cohen HJ. In search of the underlying mechanisms of frailty [editorial] J Gerontol A Biol Sci Med Sci. 2000;55(12):M706–M708. doi: 10.1093/gerona/55.12.m706. [DOI] [PubMed] [Google Scholar]
- 28.Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106(5):506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 29.Pieper CF, Rao KM, Currie MS, Harris TB, Chen HJ. Age, functional status, and racial differences in plasma D-dimer levels in community-dwelling elderly persons. J Gerontol A Biol Sci Med Sci. 2000;55(11):M649–M657. doi: 10.1093/gerona/55.11.m649. [DOI] [PubMed] [Google Scholar]
- 30.Goldschmidt-Clermont PJ, Peterson ED. On the memory of a chronic illness. Sci Aging Knowledge Environ. 2003 Nov 12;2003(45):re8. doi: 10.1126/sageke.2003.45.re8. [DOI] [PubMed] [Google Scholar]
- 31.Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003 Nov 11;108(19):2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. Epub 2003 Oct 20. [DOI] [PubMed] [Google Scholar]
- 32.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98(8):731–733. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- 33.Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-α with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57(5):M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 34.Semba RD, Margolick JB, Leng S, Walston J, Ricks MO, Fried LP. T cell subsets and mortality in older community-dwelling women. Exp Gerontol. 2005;40(12):81–87. doi: 10.1016/j.exger.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Leng SX, Cappola AR, Andersen RE, et al. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16(2):153–157. doi: 10.1007/BF03324545. [DOI] [PubMed] [Google Scholar]
- 36.Leng SX, Yang H, Walston JD. Decreased cell proliferation and altered cytokine production in frail older adults. Aging Clin Exp Res. 2004;16(3):249–252. doi: 10.1007/BF03327392. [DOI] [PubMed] [Google Scholar]
- 37.Cohen HJ, Harris T, Pieper CF. Coagulation and activation of inflammatory pathways in the development of functional decline and mortality in the elderly. Am J Med. 2003;114(3):180–187. doi: 10.1016/s0002-9343(02)01484-5. [DOI] [PubMed] [Google Scholar]
- 38.Bruunsgaard H, Andersen-Ranberg K, Hjelmborg JB, Pedersen BK, Jeune B. Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am J Med. 2003;115(4):278–283. doi: 10.1016/s0002-9343(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 39.Walston J, McBurnie MA, Newman A, et al. Cardiovascular Health Study Investigators. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162(20):2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 40.van den Biggelaar AH, Huizinga TW, de Craen AJ, et al. Impaired innate immunity predicts frailty in old age: the Leiden 85-plus study. Exp Gerontol. 2004;39(9):1407–1414. doi: 10.1016/j.exger.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 41.Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. 1999;107(2):123–136. doi: 10.1016/s0047-6374(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 42.Waters DL, Baumgartner RN, Garry PJ. Sarcopenia: current perspectives. J Nutr Health Aging. 2000;4(3):133–139. [PubMed] [Google Scholar]
- 43.Cappola AR, Bandeen-Roche K, Wand GS, Volpato S, Fried LP. Association of IGF-I levels with muscle strength and mobility in older women. J Clin Endocrinol Metab. 2001;86(9):4139–4146. doi: 10.1210/jcem.86.9.7868. [DOI] [PubMed] [Google Scholar]
- 44.Nair KS, Rizza RA, O'Brien P, et al. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355(16):1647–1659. doi: 10.1056/NEJMoa054629. [DOI] [PubMed] [Google Scholar]
- 45.Ottenbacher KJ, Ottenbacher ME, Ottenbacher AJ, Acha AA, Ostir GV. Androgen treatment and muscle strength in elderly men: a meta-analysis. J Am Geriatr Soc. 2006;54(11):1666–1673. doi: 10.1111/j.1532-5415.2006.00938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh M, Reeder GS, Jacobsen SJ, Weston S, Killian J, Roger VL. Scores for post-myocardial infarction risk stratification in the community. Circulation. 2002;106(18):2309–2314. doi: 10.1161/01.cir.0000036598.12888.de. [DOI] [PubMed] [Google Scholar]
