Abstract
The fear conditioning paradigm is used to investigate the roles of various genes, neurotransmitters, and substrates in the formation of fear learning related to contextual and auditory cues. In the brain, nitric oxide (NO) produced by neuronal nitric oxide synthase (nNOS) functions as a retrograde neuronal messenger that facilitates synaptic plasticity, including the late phase of long-term potentiation (LTP) and formation of long-term memory (LTM). Evidence has implicated NO signaling in synaptic plasticity and LTM formation following fear conditioning, yet little is known about the role of the nNOS gene in fear learning. Using knockout (KO) mice with targeted mutation of the nNOS gene and their wild-type (WT) counterparts, the role of NO signaling in fear conditioning was investigated. Plasma levels of the stress hormone corticosterone were measured to determine the relationship between physiological and behavioral response to fear conditioning. Contextual fear learning was severely impaired in male and female nNOS KO mice compared with WT counterparts; cued fear learning was slightly impaired in nNOS KO mice. Sex-dependent differences in both contextual and cued fear learning were not observed in either genotype. Deficits in contextual fear learning in nNOS KO mice were partially overcome by multiple trainings. A relationship between increase in plasma corticosterone levels following footshock administration and the magnitude of contextual, but not cued freezing was also observed. Results suggest that the nNOS gene contributes more to optimal contextual fear learning than to cued fear learning, and therefore, inhibition of the nNOS enzyme may ameliorate context-dependent fear response.
Anxiety disorders, such as post-traumatic stress disorder (PTSD), constitute the most prevalent mental illnesses in the United States, costing nearly one-third of the country's total health bill (Greenberg et al. 1999). The treatment of these disorders requires overcoming complications such as reluctance to seek mental health treatment and an extremely high comorbidity rate with other affective disorders, reaching 80% (Brady 1997; Solomon and Davidson 1997). Emerging evidence suggests that dysfunctions underlying acquired anxiety and PTSD include an abnormal reaction to stress, which is mediated by specific neurochemical and neuroanatomical substrates (Yehuda and McFarlane 1995; Adamec 1997). Pharmacotherapies that target neuronal signaling molecules, such as nitric oxide (NO), may play a role in the treatment of these disorders.
In the brain, N-methyl-d-aspartate receptor (NMDAR) activation and calcium influx into the cell activates the neuronal nitric oxide synthase (nNOS) enzyme to produce NO, which has the role of retrograde messenger (Snyder 1992). NO is involved in memory formation and synaptic plastic events such as late-phase long-term potentiation (LTP) (Lu et al. 1999; Arancio et al. 2001; Puzzo et al. 2006). Behavioral evidence in invertebrates (Lewin and Walters 1999; Muller 2000; Kemenes et al. 2002; Matsumoto et al. 2006) and vertebrates (Medina and Izquierdo 1995; Rickard et al. 1998; Ota et al. 2008) suggest that NO has a major role in consolidation of long-term memory (LTM). Recently, studies have shown that site-specific pharmacological blockade of NO signaling in rats impairs contextual (Resstel et al. 2008) and cued (Schafe et al. 2005) fear learning. However, the role of the nNOS gene in fear conditioning has not been investigated.
In the present study, fear conditioning was investigated in homozygous nNOS knockout (KO) and wild-type (WT) mice. In the fear-conditioning paradigm, the association of a footshock (unconditioned stimulus; US), with a specific context and a neutral stimulus (auditory cue) results in learned fear. Re-exposure to the conditioning context and to the previously neutral auditory cue (conditioned stimulus; CS) elicits a freezing response in the absence of the aversive US. Thus, the fear-conditioning paradigm includes both contextual and cued fear learning components, which can be measured in separate tests. Fear conditioning recruits both the amygdala (emotional cue learning) and the hippocampus (spatial/contextual learning) (Phillips and LeDoux 1992; Goosens and Maren 2004; Mei et al. 2005). The involvement of these brain regions in fear learning and anxiety has been confirmed by animal and human imaging studies (LeDoux 1998; Rauch et al. 2006).
We report that nNOS KO mice showed a severe deficiency in contextual fear learning and a less marked deficit in cued fear learning compared with WT mice after a single fear-conditioning session. This deficiency was partially improved by multiple (four) fear-conditioning sessions. In addition, we observed that plasma levels of corticosterone, the primary stress hormone in rodents, are related to contextual fear learning ability.
Results
nNOS immunoreactive neurons are found in the hippocampus and amygdala of WT but not nNOS KO mice
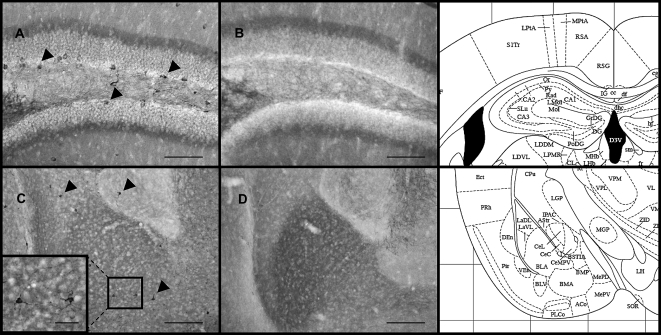
In WT mice, nNOS-immunoreactive neurons are present in the dentate gyrus region of the hippocampus (Fig. 1A) and in the lateral, basal, and central nuclei of the amygdala (Fig. 1C). nNOS-immunoreactive neurons were absent in KO mice (Fig. 1B,D). Given the role of nNOS in the formation of LTM in the hippocampus and amygdala for contextual and cued fear learning, we assumed that the absence of nNOS in KO mice may influence fear learning.
Figure 1.
