Abstract
Mice in a colony used for pancreatic cancer research and maintained in a barrier animal facility presented with vulvar masses. A census and examination of all colony animals was conducted on 17 February 2006; line, gender, and mass location were recorded; a slide caliper was used to measure the width, length, and height of each mass; and the volume of each mass was calculated. Progeny female mice from crossbreeding of the B6.FVB-Tg(Ipf1-cre)1Tuv and B6;129-Kras2tm4Tyj (KRASG12D/+) strains presented with external vulvar and periauricular papillomas. The papillomas were present in 41.2% of all female crossbred mice and ranged in size from 8 to 36 mm3. Age of mice and tumor size were not correlated. Compared with the B6.FVB-Tg(Ipf1-cre)1Tuv line, the crossbred female mice were more likely to have a vulvar mass, with an odds ratio of 29.3, 95% confidence interval (1.5, 563.9) and a positive predictive value of 42.9%. Diagnostic evaluation, including electron microscopy, light microscopy, serology, and bacteriology, did not reveal a viral or other infectious etiology. Therefore, we speculate that interaction between the genetic background of the mice and the introduced Kras oncogene may be responsible for these papillomas.
During weekly husbandry duties, an animal caretaker noticed swellings in the vulvar area of several mice in a breeding colony housed in our AAALAC-accredited animal facility (Mayo Clinic, Rochester, MN). The breeding colony was used to supply mice for pancreatic cancer studies. Clinical examination of these mice revealed enlarged, vulvar masses.
Infectious, genetic, and environmental etiologies were considered. The most common infectious cause of papillomas in most species is viral.4,5,8,9,17,18,20,21,24 Furthermore, in postpubertal genetically engineered mice, vulvar swelling can represent the phenotypic effect of a genetic factor.10 Finally, the vulvar swellings might be due to an environmental factor. For example, in prepubertal gilts, mycotoxins, such as zearalenone, in the feed can result in vulvar swelling.2 In an effort to determine the origin of these papillomas, we conducted an epidemiologic investigation to describe the incidence and distribution of the masses. Diagnostic evaluations were conducted to determine the probable cause of the papillomas in our inhouse breeding colony.
Materials and Methods
Animals.
The mice (Mus musculus) were housed in an AAALAC-accredited animal facility. All procedures were approved by the institutional animal care and use committee, and mice were housed in accordance with the Guide for the Care and Use of Laboratory Animals.15 The colony was composed of 2 lines, B6.FVB-Tg(Ipf1-cre)1Tuv and B6;129-Kras2tm4Tyj,7 which were being crossbred to produce mice for a pancreatic cancer study. These lines were generated at Mayo Clinic in our transgenic core through pronuclear injection of the Cre and Kras constructs and backcrossing of the mice onto the C57BL/6J strain. The mice were housed in autoclaved, polycarbonate, static microisolation caging (Allentown Caging Equipment, Allentown, NJ) with woodchip bedding (Sani-Chips, Montville, NJ). The mice were fed irradiated diet (PicoLab diet 5053, PMI Nutrition International, Richmond, IN) and UV-treated tap water in autoclaved water bottles. The room was kept on a 12:12-h light:dark cycle (lights on, 0600 h), and temperature was maintained at 22 ± 0.7 °C with relative humidity between 30% to 70%.
During weekly cage changing, dirty bedding was sampled randomly from 10 cages on each side of the rack and placed in the respective sentinel cages. Quarterly, sentinel mice (1 from each side of the rack) were sedated with CO2 and exsanguinated through cardiocentesis to collect serum samples. Extra sentinel mice were used in the event that additional testing was required. All serum samples were sent to Charles River Laboratories (Wilmington, MA) for serologic testing. Endo- and ectoparasite screens were conducted inhouse by our veterinary technicians.
The colony was serologically negative for Sendai virus, pneumonia virus of mice, mouse hepatitis virus, mice minute virus, Theiler murine encephalomyelitis virus, reovirus 3, Mycoplasma pulmonis, lymphocytic choriomeningitis virus, Ectromelia, K virus, polyoma virus, mouse adenovirus type 1, mouse adenovirus type 2, enzootic diarrhea of infant mice, mouse cytomegalovirus, hantavirus, Encephalitozoon cuniculi, cilia-associated respiratory bacillus, mouse parvovirus, and mouse T lymphotropic virus. The colony was also negative for endo- and ectoparasites.
Epidemiologic analysis.
