Abstract
CD45R/B220 antigen (B220) is a common mouse panB-cell marker used for paraffin-embedded tissues. However, antiB220 has limited specificity in diagnostic pathology because the B220 antigen is expressed on subsets of cytotoxic T lymphocytes and natural killer cells, on plasmacytic dendritic cells, and on T lymphocytes of mice with the lymphoproliferative disorder associated with Fas (lymphoproliferative mutant mouse, B6.MRL-Faslpr/J) or Fas ligand (generalized lymphoproliferative disease mutant mouse, C3H/HeJ-Faslgld/J or B6Smn.C3-Faslgld/J). In addition, mouse B lymphocytes vary in the amount of B220 expressed, and some subsets of mouse B lymphocytes do not express B220 at all. In comparison, Pax5 expression (detected by immunohistochemistry using antiPax5) offers greater specificity and sensitivity because of its earlier expression during B-cell differentiation, its ability to detect all committed B cells, and its restriction to the B-cell lineage. Here we describe the use of an antibody to human Pax5 in diagnostic pathology with formalin-fixed, paraffin-embedded mouse tissue.
Abbreviations: B200, CD45R/B220 antigen; IHC, immunohistochemistry; LBL-NOS, lymphoblastic lymphoma, not otherwise specified; SJCRH, St Jude Children's Research Hospital; SMZL, splenic marginal zone lymphoma; Tdt, terminal deoxynucleotidyl transferase
CD45R/B220 antigen (B220) is commonly used as a mouse pan B-cell marker in paraffin-embedded tissues. However, antiB220 has limited specificity in diagnostic pathology, in that B220 antigen is expressed on natural killer cells and subsets of cytotoxic T lymphocytes that are not restricted according to major histocompatibility complex expression, on plasmacytic dendritic cells,2,4,21,27 and on T lymphocytes of mice with the lymphoproliferative disorder associated with mutations in the Fas or Fasl genes.16,18 In addition, mouse B lymphocytes vary in their level of B220 expression, and some subsets of mouse B lymphocytes do not express B220 at all.9,13,17,22,23
The human PAX5 gene encodes B-cell–specific activator protein, a nuclear transcription factor also known as Pax5. The mouse Pax5 protein plays a central role in B-lymphocyte development and differentiation and influences the balance between immunoglobulin secretion and B-cell proliferation.1,19 Nuclear expression of Pax5 begins at the early proB cell stage, persists throughout B-cell differentiation, and is downregulated at the onset of plasma cell differentiation.5 B-cell genes other than PAX5 that are expressed in early B-cell development are CD19, CD43, and CD79a; the latter 2 are upregulated by Pax5. Like B220, CD19 is not expressed in the early proB stage,13,17 and commercial antiCD19 is not available for use with mouse formalin-fixed, paraffin-embedded tissue. CD43 is expressed in all major blood cell lineages but is downregulated in mature B cells and erythrocytes. CD43 is expressed at the early proB cell stage but is transcriptionally downregulated at the preB (large preBll) cell stage, when the cells express intracellular Igµ.14,25 Consequently, CD43 has limited use as a panB-cell marker. CD79a is less specific than Pax5 for B-lymphoblastic lymphomas and leukemias in patients,26,30 and whether the commercial mouse monoclonal antihuman CD79a works in formalin-fixed, paraffin-embedded mouse tissue is unclear.
Immunohistochemistry (IHC) studies have demonstrated that in normal mice, the CD3-expressing T cells of the splenic periarterial lymphatic sheath, lymph node paracortex region, and thymus do not express Pax5. In contrast, the B220-expressing B cells that make up lymph node and splenic follicles, including their germinal centers and marginal zone, express Pax5.7,33 Therefore, we used a commercially available antihuman Pax5 antibody to determine the B lineage of lymphoproliferations and lymphomas in formalin-fixed, paraffin-embedded mouse tissues. In this report, we use individual cases to illustrate the utility of antiPax5 antibody for demonstrating the T lineage origin of the lymphoproliferations in Fas and Fasl mutant mice; the T- or dual-lineage makeup of lymphomas expressing CD3 and B220, and the B-lineage nature of lymphomas that do not express CD3 or B220.
Materials and Methods
Archive material.
