Opiate analgesics have been used by humans for thousands of years and are the longest continuously used class of medications. The recent increased interest in opiates (drugs derived from opium) and opioids (more generally, any natural or synthetic drug that binds to an opioid receptor) has evolved largely from 5 directions: (1) advances in the design of new opioid receptor agonist and antagonist drugs; (2) expansion and innovation in methods of drug delivery; (3) increased public awareness of pain management options and the appropriateness of aggressively treating pain (eg, declaration of pain as the “fifth vital sign”1 and pain relief as a fundamental human right2); (4) growing recognition of the serious consequences of opioid misuse, misadventure, and addiction; and (5) medicolegal aspects of practitioners' prescribing practices and legal prosecution for “overprescribing.” These and related issues are addressed in 4 articles and 1 additional editorial in the current issue of Mayo Clinic Proceedings. Specifically, Passik3 discusses long-term prescription opioid therapy; Argoff and Silvershein4 address the use of long- vs short-acting opioids for treating chronic noncancer pain; Smith5 reviews opioid metabolism; and Berge et al6 discuss chemical dependency in physicians, with a focus on opioids. In their editorial, Oreskovich and Caldeiro7 discuss whether select groups of health care professionals, such as anesthesiologists, have unacceptably poor outcomes after initial opioid addiction and whether this should dictate policies of rehabilitation and possible return to clinical practice.
Before 1960, most clinical opioids were either naturally occurring opiates (morphine) or minimally altered structural analogues of naturally occurring drugs (meperidine, methadone). Thus, drug choices were few. This changed dramatically when Paul Janssen8 created and introduced into clinical practice the phenylpiperidine opioid fentanyl, which has almost 100 times the potency of morphine and, at small doses, a shorter duration of effect. Concurrent with the introduction of fentanyl and other novel opioids, laboratory-based investigators began exploring the receptors responsible for opioid effects and discovered a veritable treasure trove (eg, delta, kappa, mu) of receptor subtypes. As reviewed in part by the aforementioned authors in this issue of the Proceedings, the new opioid agonists vary greatly in duration (ranging from long-acting drugs such as methadone, which lasts hours to days,3,4 to remifentanil, with a duration of only a few minutes), potency, receptor selectivity, and clinical application.
See also pages 576, 593, 602, 613, and 625
Innovations in chemistry have also led to therapeutic opioid antagonists. For example, naloxone and naltrexone, respectively, provide intermediate- and long-term reversal of central nervous system and systemic opioid effects. The former is often given to reverse opioid sedation and respiratory depression, and the latter is often used long term to treat opioid addiction.9 More recently, the opioid receptor antagonist methylnaltrexone, which does not cross the blood-brain barrier, has been approved for concurrent use in patients receiving μ-opioid analgesics to reverse constipation without reversing analgesia.10
Novel drug delivery techniques have augmented traditional intravenous and oral routes of administration of opioids to enhance patients' relief of pain. Building on the findings by Yaksh and Rudy11 that small intrathecal doses of opiates could produce profound analgesic effects in animal models, Wang et al12 of Mayo Clinic reported in 1979 the impressive analgesic effects of intrathecal morphine in humans. This article opened the door for administering multiple additional opioids and other compounds (eg, clonidine) into the intrathecal and epidural spaces to provide analgesia. Subsequent advances in opioid delivery techniques included intravenous infusions, patient-controlled dispensing systems (using various routes of administration), time-release transdermal skin patches, oral transmucosal preparations (ie, lozenges, tablets, and films), nasal sprays, and inhaled aerosols.
