Abstract
The frequent occurrence of fatigue and disturbed sleep in cancer survivors and the negative effect of these symptoms on quality of life and clinical outcome underscore the need to identify mechanisms that cause cancer-related fatigue, with a view toward developing more effective treatments for this problem. Human studies of fatigue and disturbed sleep are limited by high inter-individual genetic and environmental variability, difficulties with behavioral or reporting compliance, and the subjective nature of the problems. Although animal models also must overcome the barrier of assessing fatigue and sleep disturbance in the absence of obvious objective clinical markers, animal studies are easier to control and standardize than are studies of people. Moreover, animal models are crucial to the identification and understanding of underlying disease mechanisms. This review describes the need for, the feasibility of, and several possible approaches to measuring fatigue in animal models of cancer and to relating such measures to disturbed sleep, immune function, and other potential mechanisms. Developing and using animal models to better understand fatigue and disturbed sleep related to cancer and its treatment has an enormous potential to expand the knowledge base and foster hypotheses necessary for the future development and testing of interventions.
Abbreviation: HR-QOL, health-related quality of life, LLC1, Lewis lung carcinoma 1
Cancer-related Fatigue and Sleep Disorders: The Need for Further Research
Human fatigue is a multidimensional symptom that is characterized by considerable variation in definition, description, and perception on the part of both the patient and the healthcare provider.117 Because of this variation, the assessment of fatigue requires consideration of a number of its features, including perceived severity, degree of interference with normal activities, daily pattern of occurrence or exacerbation, associated distress, and progression or regression over time.131 Cancer-related fatigue is defined as a subjective report of tiredness that is associated with cancer or its treatment, is persistent, extends in duration or severity beyond that which might be expected based on a subject's recent physical activity, and is severe enough to cause distress and interfere with usual functioning.119 Patients with cancer often describe fatigue based on 4 physical changes: decreased physical performance, unusual or extreme tiredness, feelings of weakness, and unusual need for rest.65,176
Fatigue and disturbed sleep are common short- and long-term problems experienced by many cancer patients and survivors.7,14,17,86,127 Fatigue is reported by as many as 40% of cancer patients at the time of diagnosis, up to 90% of those treated with radiation, and up to 80% of those treated with chemotherapy; furthermore, fatigue continues for months or years after completion of treatment in about a third of survivors of cancer.74 Fatigue is common in many types of malignancies, including leukemia, lung carcinoma, and breast cancer, and is reported to occur before, during, and after therapy5,17,52,81,97,110,123 One recent study115 identified greater likelihood of objectively reported fatigue, sleep disturbance, and daytime sleepiness among a large population of adult survivors of various childhood cancers, including acute lymphocytic leukemia, central nervous system tumors, Hodgkin lymphoma, soft-tissue sarcomas, and bone tumors, as compared with their siblings.
Fatigue is a common problem for breast cancer patients, who often develop fatigue in association with both the disease and its treatment.5,26,113 For example, patients with breast cancer score significantly higher on the Fatigue Symptom Inventory prior to adjuvant treatment, regardless of whether the cancer is invasive.2 Among breast cancer survivors, fatigue is reported by up to 90% of patients who received chemotherapy or radiation,81 with residual fatigue often persisting after or between treatments.7 However, the relationship of fatigue to therapy is not consistent across studies. Some studies report that women with breast cancer experience both disturbed sleep and fatigue before they begin chemotherapy,5 others find higher levels of fatigue among patients who received chemotherapy105,174 or both chemotherapy and radiation,30 and still others find no difference in level of fatigue as a function of type of treatment.7,20 Cancer-related fatigue can persist long after remission. For example, 1 study linked cancer therapy to fatigue that began beginning early in the recovery period after treatment and in some cases persisted for as long as 10 y.30,31
Cancer-related fatigue may be in part related to disturbed sleep, although the precise nature of the relationship and the most prevalent types of sleep disorders in cancer survivors have yet to be determined.4 The International Classification of Sleep Disorders can be used to diagnose primary sleep disorders in cancer patients and survivors.19 Insomnia and daytime sleepiness (hypersomnia) are prevalent and often chronic problems in cancer patients, with sleep–wake disturbances affecting between 30% and 60% and persisting in nearly 30% of breast cancer patients for months after surgery.63,84,143 Women also list sleep problems as among the most distressing symptoms experienced during chemotherapy.20 Cancer patients often report altered diurnal rhythms, the ability to sleep better during the day, or being able to sleep for only 3 or 4 h before awakening.176 Problems with sleep, including daytime sleepiness and sleep disturbances, also influence perceptions of fatigue.17,18 Patients are more likely to experience sleep disturbances, insomnia, or depression after diagnosis of cancer if they displayed 1 of those symptoms prior to diagnosis or treatment.30,31,142
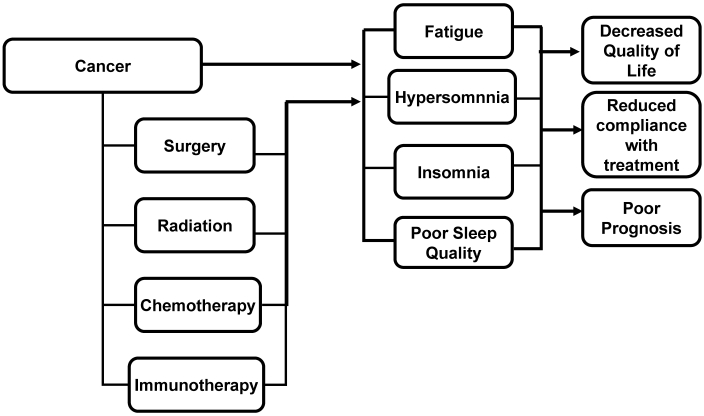
Numerous factors have been invoked to explain the occurrence of fatigue and sleep disorders in cancer patients and survivors. Factors associated with the development of fatigue and disturbed sleep include type of treatment (for example, surgery, chemotherapy, radiation therapy, immunotherapy; Figure 1), comorbid conditions (for example, anemia, malnutrition, heart failure), ancillary medications (for example, opioids, steroids), anxiety, depression, physical inactivity, and pain.4,77,117,131,162 Disturbed sleep that may contribute to fatigue also can develop due to environmental factors (for example, excess noise and light) and dietary factors (for example, caffeine and alcohol consumption).19,118 Therefore, not surprisingly, sleep disorders can cause fatigue, daytime sleepiness may be difficult to differentiate from fatigue, and epidemiologic studies demonstrate a clustering of fatigue and sleep disorders in cancer survivors.87,138,171
Figure 1.
Effects of cancer and cancer therapy on sleep and fatigue. Cancer and its various treatments have the capacity to cause fatigue via effects of sleep. In addition, fatigue may be associated with an increased need for or desire to sleep in cancer patients. These problems can have adverse consequences for the patient.
Circadian rhythms, which are also known as diurnal rhythms, are the normal daily ‘approximately 24-h’ cycles of physiologic processes and behaviors; disruptions in normal circadian rhythm disruptions are postulated to contribute to cancer itself, as well as to cancer-related fatigue.140 Circadian rhythms influence cancer development and treatment through the activity of so-called ‘clock genes,’ which modulate the timing and progression of the cell cycle, cell division, and apoptosis.23,40,107 Consistent with a role for clock genes and associated processes in reducing cancer risk and controlling tumor growth, prolonged exposure to shift work and repeated jet lag are associated with increased risk of cancer in people.59,89,114,132,135,144 In addition, alterations in behavioral and hormonal diurnal rhythms are associated with risk of death in patients with metastatic colorectal and breast cancer, respectively.112,148 In addition to clock genes, disruption of normal diurnal cycles of other hormones, notably melatonin, might influence cancer risk, tumor growth, and potentially fatigue.
Numerous pharmacologic and nonpharmacologic therapies have been recommended for treating both fatigue and sleep disturbances in general and as they develop in association with cancer, but these treatments achieve limited success.117 Fatigue and disturbed sleep negatively impact quality of life (QOL) and may predispose patients to poor psychosocial outcomes, reduce compliance with treatment, and lead to interrupted or premature withdrawal from treatment, thereby potentially affecting clinical outcome and even survival (Figure 1).4,63,113,117,147 The prevalence of fatigue and disturbed sleep in cancer patients underscores the need to assess more fully potential underlying mechanisms of fatigue with a view toward developing improved treatments for these conditions, thereby improving QOL (and perhaps treatment compliance and clinical outcomes) for cancer patients.
Cancer and fatigue: associated conditions.