Representative nNOS-immunoreactive neurons in the dentate gyrus and amygdala of male WT and nNOS KO mice. nNOS-immunoreactive neurons were present in the dentate gyrus of WT (A), but not nNOS KO mice (B); scale bar = 100 μm, magnification = 20×. Likewise, nNOS-immunoreactive neurons are present in the lateral, basal, and central nuclei of the amygdala of WT mice (C), but not KO mice (D). C and D scale bar = 200 μm, magnification = 10×. The boxed region in C shows nNOS-immunoreactive neurons in the basal nucleus of the amygdala; scale bar = 60 μm, magnification = 60×. Schematic diagrams of representative brain regions from the mouse brain atlas ( from Paxinos and Franklin 2001 and reprinted with permission from Academic Press ©2001) show the approximate antero-posterior level (−1.43 to Bregma) at which select brain regions were analyzed and nNOS-immunoreactive neurons were found.
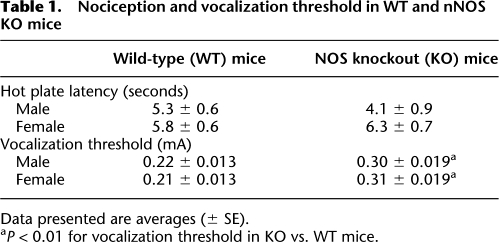
Pain response thresholds in WT and nNOS KO mice
To determine whether WT and nNOS KO mice display differences in response to painful stimuli that could influence the fear-conditioned response, two tests were performed. First, nociceptive response was assessed using the hot plate test, which involves supraspinal mechanisms of the nociceptive system. Second, the minimum footshock intensity required to elicit an audible vocalization response was determined. As shown in Table 1, hot plate test latencies did not differ significantly between the groups. Two-way ANOVA (sex × genotype) of the results revealed no sex-dependent effect (F(1,37) = 3.658; P = 0.064) and no genotype-dependent effect (F(1,37) = 0.376; P = 0.543); post-hoc analysis revealed no significant differences between the groups. These findings suggest that nNOS KO mice have no impairments in nociceptive response compared with WT mice. Results of vocalization threshold tests revealed no differences between sexes of each genotype, but a higher threshold in the KO mice compared with WT counterparts (Table 1). A two-way ANOVA (sex × genotype) revealed no main effect of sex (F(1,26) = 0.24; P = 0.878), but a main effect of genotype (F(1,26) = 32.775; P < 0.001). The interaction of sex × genotype was not significant (F(1,26) = 0.599; P = 0.446). Post-hoc tests revealed significant differences between WT and KO mice of both sexes (P < 0.01). Importantly, the shock intensity used for fear conditioning (0.75 mA) was much higher than the observed vocalization thresholds (0.31 mA).
Table 1.
Nociception and vocalization threshold in WT and nNOS KO mice
Data presented are averages (± SE).
aP < 0.01 for vocalization threshold in KO vs. WT mice.
Fear conditioning by a single training reveals fear learning impairments in nNOS KO mice
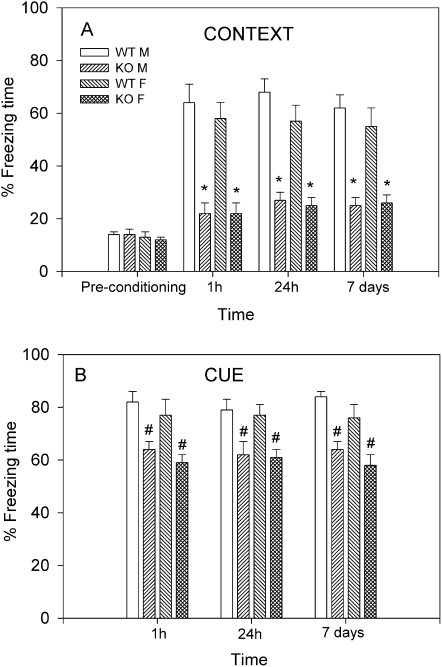
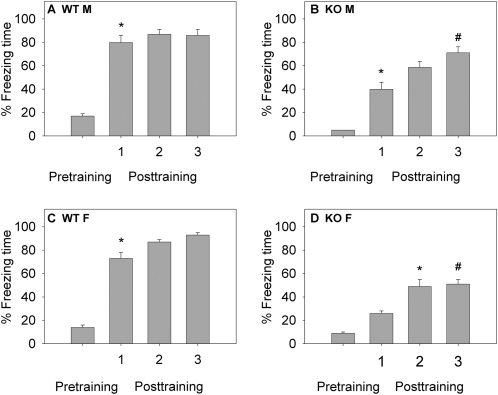
Levels of pretraining (basal) freezing to the training context did not differ between genotypes and sexes (16 ± 2%; Fig. 2A). Post-training contextual freezing (Fig. 2A) and cued freezing (Fig. 2B) were analyzed separately by three-way ANOVA (sex × genotype × time). The time component of the analysis corresponded to 1 h (short-term memory; STM), 24 h, and 7 d (LTM) used for testing. For contextual freezing, there was no significant sex-dependent effect (F(1,108) = 2.32; P = 0.13), a significant genotype effect (F(1,108) = 519.84; P < 0.001), and no significant time effect (F(2,108) = 0.39; P = 0.67); interactions between all three variables were not significant (F(2,108) = 0.22; P = 0.82). Post-hoc tests showed a significant genotype effect in males (t = 15.2; P < 0.001) and females (t = 17.08; P < 0.001) at all three time points (Fig. 2A). In the STM tests for contextual freezing, WT levels were 64 ± 7% for males and 58 ± 6% for females. For KO mice, freezing levels in the STM tests reached 22 ± 4% for males and 23 ± 4% for females. Freezing levels in WT mice were maintained in the 24-h (males: 68 ± 5%; females: 57 ± 6%) and 7-d (males: 62 ± 5%; females: 55 ± 7%) tests of LTM. KO mice displayed significantly lower percent freezing times in the respective 24-h and 7-d LTM tests, reaching 27 ± 3% and 25 ± 3% for males, and 25 ± 3% and 26 ± 3% for females (P < 0.001; Fig. 2A). These results show that nNOS KO mice display reduced contextual freezing in both STM and LTM tests compared with WT counterparts.
Figure 2.