A census and examination of colony animals was conducted. In February 2006, the colony contained 36 (male, n = 19; female, n = 17) crossbred mice. Mouse identification, line, gender, age, and mass location were recorded; a slide caliper was used to measure the width, length, and height of each mass; and the volume of each mass was calculated (Table 1). We evaluated the colony again in February 2007, when the colony contained 26 (male, n = 13; female, n = 13) crossbred mice.
Table 1.
Characteristics of B6.FVB-Tg(Ipf1-cre)1Tuv × B6;129-Kras2tm4Ty crossbred mice with papillomas
| Mouse | Gender | Agea (d) | Location of papilloma(s) | Tumor volume (mm3) |
| 2061 | Male | 353 | ear (unilateral) | 24 |
| 2076 | Female | 339 | vulva | 24 |
| 2089 | Female | 339 | vulva | 8 |
| 2319 | Female | 259 | vulva | 24 |
| 2329 | Female | 235 | vulva | 24 |
| 2331 | Female | 235 | vulva | 36 |
| 2335 | Female | 235 | vulva, ear (bilateral) | 24, 18, 36 |
| 2339 | Female | 235 | vulva | 12 |
Age on the date of papilloma measurement (2 February 2006).
Histopathology.
Two female mice with vulvar masses were euthanized with CO2; tissues including brain, heart, lungs, liver, spleen, kidneys, gastrointestinal tract, reproductive tract, and the vulvar mass were harvested and fixed in 10% neutral buffered formalin. After fixation, tissues were cut and embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Tissue samples were evaluated by a board-certified veterinary pathologist (RJM).
Electron microscopy.
To assist in ruling out a viral etiology, 2 additional female mice were euthanized, and the vulvar masses were fixed in 2% glutaraldehyde, processed according to standard procedures for transmission electron microscopy, and evaluated for viral particles.
PCR.
Sections of fixed tissue from the vulvas of the 2 mice submitted for histopathology were submitted to Charles River Laboratories for PCR testing for polyoma and K viruses. Briefly, unstained tissue sections were removed from the glass slides with a scalpel (paired samples were pooled), and DNA was extracted (DNeasy mini kit, Qiagen, Valencia, CA ). Second, extracted DNA was tested for the presence of murine polyoma virus and K virus by separate TaqMan PCR assays (Applied Biosystems, Foster City, CA) with primer and probe sets targeting conserved sequences in the VP1 gene (polyoma virus) and noncoding terminal sequence (K virus). Third, a TaqMan PCR assay targeting the mouse apolipoprotein B (apoB) gene was performed on each sample to verify that DNA suitable for amplification was extracted. Finally, after amplification in 96-well plates, the PCR reactions were read on a microplate fluorometer. Fluorescence values were compared with those from negative and positive control reactions (calf thymus DNA and 100 copies of plasmid-cloned virus target sequence, respectively).
Detection of KrasG12D transcription.
Two female mice were euthanized by using CO2; 2 papillomas were excised and snap-frozen. Pancreatic tumor tissue from a B6;129-Kras2tm4Tyj mouse was used as a positive control, and wild-type mouse pancreas was used as a negative control. RNA was extracted (Trizol, Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. cDNA was generated by using a kit (First-strand Synthesis Kit, Invitrogen). Primers for KrasG12D mutant transcripts were used for PCR (Kras1, 5′AGG CCT GCT GAA AAT GAC TG3′; Kras7, 5′CCC TCC CGA GTT CTC ATG TA3′) to amplify a 243-bp product from both wild-type and mutant transcripts. The KrasG12D allele (but not the wild-type allele) contains a HinDIII site, yielding a 213-bp and 30-bp product.1 CR products were verified on a 1% agarose gel. Products were sequenced to detect the G→A mutation in the cDNA that is indicative of the KrasG12D mutation.
Statistical analysis.
Spearman correlation coefficients were used to evaluate correlations of age with mass size. An odds ratio with 95% confidence intervals and a positive predictive value were calculated to compare the likelihood of vulvar papillomas in crossbred female mice with that in the B6.FVB-Tg(Ipf1-cre)1Tuv line. All statistics were calculated by using SAS software (version 8.2, SAS Institute, Cary, NC). A P value of less than 0.05 was considered statistically significant.