Peripheral lymphoid and nonlymphoid organs were obtained at the time of necropsy from MRL/MpJ-Faslpr/J, C3H/HeJ-Faslgld/J, B6Smn.C3-Faslgld/J, and Cft.C3H-Faslgld /J mice during routine disease surveillance at The Jackson Laboratory (Bar Harbor, ME) and from the pathology department archives at St Jude Children's Research Hospital (SJCRH, Memphis, TN). The SJCRH archival tissues were from the institution's colonies of mice with B6.129 backgrounds and bred for targeted gene deletions associated with the Arf–Mdm2–p53 pathway. Tissue was fixed in either Fekete acid–alcohol–formalin solution (The Jackson Laboratory)29 or 10% neutral buffered formalin (SJCRH), embedded in paraffin, and processed routinely; 4-μm sections were prepared and stained with hematoxylin and eosin or used for immunohistochemistry as described in the following section. The histopathology of all cases was reviewed by 1 of the authors (JER), and lymphomas were classified according to the guidelines proposed by the Mouse Models of Human Cancers Consortium.20 The tissues were obtained from mouse projects approved by the institutional animal care and use committees at The Jackson Laboratory and SJCRH.
Immunohistochemistry.
Immunoperoxidase labeling was performed on tissue fixed in Fekete acid–alcohol–formalin solution or 10% neutral buffered formalin and paraffin-embedded. Briefly, 4-μm sections were used for immunoperoxidase analysis after heating for 1 h at 60 °C, deparaffinization, and rehydration. After antigen retrieval for 30 min in Target Retrieval solution (Dako, Carpinteria, CA; CD3, CD43, IgM, κ light chain), for 15 min in citrate (Zymed, San Francisco, CA; CD45/B200) or 30 min in citrate (terminal deoxynucleotidyl transferase [Tdt], Pax5), IHC was performed by using the avidin–biotin peroxidase complex technique in an automated immunostaining module. The antibodies and dilutions used were: rat antimouse CD45R/B220, 1:200 (clone RA3-6B2); rat antimouse IgM, 1:60 (clone II/41, PharMingen, San Diego, CA); goat polyclonal antihuman CD3, 1:400 (Santa Cruz Biotechnology, Santa Cruz, CA); rat antimouse CD43, 1:20 (clone S7, PharMingen); rabbit polyclonal antihuman Tdt, 1:20 (Supertechs, Bethesda, MD); goat polyclonal antihuman Pax5, 1:100 (Santa Cruz Biotechnology); and goat polyclonal antimouse κ light chain, 1:2000 (Southern Biotechnology Associates, Birmingham, AL). Normal spleen and thymus served as positive lymphocyte antigen controls; these tissues were processed and stained with the subject specimens. For negative control specimens, isotype and concentration matches were substituted for primary antibodies.
Results
Lymphoproliferations with CD3 and B220 expression.
Mice homozygous null for either the Faslpr or the Faslgld gene develop lymphadenopathy due to proliferation or decreased apoptosis of abnormal T cells, which express CD3 and B220.16,18 The lymphoid tissues of 5 Faslgld/Faslgld (B6Smn.C3-Faslgld/J, n = 2; C3H/HeJ-Faslgld/J, n = 2; Cft.C3H-Faslgld/J, n = 1) mice and 2 Faslpr/Faslpr (MRL/MpJ-Faslpr/J) mice were labeled with CD3, B220, Pax5, CD43, and IgM antibodies. The lymphocytes associated with the diffuse hyperplastic lymphocyte proliferation in the lymphoid tissues of all 5 Faslgld/Faslgld and the 2 Faslpr/Faslpr mutant mice were small with mature chromatin and expressed surface CD3, B220, and CD43, but they did not express nuclear Pax5 or immunoglobulin (Figure 1, Table 1).
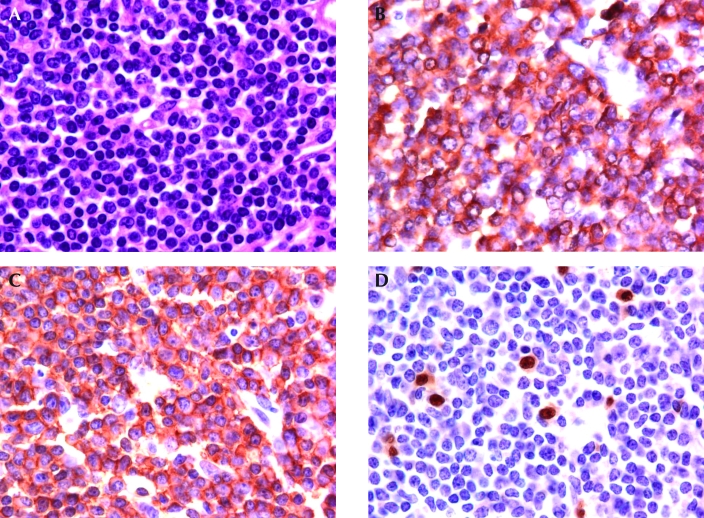
Figure 1.