Short- and long-term memory (STM; LTM) of fear conditioning in male and female WT and nNOS KO mice. Mice (n = 10–16/group) underwent a single training; STM was determined 1 h post-training and LTM was determined 24 h and 7 d post-training. (A) Results of contextual freezing are expressed as percent of total time spent freezing to the training context. During pretraining (basal), no significant differences between genotypes in percent freezing were observed. For the post-training phase, Bonferroni post-hoc tests showed significant genotype effect in males (t = 15.2; [*] P < 0.001) and females (t = 17.08; [*] P < 0.001) at all three time points, suggesting impairments in contextual fear learning in KO mice. (B) Results of cued freezing are expressed as percent freezing during auditory cue re-exposure in a different context. Bonferroni multiple comparisons showed no significant differences between WT and KO mice at all three time points. Only the least-restricted post-hoc LSD test showed significant differences between WT and KO mice ([#] P < 0.05).
Results of cued freezing were analyzed by three-way ANOVA (sex × genotype × time), which revealed no sex-dependent effect (F(1,133) = 3.83; P = 0.052), a significant genotype effect (F(1,133) = 57.9; P < 0.001), and no significant time effect; no interactions between variables were significant. Notably, Bonferroni post-hoc tests showed no significant differences between WT and KO mice of either sex in cued freezing levels for any of the time points investigated (P > 0.05; Fig. 2B). Because the ANOVA showed overall significance, and Bonferroni post-hoc analysis did not show pairwise significant differences, Scheffe, Sidak, and Tukey, and LSD post-hoc tests were performed. Only the LSD post-hoc test, which does not take into account multiple comparisons, showed significant differences between WT and KO mice at the three time points ([#] P < 0.05; Fig. 2B).
Plasma corticosterone levels after a single training predicts fear learning ability
The neuroendocrine response to trauma in humans and animals is known to effect fear-learning ability (Charney et al. 1993). In order to determine whether the blood-drawing procedure influenced the learned freezing response, corticosterone levels were measured in half of the subjects (five to eight) that had undergone fear conditioning. Results showed no differences in the magnitude of contextual and cued freezing between subjects (male and female WT and KO mice) that underwent blood drawing and those that did not. Therefore, behavioral results of subjects that did and did not undergo blood drawing were combined (Fig. 2). For day 7 tests (post-context and post-auditory cue), blood samples were drawn from different groups to avoid possible effects of repeated blood drawing.
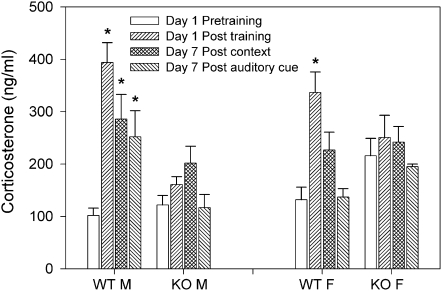
The results of the corticosterone assays (Fig. 3) were analyzed by three-way ANOVA (sex × genotype × time). There was no significant sex-dependent effect (F(1,115) = 0.21; P = 0.648), a significant genotype effect (F(1,115) = 8.948; P = 0.003), a significant time effect (F(3,115) = 21.688; P < 0.001), and significant interaction between sex and genotype (F(1,115) = 16.301; P < 0.001) and between genotype and time (F(3,115) = 11.389; P < 0.001). Post-hoc tests showed no significant differences between pretraining (basal) corticosterone levels across all groups (Fig. 3). In WT males, significant increases in corticosterone levels from pretraining were observed on day 1 post-training (15 min) and day 7 following re-exposure to the context and cue (15 min after each) (P < 0.001; Fig. 3). In KO males, no significant differences between pretraining and post-training corticosterone levels were observed (P > 0.05); likewise, on day 7 context and auditory cue re-exposure had no significant effect on corticosterone (Fig. 3). In WT females, a significant increase in corticosterone levels was observed on day 1 post-training (P < 0.01), but not on day 7 following context or cue re-exposure (Fig. 3). In KO females, like their male counterparts, a single fear-conditioning session had no significant effect on corticosterone levels 15 min post-training or 7 d later (Fig. 3). The results suggest that (1) increased corticosterone release following fear conditioning is related to successful contextual fear learning, and (2) re-exposure to context and cue associated with footshock causes marked increase in corticosterone long after the footshock experience in WT males, but not females.
Figure 3.
Corticosterone levels before and after a single training. In WT males a significant increase in corticosterone levels was observed on day 1, 15 min post-training ([*] P < 0.001), and on day 7 post-context or auditory cue re-exposure ([*] P < 0.05). In KO males no significant differences in corticosterone levels were observed. In WT females a significant increase in corticosterone was observed only on day 1, 15 min post-training ([*] P < 0.001). In KO females no significant differences between corticosterone levels pre- and post-training were observed.
Multiple trainings improves primarily contextual fear learning in nNOS KO mice
To investigate whether a more robust training strategy would improve fear learning in nNOS KO mice, WT and KO mice underwent multiple (four) trainings with an intertrial interval (ITI) of 10–12 min. Mice were tested for contextual STM (Fig. 4); the percent freezing time was measured both pretraining and during a 2-min period in context-A (pre-shock) 10 min after the delivery of the first, second, and third footshock. Only contextual STM was investigated because of the magnitude of the learning deficit in nNOS KO mice. Results of both contextual and cued LTM testing are shown in Figure 5.
Figure 4.
Contextual STM following multiple trainings. (A) Contextual STM in WT males (n = 8). (B) Contextual STM in KO males (n = 9). (C) Contextual STM in WT females (n = 8). (D) Contextual STM in KO females (n = 10). Mice underwent four trainings (10–12 min intertrial interval); contextual freezing was measured pretraining and then 10 min following the first, second, and third training. Post-hoc tests showed significant differences in percent freezing between pretraining and the first post-training tests for males of both genotypes and WT females ([*] P < 0.001); KO females showed significant differences in percent freezing between pretraining and the second post-training test ([*] P < 0.001). Significant differences between percent freezing after the first and the third training session in KO mice were observed, suggesting multiple trainings significantly increased contextual STM compared with a single training ([#] P < 0.05).