Fatigue during cancer may be associated with the disease itself, the therapy, previous health conditions made worse by the disease or treatment, or psychologic responses to the diagnosis or treatment of cancer. Some other potential causes of fatigue during cancer and cancer treatment include functional impairments of cancerous or related organ systems and tissues (for example, brain, muscle, gut, liver, bone marrow, vasculature, lung kidneys, pancreas, thyroid), associated neurotransmitter and hormone dysregulation, and general problems such as anorexia, cachexia, depression, and pain.7,30,34,140 In addition, socioeconomic factors may influence the development of fatigue. For example, breast cancer patients were more likely to be fatigued 6 months after treatment if unmarried, in a lower income bracket, more fearful of fatigue, obese, or more sedentary.53 In response to a standardized social stressor, fatigued breast cancer survivors showed a significantly blunted cortisol response as compared with nonfatigued survivors, suggesting that the enduring fatigue is associated with dysregulation in hypothalamic–pituitary–adrenal axis responsiveness.24 Fatigue has been reported to predict survival time of terminal cancer patients.67
Depression is a strong predictor of fatigue in cancer patients, including those whose fatigue persists long after treatment,30,31 and both fatigue and depression may improve in response to the same therapy. For example, psychologic and educational intervention focused on coping with common fears improved fatigue, energy, cancer-specific distress, and depression in cancer survivors.154 Another potential treatment for both depression and fatigue is light therapy, which both strengthens diurnal rhythms and improves mood.100 Patients who survive cancer eventually experience less interaction with a medical team, decreased emotional support, and perhaps fear of cancer recurrence; such factors have been linked to increased depression and fatigue.10,109,154,167,168
Cancer patients often describe their fatigue as a lack of energy, which could indicate that fatigue is caused by changes in metabolism and energy generation, particularly in skeletal muscles.64 In addition, patients undergoing chemotherapy often develop anorexia, and reduced food intake could reduce energy availability and contribute to fatigue.113 Cancer-related anorexia and cachexia affect about half of all patients with cancer, often preceding death.96,104,141 However, reduced food intake is not the sole cause of cancer-related weight loss, because increasing caloric intake may not reverse the weight loss.156
Anemia affects as many as half of all cancer patients, with potential adverse effects on QOL, yet this condition is often undiagnosed and untreated.68,76,88,111 Studies using validated survey instruments to assess fatigue and QOL in patients receiving chemotherapy find a relationship between chemotherapy-induced anemia, fatigue, and QOL, with improvements in all measures achieved by treatment for anemia.38 A recent review of 18 clinical trials found statistically and clinically significant improvements in both hemoglobin and health-related QOL (particularly with regard to fatigue) in cancer patients receiving anemia treatment as compared with those receiving placebo or standard of care.86 For example, anemic cancer patients who were treated with recombinant human erythropoietin α reported increased energy and improved QOL.50,68
Some chemotherapeutic agents cause neurotoxicity, which could induce neuromuscular weakness that might contribute to feelings of fatigue. However, the interactions of neurotoxicity and fatigue can be complex. For example, the chemotherapeutic drugs known as taxanes produce their anticancer effects by stabilizing microtubules and thereby interfering with mitosis. By stabilizing microtubules, taxanes also interfere with neuronal function. A comparison of the taxanes paclitaxel and docetaxel in Wistar rats showed that both agents reduced nerve conduction velocity to a similar degree in a dose-dependent manner, yet histologic nerve damage was more severe after paclitaxel administration and for both drugs was less severe than expected based on the neurophysiologic findings.128 These results were interpreted to suggest that taxanes exert their neurotoxic effect not only via microtubular stabilization in peripheral nerves but also through other unknown mechanisms.128 Paclitaxel neuropathy has been associated with hyperalgesia and allodynia in rats in the absence of motor impairment.130
Patients receiving treatment for cancer often show diminished physical and functional wellbeing as compared with patients not receiving treatment.33 Planned postintervention rehabilitation can be used to improve strength and cardiovascular conditioning and reduce pain and fatigue after treatment.151 Maintaining an exercise program of moderate intensity, individualized to the patient's specific needs, reduced fatigue significantly both during and after cancer treatment.145 A meta-analysis of 41 trials that used fatigue as an outcome measure for psychologic and activity-based interventions in adult cancer patients suggested some efficacy of nonpharmacologic interventions to manage cancer-related fatigue, although a paucity of research with heightened fatigue as an eligibility criterion weakened the strength of the conclusions.80
Cytokines, inflammation, and fatigue during cancer and cancer therapy.
Acute inflammation is a complex, tightly regulated, self-limiting process that is critical for survival. However, some health conditions are associated with inflammation that does not resolve or self-limit, but rather becomes chronic. Substantial evidence supports a causal link between chronic inflammation and the initiation and progression of cancer.12,13 In addition, tumors cancers often contain an infiltrate of inflammatory cells that, together with neoplastic cells, express a complex array of cytokines and chemokines.12,43,72,93,174 Cytokines orchestrate communication between tumor cells, stromal cells, and tumor-infiltrating immune cells, whereas chemokines coordinate trafficking of immune cells into areas of inflammation. The immune cells that enter the tissue in response to chemokines in turn secrete additional cytokines and other tumor promoting mediators. These processes can influence tumor progression. For example, infiltration by large numbers of macrophages is associated with poor prognosis in some human cancers,99 and cytokine production by tumor cells has been associated with aggressive tumors that grow more rapidly and are more likely to metastasize.3
Numerous studies indicate that inflammation and the immune response can influence sleep.161 Research that began in the 1970s demonstrates that cytokines, particularly the proinflammatory cytokines IL1β and TNFα, are powerful modulators of sleep-wake behavior. In general, substances or manipulations that induce IL1β or TNFα increase sleep, whereas substances that inhibit the synthesis or actions of these cytokines reduce sleep.92,122 In rats, mRNA and protein concentrations of IL1β and TNFα vary diurnally in brain, with peaks occurring during the sleep phase.32,61 In humans, plasma concentrations of TNFα peak during sleep.70 Administration of exogenous TNFα or IL1β increases both time spent in slow-wave sleep and electroencephalographic slow-wave amplitudes during sleep (a measure of the depth of sleep) in a variety of species.49,58 Accumulating evidence suggests that IL6 also modulates sleep. IL6 is elevated during conditions associated with excessive daytime sleepiness (for example, narcolepsy, obstructive sleep apnea).166 IL6 concentrations vary in phase with sleep–wake behavior in humans and rats.69,165 In healthy volunteers, prolonged wakefulness increases IL6 concentrations in plasma,149 and administration of IL6 increases sleep.153
Tumor growth and therapy, including chemotherapy, immunotherapy, and radiotherapy, typically are associated with inflammation and the production of an associated array of proinflammatory and sleep-modulatory cytokines, including TNFα, IL1β, and IL6.13,16,41,120 For example, the chemotherapeutic drugs paclitaxel, tamoxifen, and cisplatin increase serum levels of sleep-modulatory cytokines in cancer patients.94,133 These and other cytokines have been also associated with chronic fatigue.39,94,126 In persistent cancer-related fatigue, abnormal or unresolved inflammatory activation may persist beyond the period of treatment and into the remission phase. For example, a study of breast cancer survivors with persistent fatigue revealed that their immune systems remained activated for as long as 5 y after diagnosis, with circulating levels of inflammatory cytokines that were up to 5 times higher in breast cancer survivors who had fatigue as compared with those who did not.42 Cancer survivors with fatigue also may have an altered glucocorticoid response to stress;27 this altered response might contribute to both altered immune activation and problems with sleep. Two immunologic markers, the ratio of soluble IL6 receptor to monocyte-associated IL6 receptor and low numbers of circulating CD69+ T lymphocytes, were highly diagnostic of fatigue in a cross-validated study of breast cancer survivors.42 Collectively, these data demonstrate that sleep-modulatory cytokines are likely to be perturbed in cancer patients and support the possibility that cytokines contribute to their sleep disruption and fatigue.
Inflammation also has been associated with other common symptoms and clinical correlates of cancer. For example, proinflammatory cytokines can cause anorexia, anhedonia and depression, and may perturb CNS systems that regulate energy balance, the hypothalamic–pituitary–adrenal axis, the sympathetic nervous system, and the immune system.6,104,141 Rapid induction of proinflammatory cytokines can also suppress the formation of red blood cells, leading to mild or moderate anemia.69 Inflammatory cytokines have also been linked to serotonin dysregulation, fatigue and depression.140,172 For example, administration of the cytokine interferon α as a cancer chemotherapeutic agent causes anorexia and fatigue that are not easily controlled with antidepressant medication.37,103,134 A weighted meta-analysis of cancer studies measuring inflammatory markers and fatigue showed significant positive correlation between fatigue and circulating levels of IL6, IL1 receptor antagonist, and neopterin but not IL1β or TNFα.146 A study of patients with metastatic colorectal cancer revealed significant correlations among serum levels of IL6, circadian patterns of activity, serum cortisol, and tumor-related symptoms, including fatigue, supporting the hypothesis that some cancer symptoms are related to tumor- or host-generated cytokines and could reflect cytokine effects on the circadian timing system.136
Data from both animal and human studies suggest that short-term sleep loss may be accompanied by enhanced nonspecific host defense mechanisms, whereas chronic or prolonged sleep loss, as may occur in cancer patients, can result in immune impairment.78,91,137,158 The contention that sleep loss impairs immune competence is most strongly supported by observations that chronic unremitting sleep deprivation of rats results in intestinal bacterial proliferation, microbial penetration into lymph nodes, and septicemia, culminating in about 3 wk in death.55-57 In the only animal study we are aware of that evaluates the effects of sleep loss on cancer, total sleep deprivation retarded the growth of an allogeneic tumor in rats, implying immune enhancement.21 However, this study did not evaluate the potential impact of other physiologic factors (for example, significant weight loss and metabolic changes that occur in sleep-deprived animals as compared with the control group). Therefore, the direct effects of impaired sleep or sleep loss on tumor growth and progression are essentially unknown, as are their effects on the response to chemotherapy.
Studies of the relationship between normal sleep duration and cancer risk have generated mixed conclusions. For example, 1 study of 4033 women with invasive breast cancer and 5314 community women without breast cancer found that greater sleep duration was modestly associated with increased risk of breast cancer, although short duration of sleep (less than 7 h/night) was not.108 In contrast, an analysis of data that were collected from twins over a 20-y period found a significantly lower risk of developing breast cancer in women who reported sleeping more than 9 h/night,164 whereas a study of 4223 incident cases of breast cancer among 77,418 women found no convincing evidence for an association between sleep duration and the incidence of breast cancer.129
The study of symptoms in humans and animals.