Representative histology and immunohistochemistry of the lymphoproliferative disorder in Fas and Fasl mutant mice. (A) Lymph node of a Fasl mutant mouse, consisting of small mature lymphocytes with uniform nuclei and condensed chromatin. Hematoxylin and eosin stain. (B) The bulk of the lymphocytes in the lymph node express CD3. (C) A section of the lymph node shown in panels A and B. The bulk of the lymphocytes that express CD3 also express CD45R/B220. (D) The lymphocytes of the Fasl mutant mouse lymph node that expressed both CD3 and CD45R/B220 do not express Pax5, consistent with the lymphocytes being of T-cell lineage. A few normal B lymphocytes that express Pax5 are scattered through the abnormal lymphoproliferative population. Magnification, ×80.
Table 1.
Lymphocyte antigens detected by immunohistochemistry in mouse lymphoproliferations
| Lymphoid classification | Na | CD3 | CD45R/B22 | Pax5 | CD43 | Tdt | cIgµ |
| Faslpr; Fasl gld lymphoproliferation disorder | 7b | 7 | 7 | 0 | 7c | 0 | 0 |
| Lymphoma with CD3 and B220 expression (biphenotypic) | 6 | 6 | 6 | 0 | 0 | 0 | 0 |
| Pro-B lymphoblastic lymphoma | 3 | 0 | 0 | 3 | 3 | 3 | 0 |
| Pre-B lymphoblastic lymphoma | 7 | 0 | 0 | 7 | 0 | 0 | 7 |
| Splenic marginal zone lymphoma | 18 | 0 | 10 | 18 | 0 | 0 | 9 |
Tdt, terminal deoxynucleotidyl transferase
Number of mice examined.
Number of mice with antigen expression by immunohistochemistry.
50% to 60% of the lymphocytes had detectable CD43 expression.
Lymphomas with CD3 and B220 expression.
Lymphomas expressing CD3 and B220 in mice have been genetically determined to be of T-cell lineage.31 Six cases of lymphoma with cells that expressed CD3 and B220 were retrieved from the SJCRH institutional tissue archive of genetically manipulated mice (Table 1). The lymphoma in all 6 cases was disseminated, involving lymphoid and nonlymphoid organs. The lymphomas had high mitotic rates; the nuclei of these cells were 2 to 2.5 times the size of those from normal lymphocytes and had inconspicuous or distinct nucleoli (Figure 2 A). In some tissues, 2 populations of cells were apparent: 1 that expressed both CD3 and B220 (Figure 2 B, C) and 1 that expressed only CD3. However, in other tissues, 2 distinct populations were less clearly distinguishable. In our 6 cases, the lymphoma cells expressing CD3 and B220 did not express Pax5 (Figure 2 D) or immunoglobulins (Table 1), and CD43 was expressed only by lymphocytes expressing CD3 in the absence of B220 expression.
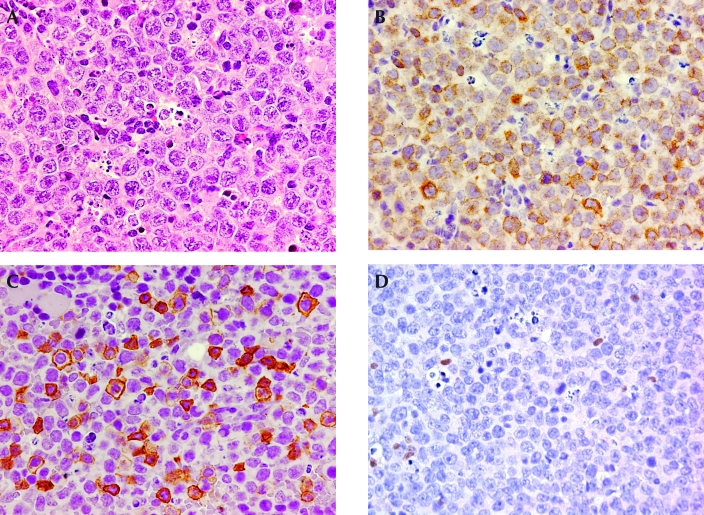
Figure 2.