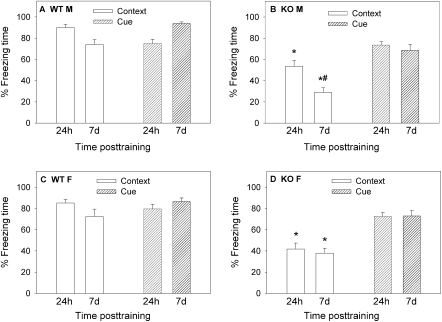
Figure 5.
Long-term memory (LTM) of contextual and cued fear conditioning following multiple trainings. Mice that had been tested for STM of contextual fear (Fig. 4) were tested after 24 h and 7 d for contextual and cued freezing. (A) Contextual and cued LTM in WT males (n = 8). (B) Contextual and cued LTM in KO males (n = 9). (C) Contextual and cued LTM in WT females (n = 8). (D) Contextual and cued LTM in KO females (n = 10). Contextual freezing: nNOS KO males (B) showed lower contextual freezing than their WT counterparts (A) in both the 24 h and 7 d tests ([*] P < 0.001). In KO males (B) there was a significant decrease in contextual freezing on day 7 compared with 24 h post-training ([#] P < 0.05). nNOS KO females (D) showed lower contextual freezing than WT counterparts (C) in both the 24-h and 7-d tests ([*] P < 0.001). Cued freezing: Bonferroni multiple comparison tests showed no significant differences in the level of cued freezing among genotypes, sexes, and time-elapsed post-training.
STM testing after multiple trainings
Results of STM tests (Fig. 4) for all groups were analyzed by a three-way ANOVA (sex × genotype × time). There was no significant sex-dependent effect (F(1,120) = 2.152; P = 0.145), a significant genotype-dependent effect (F(1,120) = 200.3; P < 0.001), a significant time-dependent effect (F(1,120) = 160.11; P < 0.001), and a significant interaction between genotype and time (F(3,120) = 12.939; P < 0.001). Results within groups were analyzed by repeated measures ANOVA to determine the effect of each training session on subsequent contextual freezing. Results for all groups showed an overall significant training effect (P < 0.001). Post-hoc tests were used to determine differences in percent freezing after each training session. In WT males and females, there was a significant difference between pretraining and the first training tests (P < 0.001), but no other significant differences were observed (Fig. 4A,C). In KO males, a significant difference between pretraining and the first training session was observed (P < 0.001) as well as between the first (42 ± 4%) and the third (65 ± 6%) training sessions (P = 0.004; Fig. 4B). In KO females, there were significant differences between pretraining and the second training (P < 0.001) as well as between the first and the third (P = 0.018) training sessions (Fig. 4D). These results show that WT mice ceased improvements in contextual STM after a single training, while nNOS KO mice exhibited improvements after multiple trainings that exceeded freezing from a single training.
LTM testing after multiple trainings
Mice were tested for contextual and cued freezing 24 h and 7 d (LTM) after the multiple trainings (Fig. 5). Results for the LTM tests were analyzed by four-way ANOVA: sex × genotype × time × test (contextual or cued freezing). There was no significant sex-dependent effect (F(1,124) = 1.25; P = 0.266), a significant genotype-dependent effect (F(1,124) = 103.316; P < 0.001), no significant time-dependent effect (F(1,124) = 1.49; P = 0.223), and a significant test-dependent effect (F(1,124) = 62.877; P < 0.001). The following significant interactions were observed: genotype × time (F(1,124) = 4.927; P = 0.028); genotype × test (F(1,124) = 24.121; P < 0.001); time × test (F(1,124) = 11.707; P = 0.001).
Contextual LTM
For both the 24-h test (males: WT = 90 ± 3%; KO = 53 ± 6%) and the 7-d test (males: WT = 74 ± 5%; KO = 29 ± 4%), a significant genotype difference was observed (P < 0.001; Fig. 5A,B). These results suggest that contextual fear learning in KO males remains impaired even after four training sessions. However, it should be noted that in the 24-h test for KO males (53 ± 6%; Fig. 5B), contextual fear learning was twofold higher than that observed after a single training (27 ± 3%; P = 0.001; Fig. 2A) and not significantly different from the percent freezing of WT males 24 h following a single training session (68 ± 5%; P > 0.05; Fig. 2A). For WT and KO females, a similar trend was observed. KO females showed significant improvements in contextual LTM after the multiple trainings (42 ± 6%; Fig. 5D) compared with a single training (25 ± 3%; P = 0.003; Fig. 2A) and not significantly different from percent freezing of WT females 24 h following a single training session (58 ± 6%; P > 0.05; Fig. 2A). Thus, in nNOS KO mice the results from contextual LTM tests 24 h after multiple trainings were similar to the results in WT mice after a single training.
For the 7-d tests in nNOS KO mice, contextual freezing after multiple trainings was significantly improved in females (P < 0.05; Fig. 5D) but not males (Fig. 5B), suggesting that in males the improvement in contextual LTM was transient (24 h, but not 7 d).
Cued LTM
Post-hoc comparisons for the magnitude of cued freezing after the four training sessions revealed no significant differences between WT and KO males (Fig. 5A,B) and WT and KO females (Fig. 5C,D) at both time points. Comparisons between the results of cued freezing after the single and multiple trainings revealed significant improvements in the 7-d test in WT males (t = 3.435; P < 0.003) but not in KO males (P > 0.05). This finding suggests that cued freezing in nNOS KO males following single and multiple trainings was similar. In nNOS KO females, but not in the WT counterparts, there was a significant difference between cued freezing following single and multiple trainings in the 24-h and 7-d tests (P < 0.05), suggesting that multiple trainings improved cued fear learning in nNOS KO females.