Despite the importance of symptoms with regard to health-related quality of life (HR-QOL), the subjective and ambiguous nature of assessing symptoms has constrained research on this important topic. As a result, little in known about the causes of, and even less about the prevention or alleviation of, the prevalent and debilitating symptoms that reduce human HR-QOL during prolonged or chronic disease. The use of animal models to study subjective human symptoms (for example, fatigue) is particularly challenging as compared with conditions that can be evaluated based on objective clinical markers. However, studying symptoms in human populations is also difficult. Even those human studies with well-defined patient and control groups must accommodate high genetic and environmental heterogeneity and difficulty in assuring behavioral or reporting compliance with study design. For example, in human populations, the imposition of day-to-day demands can cause voluntary curtailment of sleep, and elimination of substances like caffeine and alcohol cannot be assured. Because of such considerations, the crucial determinants and associated symptoms and signs of disease that develop in humans vary widely across persons, and studies of mechanisms are largely retrospective and correlative.
In contrast to human populations, inbred strains of laboratory mice have well-defined and reproducible genetics, can be maintained in identical environments, can be exposed to challenges at defined doses and ages, can be subjected to standardized treatments, and can undergo euthanasia for sample collection of times of interest. Furthermore, the large variety of readily available, genetically defined inbred strains of mice provides a simple surrogate for human genetic diversity. However, the study of symptoms of disease in animals often triggers questions such as “How do you know it's really fatigue?” Questions of this type are well-intended and important in their implications, yet they seem to overlook an important feature of clinical medicine: healthcare providers routinely rely on subjective behavioral signs to diagnose and successfully manage health problems in their patients. For the pediatrician, parental report and observed behavioral changes are crucial for evaluating preverbal children. Similarly, because veterinary patients cannot self-report their condition, veterinarians rely extensively on observed and owner-reported behavioral changes when diagnosing and treating animals. These behavioral assessments constitute a vital supplement to objective measurable signs of illness (for example, fever, weight loss, clinical chemistry values). Such behavioral approaches to medicine are also bulwarks for medical and nursing management of psychiatric and mentally handicapped patients. Behaviorists (and parents and animal owners) know that children and animals can respond in nonverbal ways that convey their ‘feelings.’ For example, children may become noticeably less active and lose their appetites when they are ill.
So-called ‘sickness behaviors’ (for example, fatigue, anorexia, anhedonia, reduced social interaction) have been linked to facets of the host response to immune or inflammatory challenge in both people and animals.46,47,170 As reviewed in preceding sections, ample evidence links facets of the host defense response to altered sleep or fatigue in animals and people.101,159 Given this relationship, prolonged production of even low levels of sleep-promoting or sleep-disruptive cytokines (for example, IL1β, IL6, and TNFα) may underlie fatigue associated with chronic illness. For example, chronic low-level or nonresolving immune activation has been associated with chronic fatigue in breast cancer survivors.25,29 However, fatigue remains largely unexplored under either acute or chronic conditions. Therefore, establishing models for immune-related or cancer-related fatigue in animals could provide a means of studying (and eventually understanding) how immune factors may cause fatigue regardless of the etiology of the immune stimulation.
Approaches to measuring fatigue in rodents during cancer and cancer therapy.
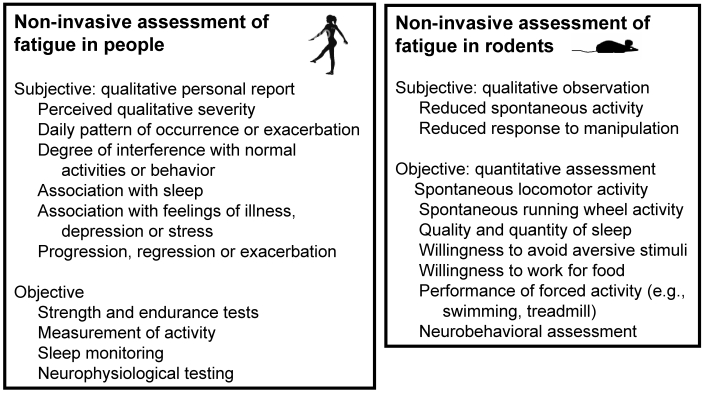
The assessment of sleep in mammals, including people, is based on evaluation of the electroencephalogram, the electromyogram, and other physiological measures in a process known as polysomnography.159 These generally accepted measures allow the quantitative evaluation of sleep for both clinical (human) and experimental (human and animal) purposes. However, in contrast to sleep, the subtle and subjective nature of fatigue makes it a difficult variable to assess in both people and animals.75 This difficulty can be mitigated both clinically and experimentally by the use of objective performance measures to quantify fatigue (Figure 2).
Figure 2.
Assessment of fatigue in people and mice. Several approaches are available for the assessment of fatigue in both people and rodents. Subjective approaches involve responses to questions (people) or observation (research evaluation of rodents and, in human cancer patients, oral reports by care-givers). Objective approaches involve the acquisition of quantitative data regarding performance, anatomic variations (e.g., muscle atrophy), or physiological functions.
One approach to meeting this challenge in animals is to evaluate various forms of forced or spontaneous activity. A commonly used method is the measurement of voluntary activity on a running wheel. For example, mice given an antigenic challenge showed normal amounts of wheel running during the initial 2 h after dark onset, but this active period was followed by a subsequent reduction in running during the remainder of the dark (active) phase.125 This pattern may be analogous to that of people who begin the day with normal energy but then experience unusual and increasing fatigue as the day progresses. The use of running wheel activity as an index of fatigue in mice can be broadened by concurrent measurement of horizontal locomotion on the cage floor. Horizontal locomotion, which incorporates the activity necessary for essential maintenance behaviors such as feeding, drinking, and grooming, can be viewed as largely obligatory and to some degree essential for life. In contrast, running wheel activity is voluntary, is not essential for survival, and perhaps can even be viewed as recreational. Comparison of running wheel activity and normal (obligatory) locomotion in the home cage offers the advantages of simplicity and spontaneity of behavior. Furthermore, conditions or treatments that alter spontaneous activity on the running wheel do not necessarily alter normal locomotor (obligatory) activity. Rodents with overtly imperceptible illness may reduce running wheel but not locomotor activity, thus providing an objective yet nonverbal measure of well being that is consistent with fatigue.
Other approaches to quantifying fatigue could include, for example, measuring motivated behavior by requiring the animal to press a bar to obtain food, monitoring the animal's willingness to avoid or terminate a mildly aversive stimulus, such as a vibration, shock, or air puff, or using a forced activity such as swimming. However, these approaches introduce other complicating factors such as, respectively, the need for food restriction and the effect of cancer-related changes in appetite, sensory or motor impairments associated with the tumor or its treatment, or exposure of the animal to severe stress due to life-threatening situations. A recent model used both timed swimming tests and assessment of postswim grooming and rearing to differentiate muscle strength, motor impairments, and postexertional fatigue.35 In this model, mice that lacked vitamin D receptors were similar to controls in preswim activity and in the time taken to swim a 1-m distance to reach a visible platform; however, in contrast to normal mice, which displayed high levels of grooming and rearing after the swim, the deficient mice were far less active, suggesting postexertional fatigue.35
Changes in sleep may provide indirect indications of fatigue. However, the relationship between sleep and fatigue is complex in that behavioral activity and sleep can either reflect fatigue (that is, less activity and more sleep in fatigued subjects) or contribute to fatigue (that is, increased physical activity and inadequate sleep can cause fatigue). To our knowledge, electroencephalography-based assessment of sleep in animals undergoing cancer or chemotherapy has not been reported. However, studies in mice with infectious or inflammatory disease indicate how such evaluations can contribute to assessment of fatigue. For example, C57BL/6 mice develop both increased sleep and reduced locomotor activity during the active phase after inoculation with influenza virus, whereas infected BALB/cByJ mice show poor-quality or reduced sleep during their normal diurnal rest phase as well as reduced activity and impaired sleep during the dark phase.157,160 In another example, both C57BL/6J and BALB/cByJ mice develop reduced locomotor activity after injection with synthetic double-stranded RNA (poly I:C) or Newcastle disease virus, but only the C57BL/6J mice show an associated significant increase in sleep.157
Rodents also can be used to study the association between inflammation and symptom development during cancer or cancer treatment. For example, in mice, Lewis lung carcinoma 1 (LLC1) tumor and the chemotherapeutic drug etoposide both increase serum IL6,175 which has been associated with anorexia in rodents.90 However, whereas the drug reduced food intake for 24 h after administration, the tumor alone did not significantly influence food intake.175 Etoposide also reduced hemoglobin levels in nontumor-bearing mice, although the reduction was not severe enough to be classified as anemia; mice with LLC1 developed anemia with or without etoposide treatment.175 To our knowledge, this study is the only 1 to date that has evaluated spontaneous behavioral activity or the development of fatigue in the context of cancer and chemotherapy in mice. In this study, administration of the chemotherapeutic agent etoposide reduced voluntary wheel running in both tumor-bearing and nontumor-bearing mice; furthermore, the reductions in wheel running were significantly correlated with serum concentrations of IL6175
A crucial factor in interpreting running wheel data is the animal's physical ability to perform the behavior. For example, the chemotherapeutic drugs known as taxanes stabilize cellular microtubules and in that manner impair both cell division and neuronal transport. The former feature contributes to their chemotherapeutic efficacy, whereas the latter causes the side effect of peripheral neuropathy in a substantial percentage of patients.95,105 In 1 study, 2 of 5 mice that received the maximum tolerated dose of a taxane developed a transient paralysis in 1 hindlimb 6 d after the last dose121—this effect obviously would impair running ability. Because neurologic side effects may mask or mimic fatigue, rodents that show reduced wheel running or locomotor activity should undergo a comprehensive neurologic evaluation before investigators conclude that the fatigue is not due to neurologic impairment. This assessment can be accomplished by first evaluating qualitative measures of general wellbeing such as coat condition, posture, salivation, lacrimation, and vocalization. An appropriate panel of quantitative behavioral markers (exemplified in Table 1) should then be evaluated.11,44,45 Open-field activity can be used to assess grossly observable signs of impaired mobility, including that which may be attributable to tumor location or size. Various tests of grip strength, balance, and motor coordination can differentiate fatigue from vestibular and neurotoxic side effects of chemotherapeutic drugs.