Representative histology and immunohistochemistry of T-cell lymphomas expressing CD45R/B220. (A) This section of lymphoma shows lymphocytes with large vesicular nuclei and prominent nucleoli. Hematoxylin and eosin stain. (B) Overall, the population of lymphoma lymphocytes expresses CD3. (C) At least 50% of the lymphoma lymphocytes that express CD3 also express CD45R/B220. (D) Lymphoma lymphocytes that express CD45R/B220 do not express Pax5, suggesting that the mouse has 2 T-cell lymphomas, 1 of which expresses CD3 and CD45R/B220. A few normal B lymphocytes that express Pax5 are scattered through the tumor. Magnification: ×50 (A); ×40 (B–D).
Lymphomas that lack B220 expression.
A review of the lymphomas in the SJCRH tissue archive that did not express CD3 or B220 identified 2 morphologically distinct sets of lymphomas. One set of 10 lymphomas was morphologically consistent with lymphoblastic lymphoma (LBL, Figure 3 A); the other set of 8 lymphomas involved only the spleen and was morphologically similar to the 10 splenic marginal zone lymphomas (SMZL, Figure 4 A, B)11,34 in the tissue archive.
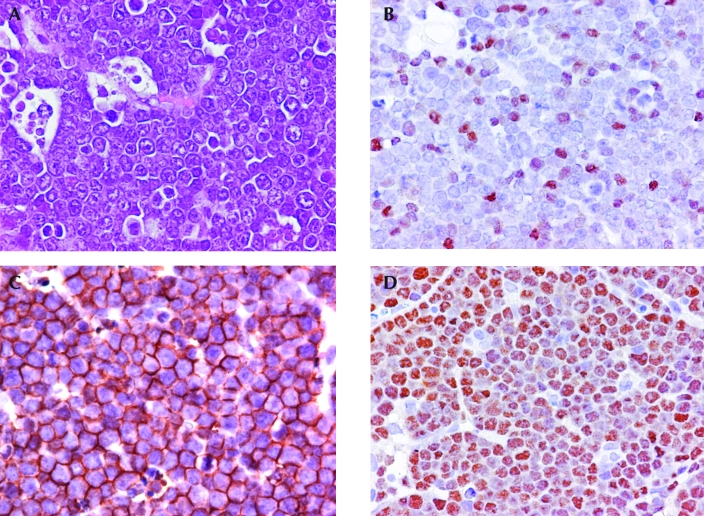
Figure 3.
Representative histology and immunohistochemistry of mouse lymphomas that do not express CD3 or B220. (A) Section of a lymph node from a mouse with lymphoblastic lymphoma. At the left, macrophages with tingible bodies are associated with the tumor lymphocytes, which have small- to medium-sized nuclei and inconspicuous or small nucleoli. Hematoxylin and eosin stain. (B) Lymphoma cells showing varying intensity of expression of terminal deoxynucleotidyl transferase, consistent with a lymphoblastic lymphoma. (C) Overall the lymphoma cell population has strong expression of CD43, consistent with a lymphoblastic lymphoma. (D) Overall the lymphoma cell population shows strong nuclear expression of Pax5, indicating a B-lymphoblastic lymphoma. Magnification, ×80.
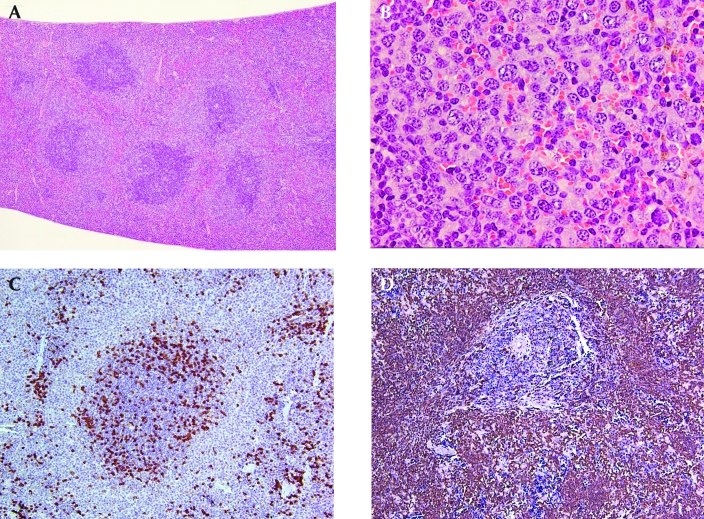
Figure 4.