Multiple trainings resulted in increased corticosterone levels in nNOS KO mice
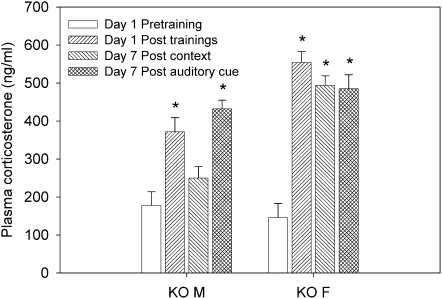
Corticosterone was measured in nNOS KO mice following multiple trainings in order to determine whether levels would be predictive of contextual fear learning ability as had been observed after a single training session (Fig. 3). Only nNOS KO mice were tested, because WT mice showed significant increases in corticosterone after a single training, while nNOS KO mice did not (Fig. 3). As before, corticosterone measurements were taken on day 1: 15 min before and after training, and 7 d later: 15 min post-context or auditory cue re-exposure (Fig. 6). Results were analyzed by two-way ANOVA (sex × time) and revealed a significant sex-dependent effect (F(1,53) = 13.968; P < 0.001), a significant time effect (F(3,53) = 24.984; P < 0.001), and a significant interaction between sex and time (F(3,53) = 4.33; P = 0.008). Post-hoc tests showed significant post-training (day 1) elevations in corticosterone in male and female nNOS KO mice (P < 0.001). On day 7, a significant increase in corticosterone was observed in male nNOS KO mice following cue re-exposure (P < 0.001) and in females following both cue and context re-exposure (P < 0.001). The results show that the multiple training sessions increased corticosterone levels in nNOS KO mice (Fig. 6), which coincided with improvements in contextual freezing with this training strategy (Fig. 5).
Figure 6.
Corticosterone levels before and after multiple trainings in nNOS KO mice. In male KO mice, there was a significant increase in plasma corticosterone 15 min after the four training sessions as well as on day 7 after re-exposure to the auditory cue ([*] P < 0.001). In female KO mice, there was a significant increase in plasma corticosterone 15 min after the four training sessions, and on day 7 after re-exposure to context or auditory cue ([*] P < 0.001).
Discussion
Results of the present study show the following: (1) The absence of the nNOS gene causes deficits in fear learning, a finding that supports pharmacological evidence implicating NO signaling in fear learning (Schafe et al. 2005; Resstel et al. 2008). (2) Multiple trainings improved primarily contextual fear learning ability in nNOS KO mice. (3) The physiological response to unconditioned and conditioned fearful stimulus is similar and appears to be related to optimal fear-learning ability as determined by measuring plasma corticosterone levels.
NO and fear conditioning
At present, only a few studies have investigated the role of NO signaling in fear conditioning. Two studies have found that systemic administration of NOS inhibitors did not prevent fear learning in rodents (Maren 1998; Johnson et al. 2000). However, recently it has been reported that blockade of NO signaling in the ventral portion of the medial prefrontal cortex resulted in contextual fear-learning deficits (Resstel et al. 2008), and that blockade of NO signaling in the lateral amygdala resulted in reduced LTP and impaired cued fear learning in rats (Ota et al. 2008). Our findings show that global deletion of the nNOS gene in mice resulted in a more severe impairment in contextual than cued fear learning. Fear conditioning is believed to recruit both the amygdala (emotional cued learning) and the hippocampus (spatial/contextual learning) (Phillips and LeDoux 1992; Goosens and Maren 2004; Mei et al. 2005). Given that nNOS-immunoreactive neurons are absent in both the hippocampus and amygdala of nNOS KO mice (Fig. 1), it is unclear why contextual fear learning was more severely impaired than the auditory-cued learning. This may suggest that neuroadaptations in the nNOS KO mice in the amygdala—more so than in the hippocampus—resulted in NO-independent fear learning. Protein expression analysis of nNOS KO mice has revealed aberrant protein expression in the hippocampus (Kirchner et al. 2004) that may underlie the deficits in contextual fear learning observed in the present study, as well as other cognitive deficits (Weitzdoerfer et al. 2004).
Pain response thresholds in WT and nNOS KO mice
The finding that nNOS KO mice showed deficits in conditioned fear learning raises the question of whether nNOS KO mice are less sensitive to painful stimulus. The results from the hot plate test suggest that nociceptive responses are intact in nNOS KO mice (Table 1), confirming an earlier report (Azad et al. 2001). We did observe a difference in vocalization thresholds between WT and nNOS KO mice, where nNOS KO mice of both sexes required higher shock intensity before audible vocalization responses were made (Table 1). Whether the KO mice are less sensitive to the painful footshock or are less likely to vocalize in response to pain is not clear; however, the intensity of the footshock during training was 2.5-fold higher than that required for vocalization. Further, KO mice exhibited normal cued fear learning (Figs. 2B, 5B,D).
Previous studies suggested that nNOS KO mice have blunted stress response and less anxiety-like behavior compared with WT counterparts following restraint (Bilbo et al. 2003). Hence, it may be argued that the absence of the nNOS gene contributes to an “anxiolytic effect” and, consequently, to impairment in fear conditioning. However, the finding that cued fear learning in the absence of the nNOS gene was only slightly reduced (following single training) or near optimal (following multiple trainings) suggests that blunted stress response by itself cannot explain the severe impairment in contextual fear conditioning.
Multiple trainings
We investigated whether an increased training intensity (from one to four training sessions) would enable the nNOS KO mice to overcome the fear-learning deficits. We found that four trainings improved the contextual learning ability of nNOS KO mice in both STM and LTM tests. In KO mice, contextual STM improved after each training session (Fig. 4B,D), while in WT mice, contextual freezing was maximized by the first training session (Fig. 4A,C). The four training sessions also increased LTM for contextual fear (24 h) in KO mice (males: 53 ± 6%; females: 42 ± 4%) compared with results of a single training session (males: 27 ± 3%; females: 25 ± 3%). Nevertheless, results of LTM tests still showed that context re-exposure elicited less freezing in KO mice of both sexes compared with WT (Fig. 5). Notably, however, LTM of cued fear learning after four training sessions was remarkably similar for both sexes in WT (82%–88%) and KO (78%–83%) mice (Fig. 5) and was similar to the magnitude after a single training (Fig. 2B).