- 47.Sachdev M, Sun JL, Tsiatis AA, Nelson CL, Mark DB, Jollis JG. The prognostic importance of comorbidity for mortality in patients with stable coronary artery disease. J Am Coll Cardiol. 2004;43(4):576–582. doi: 10.1016/j.jacc.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 48.Gill TM, Williams CS, Tinetti ME. Assessing risk for the onset of functional dependence among older adults: the role of physical performance. J Am Geriatr Soc. 1995;43(6):603–609. doi: 10.1111/j.1532-5415.1995.tb07192.x. published correction appears in J Am Geriatr Soc. 1995;43(10):1172. [DOI] [PubMed] [Google Scholar]
- 49.Puts MT, Lips P, Deeg DJ. Sex differences in the risk of frailty for mortality independent of disability and chronic diseases. J Am Geriatr Soc. 2005;53(1):40–47. doi: 10.1111/j.1532-5415.2005.53008.x. [DOI] [PubMed] [Google Scholar]
- 50.Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hebert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people [letter] Lancet. 1999;353(9148):205–206. doi: 10.1016/S0140-6736(98)04402-X. [DOI] [PubMed] [Google Scholar]
- 51.Vanitallie TB. Frailty in the elderly: contributions of sarcopenia and visceral protein depletion. Metabolism. 2003;52(10):22–26. doi: 10.1016/s0026-0495(03)00297-x. suppl 2. [DOI] [PubMed] [Google Scholar]
- 52.Lee SJ, Lindquist K, Segal MR, Covinsky KE. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA. 2006;295(7):801–808. doi: 10.1001/jama.295.7.801. published correction appears in JAMA. 2006;295(16):1900] [DOI] [PubMed] [Google Scholar]
- 53.Krumholz HM, Chen J, Chen YT, Wang Y, Radford MJ. Predicting oneyear mortality among elderly survivors of hospitalization for an acute myocardial infarction: results from the Cooperative Cardiovascular Project. J Am Coll Cardiol. 2001;38(2):453–459. doi: 10.1016/s0735-1097(01)01395-x. [DOI] [PubMed] [Google Scholar]
- 54.Lichtman JH, Krumholz HM, Wang Y, Radford MJ, Brass LM. Risk and predictors of stroke after myocardial infarction among the elderly: results from the Cooperative Cardiovascular Project. Circulation. 2002;105(9):1082–1087. doi: 10.1161/hc0902.104708. [DOI] [PubMed] [Google Scholar]
- 55.Boockvar KS, Meier DE. Palliative care for frail older adults: “there are things I can't do anymore that I wish I could …. JAMA. 2006;296(18):2245–2253. doi: 10.1001/jama.296.18.2245. [DOI] [PubMed] [Google Scholar]
- 56.Binder EF, Schechtman KB, Ehsani AA, et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc. 2002;50(12):1921–1928. doi: 10.1046/j.1532-5415.2002.50601.x. [DOI] [PubMed] [Google Scholar]
- 57.Binder EF, Yarasheski KE, Steger-May K, et al. Effects of progressive resistance training on body composition in frail older adults: results of a randomized, controlled trial. J Gerontol A Biol Sci Med Sci. 2005;60(11):1425–1431. doi: 10.1093/gerona/60.11.1425. [DOI] [PubMed] [Google Scholar]
- 58.Butler RN. Fighting frailty: prescription for healthier aging includes exercise, nutrition, safety, and research [editorial] Geriatrics. 2000;55(2):20. [PubMed] [Google Scholar]
- 59.Fiatarone MA, O'Neill EF, Doyle N, et al. The Boston FICSIT study: the effects of resistance training and nutritional supplementation on physical frailty in the oldest old. J Am Geriatr Soc. 1993;41(3):333–337. doi: 10.1111/j.1532-5415.1993.tb06714.x. [DOI] [PubMed] [Google Scholar]
- 60.Wolf SL, Kutner NG, Green RC, McNeely E. The Atlanta FICSIT study: two exercise interventions to reduce frailty in elders. J Am Geriatr Soc. 1993;41(3):329–332. doi: 10.1111/j.1532-5415.1993.tb06713.x. [DOI] [PubMed] [Google Scholar]