Representative histology and immunohistochemistry of splenic marginal zone lymphomas that do not express CD45R/B220. (A) Splenic marginal zone lymphoma with multiple follicles that have expanding marginal zones that extend into the red pulp and bridge the follicles. Hematoxylin and eosin stain; magnification, ×4. (B) Splenic marginal zone lymphoma in the red pulp. The lymphoma cells have small to medium round or irregular nuclei and abundant pale cytoplasm. A mitotic figure is evident in the upper right corner. Hematoxylin and eosin stain; magnification, ×50. (C) The lymphoma cells in the marginal zone surrounding the follicle and in the red pulp do not express CD45R/B220. Magnification, ×10. (D) The lymphoma cells in the marginal zone surrounding the follicle and filling the red pulp express Pax5 with strong intensity. Magnification, ×10.
Approximately 55% of preB and 65% of early preB (proB) childhood lymphoblastic leukemias reportedly do not express the panB-cell CD20 marker.6 The frequency of mouse lymphoblastic lymphomas that do not express the panB-cell B200 antigen is not well documented. The 10 mouse CD3− LBL lacking B220 expression initially were classified as ‘LBL, not otherwise specified’ (LBL-NOS). AntiPax5 labeling showed diffuse nuclear Pax5 expression in all 10 of the LBL (Figure 3 D, Table 1); 3 of the 10 LBL also expressed Tdt and CD43 (Figure 3 B, C), but they lacked cytoplasmic Igμ (Table 1). The other 7 LBL-NOS did not express Tdt, CD43, or light chain immunoglobulin, but the cells expressed cytoplasmic Igμ with weak to moderate intensity (Table 1).
The 18 lymphomas with the distribution pattern and cytologic morphology of SMZL (Figure 4 A, B) had 2 B220 immunophenotypic profiles. Whereas 10 of the SMZL expressed B220, 8 did not (Figure 4 C). The cytologic morphology of the 2 sets of lymphomas was similar. The lymphomas consisted of small or medium sized lymphocytes with either round or irregular nuclei and a moderate amount of pale cytoplasm (Figure 4 B). Although B220 expression was not detected in 8 of the 18 lymphomas with SMZL morphology (Figure 4 C), nuclear Pax5 expression was evident in all 18 by IHC with antiPax5 antibody (Figure 4 D, Table 1).
Discussion
B220 antigen is the standard panB-cell marker for identifying mouse B-cell lymphoproliferations and lymphomas. However, the B220 antigen is sometimes expressed on T lymphocytes and T-cell lymphomas, and it is often not detected in some B-cell lymphomas.4,11,16,18,27,31 Consequently, antibody to B220 is not ideal for diagnosis of mouse lymphoproliferative disorders and B-cell lymphomas. In normal mice, antiPax5 antibody labels the lymphocytes in the spleen and lymph node that express CD45R/B220.7,33 These observations suggest that antibody to Pax5 may be superior to antiB220 in identifying abnormal B-cell lymphoproliferations. However, to date, antiB220 and antiPAX5 have not been compared extensively in the diagnosis of mouse hematopoietic disorders.
The present study confirms that proliferating lymphocytes of Fas and Fasl mutant mice express both CD3 and B220. To our knowledge, this is the first study to demonstrate that a high percentage of the lymphocytes expressing CD3 and B220 also express CD43, whereas none tested express Pax5 or immunoglobulin. The expression of CD43 on CD3+B220+ lymphocytes suggests that they may be T lymphocytes, although CD43 is not a lineage-specific antigen. Further, the lack of Pax5 and immunoglobulin expression in these lymphocytes is consistent with the T-cell lineage of the proliferating lymphocytes in Fas and Fasl mutant mice. With the extensive development of genetically engineered mice, mice with various genetic mutations in the Fas apoptotic pathway may be developed intentionally (Fastm1Dlo, Fasltm1.1Lest)15,28 or inadvertently, and the use of antiPax5 antibody will be useful in diagnosing this type of lymphoproliferative disorder.
The lack of Pax5 expression and detectable immunoglobulins in the CD3+B220+ lymphomas suggests that the 6 mice presented have 2 distinct T-cell lymphomas, 1 expressing only CD3 and 1 expressing both CD3 and B220. The lymphoma expressing only CD3 also may express B220 but at a level below the limit of detection by IHC. However, multiple neoplasms frequently develop in genetically manipulated mice.10,35 The CD43 antigen is not lineage-specific and is only weakly expressed on cortical thymocytes. Therefore, the lack of detectable CD43 expression in the CD3+B220+ lymphoma lymphocytes further suggests that these lymphomas may represent a T-cell stage with weak CD43 expression that is below the limit of IHC detection. In addition, these 2 immunophenotypic lymphoma populations may have originated from the same clone, with 1 population arising from the other. Tissue was not available for clonal analysis, but clonal analysis is not always helpful in the diagnosis of lymphoid neoplasia in genetically targeted mice.20 Although the immunophenotype of 1 population was similar to that of Fas and Fasl mutant mice, the proliferations in all 6 cases were considered to be neoplastic rather than benign lymphoproliferations on the basis of the high mitotic rates, large nuclei, prominent nucleoli, and extensive involvement of nonlymphoid organs.