Corticosterone
In rodents, corticosterone has been implicated in LTM formation of contextual fear memory (Pugh et al. 1997; Thompson et al. 2004) and tone-specific cued fear memory (Hui et al. 2004; Roozendaal et al. 2006; Marchand et al. 2007). We aimed to investigate how nNOS gene deletion might affect the physiological response to fear conditioning. The results from the plasma corticosterone measurements implicated a relationship between plasma corticosterone and successful contextual fear learning for the following reasons. First, elevated corticosterone was observed 15 min after a single training session in WT mice (Fig. 3), which exhibited successful STM and LTM of contextual fear learning (Fig. 2), and not in KO mice (Fig. 3), which did not exhibit successful contextual fear learning (Fig. 2). Second, 15 min after the four trainings, KO mice showed elevated corticosterone (Fig. 6) concomitant with significant improvements in STM (Fig. 4) and LTM (Fig. 5) of contextual fear learning. Third, 7 d following the multiple trainings, male KO mice showed relatively low corticosterone release (Fig. 6) following context re-exposure and a low magnitude of context-dependent freezing response (29 ± 4%; Fig. 5B). Conversely, female KO mice showed significantly elevated corticosterone (Fig. 6) and a relatively higher context-dependent freezing response (40 ± 3%; Fig. 5D). Overall, it is notable that the context and the auditory cue associated with a painful stimulus elicited a physiological response (increase in plasma stress hormones) 7 d later, which was similar in magnitude to that observed immediately following exposure to the painful stimulus (Fig. 6). This finding suggests that measurements of the neuroendocrine response to cues associated with stressful stimulus may provide insight into the effectiveness of clinical treatments for anxiety disorders such as PTSD.
In summary, the present study demonstrates a role for the nNOS gene in contextual and cued fear learning. Our results suggest that the nNOS gene deletion disrupts primarily contextual fear learning, which recruits the hippocampus, and to a lesser extent, cued fear learning, which recruits the amygdala. Also, elevation in plasma stress hormones (e.g., corticosterone in rodents) may be predictive of optimal contextual fear learning. Hence, pharmacological inhibition of nNOS and drugs that block release or receptor binding sites of stress hormones may be potential therapeutics against contextual fear conditioning.
Materials and Methods
Subjects
nNOS KO mice were generated on a mixed B6;129S genetic background; the targeted deletion of the α subunit of nNOS resulted in >95% reduction in brain nNOS catalytic activity (Huang et al. 1993). Adult male and female homozygous nNOS KO mice (B6;129S4-Nos1; 6–8 wk old), and the parental strains of their hybrid WT counterparts (C57BL/6J and 129/SvImJ) were purchased from Jackson Laboratories. Our breeding colony, littermate selection, and animal care have been described earlier (Balda et al. 2006). Animal care was in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council 1996) and was approved by the University of Miami Animal Care and Use Committee. Adult (8–10 wk old) WT and nNOS KO mice of both sexes were investigated.
Immunohistochemistry of nNOS
Adult male WT and nNOS KO mice (n = 3/group) were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg), then perfused with sodium phosphate-buffered saline (PBS), followed by 4% p-formaldehyde. Serial coronal sections (50 μm) were cut with a Vibratome 1000 (TPI, Inc.) and treated as described previously (Balda et al. 2006). Sections were incubated (72 h; 4°C) with a rabbit anti-nNOS polyclonal antibody (1:3000, Santa Cruz Biotechnology), then further developed according to Vectastain Elite ABC kit directions (Vector Laboratories), using diaminobenzidine (DAB) as a chromogen. nNOS-immunoreactive cells were detected with a compound light microscope (Olympus BX51; C. Squared Co.) attached to a cooled monochrome camera (Retiga 2000R), using Image-Pro Plus software.
Pain response thresholds in WT and nNOS KO mice
To investigate genotype sensitivity to noxious stimuli, two experiments were performed: (1) the hot plate test and (2) footshock-induced vocalization threshold.
Hot plate test
Male and female WT and KO mice (n = 6–8/group) were placed individually inside a heated (55 ± 0.5°C) glass cylinder (15 cm diameter × 25 cm high), and the time taken for mice to show the first sign of discomfort (licking paws, flinching, or jumping) was recorded by two observers. A cut-off time of 30 sec was used to prevent tissue damage.
Vocalization thresholds
Individual mice (n = 6–8/group) were placed in the fear conditioning apparatus and electric footshocks (2 sec) of increasing intensity were delivered until an audible vocalization response was heard by two observers. The shock intensity began at 0.1 mA and was increased in increments of 0.05 mA; 2 min elapsed between each shock.
Fear conditioning
Apparatus
Fear conditioning training and testing occurred in Plexiglas chambers (30.5 × 30.5 × 43.5 cm; Noldus Information Technology, Inc.). Each chamber was equipped with a stainless-steel rod floor through which the electric shock was delivered, and an upper control panel containing a video camera, a sound emitter, and a white light illuminating one corner of the chamber. The chambers were housed in custom-built sound-attenuating cubicles that gave the appearance of black walls to the chamber.
Conditioning and testing
A fear-conditioning training session consisted of placement in the training context (context A), and after 2 min an auditory cue (2.3 kHz; 70 dB) sounded for 30 sec, which coterminated with a 2-sec footshock (0.75 mA). Mice were returned 30 sec later to the home cage. In the multiple trainings experiments, animals underwent four of the previously described training sessions with an intertrial interval (ITI) of 10–12 min, during which time mice were returned to the home cage. Contextual fear conditioning was measured in context A and consisted of digitally recording the animal's percentage of total time spent “freezing” while in the chamber for 3 min; freezing, defined as a complete lack of movement beside respiration (<5% mobility), was calculated by EthoVision v3.1 software (Noldus Information Technology, Inc.) and expressed as a percentage of total time in the chamber. Cued fear conditioning was measured in a different context (context B), and the previously used tone sounded for 2 min after an initial habituation period of 3 min. Context B utilized a smooth white foam floor covering the shock grid, four opaque white walls, and olfactory enrichment of pure orange extract affixed to the chamber ceiling. Hence, visual, tactile, and olfactory cues were used to differentiate context A from context B. Context- and cue-dependent freezing were tested 1–2 h after training for STM, and 24 h and 7 d later for LTM. LTM tests for contextual and cued freezing levels were determined in all subjects; the interval between context- and cue-dependent freezing was 4 h.