Results
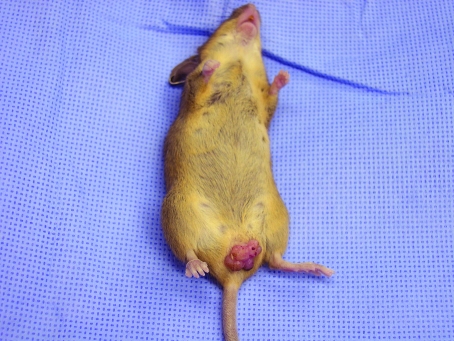
Evaluation revealed that only mice resulting from crossbreeding of the lines B6.FVB-Tg(Ipf1-cre)1Tuv and B6;129-Kras2tm4Tyj developed the vulvar masses. Of the 17 female mice, 7 were affected with a vulvar mass, for a prevalence of 41.2%. In addition, periauricular masses were noted unilaterally (n = 1) and bilaterally (n = 1). In addition, single male mouse in the colony had a periauricular mass. Grossly, vulvar masses were bilateral, oblong to round, elevated, with sessile formation, and well delineated from the surrounding tissue (Figure 1). Evaluation of the B6.FVB-Tg(Ipf1-cre)1Tuv colony animals (male, 10; female, 20) did not reveal any masses externally; at the time of this evaluation, the colony contained no B6;129-Kras2tm4Tyj mice. However, a search of our rodent health database and the investigator's colony records did not reveal any entries regarding vulvar papillomas in this line. We evaluated the colony a year later and found 11 of the 13 female mice had with a vulvar mass (prevalence, 84.6%). In addition, 1 male mouse presented with a periorbital mass above the left eye (prevalence, 7.8%).
Figure 1.
Gross appearance of crossbred (B6.FVB-Tg[Ipf1-cre]1Tuv × B6;129-Kras2tm4Ty) female mouse with vulvar mass. Vulvar masses were bilateral, oblong to round, elevated, with sessile formation, and well delineated from the surrounding tissue.
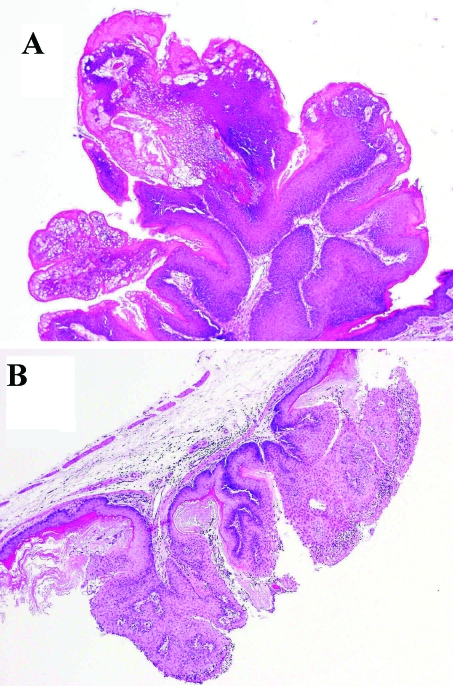
Subsequent histologic evaluation revealed the masses were papillomas (Figure 2 A). No inclusions were noted during histologic evaluation. Ovarian tissue in crossbred mice showed marked, bilateral decreased follicular activity. In addition to the vulvar papilloma, a papilloma was identified in the stomach of 1 mouse (Figure 2 B) on histologic evaluation. However, this gastric papilloma was not visible grossly. Histologically, papillomas were characterized by increased squamous hyperplasia with a fibrovascular core, with few inflammatory cells and mitotic figures.
Figure 2.
Histologic features of (A) vulvar papilloma and (B) stomach papilloma of index case. Papillomas were characterized by increased squamous hyperplasia with a fibrovascular core, with few inflammatory cells and mitotic figures. Magnification, ×125.
To further investigate the origin of the vulvar lesions, a live crossbred mouse from the colony was submitted to Charles River Laboratories for a comprehensive evaluation. This animal was serologically negative for the previously mentioned viruses. However, nasal aspirate cultures were positive for Pasteurella pneumotropica, PCR testing was positive for Helicobacter spp., and examination for endoparasites revealed trichomonads. Gross and histopathology findings included a vulvar papilloma, eosinophilic crystalline pneumonia, which was negative for Pneumocystis spp., and an epidermal cyst on the tail, all of which were considered incidental and of no colony health significance. Gastric papillomas were not seen grossly or histologically on this mouse. Although multiple fecal samples were submitted to the University of Missouri Research Animal Diagnostic Laboratory (Columbia, MO) for speciation of Helicobacter organisms, all of the samples were negative for these bacteria. In light of these results, the papillomas are not likely to be due to Helicobacter infection and the gastric papilloma seen in the 1 mouse submitted to our veterinary pathologist (RJM) likely was likely an incidental finding. The Helicobacter status of that animal is unknown.