Table 1.
Quantitative assessment of mice for neuromuscular and vestibular toxicity
| Test | Measure |
| Open field activity | Pattern of exploration, amount of horizontal activity, amount of rearing, pattern of change with time |
| Balance beam | Time mouse remains on beam; ability and time necessary to walk to enclosed shelter |
| Vertical pole | Angle above horizontal at which mouse falls off |
| Wire hang | Time mouse maintains grip (normal mice can maintain grip for 60 s, which can be used as a maximum) |
| Inverted screen | Time it takes for the mouse to turn to face upward or to fall off screen (normal mice will turn upward within 30 s, which can be used as a maximum) |
Gene–environment interactions can also influence the assessment of fatigue and other cancer-related variables in mice. For example, physiologic concentrations of the pineal hormone melatonin show preventive, oncostatic, and inhibitory effects in various experimental models of cancer,82 and light-induced suppression of the normal nighttime production of melatonin has been implicated in increased cancer risk for those exposed to chronic shift work or repeated jet lag.8,15,48 However, many commonly used strains of rodents, including C57BL/6 and BALB/c, are deficient in production of melatonin, whereas other, including C3H/He and CBA, show pronounced diurnal rhythms of melatonin.54,66,139,169 Therefore, choosing the appropriate mouse strain is essential to studying the potential role of melatonin in controlling cancer in mice. In addition, cancer can cause severe skeletal muscle wasting that can contribute to fatigue. Rats have been used to determine whether physical conditioning can reduce cancer-related muscle wasting73,124 and to differentiate the effects of interventions on chemotherapy-induced inactivity and anorexia.102
General considerations in the use of rodents to study cancer and cancer-related fatigue.
The utility of any specific animal model is influenced directly by how closely it reproduces the human disease (for example, in terms of histology, physiologic effects, biochemical pathways, and metastatic pattern). Mouse models for the study of cancer can be generally classified as spontaneous, engrafted (syngeneic, allogeneic, or xenogeneic), pathogen-induced, or chemically induced. Syngeneic models rely on the implantation or injection of tumor cells into immune-competent recipients of the same genetic background. In contrast, xenograft models rely on grafting of a tumor cell line derived from 1 species (for example, human) onto a different recipient species (for example, mouse). To prevent immunologic rejection of the foreign tissue, the immune response of the xenograft host must be impaired. In mice, this result is most commonly accomplished by using mouse strains or stocks that bear the mutations nude (Foxn1nu) or severe combined immunodeficiency (SCID; Prkdcscid). Mice that are homozygous for nude mutation have thymic epithelial dysgenesis that results in T cell deficiency.79 Consequently, these mice show a reduced lymphocyte population that is comprised almost entirely of B-cells, a poor response to thymus-dependent antigens, including failure to reject allogeneic and xenogeneic skin and tumor grafts, and reduced B cells and bone marrow stem cells, due in part to the T-cell defect. Mice that bear the SCID mutation have a spontaneous mutation in protein kinase, DNA-activated, catalytic polypeptide (Prkdc), which is instrumental in recombining the variable, diversity, and joining segments of immunoglobulin and T-cell receptor genes. Therefore, mice homozygous for the SCID mutation show absence of mature functional T cells and B cells, a normal hematopoietic microenvironment, and normal antigen-presenting, myeloid, and NK functions.79 SCID mice accept allogeneic and xenogeneic grafts, making them ideal for implantation of human tumor cell lines. Although the defective adaptive immune responses of nude and SCID mice allow the engraftment of primary human tumor cells or cell lines, these strains have normal, or even increased, innate immune responses (for example, natural killer cells, antigen presenting cells, macrophages, and complement). Therefore, the nature of the host immune response to tumor antigens, particularly with respect to the associated profile of cytokine production, almost certainly differs in immune impaired mice as compared with normal mice.
Xenograft and syngeneic models both offer the advantages of experimental reproducibility, a precise known onset of tumor formation, rapid tumor development, and easy tumor visualization. However, these models lack the stepwise genetic changes that accompany spontaneous tumor development and progression, as well as the native tumor stroma. In addition, the phenotypes of implanted cell lines are often altered by culture conditions and genetic drift and therefore may not accurately reproduce the phenotype of the tumor of origin. Finally, general technical caveats of cell culture systems, such as viral or Mycoplasma infection, mislabeling of individual cell lines, and uncertainty regarding the actual identity of the tumor of origin, must be considered when using xenograft and syngeneic tumor models.60,62 The normal immune competence of recipient mice used in syngeneic models makes them more likely than xenograft hosts to accurately reproduce the normal host reaction to a tumor, particularly if the tumor is implanted orthotopically. Because immune mediators, including cytokines and chemokines, are implicated in the development of cancer-related fatigue,28,30,94,113 syngeneic models are probably better for studying such symptoms.
Genetically engineered mouse models, which provide in situ tumor development in an immune competent host, are an alternative to traditional preclinical xenograft models. In general, these mice are engineered to overexpress a transgene (oncogene or point mutation), contain a genetic knock-in of a point mutation, or have complete or conditional deletion of a specific gene (for example, using the cre–lox system).1,163 However, because the genomic alteration is present in the germ line or in a large proportion of somatic cells, most genetically engineered mouse models are more representative of human cancer predisposition syndromes rather than of random tumorigenesis. This situation contrasts with most human cancers, in which gene alterations are typically rare and stochastic.85 In addition, relatively few genetically engineered models develop metastases, and those that do tend to display metastases with different tissue specificity than occurs in human cancer.85,152 Despite these limitations, several genetic engineered models of breast cancer have been developed that replicate the molecular events that occur in human breast cancer.22,51,152 However, even these models differ in important ways from human breast cancer (for example, in terms of estrogen receptor status and metastatic pattern).152 Such differences could influence the tendency or mechanisms by which the host develops symptoms such as fatigue.
Another topic that merits discussion in the context of this review is the comparative differences in sleep among rodents, humans, and other animal species. Most notably, many rodents are nocturnal. Thus, their primary sleep period occurs during the light phase. Furthermore, the sleep architecture of rodents is often highly fragmented compared with that of humans and other species, whose sleep patterns are often characterized by long bouts of consolidated sleep. In addition, different strains of rodents vary widely in terms of both physiologic and behavioral characteristics, including patterns of sleep. However, a discussion of strain variation in activity and sleep among mouse strains is beyond the scope of this review. Interested readers are referred elsewhere for comprehensive discussions of species and rodent strain differences in activity, sleep, and sleep architecture.36,98,150,155,159
Finally, despite the widespread use of rodent models in cancer research, several recent reviews discuss the context in which such models are generally valuable and provide caveats concerning their use, value, and interpretation.9,71,83,116 Interested readers are referred to these reviews for additional information when designing similar studies.
Summary
Many types of physiologic and behavioral measures and clinical samples are difficult, extremely expensive, or impossible to collect from people. These impediments complicate the study of complex multigenic and multifactorial human health conditions, particularly as related to symptoms. The growing and imperative importance of learning more about perceived symptoms in the context of healthy aging, prolonged or chronic disease, and maximizing HR-QOL calls for an immediate and imaginative approach to this challenge. To that end, inbred mice offer a valuable surrogate for human populations, supporting the identification of genes and mechanisms that differentially predispose different genetically distinct subjects to so-called ‘sickness behaviors.’ The ready availability of numerous strains of inbred mice, with their identical genomes and standardized environments, provides a valuable population for studying complex diseases, as has been done successfully in the past. However, to date such studies have predominantly focused on disease pathophysiology, rather than on the pathogenesis and treatment of associated or persistent symptoms.
Despite their importance, little is known about symptoms. Enormous complexities, including logistics, expense, uncontrollable variation, and ethical concerns, discourage the application of genetic and physiologic approaches to the study of these issues directly in humans. In contrast, exploring promising animal models can provide the insights needed to foster hypothesis-based studies of the mechanisms that underlie apparently similar symptoms in humans. The potential for discovery is enormous. The development of valid models for the study of disease-related symptoms that impair QOL has obvious widespread significance.