Measurements of plasma corticosterone
To investigate the acute effect of footshock on plasma corticosterone levels, blood samples (80 μL) were drawn from the retro-orbital venous plexus using heparinized microcapillary tubes on day 1, 15 min before and 15 min after the last cue–shock pairing. To investigate the physiological response to the conditioned stimuli (context and cue) associated with the footshock, on day 7 mice were tested for context- or cue-dependent freezing, and blood samples were drawn 15 min after re-exposure to the context (half of the subjects) and the auditory cue (half of the subjects). This schedule prevented multiple blood drawings from the same mouse. Samples were treated according to instructions provided in a Corticosterone EIA kit (Immunodiagnostic Systems, Ltd.), and read on a spectrophotometer at 450 nm.
Statistical analysis
Results of hot plate test and vocalization threshold were analyzed by two-way ANOVA with sex and genotype as between-subject factors. In the single-trial fear-conditioning experiments, differences in percent freezing were analyzed by three-way ANOVA (sex × genotype × time; 1 h, 24 h, and 7 d). Results of contextual STM tests following multiple trainings were analyzed by repeated measures ANOVA with the number of the training session as the repeated measure. Results of LTM tests were analyzed by four-way ANOVA (sex × genotype × time × type of test; context or cue). ANOVAs were routinely followed by Bonferroni post-hoc analyses to compare between specific groups. In one case where cued fear learning was analyzed (Fig. 2B), overall ANOVA showed a significant genotype effect, but Bonferroni post-hoc test between pairs did show significant difference between genotypes. Therefore, other post-hoc tests were conducted: Scheffe, Sidak, Tukey, and LSD. Comparisions of the results of the single and multiple training experiments were performed by two-tailed student's t-test. All results are shown as the mean and standard error of the mean. In all cases a P-value of <0.05 was considered statistically significant.
Acknowledgment
This work was supported by grant RO1DA019107 from the National Institute on Drug Abuse, National Institutes of Health.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.1329209.
References
- Adamec R. Transmitter systems involved in neural plasticity underlying increased anxiety and defense–implications for understanding anxiety following traumatic stress. Neurosci Biobehav Rev. 1997;21:755–765. doi: 10.1016/s0149-7634(96)00055-3. [DOI] [PubMed] [Google Scholar]
- Arancio O, Antonova I, Gambaryan S, Lohmann SM, Wood JS, Lawrence DS, Hawkins RD. Presynaptic role of cGMP-dependent protein kinase during long-lasting potentiation. J Neurosci. 2001;21:143–149. doi: 10.1523/JNEUROSCI.21-01-00143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad SC, Marsicano G, Eberlein I, Putzke J, Zieglgansberger W, Spanagel R, Lutz B. Differential role of the nitric oxide pathway on Δ9-THC-induced central nervous system effects in the mouse. Eur J Neurosci. 2001;13:561–568. doi: 10.1046/j.1460-9568.2001.01431.x. [DOI] [PubMed] [Google Scholar]
- Balda MA, Anderson KL, Itzhak Y. Adolescent and adult responsiveness to the incentive value of cocaine reward in mice: Role of neuronal nitric oxide synthase (nNOS) gene. Neuropharmacology. 2006;51:341–349. doi: 10.1016/j.neuropharm.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Hotchkiss AK, Chiavegatto S, Nelson RJ. Blunted stress responses in delayed type hypersensitivity in mice lacking the neuronal isoform of nitric oxide synthase. J Neuroimmunol. 2003;140:41–48. doi: 10.1016/s0165-5728(03)00175-9. [DOI] [PubMed] [Google Scholar]
- Brady KT. Posttraumatic stress disorder and comorbidity: Recognizing the many faces of PTSD. J Clin Psychiatry. 1997;58((Suppl 9)):12–15. [PubMed] [Google Scholar]
- Charney DS, Deutch AY, Krystal JH, Southwick SM, Davis M. Psychobiologic mechanisms of posttraumatic stress disorder. Arch Gen Psychiatry. 1993;50:295–305. doi: 10.1001/archpsyc.1993.01820160064008. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. NMDA receptors are essential for the acquisition, but not expression, of conditional fear and associative spike firing in the lateral amygdala. Eur J Neurosci. 2004;20:537–548. doi: 10.1111/j.1460-9568.2004.03513.x. [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Sisitsky T, Kessler RC, Finkelstein SN, Berndt ER, Davidson JR, Ballenger JC, Fyer AJ. The economic burden of anxiety disorders in the 1990s. J Clin Psychiatry. 1999;60:427–435. doi: 10.4088/jcp.v60n0702. [DOI] [PubMed] [Google Scholar]
- Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- Hui GK, Figueroa IR, Poytress BS, Roozendaal B, McGaugh JL, Weinberger NM. Memory enhancement of classical fear conditioning by post-training injections of corticosterone in rats. Neurobiol Learn Mem. 2004;81:67–74. doi: 10.1016/j.nlm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Johnson DM, Baker JD, Azorlosa JL. Acquisition, extinction, and reinstatement of Pavlovian fear conditioning: The roles of the NMDA receptor and nitric oxide. Brain Res. 2000;857:66–70. doi: 10.1016/s0006-8993(99)02388-4. [DOI] [PubMed] [Google Scholar]