Transmission electron microscopy was used to evaluate multiple areas of cellular debris and damage for the presence of viral particles in the vulvar tissue. The granules present were too large to be viral particles, although similar in shape to viral particles. Pyknotic nuclei and transition zones with polymorphic nucleated cells were present (data not shown). Areas of cellular debris and damage were evaluated because viruses tend to replicate and rupture cells, so these areas would have the highest likelihood of viral particles. Extensive transmission electron microscopic scanning revealed an absence of viral particles. Furthermore, all samples that were submitted for PCR testing for polyoma and K viruses returned negative results for both viruses. All the samples showed strong signals for the apoB assay, so we are confident of their negative virus status.
The positive control as well as both papillomas tested expressed the KrasG12D allele when assayed by both restriction digestion and sequencing, whereas the negative control expressed only the wild-type allele (data not shown).
For crossbred mice, the median (25th and 75th percentile) age of the mice was 235 d (235 d, 319 d) from birth to the date of papilloma measurement, and the median papilloma volume was 24 mm3 (20 mm3, 24 mm3). Analysis of the collected data revealed no correlation between the age of the animal and size of the papilloma (P = 0.30). The odds of vulvar papilloma was increased in crossbred female mice compared with B6.FVB-Tg(Ipf1-cre)1Tuv female mice (odds ratio, 29.3; 95% confidence interval, 1.5–563.9), with a positive predictive value of 42.9%.
Discussion
Vulvar papillomas were identified in more than 40% of progeny female mice in an inhouse breeding colony producing experimental animals for a pancreatic cancer study and in 84.6% of the colony when the animals were evaluated approximately 1 y later. An epidemiologic investigation revealed that all affected mice were of the crossbred line. Diagnostic evaluation of these animals revealed negative serology for common rodent viruses and transmission electron microscopy of the tumor was negative for viral particles. The presence of vulvar papillomas was strongly associated with crossbred female progeny from 2 lines.
Papillomas are a benign epithelial neoplasm consisting of villous or arborescent outgrowths of fibrovascular stroma covered by neoplastic cells. Viruses are the most common cause of papillomas and affect numerous species, including birds,20,21 turtles,4,5 fish,9 humans,8,18 hamsters,17 and rabbits.24 Some animal species (for example, rabbits16,24) are studied as models of human papillomaviral disease. Although mice are infected by viruses in the Papovaviradae family, papillomas have not been reported in infected mice. An important consideration in our case, as noted recently elsewhere,3 is that in genetically engineered mice, the interaction between the microbe and host and the resulting manifestation of disease may be different from that in inbred and outbred mice. Therefore infection with a virus from the Papovaviradae family may have altered the phenotype of our crossbred line, resulting in vulvar papillomas and confounding interpretation of experimental results and conclusions about the function of the mutated gene.
Vulvar masses are rare in mice. A search of the Mouse Tumor Biology Database21 revealed only 3 references. Most recently, spontaneous vulvar carcinomas were identified in 129/J mice that were part of an inhouse production colony.12 A second publication described a malignant vulvar wart in a C57 Black breeding mouse.11 The third reference was a review of characteristic tumors in mice; the authors noted that the incidence of skin papillomas in hairless HR/De mice was approximately 9%,13 compared with less than 1% in C3H3B/De and C3Hf/He mice.13 The reported incidence of skin papillomas in haired HR/De mice was 2% in female mice and 4% in male mice.13 This review also stated that papillomas and squamous cell carcinomas of the vulva were seen in strain 129 female mice at The Jackson Laboratory.13
Additional factors may have contributed to the development of the vulvar masses in these mice. Although papilloma size was not correlated with age, this result may reflect the small size of our colony. Because only prevalent cases were examined, we were unable to determine the age at which the papillomas first appeared. The age of the mice at the time of papilloma measurement ranged from 235 to 353 d. The prevalence of papillomas increased from 41.2% to 84.6% 1 yr later. A review of the investigator's colony records revealed that the papillomas began to appear when mice were 2 to 3 mo of age. Female mice are considered sexually mature at 7 to 8 wk.22 Therefore, exposure to sex hormones, such as luteinizing hormone, follicle stimulating hormone, and estradiol, during sexual maturity may have induced cells of the vulva to become hyperplastic. The vulva and lining of the gastrointestinal tract are derived from epithelial cells, so the fact that papillomas were found in these different locations is not surprising. Some mice had papillomas in the skin around the ears and eyes, and 1 mouse had a gastric papilloma.