References
- 1.Adams JM, Cory S. 1991. Transgenic models of tumor development. Science 254:1161–1167 [DOI] [PubMed] [Google Scholar]
- 2.Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, Hanscom BS, Mulrooney TJ, Schwartz GN, Kaufman PA. 2007. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat 3 Aug [Epub]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alikunju S, Pillarisetti S. 2006. Selected players in the inflammation cascade and drugs that target these inflammation genes against metastasis. Anticancer Agents Med Chem 6:461–468 [DOI] [PubMed] [Google Scholar]
- 4.Ancoli-Israel S. 2001. The relationship between fatigue and sleep in cancer patients: a review. Eur J Cancer Care (Engl) 10:245–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ancoli-Israel S, Liu L, Marler MR, Parker BA, Jones V, Sadler GR, Dinsdale J, Cohen-Zion M, Fiorentino L. 2006. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer 14:201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andreasson A, Arborelius L, Erlanson-Albertsson C, Lekander M. 2007. A putative role for cytokines in the impaired appetite in depression. Brain Behav Immun 21:147–152 [DOI] [PubMed] [Google Scholar]
- 7.Andrykowski MA, Curran SL, Lightner R. 1998. Off-treatment fatigue in breast cancer survivors: a controlled comparison. J Behav Med 21:1–18 [DOI] [PubMed] [Google Scholar]
- 8.Anisimov VN. 2006. Light pollution, reproductive function, and cancer risk. Neuroendocrinol Lett 27:35–52 [PubMed] [Google Scholar]
- 9.Anisimov VN, Ukraintseva SV, Yashin AI. 2005. Cancer in rodents: does it tell us about cancer in humans? Nat Rev Cancer 5:807–819 [DOI] [PubMed] [Google Scholar]
- 10.Arnold EM. 1999. The cessation of cancer treatment as a crisis. Soc Work Health Care 29:21–38 [DOI] [PubMed] [Google Scholar]
- 11.Bailey KR, Rustay NR, Crawley JN. 2006. Behavioral phenotyping of transgenic and knockout mice: practical concerns and potential pitfalls. ILAR J 47:124–131 [DOI] [PubMed] [Google Scholar]
- 12.Balkwill F, Charles KA, Mantovani A. 2005. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 7:211–217 [DOI] [PubMed] [Google Scholar]
- 13.Balkwill F, Coussens LM. 2004. Cancer: an inflammatory link. Nature 431:405–406 [DOI] [PubMed] [Google Scholar]
- 14.Barton-Burke M. 2006. Cancer-related fatigue and sleep disturbances. Am J Nurs 106 Suppl.:72–77 [DOI] [PubMed] [Google Scholar]
- 15.Bartsch C, Bartsch H. 2006. The antitumor activity of pineal melatonin and cancer enhancing life styles in industrialized societies. Cancer Causes Control 17:559–571 [DOI] [PubMed] [Google Scholar]
- 16.Ben-Baruch A. 2006. Inflammation-associated immune suppression in cancer: the roles played by cytokines, chemokines and additional mediators. Semin Cancer Biol 16:38–52 [DOI] [PubMed] [Google Scholar]
- 17.Berger AM, Farr LA, Kuhn BR, Fischer P, Agrawal S. 2007. Values of sleep/wake, activity/rest, circadian rhythms, and fatigue prior to adjuvant breast cancer chemotherapy. J Pain Symptom Manage 33:398–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger AM, Mitchell SA. 2008. Modifying cancer-related fatigue by optimizing sleep quality. J Natl Compr Canc Netw 6:3–13 [DOI] [PubMed] [Google Scholar]
- 19.Berger AM, Parker KP, Young-McCaughan S, Mallory GA, Barsevick AM, Bck SL, Carpenter JS, Carter PA, Farr LA, Hinds PS, Lee KA, Miaskowski C, Mock V, Payne JK, Hall M. 2005. Sleep wake disturbances in people with cancer and their caregivers: state of the science. Oncol Nurs Forum 32:E98–E126 [DOI] [PubMed] [Google Scholar]
- 20.Berglund G, Bolund C, Fornander T, Rutqvist LE, Sjoden PO. 1991. Late effects of adjuvant chemotherapy and postoperative radiotherapy on quality of life among breast cancer patients. Eur J Cancer 27:1075–1081 [DOI] [PubMed] [Google Scholar]
- 21.Bergmann BM, Rechtschaffen A, Gilliland MA, Quintans J. 1996. Effect of extended sleep deprivation on tumor growth in rats. Am J Physiol 271:R1460–R1464 [DOI] [PubMed] [Google Scholar]
- 22.Berton TR, Matsumoto T, Page A, Conti CJ, Jorcano JL, Johnson DG. 2003. Tumor formation in mice with conditional inactivation of Brca1 in epithelial tissues. Oncogene 22:5415–5426 [DOI] [PubMed] [Google Scholar]
- 23.Bjarnason GA, Jordan R. 2000. Circadian variation of cell proliferation and cell cycle protein expression in man: clinical implications. Prog Cell Cycle Res 4:193–206 [DOI] [PubMed] [Google Scholar]
- 24.Bower JE. 2005. Prevalence and causes of fatigue after cancer treatment: the next generation of research. J Clin Oncol 23:8280–8282 [DOI] [PubMed] [Google Scholar]
- 25.Bower JE. 2007. Cancer-related fatigue: links with inflammation in cancer patients and survivors. Brain Behav Immun 21:863–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bower JE. 2008. Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol 26:768–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bower JE, Ganz PA, Aziz N. 2005. Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosom Med 67:277–280 [DOI] [PubMed] [Google Scholar]
- 28.Bower JE, Ganz PA, Aziz N, Fahey JL. 2002. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med 64:604–611 [DOI] [PubMed] [Google Scholar]
- 29.Bower JE, Ganz PA, Aziz N, Olmstead R, Irwin MR, Cole SW. 2007. Inflammatory responses to psychological stress in fatigued breast cancer survivors: relationship to glucocorticoids. Brain Behav Immun 21:251–258 [DOI] [PubMed] [Google Scholar]
- 30.Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, Meyerowitz BE, Belin TR. 2006. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer 106:751–758 [DOI] [PubMed] [Google Scholar]
- 31.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. 2000. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol 18:743–753 [DOI] [PubMed] [Google Scholar]
- 32.Bredow S, Guba-Thakurta N, Taishi P, Obal F, Krueger JM. 1997. Diurnal variations of tumor necrosis factor α mRNA and α-tubulin mRNA in rat brain. Neuroimmunomodulation 4:84–90 [DOI] [PubMed] [Google Scholar]
- 33.Bremberg ER, Brandberg Y, Hising C, Friesland S, Eksborg S. 2007. Anemia and quality of life including anemia-related symptoms in patients with solid tumors in clinical practice. Med Oncol 24:95–102 [DOI] [PubMed] [Google Scholar]
- 34.Broeckel JA, Jacobsen PB, Horton J, Balducci L, Lyman GH. 1998. Characteristics and correlates of fatigue after adjuvant chemotherapy for breast cancer. J Clin Oncol 16:1689–1696 [DOI] [PubMed] [Google Scholar]
- 35.Burne TH, Johnston AN, McGrath JJ, Mackay-Sim A. 2006. Swimming behaviour and post-swimming activity in Vitamin D receptor knockout mice. Brain Res Bull 69:74–78 [DOI] [PubMed] [Google Scholar]
- 36.Campbell SS, Tobler I. 1984. Animal sleep: a review of sleep duration across phylogeny. Neurosci Biobehav Rev 8:269–300 [DOI] [PubMed] [Google Scholar]
- 37.Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. 2002. Neurobehavioral effects of interferon-α in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology 26:643–652 [DOI] [PubMed] [Google Scholar]
- 38.Cella D. 2006. Quality of life and clinical decisions in chemotherapy-induced anemia. Oncology 20:25–28 [PubMed] [Google Scholar]
- 39.Chao CC, Gallagher M, Phair J, Peterson PK. 1990. Serum neopterin and interleukin-6 levels in chronic fatigue syndrome. J Infect Dis 162:1412–1413 [DOI] [PubMed] [Google Scholar]
- 40.Chen-Goodspeed M, Lee CC. 2007. Tumor suppression and circadian function. J Biol Rhythms 22:291–298 [DOI] [PubMed] [Google Scholar]
- 41.Cleeland CS, Bennett GJ, Dantzer R, Dougherty PM, Dunn AJ, Meyers CA, Miller AH, Payne R, Reuben JM, Wang XS, Lee BN. 2003. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer 97:2919–2925 [DOI] [PubMed] [Google Scholar]
- 42.Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. 2006. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res 12:2759–2786 [DOI] [PubMed] [Google Scholar]
- 43.Colombo MP, Mantovani A. 2005. Targeting myelomonocytic cells to revert inflammation-dependent cancer promotion. Cancer Res 65:9113–9116 [DOI] [PubMed] [Google Scholar]
- 44.Cory-Slechta DA, Crofton KM, Foran JA, Rose JF, Sheets LP, Weiss B, Mileson B. 2001. Methods to identify and characterize developmental neurotoxicity for human health risk assessment. I: Behavioral effects. Environ Health Perspect 109 Suppl. 1:79–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crawley JN. 2003. Behavioral phenotyping of rodents. Comp Med 53:140–146 [PubMed] [Google Scholar]
- 46.Dantzer R. 2005. Somatization: a psychoneuroimmune perspective. Psychoneuroendocrinology 30:947–952 [DOI] [PubMed] [Google Scholar]