B220, a common panB-cell marker used to identify mouse B lymphocytes, is not expressed on B cells during their earliest developmental stages,25 and FACS analysis does not detect B220 on the surface of 20% of SMZL in mice.11 Similarly, CD20, a common panB-cell marker used to identify human B lymphocytes, frequently is not detected by FACS analysis on the cell surfaces of human B-cell lymphomas.6 These observations suggest that some B-cell antigens such as CD20 and B220, are not detected because they are not expressed or because they are expressed at a low level rather than because the tissues were fixed in formalin and embedded in paraffin.
The detection of Pax5 expression in the 10 LBL that did not express CD3 or B220 clearly indicates that these lymphomas were of B-cell lineage. Subsequently, the LBL-NOS were reclassified as either early preB (proB) LBL or preB LBL on the basis of the antigen expression scheme of B-cell ontogeny.25 Mouse B cells and T cells express both Tdt and CD43 early during B-cell and T-cell ontogeny.25 The 3 lymphomas expressing Pax5, Tdt, and CD43 but lacking cytoplasmic Igμ were classified as early preB (proB) lymphoblastic lymphoma. The other 7 LBL having Pax5 expression and variable cytoplasmic Igμ expression but lacking Tdt, CD43, and immunoglobulin light chain expression were classified as preB LBL.
B220 in SMZL has been reported as undetectable by flow cytometry, which is more sensitive than IHC.11 The B-cell nature of the 8 B220− lymphomas with SMZL morphology was clearly demonstrated by IHC using antiPax5 antibody. The detection of IgM or κ light chain immunoglobulin also would be helpful in these situations. IgM and κ light chain are detected by flow cytometry in a high percentage of mouse SMZL.11,31 However, IHC of paraffin-embedded tissues frequently is not sufficiently sensitive to detect immunoglobulin on lymphocytes.12 Furthermore, immunoglobulin IHC is complicated by nonspecific background uptake of immunoglobulin by a variety of cells and by staining of extracellular serum or stromal immunoglobulin molecules. Our study shows that these problems can be alleviated by using antiPax5 to confirm the B-cell nature of B220− SMZL. A recent report describes 2 types of SMZL in human patients: 1 type consists of nonmutated cells believed to originate from naïve marginal zone B cells, and the other type consists of mutated cells thought to arise from memory marginal zone B cells.3,24 The mouse splenic marginal zone comprises a heterogeneous B-cell population consisting of memory postgerminal-center B cells, naïve transitional B cells from the bone marrow, and recirculation B cells.32 A naïve marginal zone cell population was shown to be negative for the B220 and CD5 expression that is characteristic of B1b cells.8,21 Mouse marginal zone lymphomas are CD5− by IHC.11 Adult mouse B1b cells arise from the bone marrow and are found in a variety of tissues, including the spleen. B1b cells are responsible for the innate immune response and respond readily to a variety of T-independent antigens. Marginal zone lymphomas that express B220 are reported to have immunoglobulin heavy chain rearrangements,34 which indicate that these lymphoma cells are mutated memory cells. The mutational status of the B220− SMZL in the present and other studies remains undetermined.
The described findings indicate that antibody to the B220 antigen has limitations for diagnosis of mouse lymphoproliferative disorders and B-cell lymphomas. The findings demonstrate the value and superiority of antiPax5 to anti-B220 in determining whether lymphoproliferations and lymphomas that express CD3 and B220 are of B-cell or T-cell lineage and in confirming that lymphomas not expressing B220 are of B-cell lineage.
Acknowledgments
We thank Dorothy Bush for the excellent immunohistochemistry and the SJCRH histotechnicians for their technical assistance. JER is grateful to the American Lebanese Syrian Associated Charities (ALSAC) of SJCRH for their generous support.