Excluding a viral etiology, the most likely cause of the vulvar papillomas is genetic. The development of a genetically engineered mouse model that targets the endogenous Kras locus with an oncogenic KrasG12D allele represents an advance in the study of pancreatic cancer. This gene is activated in the pancreas by Cre-mediated recombination. Cre is expressed in this mouse model as a Pdx1 promoter-driven transgene, which results in mice that develop pancreatic intraductal lesions and eventually develop into invasive, metastatic adenocarcinoma.6 In our mice, the most likely explanation for the development of papillomas is that the Pdx1 promoter is strongly activated in vulvar cells as well as in the pancreas. Given that Pdx1 is a developmental transcription factor, this outcome is certainly possible. This strong activation of the Pdx1 promoter leads to transcription of the KrasG12D oncogene in these cells and may result in proliferation and benign papilloma formation. Because these papillomas were present only in the crossbred line, it appears that the activated Kras2 gene is driving the process.
Other studies documenting this condition in mice are sparse; unlike vulvar adenocarcinomas,12 these papillomas were benign. In addition, animals with vulvar carcinomas had hemorrhagic discharge, which our mice did not. Microscopic changes were noted in the ovaries and uterus of mice with the vulvar carcinomas.12 Similarly, mice in our study had ovarian changes of marked, bilateral decreased follicular activity. Other similarities included a high prevalence of vulvar lesions: 44% of mice in the cited inhouse breeding colony12 compared with 41.2% and 84.6% in our colony. In both colonies, mice were serologically negative for murine viruses responsible for causing tumors.12 Electron microscopy failed to reveal viral particles in either colony.12 In both colonies, mice were on a strain 129 background.12 Examination of other mouse lines, with the exception of strain 129 females at The Jackson Laboratory,13 yielded a low incidence of epithelial tumors in mice in general.11 The B6;129-Kras2tm4Tyj line is on a B6;129 genetic background,14 whereas the B6.FVB-Tg(Ipf1-cre)1Tuv is on a strain 129 background; female mice in both of these parental lines lacked vulvar papillomas. However, crossing these lines resulted in a large percentage of mice with vulvar papillomas. This scenario is similar to that of mice at Jackson Labs that developed vulvar lesions. Furthermore, genetic variation among 129 substrains, as well as contamination of the 129/SvJ inbred strain, have been well documented,19,23 resulting in differences when comparing phenotypes resulting from genes targeted in various 129-derived embryonic stem cell lines.
Our investigation had several limitations. We did not test the diet for mycotoxin activity, which may have played a role in the development of the vulvar papillomas. However, none of the other mice in the facility, all of which received the same diet, developed these masses. In addition, hormone assays were not conducted; this area of investigation may help to elucidate the role of sex steroids in the pathogenesis of these papillomas. Furthermore, our study attempted to rule out potential causes of papillomas, and we were left with genetic mechanisms as the most likely etiology. However, given the limits of our current investigation, we cannot definitively assert that the genetic background of the mice is the cause of the papillomas.
In summary, female B6.FVB-Tg(Ipf1-cre)1Tuv × B6;129-Kras2tm4Tyj mice had a high prevalence of vulvar papillomas. Diagnostic evaluation failed to reveal a viral etiology. Molecular data confirmed activation of the Kras oncogene in the vulvar tissue. We therefore speculate that the interaction between the transduced Kras oncogene and the genetic background of the mice may be responsible for these papillomas.
Acknowledgments
The authors thank the Department of Comparative Medicine Veterinary Technicians for their assistance in collecting, fixing, and shipping the mouse tissues. We thank Mr Douglas J Jazdzewski for editorial review of a preliminary version of this manuscript. We wish to thank Ms Leslie Dixon for her assistance in processing, cutting, and staining the tissues for histologic evaluation.
This project is supported by a research grant from the National Institutes of Health SPORE in Pancreatic Cancer, grant P50 CA102701, and the Mayo Foundation.