- 47.Dantzer R, Kelley KW. 2007. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun 21:153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davis S, Mirick DK. 2006. Circadian disruption, shift work and the risk of cancer: a summary of the evidence and studies in Seattle. Cancer Causes Control 17:539–545 [DOI] [PubMed] [Google Scholar]
- 49.De Sarro G, Gareri P, Sinopoli A, David E, Rotiroti D. 1997. Comparative, behavioural and electrocortical effects of tumor necrosis factor-α and interleukin-1 microinjected into the locus coeruleus of rat. Life Sci 60:555–564 [DOI] [PubMed] [Google Scholar]
- 50.Demetri GD, Gabrilove JL, Blasi MV, Hill RJ, Glaspy J. 2002. Benefits of epoetin α in anemic breast cancer patients receiving chemotherapy. Clin Breast Cancer 3:45–51 [DOI] [PubMed] [Google Scholar]
- 51.Deng CX, Scott F. 2000. Role of the tumor suppressor gene Brca1 in genetic stability and mammary gland tumor formation. Oncogene 19:1059–1064 [DOI] [PubMed] [Google Scholar]
- 52.Dimsdale JE, Ancoli-Israel S, Ayalon L, Elsmore TF, Gruen W. 2007. Taking fatigue seriously. II: Variability in fatigue levels in cancer patients. Psychosomatics 48:247–252 [DOI] [PubMed] [Google Scholar]
- 53.Donovan KA, Small BJ, Andrykowski MA, Munster P, Jacobsen PB. 2007. Utility of a cognitive-behavioral model to predict fatigue following breast cancer treatment. Health Psychol 26:464–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ebihara S, Marks T, Hudson DJ, Menaker M. 1986. Genetic control of melatonin synthesis in the pineal gland of the mouse. Science 231:491–493 [DOI] [PubMed] [Google Scholar]
- 55.Everson CA. 1993. Sustained sleep deprivation impairs host defense. Am J Physiol 265:R1148–R1154 [DOI] [PubMed] [Google Scholar]
- 56.Everson CA. 2005. Clinical assessment of blood leukocytes, serum cytokines, and serum immunoglobulins as responses to sleep deprivation in laboratory rats. Am J Physiol Regul Integr Comp Physiol 289:R1054–R1063 [DOI] [PubMed] [Google Scholar]
- 57.Everson CA, Toth LA. 2000. Systemic bacterial invasion induced by sleep deprivation. Am J Physiol Regul Integr Comp Physiol 278:R905–R916 [DOI] [PubMed] [Google Scholar]
- 58.Fang J, Wang Y, Krueger JM. 1997. Mice lacking the TNF 55 kDa receptor fail to sleep more after TNFα treatment. J Neurosci 17:5949–5955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Filipski E, Li XM, Levi F. 2006. Disruption of circadian coordination and malignant growth. Cancer Causes Control 17:509–514 [DOI] [PubMed] [Google Scholar]
- 60.Finkelstein SD, Black P, Nowak TP, Hand CM, Christensen S, Finch PW. 1994. Histological characteristics and expression of acidic and basic fibroblast growth factor genes in intracerebral xenogeneic transplants of human glioma cells. Neurosurgery 34:136–144 [PubMed] [Google Scholar]
- 61.Floyd RA, Krueger JM. 1997. Diurnal variation of TNF α in the rat brain. Neuroreport 3:915–918 [DOI] [PubMed] [Google Scholar]
- 62.Fomchenko EI, Holland EC. 2006. Mouse models of brain tumors and their applications in preclinical trials. Clin Cancer Res 12:5288–5297 [DOI] [PubMed] [Google Scholar]
- 63.Fortner BV, Stepanski EJ, Wang SC, Kasprowicz S, Durrence HH. 2002. Sleep and quality of life in breast cancer patients. J Pain Symptom Manage 24:471–480 [DOI] [PubMed] [Google Scholar]
- 64.Giordano A, Calvani M, Petillo O, Carteni M, Melone MR, Peluso G. 2003. Skeletal muscle metabolism in physiology and in cancer disease. J Cell Biochem 90:170–186 [DOI] [PubMed] [Google Scholar]
- 65.Glaus A, Crow R, Hammond S. 1996. A qualitative study to explore the concept of fatigue/tiredness in cancer patients and in healthy individuals. Eur J Cancer Care (Engl) 5:8–23 [DOI] [PubMed] [Google Scholar]
- 66.Goto M, Oshima I, Tomita T, Ebihara S. 1989. Melatonin content of the pineal gland in different mouse strains. J Pineal Res 7:195–204 [DOI] [PubMed] [Google Scholar]
- 67.Gripp S, Moeller S, Bolke E, Schmitt G, Matuschek C, Asgari S, Asgharzadeh F, Roth S, Budach W, Franz M, Willers R. 2007. Survival prediction in terminally ill cancer patients by clinical estimates, laboratory tests, and self-rated anxiety and depression. J Clin Oncol 25:3313–3320 [DOI] [PubMed] [Google Scholar]
- 68.Groopman JE, Itri LM. 1999. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst 91:1616–1634 [DOI] [PubMed] [Google Scholar]
- 69.Guan Z, Vgontzas AN, Omori T, Peng X, Bixler EO, Fang J. 2005. Interleukin-6 levels fluctuate with the light-dark cycle in the brain and peripheral tissues in rats. Brain Behav Immun 19:526–529 [DOI] [PubMed] [Google Scholar]
- 70.Gudewill S, Pollmächer T, Vedder H, Schreiber W, Fassbender K, Holsboer F. 1992. Nocturnal plasma levels of cytokines in healthy men. Eur Arch Psychiatry Clin Neurosci 242:53–56 [DOI] [PubMed] [Google Scholar]
- 71.Gutmann DH, Hunter-Schaedle K, Shannon KM. 2006. Harnessing preclinical mouse models to inform human clinical cancer trials. J Clin Invest 116:847–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hagemann T, Wilson J, Kulbe H, Li NF, Leinster DA, Charles K, Klemm F, Pukrop T, Binder C, Balkwill FR. 2005. Macrophages induce invasiveness of epithelial cancer cells via NF-κ B and JNK. J Immunol 175:1197–1205 [DOI] [PubMed] [Google Scholar]
- 73.Hayward R, Ruangthai R, Schneider CM, Hyslop RM, Strange R, Westerlind KC. 2004. Training enhances vascular relaxation after chemotherapy-induced vasoconstriction. Med Sci Sports Exerc 36:428–434 [DOI] [PubMed] [Google Scholar]
- 74.Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. 2007. Cancer-related fatigue: the scale of the problem. Oncologist 12 Suppl 1:4–10 [DOI] [PubMed] [Google Scholar]
- 75.Hossain JL, Ahmad P, Reinish LW, Kayumov L, Hossain NK, Shapiro CM. 2005. Subjective fatigue and subjective sleepiness: two independent consequences of sleep disorders? J Sleep Res 14:245–253 [DOI] [PubMed] [Google Scholar]
- 76.Hurter B, Bush NJ. 2007. Cancer-related anemia: clinical review and management update. Clin J Oncol Nurs 11:349–359 [DOI] [PubMed] [Google Scholar]
- 77.Hwang SS, Chang VT, Rue M, Kasimis B. 2003. Multidimensional independent predictors of cancer-related fatigue. J Pain Symptom Manage 26:604–614 [DOI] [PubMed] [Google Scholar]
- 78.Irwin M. 2002. Effects of sleep and sleep loss on immunity and cytokines. Brain Behav Immun 16:503–512 [DOI] [PubMed] [Google Scholar]
- 79.The Jackson Laboratory Cancer research and the laboratory mouse [Internet]. Bar Harbor (ME): The Jackson Laboratory; c2008. Available from: http://jaxmice.jax.org/manual/cancer.pdf [Google Scholar]
- 80.Jacobsen PB, Donovan KA, Vadaparampil ST, Small BJ. 2007. Systematic review and meta-analysis of psychological and activity-based interventions for cancer-related fatigue. Health Psychol 26:660–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jereczek-Fossa BA, Marsiglia HR, Orecchia R. 2002. Radiotherapy-related fatigue. Crit Rev Oncol Hematol 41:317–325 [DOI] [PubMed] [Google Scholar]
- 82.Jung B, Ahmad N. 2006. Melatonin in cancer management: progress and promise. Cancer Res 66:9789–9793 [DOI] [PubMed] [Google Scholar]
- 83.Kamb A. 2005. What's wrong with our cancer models? Nat Rev Drug Discov 4:161–165 [DOI] [PubMed] [Google Scholar]
- 84.Kenefick AL. 2006. Patterns of symptom distress in older women after surgical treatment for breast cancer. Oncol Nurs Forum 33:327–335 [DOI] [PubMed] [Google Scholar]
- 85.Kim JB, O'Hare MJ, Stein R. 2004. Models of breast cancer: is merging human and animal models the future? Breast Cancer Res 6:22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kimel M, Leidy NK, Mannix S, Dixon J. 2008. Does epoetin α improve health-related quality of life in chronically ill patients with anemia? Summary of trials of cancer, HIV/AIDS, and chronic kidney disease. Value Health 11:57–75 [DOI] [PubMed] [Google Scholar]
- 87.Kirkova J, Walsh D. 2007. Cancer symptom clusters—a dynamic construct. Support Care Cancer 15:1011–1013 [DOI] [PubMed] [Google Scholar]
- 88.Knight K, Wade S, Balducci L. 2004. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med 116 Suppl 7A:11S–26S [DOI] [PubMed] [Google Scholar]
- 89.Kojo K, Pukkala E, Auvinen A. 2005. Breast cancer risk among Finnish cabin attendants: a nested case-control study. Occup Environ Med 62:488–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Konsman JP, Dantzer R. 2001. How the immune and nervous systems interact during disease-associated anorexia. Nutrition 17:664–668 [DOI] [PubMed] [Google Scholar]
- 91.Krueger JM, Fang J, Majde JA. 2001. Sleep in health and disease. Ader R, Felten DL, Cohen N. Psychoneuroimmunology. New York: Academic Press; p 667–685 [Google Scholar]
- 92.Krueger JM, Toth LA. 1994. Cytokines as regulators of sleep. Ann N Y Acad Sci 739:299–310 [DOI] [PubMed] [Google Scholar]