- Kemenes I, Kemenes G, Andrew RJ, Benjamin PR, O'Shea M. Critical time-window for NO-cGMP-dependent long-term memory formation after one-trial appetitive conditioning. J Neurosci. 2002;22:1414–1425. doi: 10.1523/JNEUROSCI.22-04-01414.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner L, Weitzdoerfer R, Hoeger H, Url A, Schmidt P, Engelmann M, Villar SR, Fountoulakis M, Lubec G, Lubec B. Impaired cognitive performance in neuronal nitric oxide synthase knockout mice is associated with hippocampal protein derangements. Nitric Oxide. 2004;11:316–330. doi: 10.1016/j.niox.2004.10.005. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Fear and the brain: Where have we been, and where are we going? Biol Psychiatry. 1998;44:1229–1238. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- Lewin MR, Walters ET. Cyclic GMP pathway is critical for inducing long-term sensitization of nociceptive sensory neurons. Nat Neurosci. 1999;2:18–23. doi: 10.1038/4520. [DOI] [PubMed] [Google Scholar]
- Lu YF, Kandel ER, Hawkins RD. Nitric oxide signaling contributes to late-phase LTP and CREB phosphorylation in the hippocampus. J Neurosci. 1999;19:10250–10261. doi: 10.1523/JNEUROSCI.19-23-10250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand AR, Barbelivien A, Seillier A, Herbeaux K, Sarrieau A, Majchrzak M. Contribution of corticosterone to cued versus contextual fear in rats. Behav Brain Res. 2007;183:101–110. doi: 10.1016/j.bbr.2007.05.034. [DOI] [PubMed] [Google Scholar]
- Maren S. Effects of 7-nitroindazole, a neuronal nitric oxide synthase (nNOS) inhibitor, on locomotor activity and contextual fear conditioning in rats. Brain Res. 1998;804:155–158. doi: 10.1016/s0006-8993(98)00668-4. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Unoki S, Aonuma H, Mizunami M. Critical role of nitric oxide-cGMP cascade in the formation of cAMP-dependent long-term memory. Learn Mem. 2006;13:35–44. doi: 10.1101/lm.130506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JH, Izquierdo I. Retrograde messengers, long-term potentiation and memory. Brain Res Brain Res Rev. 1995;21:185–194. doi: 10.1016/0165-0173(95)00013-5. [DOI] [PubMed] [Google Scholar]
- Mei B, Li C, Dong S, Jiang CH, Wang H, Hu Y. Distinct gene expression profiles in hippocampus and amygdala after fear conditioning. Brain Res Bull. 2005;67:1–12. doi: 10.1016/j.brainresbull.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Muller U. Prolonged activation of cAMP-dependent protein kinase during conditioning induces long-term memory in honeybees. Neuron. 2000;27:159–168. doi: 10.1016/s0896-6273(00)00017-9. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. National Academies Press; Washington, DC: 1996. [Google Scholar]
- Ota KT, Pierre VJ, Ploski JE, Queen K, Schafe GE. The NO-cGMP-PKG signaling pathway regulates synaptic plasticity and fear memory consolidation in the lateral amygdala via activation of ERK/MAP kinase. Learn Mem. 2008;15:792–805. doi: 10.1101/lm.1114808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin BJ. The mouse brain in stereotaxic coordinates. 2nd ed. Academic Press; San Diego, CA: 2001. [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Tremblay D, Fleshner M, Rudy JW. A selective role for corticosterone in contextual-fear conditioning. Behav Neurosci. 1997;111:503–511. [PubMed] [Google Scholar]
- Puzzo D, Palmeri A, Arancio O. Involvement of the nitric oxide pathway in synaptic dysfunction following amyloid elevation in Alzheimer's disease. Rev Neurosci. 2006;17:497–523. doi: 10.1515/revneuro.2006.17.5.497. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research–past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Correa FM, Guimaraes FS. The expression of contextual fear conditioning involves activation of an NMDA receptor-nitric oxide pathway in the medial prefrontal cortex. Cereb Cortex. 2008;18:2027–2035. doi: 10.1093/cercor/bhm232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard NS, Ng KT, Gibbs ME. Further support for nitric oxide-dependent memory processing in the day-old chick. Neurobiol Learn Mem. 1998;69:79–86. doi: 10.1006/nlme.1997.3806. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Hui GK, Hui IR, Berlau DJ, McGaugh JL, Weinberger NM. Basolateral amygdala noradrenergic activity mediates corticosterone-induced enhancement of auditory fear conditioning. Neurobiol Learn Mem. 2006;86:249–255. doi: 10.1016/j.nlm.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Bauer EP, Rosis S, Farb CR, Rodrigues SM, LeDoux JE. Memory consolidation of Pavlovian fear conditioning requires nitric oxide signaling in the lateral amygdala. Eur J Neurosci. 2005;22:201–211. doi: 10.1111/j.1460-9568.2005.04209.x. [DOI] [PubMed] [Google Scholar]
- Snyder SH. Nitric oxide and neurons. Curr Opin Neurobiol. 1992;2:323–327. doi: 10.1016/0959-4388(92)90123-3. [DOI] [PubMed] [Google Scholar]
- Solomon SD, Davidson JR. Trauma: Prevalence, impairment, service use, and cost. J Clin Psychiatry. 1997;58((Suppl 9)):5–11. [PubMed] [Google Scholar]
- Thompson BL, Erickson K, Schulkin J, Rosen JB. Corticosterone facilitates retention of contextually conditioned fear and increases CRH mRNA expression in the amygdala. Behav Brain Res. 2004;149:209–215. doi: 10.1016/s0166-4328(03)00216-x. [DOI] [PubMed] [Google Scholar]
- Weitzdoerfer R, Hoeger H, Engidawork E, Engelmann M, Singewald N, Lubec G, Lubec B. Neuronal nitric oxide synthase knock-out mice show impaired cognitive performance. Nitric Oxide. 2004;10:130–140. doi: 10.1016/j.niox.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Yehuda R, McFarlane AC. Conflict between current knowledge about posttraumatic stress disorder and its original conceptual basis. Am J Psychiatry. 1995;152:1705–1713. doi: 10.1176/ajp.152.12.1705. [DOI] [PubMed] [Google Scholar]