References
- 1.Adams B, Dorfler P, Aguzzi A, Kozmik Z, Urbanek P, Maurer-Fogy I, Busslinger M. 1992. Pax5 encodes the transcription factor BSAP and is expressed in B lymphocytes, the developing CNS, and adult testis. Genes Dev 6:1589–1607 [DOI] [PubMed] [Google Scholar]
- 2.Asselin-Paturel C, Brizard G, Pin JJ, Briere F, Trinchieri G. 2003. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J Immunol 171:6466–6477 [DOI] [PubMed] [Google Scholar]
- 3.Bahler DW, Pindzola JA, Swerdlow SH. 2002. Splenic marginal zone lymphomas appear to originate from different B cell types. Am J Pathol 161:81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballas ZK, Rasmussen W. 1993. Lymphokine-activated killer cells. VII. IL4 induces an NK1.1+CD8α+β−TCRαβ B220+ lymphokine-activated killer subset. J Immunol 150:17–30 [PubMed] [Google Scholar]
- 5.Barberis A, Widenhorn K, Vitelli L, Busslinger M. 1990. A novel B-cell lineage-specific transcription factor present at early but not late stages of differentiation. Genes Dev 4:849–859 [DOI] [PubMed] [Google Scholar]
- 6.Behm F. 2006. Immunophenotyping. Pui C-H. Childhood leukemias. New York: Cambridge University Press; p. 150–209 [Google Scholar]
- 7.Cattoretti G, Shaknovich R, Smith PM, Jack HM, Murth VV, Alobeid B. 2006. Stages of germinal center transit are defined by B cell transcription factor coexpression and relative abundance. J Immunol 177:6930–6939 [DOI] [PubMed] [Google Scholar]
- 8.de Andres B, Cortegano I, Serrano N, Del RB, Martin P, Gonzalo P, Marcos MA, Gaspar ML. 2007. A population of CD19highCD45R /lowCD21low B lymphocytes poised for spontaneous secretion of IgG and IgA antibodies. J Immunol 179:5326–5334 [DOI] [PubMed] [Google Scholar]
- 9.Driver DJ, Heyzer-Williams LJ, Cool M, Stetson DB, Heyzer-Williams MG. 2001. Development and maintenance of a B220-memory B cell compartment. J Immunol 167:1393–1405 [DOI] [PubMed] [Google Scholar]
- 10.Eischen CM, Rehg JE, Korsmeyer SJ, Cleveland JL. 2002. Loss of Bax alters tumor spectrum and tumor numbers in ARF-deficient mice. Cancer Res 62:2184–2191 [PubMed] [Google Scholar]
- 11.Fredrickson TN, Lennert K, Chattopadhyay SK, Morse HC, 3rd, Hartley JW. 1999. Splenic marginal zone lymphomas of mice. Am J Pathol 154:805–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gocke C. 2007. Immunohistology of non-Hodgkin lymphoma. Dabbs D. Diagnostic immunohistochemistry, 2nd ed Philadelphia: Churchhill Livingstone; p. 137–161 [Google Scholar]
- 13.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. 1991. Resolution and characterization of proB and pre-proB cell stages in normal mouse bone marrow. J Exp Med 173:1213–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jimi E, Phillips RJ, Rincon M, Voll R, Karasuyama H, Flavell R, Ghosh S. 2005. Activation of NFκB promotes the transition of large, CD43+ preB cells to small. Int Immunol 17:815–825 [DOI] [PubMed] [Google Scholar]
- 15.Karray S, Kress C, Cuvellier S, Hue-Beauvais C, Damotte D, Babinet C, Levi-Strauss M. 2004. Complete loss of Fas ligand gene causes massive lymphoproliferation and early death, indicating a residual activity of gld allele. J Immunol 172:2118–2125 [DOI] [PubMed] [Google Scholar]
- 16.Kobata T, Takasaki K, Asahara H, Hong NM, Masuko-Hongo K, Kato T, Hirose S, Shirai T, Kayagaki N, Yagita H, Okumura K, Nishioka K. 1997. Apoptosis with FasL+ cell infiltration in the periphery and thymus of corrected autoimmune mice. Immunology 92:206–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krop I, de Fougerolles AR, Hardy RR, Allison M, Schlissel MS, Fearon DT. 1996. Self-renewal of B1 lymphocytes is dependent on CD19. Eur J Immunol 26:238–242 [DOI] [PubMed] [Google Scholar]
- 18.Laouar Y, Ezine S. 1994. In vivo CD4+ lymph node T cells from lpr mice generate CD4−CD8−B220+TCRβlow cells. J Immunol 153:3948–3955 [PubMed] [Google Scholar]