- 93.Kulbe H, Hagemann T, Szlosarek PW, Balkwill FR, Wilson JL. 2005. The inflammatory cytokine tumor necrosis factor-alpha regulates chemokine receptor expression on ovarian cancer cells. Cancer Res 65:10355–10362 [DOI] [PubMed] [Google Scholar]
- 94.Lee BN, Dantzer R, Langley KE, Benentt GJ, Dougherty PM, Dunn AJ, Meyers CA, Miller AH, Payne R, Reuben JM, Wang XS, Cleeland CS. 2004. A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuroimmunomodulation 11:279–292 [DOI] [PubMed] [Google Scholar]
- 95.Lee JJ, Swain SM. 2006. Peripheral neuropathy induced by microtubule-stabilizing agents. J Clin Oncol 24:1633–1642 [DOI] [PubMed] [Google Scholar]
- 96.Lelbach A, Muzes G, Feher J. 2007. Current perspectives of catabolic mediators of cancer cachexia. Med Sci Monit 13:RA168–RA173 [PubMed] [Google Scholar]
- 97.Levin RD, Daehler MA, Grutsch JF, Quiton J, Lis CG, Peterson C, Gupta D, Watson K, Layer D, Huff-Adams S, Desai B, Sharma P, Wallam M, Delioukina M, Ball P, Bryant M, Ashford M, Copeland D, Ohmiro M, Wood PA, Hrushesky WJ. 2005. Circadian function in patients with advanced non-small-cell lung cancer. Br J Cancer 93:1202–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lightfoot JT, Turner MJ, Daves M, Vordermark A, Kleeberger SR. 2004. Genetic influence on daily wheel running activity level. Physiol Genomics 19:270–276 [DOI] [PubMed] [Google Scholar]
- 99.Lin EY, Pollard JW. 2004. Macrophages: modulators of breast cancer progression. Novartis Found Symp 256:158–168 [PubMed] [Google Scholar]
- 100.Liu L, Marler MR, Parker BA, Jones V, Johnson S, Cohen-Zion M, Fiorentino L, Sadler GR, Ancoli-Israel S. 2005. The relationship between fatigue and light exposure during chemotherapy. Support Care Cancer 13:1010–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Majde JA, Krueger JM. 2005. Links between the innate immune system and sleep. J Allergy Clin Immunol 116:1188–1196 [DOI] [PubMed] [Google Scholar]
- 102.Malik NM, Liu YL, Cole N, Sanger GJ, Andrews PL. 2007. Differential effects of dexamethasone, ondansetron and a tachykinin NK1 receptor antagonist (GR205171) on cisplatin-induced changes in behaviour, food intake, pica and gastric function in rats. Eur J Pharmacol 555:164–173 [DOI] [PubMed] [Google Scholar]
- 103.Malik UR, Makower DF, Wadler S. 2001. Interferon-mediated fatigue. Cancer 92:1664–1668 [DOI] [PubMed] [Google Scholar]
- 104.Mantovani G, Maccio A, Lai P, Massa E, Ghiani M, Santona MC. 1998. Cytokine activity in cancer-related anorexia/cachexia: role of megestrol acetate and methoxyprogesterone acetate. Semin Oncol 25 Suppl. 6:45–52 [PubMed] [Google Scholar]
- 105.Marupudi NI, Han JE, Li KW, Renard VM, Tyler BM, Brem H. 2007. Paclitaxel: a review of adverse toxicities and novel delivery strategies. Expert Opin Drug Saf 6:609–621 [DOI] [PubMed] [Google Scholar]
- 106.Mast ME. 1998. Correlates of fatigue in survivors of breast cancer. Cancer Nurs 21:136–142 [DOI] [PubMed] [Google Scholar]
- 107.Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F, Okamura H. 2003. Control mechanism of the circadian clock for timing of cell division in vivo. Science 302:255–259 [DOI] [PubMed] [Google Scholar]
- 108.McElroy JA, Newcomb PA, Totus-Ernstoff L, Trentham-Dietz A, Hampton JM, Egan KM. 2006. Duration of sleep and breast cancer risk in a large population-based case-control study. J Sleep Res 15:241–249 [DOI] [PubMed] [Google Scholar]
- 109.McKinley ED. 2000. Under Toad days: surviving the uncertainty of cancer recurrence. Ann Intern Med 133:479–480 [DOI] [PubMed] [Google Scholar]
- 110.Meeske KA, Siegel SE, Globe DR, Mack WJ, Bernstein L. 2005. Prevalence and correlates of fatigue in long-term survivors of childhood leukemia. J Clin Oncol 23:5501–5510 [DOI] [PubMed] [Google Scholar]
- 111.Mercadante S, Gebbia V, Marrazzo A, Filosto S. 2000. Anaemia in cancer: pathophysiology and treatment. Cancer Treat Rev 26:303–311 [DOI] [PubMed] [Google Scholar]
- 112.Mormont MC, Waterhouse J, Bleuzen P, Giacchetti S, Jami A, Bigdan A, Lellouch J, Misset JL, Touitou Y, Levi F. 2000. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin Cancer Res 6:3038–3046 [PubMed] [Google Scholar]
- 113.Morrow GR, Andrews PLR, Hickok JT, Roscoe JA, Matteson S. 2002. Fatigue associated with cancer and its treatment. Support Care Cancer 10:389–398 [DOI] [PubMed] [Google Scholar]
- 114.Moser M, Penter R, Fruerwirth M, Kenner T. 2006. Why life oscillates–biological rhythms and health. Conf Proc IEEE Eng Med Biol Sci 1:424–428 [DOI] [PubMed] [Google Scholar]
- 115.Mulrooney DA, Ness KK, Neglia JP, Whitton JA, Green DM, Zwltzer LK, Robison LL, Mertens AC. 2008. Fatigue and sleep disturbance in adult survivors of childhood cancer: a report from the childhood cancer survivor study (CCSS). Sleep 31:271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Musch TI, Carroll RG, Just A, Lane PH, Talman WT. 2007. A broader view of animal research. BMJ 334:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mustian KM, Morrow GR, Carroll JK, Figueroa-Moseley CD, Jean-Pierre P, Williams GC. 2007. Integrative nonpharmacologic behavioral interventions for the management of cancer-related fatigue. Oncologist 12 Suppl 1:52–67 [DOI] [PubMed] [Google Scholar]
- 118.Mystakidou K, Parpa E, Tsilika E, Pathiaki M, Patiraki E, Galanos A, Vlahos L. 2007. Sleep quality in advanced cancer patients. J Psychosom Res 62:527–533 [DOI] [PubMed] [Google Scholar]
- 119.National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology. Cancer-related fatique. [Internet]. Fort Washington (PA): NCCN; c2008. Available from: http://www.nccn.org/professionals/physician_gls/PDF/fatigue.pdf
- 120.Neta R. 2000. The promise of molecular epidemiology in defining the association between radiation and cancer. Health Phys 79:77–84 [DOI] [PubMed] [Google Scholar]
- 121.Ng SS, Sparreboon A, Shaked Y, Lee C, Desai N, Soon-Shiong P, Figg WD, Kerbel RS. 2006. Influence of formulation vehicle on metronomic taxane chemotherapy: albumin-bound versus cremophor EL-based paclitaxel. Clin Cancer Res 12:4331–4338 [DOI] [PubMed] [Google Scholar]
- 122.Opp MR, Kapás L, Toth LA. 1992. Cytokine involvement in the regulation of sleep. Proc Soc Exp Biol Med 201:16–27 [DOI] [PubMed] [Google Scholar]
- 123.Orre IJ, Fossa SD, Murison R, Bremnes R, Dahl O, Klepp O, Loge JH, Wist E, Dahl AA. 2008. Chronic cancer-related fatigue in long-term survivors of testicular cancer. J Psychosom Res 64:363–371 [DOI] [PubMed] [Google Scholar]
- 124.Otis JS, Lees SJ, Williams JH. 2007. Functional overload attenuates plantaris atrophy in tumor-bearing rats. BMC Cancer 7:146–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ottenweller JE, Natelson BH, Gause WC, Carroll KK, Beldowicz D, Zhou XD, LaManca JJ. 1998. Mouse running activity is lowered by Brucella abortus treatment: a potential model to study chronic fatigue. Physiol Behav 63:795–801 [DOI] [PubMed] [Google Scholar]
- 126.Patarca R, Klimas NG, Lugtendorf S, Antoni M, Fletcher MA. 1994. Dysregulated expression of tumor necrosis factor in chronic fatigue syndrome: interrelations with cellular sources and patterns of soluble immune mediator expression. Clin Infect Dis 18 Suppl 1:S147–S153 [DOI] [PubMed] [Google Scholar]
- 127.Patrick DL, Ferketich SL, Frame PS, Harris JJ, Hendricks JB, Levin B, Link MP, Lustig C, McLaughlin J, Ried LD, Turrisi AT, Unutzer J, Vernon SW. 2003. National Institutes of Health State-of-the-Science Conference Statement: Symptom management in cancer: pain, depression, and fatigue. J Natl Cancer Inst 95:1110–1117 [DOI] [PubMed] [Google Scholar]
- 128.Persohn E, Canta A, Schoepfer S, Traebert M, Mueller L, Gilardini A, Galbiati S, Nicolini G, Scuteri A, Lanzani F, Giussani G, Cavaletti G. 2005. Morphological and morphometric analysis of paclitaxel and docetaxel-induced peripheral neuropathy in rats. Eur J Cancer 41:1460–1466 [DOI] [PubMed] [Google Scholar]
- 129.Pinheiro SP, Schernhammer ES, Tworoger SS, Michels KB. 2006. A prospective study on habitual duration of sleep and incidence of breast cancer in a large cohort of women. Cancer Res 66:5521–5525 [DOI] [PubMed] [Google Scholar]
- 130.Polomano RC, Mannes A, Clark US, Bennett GJ. 2001. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain 94:293–304 [DOI] [PubMed] [Google Scholar]
- 131.Portenoy RK, Itri LM. 1999. Cancer-related fatigue: guidelines for revaluation and management. Oncologist 4:1–10 [PubMed] [Google Scholar]