References
- 1.Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, Redston MS, DePinho RA. 2003. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev 17:3112–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaney BJ, Bloomfield RC, Moore CJ. 1984. Zearalenone intoxication of pigs. Aust Vet J 61:24–27 [DOI] [PubMed] [Google Scholar]
- 3.Franklin CL. 2006. Microbial considerations in genetically engineered mouse research. ILAR J 47:141–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenblatt RJ, Quackenbush SL, Casey RN, Rovnak J, Balazs GH, Work TM, Casey JW, Sutton CA. 2005. Genomic variation of the fibropapilloma-associated marine turtle herpesvirus across seven geographic areas and three host species. J Virol 79:1125–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbst L, Ene A, Su M, Desalle R, Lenz J. 2004. Tumor outbreaks in marine turtles are not due to recent herpesvirus mutations. Curr Biol 14:R697–R699 [DOI] [PubMed] [Google Scholar]
- 6.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, Tuveson DA. 2003. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 4:437–450 [DOI] [PubMed] [Google Scholar]
- 7.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. 2005. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 7:469–483 [DOI] [PubMed] [Google Scholar]
- 8.Katori H, Nozawa A, Tsukuda M. 2006. Increased expression of matrix metalloproteinase 2 and 9 and human papilloma virus infection are associated with malignant transformation of sinonasal inverted papilloma. J Surg Oncol 93:80–85 [DOI] [PubMed] [Google Scholar]
- 9.Kortet R, Vainikka A, Taskinen J. 2003. Effect of epidermal papillomatosis on survival of the freshwater fish Rutilus rutilus. Dis Aquat Organ 57:163–165 [DOI] [PubMed] [Google Scholar]
- 10.Linder CC. 2006. Genetic variables that influence phenotype. ILAR J 47:132–140 [DOI] [PubMed] [Google Scholar]
- 11.Little CC, Murray WS, Cloudman AM. 1939. The genetics of nonepithelial tumor formation in mice. Am J Cancer 36:431–450 [Google Scholar]
- 12.Locklear J, Mahler J, Thigpen JE, Goelz MF, Forsythe D. 1995. Spontaneous vulvar carcinomas in 129/J mice. Lab Anim Sci 45:604–606 [PubMed] [Google Scholar]
- 13.Murphy 1966. Biology of the laboratory mouse. Green E. New York: McGraw-Hill; p 521–562 [Google Scholar]
- 14.National Cancer Institute. Mouse respository: mouse models of human cancers consortium [Internet]. Frederick (MD): National Cancer Institute. [cited 2006 Feb 12]. Available from: http://mouse.ncifcrf.gov.
- 15.National Research Council 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press [Google Scholar]
- 16.Peng X, Lang CM, Kreider JW. 1996. Immortalization of inbred rabbit keratinocytes from a Shope papilloma and tumorigenic transformation of the cells by EJras. Cancer Lett 108:101–109 [DOI] [PubMed] [Google Scholar]
- 17.Scherneck S, Ulrich R, Feunteun J. 2001. The hamster polyomavirus—a brief review of recent knowledge. Virus Genes 22:93–101 [DOI] [PubMed] [Google Scholar]
- 18.Sharma G, DeHart J, Nuovo GJ. 2005. Correlation of histology, human papillomavirus, and viral load in laryngeal papillomas of childhood. Diagn Mol Pathol 14:230–236 [DOI] [PubMed] [Google Scholar]
- 19.Simpson EM, Linder CC, Sargent EE, Davisson MT, Mobraaten LE, Sharp JJ. 1997. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat Genet 16:19–27 [DOI] [PubMed] [Google Scholar]
- 20.Styles DK, Tomaszewski EK, Jaeger LA, Phalen DN. 2004. Psittacid herpesviruses associated with mucosal papillomas in neotropical parrots. Virology 325:24–35 [DOI] [PubMed] [Google Scholar]
- 21.Styles DK, Tomaszewski EK, Phalen DN. 2005. A novel psittacid herpesvirus found in African Grey parrots (Psittacus erithacus erithacus). Avian Pathol 34:150–154 [DOI] [PubMed] [Google Scholar]
- 22.Suckow M, Danneman P, Brayton C. 2001. The laboratory mouse. Boca Raton (FL): CRC Press [Google Scholar]
- 23.Threadgill DW, Yee D, Matin A, Nadeau JH, Magnuson T. 1997. Genealogy of the 129 inbred strains: 129/SvJ is a contaminated inbred strain. Mamm Genome 8:390–393 [DOI] [PubMed] [Google Scholar]
- 24.Wilgenburg BJ, Budgeon LR, Lang CM, Griffith JW, Christensen ND. 2005. Characterization of immune responses during regression of rabbit oral papillomavirus infections. Comp Med 55:431–439 [PubMed] [Google Scholar]