- 19.Michaelson JS, Singh M, Birshtein BK. 1996. B cell lineage-specific activator protein (BSAP). A player at multiple stages of B cell development. J Immunol 156:2349–2351 [PubMed] [Google Scholar]
- 20.Morse HC, 3rd, Anver MR, Fredrickson TN, Haines DC, Harris AW, Harris NL, Jaffe ES, Kogan SC, MacLennan IC, Pattengale PK, Ward JM. 2002. Bethesda proposals for classification of lymphoid neoplasms in mice. Blood 100:246–258 [DOI] [PubMed] [Google Scholar]
- 21.Nikolic T, Dingjan GM, Leenen PJ, Hendriks RW. 2002. A subfraction of B220(+) cells in murine bone marrow and spleen does not belong to the B cell lineage but has dendritic cell characteristics. Eur J Immunol 32:686–692 [DOI] [PubMed] [Google Scholar]
- 22.Nishimura H, Hattori S, Abe M, Hirose S, Shirai T. 1992. Differential expression of a CD45R epitope (6B2) on murine CD5+ B cells: possible difference in the posttranslational modification of CD45 molecules. Cell Immunol 140:432–443 [DOI] [PubMed] [Google Scholar]
- 23.O'Keefe TL, Williams GT, Davies SL, Neuberger MS. 1998. Mice carrying a CD20 gene disruption. Immunogenetics 48:125–132 [DOI] [PubMed] [Google Scholar]
- 24.Oscier D, Owen R, Johnson S. 2005. Splenic marginal zone lymphoma. Blood Rev 19:39–51 [DOI] [PubMed] [Google Scholar]
- 25.Osmond DG, Rolink A, Melchers F. 1998. Murine B lymphopoiesis: toward a unified model. Immunol Today 19:65–68 [DOI] [PubMed] [Google Scholar]
- 26.Pilozzi E, Pulford K, Jones M, Müller-Hermelink HK, Falini B, Ralfkiaer E, Pileri S, Pezzella F, De Wolf-Peeters C, Arber D, Stein H, Mason D, Gatter K. 1998. Coexpression of CD79a (JCB117) and CD3 by lymphoblastic lymphoma. J Pathol 186:140–143 [DOI] [PubMed] [Google Scholar]
- 27.Rolink A, ten BE, Melchers F, Fearon DT, Krop I, Andersson J. 1996. A subpopulation of B220+ cells in murine bone marrow does not express CD19 and contains natural killer cell progenitors. J Exp Med 183:187–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Senju S, Negishi I, Motoyama N, Wang F, Nakayama K, Nakayama K, Lucas PJ, Hatakeyama S, Zhang Q, Yonehara S, Loh DY. 1996. Functional significance of the Fas molecule in naive lymphocytes. Int Immunol 8:423–431 [DOI] [PubMed] [Google Scholar]
- 29.Seymour R, Ichiki T, Mikaelian I, Bogges D, Silva K, Sundberg J. 2004. Necropsy methods. Hedrich HJ, edition London: Academic Press; p 495–516 [Google Scholar]
- 30.Tiacci E, Pileri S, Orleth A, Pacini R, Tabarrini A, Frenguelli F, Liso A, Diverio D, Lo-Coco F, Falini B. 2004. PAX5 expression in acute leukemias: higher B-lineage specificity than CD79a and selective association with t(8;21)-acute myelogenous leukemia. Cancer Res 64:7399–7404 [DOI] [PubMed] [Google Scholar]
- 31.Vasmel WL, Radaszkiewicz T, Miltenburg AM, Zijlstra M, Melief CJ. 1987. Refinement and precision in the classification of murine lymphomas by genotyping with immunoglobulin and T cell receptor probes. Leukemia 1:155–162 [PubMed] [Google Scholar]
- 32.Vinuesa C, Cook M. 2007. The molecular basis of lymphoid architecture in the mouse. Fox J, Davisson M, Quimby F, Barthold S, Newcomer C, Smith A. The mouse in biomedical research, 2nd ed Burlington (MA): Academic Press; p 57–75 [Google Scholar]
- 33.Ward JM, Erexson CR, Faucette LJ, Foley JF, Dijkstra C, Cattoretti G. 2006. Immunohistochemical markers for the rodent immune system. Toxicol Pathol 34:616–630 [DOI] [PubMed] [Google Scholar]
- 34.Ward JM, Tadesse-Heath L, Perkins SN, Chattopadhyay SK, Hursting SD, Morse HC., 3rd 1999. Splenic marginal zone B-cell and thymic T-cell lymphomas in p53-deficient mice. Lab Invest 79:3–14 [PubMed] [Google Scholar]
- 35.Weber JD, Jeffers JR, Rehg JE, Randle DH, Lozano G, Roussel MF, Sherr CJ, Zambetti GP. 2000. p53-independent functions of the p19(ARF) tumor suppressor. Genes Dev 14:2358–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]