- 132.Pukkala E, Aspholm R, Auvinen A, Eliasch H, Gundestrup M, Haldorsen T, Hammar N, Hrfnkelsson J, Kyyronrn O, Linnersjo A, Rafnsson V, Storm H, Tveten U. 2002. Incidence of cancer among Nordic airline pilots over five decades: occupational cohort study. BMJ 325:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pusztai L, Mendoza TR, Reuben JM, Martinez MM, Willey JS, Lara J, Syed A, Fritsche HA, Bruera E, Booser D, Valero V, Arun B, Ibrahim N, Rivera E, Royce M, Cleeland CS, Hortobagyi GN. 2004. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine 25:94–102 [DOI] [PubMed] [Google Scholar]
- 134.Raison CL, Demetrashvili M, Capuron L, Miller AH. 2005. Neuropsychiatric adverse effects of interferon-α: recognition and management. CNS Drugs 19:105–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Reynolds P, Cone J, Layefsky M, Goldberg DE, Hurley S. 2002. Cancer incidence in California flight attendants (United States). Cancer Causes Control 13:317–324 [DOI] [PubMed] [Google Scholar]
- 136.Rich T, Innominato PF, Boerner J, Mormont MC, Iacobelli S, Baron B, Jasmin C, Levi F. 2005. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clin Cancer Res 11:1757–1764 [DOI] [PubMed] [Google Scholar]
- 137.Rogers NL, Sziba MP, Staab JP, Evans DL, Dinges DF. 2001. Neuroimmunologic aspects of sleep and sleep loss. Semin Clin Neuropsychiatry 6:295–307 [DOI] [PubMed] [Google Scholar]
- 138.Roscoe JA, Morrow GR, Hickok JT, Bushonow O, Matteson S, Rakito D, Andrews PLR. 2002. Temporal interrelationships among fatigue, circadian rhythm and depression in breast cancer patients undergoing chemotherapy treatment. Support Care Cancer 10:329–336 [DOI] [PubMed] [Google Scholar]
- 139.Roseboom PH, Namboodiri MA, Zimonjic DB, Popescu NC, Rodriguez IR, Gastel JA, Klein DC. 1998. Natural melatonin “knock-down” in C57BL/6J mice: rare mechanism truncates serotonin N-acetyltransferase. Brain Res Mol Brain Res 63:189–197 [DOI] [PubMed] [Google Scholar]
- 140.Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. 2007. Mechanisms of cancer-related fatigue. Oncologist 12 Suppl. 1:22–34 [DOI] [PubMed] [Google Scholar]
- 141.Saini A, Al-Shanti N, Stewart CE. 2006. Waste management—cytokines, growth factors and cachexia. Cytokine Growth Factor Rev 17: 475–486 [DOI] [PubMed] [Google Scholar]
- 142.Savard J, Morin CM. 2001. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol 19:895–908 [DOI] [PubMed] [Google Scholar]
- 143.Savard J, Simard S, Ivers H, Morin CM. 2005. Randomized study on the efficacy of cognitive behavioral therapy for insomnia secondary to breast cancer, part II: immunologic effects. J Clin Oncol 23:6097–6106 [DOI] [PubMed] [Google Scholar]
- 144.Schernhammer ES, Kroenke CS, Laden F, Hankinson SE. 2006. Night work and risk of breast cancer. Epidemiology 17:108–111 [DOI] [PubMed] [Google Scholar]
- 145.Schneider CM, Hsieh CC, Sprod LK, Carter SD, Hayward R. 2007. Effects of supervised exercise training on cardiopulmonary function and fatigue in breast cancer survivors during and after treatment. Cancer 110:918–925 [DOI] [PubMed] [Google Scholar]
- 146.Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. 2007. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun 21:413–427 [DOI] [PubMed] [Google Scholar]
- 147.Schwartz AL. 1999. Fatigue mediates the effects of exercise on quality of life. Qual Life Res 8:529–538 [DOI] [PubMed] [Google Scholar]
- 148.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. 2000. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst 92:994–1000 [DOI] [PubMed] [Google Scholar]
- 149.Shearer WT, Reuben JM, Mullington JM, Price NJ, Lee BN, Smith EO, Szuba MP, Van Dongen HPA, Dinges DF. 2001. Soluble TNF-α receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of space flight. J Allergy Clin Immunol 107:165–170 [DOI] [PubMed] [Google Scholar]
- 150.Siegel JM. 2005. Clues to the functions of mammalian sleep. Nature 437:1264–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Silver JK. 2007. Rehabilitation in women with breast cancer. Phys Med Rehabil Clin N Am 18:521–537, x. [DOI] [PubMed] [Google Scholar]
- 152.Singh M, Johnson L. 2006. Using genetically engineered mouse models of cancer to aid drug development: an industry perspective. Clin Cancer Res 12:5312–5328 [DOI] [PubMed] [Google Scholar]
- 153.Spath-Schwalbe E, Hansen K, Schmidt F, Schrezenmeier H, Marshall L, Burger K, Fehm HL, Born J. 1998. Acute effects of recombinant human interleukin-6 on endocrine and central nervous sleep functions in healthy men. J Clin Endocrinol Metab 83:1573–1579 [DOI] [PubMed] [Google Scholar]
- 154.Stanton AL, Ganz PA, Kwan L, Meyerowitz BE, Bower JE, Krupnick JL, Rowland JH, Leedham B, Belin TR. 2005. Outcomes from the Moving Beyond Cancer psychoeducational, randomized, controlled trial with breast cancer patients. J Clin Oncol 23:6009–6018 [DOI] [PubMed] [Google Scholar]
- 155.Tafti M. 2007. Quantitative genetics of sleep in inbred mice. Dialogues Clin Neurosci 9:273–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tisdale MJ. 2002. Cachexia in cancer patients. Nat Rev Cancer 2:862–871 [DOI] [PubMed] [Google Scholar]
- 157.Toth LA. 1996. Strain differences in the somnogenic effects of interferon inducers in mice. J Interferon Cytokine Res 16:1065–1072 [DOI] [PubMed] [Google Scholar]
- 158.Toth LA, Hughes LF. 2004. Macrophage participation in influenza-induced sleep enhancement in C57BL/6J mice. Brain Behav Immun 18:375–389 [DOI] [PubMed] [Google Scholar]
- 159.Toth LA, Jhaveri K. 2003. Sleep mechanisms in health and disease. Comp Med 53:473–486 [PubMed] [Google Scholar]
- 160.Toth LA, Rehg JE, Webster RG. 1995. Strain differences in sleep and other pathophysiological sequelae of influenza virus infection in naive and immunized mice. J Neuroimmunol 58:89–99 [DOI] [PubMed] [Google Scholar]
- 161.Trammell R, Jhaveri K, Toth LA. 2007. Inflammation and sleep. Pandi-Perumal SR, Chrousos G, Cardinali DP. Neuroimmunology of sleep. New York: Springer Press [Google Scholar]
- 162.Tsai LY, Li I, Lai Y, Liu C, Change T, Tu C. 2007. Fatigue and its associated factors in hospice cancer patients in Taiwan. Cancer Nurs 30:24–30 [DOI] [PubMed] [Google Scholar]
- 163.Van Dyke T, Jacks T. 2002. Cancer modeling in the modern era: progress and challenges. Cell 108:135–144 [DOI] [PubMed] [Google Scholar]
- 164.Verkasalo PK, Lillberg K, Stevens RG, Hublin C, Partinen M, Koskenvuo M, Kaprio J. 2005. Sleep duration and breast cancer: a prospective cohort study. Cancer Res 65:9595–9600 [DOI] [PubMed] [Google Scholar]
- 165.Vgontzas AN, Bixler EO, Lin HM, Prolo P, Trakada G, Chrousos GP. 2005. IL-6 and its circadian secretion in humans. Neuroimmunomodulation 12:131–140 [DOI] [PubMed] [Google Scholar]
- 166.Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. 1997. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab 82:1313–1316 [DOI] [PubMed] [Google Scholar]
- 167.Vickberg SM. 2003. The Concerns About Recurrence Scale (CARS): a systematic measure of women's fears about the possibility of breast cancer recurrence. Ann Behav Med 25:16–24 [DOI] [PubMed] [Google Scholar]
- 168.Vickberg SMJ. 2001. Fears about breast cancer recurrence. Cancer Pract 9:237–243 [DOI] [PubMed] [Google Scholar]
- 169.Vivien-Roels B, Malan A, Rettori MC, Delagrange P, Jeannoit JP, Pevet P. 1998. Daily variations in pineal melatonin concentraions in inbred and outbred mice. J Biol Rhythms 13:403–409 [DOI] [PubMed] [Google Scholar]
- 170.Vollmer-Conna U, Fazou C, Cameron B, Li H, Brennan C, Luck L, Davenport T, Wakefield D, Hickie I, Lloyd A. 2004. Production of pro-inflammatory cytokines correlates with the symptoms of acute sickness behaviour in humans. Psychol Med 34:1289–1297 [DOI] [PubMed] [Google Scholar]
- 171.Wang SY, Tsai CM, Chen BC, Lin CH, Lin CC. 2008. Symptom clusters and relationships to symptom interference with daily life in Taiwanese lung cancer patients. J Pain Symptom Manage 35:258–266 [DOI] [PubMed] [Google Scholar]
- 172.Wichers M, Maes M. 2002. The psychoneuroimmuno-pathophysiology of cytokine-induced depression in humans. Int J Neuropsychopharmacol 5:375–388 [DOI] [PubMed] [Google Scholar]
- 173.Wilson J, Balkwill F. 2002. The role of cytokines in the epithelial cancer microenvironment. Semin Cancer Biol 12:113–120 [DOI] [PubMed] [Google Scholar]
- 174.Woo B, Dibble SL, Piper BF, Keating SB, Weiss MC. 1998. Differences in fatigue by treatment methods in women with breast cancer. Oncol Nurs Forum 25:915–920 [PubMed] [Google Scholar]
- 175.Wood LJ, Nail LM, Perrin NA, Elesa CR, Fischer A, Druker BJ. 2006. The cancer chemotherapy drug etoposide (VP-16) induces proinflammatory cytokine production and sickness behavior-like symptoms in a mouse model of cancer chemotherapy-related symptoms. Biol Res Nurs 8:157–169 [DOI] [PubMed] [Google Scholar]
- 176.Wu HS, McSweeney M. 2007. Cancer-related fatigue: “It's so much more than just being tired.” Eur J Oncol Nurs 11:117–125 [DOI] [PubMed] [Google Scholar]