The plethora of drugs and delivery techniques has produced infinite combinations of drug selection, route of administration, and effective dose. For example, by administering opioids intrathecally, the effective analgesic dose is a mere fraction of that given intravenously and may avoid many of the adverse effects. Intraoperatively, anesthesiologists have given megadoses of fentanyl13 and morphine14 to produce general anesthesia for heart surgery. In veterinary medicine, the extremely potent opioid carfentanil, delivered in minute volumes and from a safe distance, can transiently incapacitate large animals with minimal adverse effects.15
Novel opioid delivery techniques are not without adverse effects. The most worrisome immediate opioid adverse effect has always been and will continue to be respiratory depression. In the hands of anesthesiologists or other highly trained clinicians, such adverse effects are anticipated, mitigated, and prevented. However, in the hands of those with inadequate pharmacological knowledge, unfortunate opioid events can occur to individual patients3-5 or practitioners6,7 and en masse.16 In 2002 when Russian troops attempted to immobilize 50 Chechnyan rebels and their 800 hostages inside a Moscow theater, the troops allegedly used an aerosolized potent opioid (presumably carfentanil or a related drug) as a mass “calmative”; 127 (16%) of the hostages died, and others became critically ill. These outcomes were related to the inability of the “practitioners” to manage the long-appreciated opioid adverse effect of respiratory depression.16
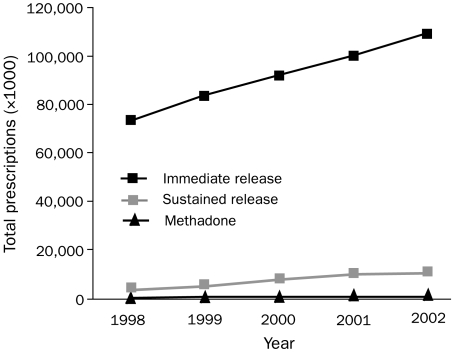
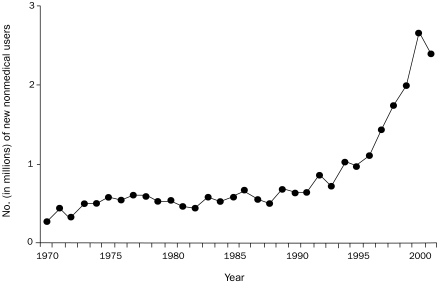
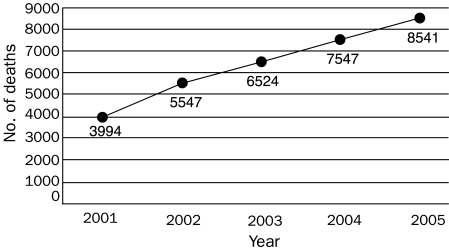
Because of the aforementioned applications of opioids, their use is at an all-time high (Figure 1)17 and will continue to expand. Greater use will increase not only pain relief and therapeutic benefit but also, unfortunately, short- and long-term toxicity, as well as secondary medicolegal issues and the prevalence of opioid abuse. Diversion of prescription opioids, away from intended patients and into the hands of recreational users, is one part of the abuse equation, and nonmedical use of prescription opioids has increased exponentially since the early 1990s (Figure 2).18 Not surprisingly, the expanded use of prescription opioids for all reasons, legitimate and illicit, has correlated with a steady increase in opioid-related deaths nationwide (Figure 3).19
FIGURE 1.
Total US prescriptions dispensed annually for all opioid analgesics from 1998-2002. Adapted from the IMS Health, National Prescription Audit Plus.17
FIGURE 2.
Annual numbers (in millions) of new nonmedical users of pain relievers who are 12 years or older, from 1970-2001. From the National Survey on Drug Use and Health, 2004.18
FIGURE 3.
Prescription opioid analgesic deaths nationwide, from 2001-2005. Adapted from the National Drug Intelligence Center: National Prescription Drug Threat Assessment 2009.19
Aside from drug diversion, many of the dilemmas with contemporary opioid use share common themes: Whom should we physicians treat, how should we treat them, and when should we treat them (or, conversely, not treat them)? As reviewed in part by Argoff and Silvershein,4 the appropriate use of short- vs long-acting opioids in the management of chronic pain presents a sometimes precarious balancing act: Failure to administer opioids when appropriate may be viewed as inadequate treatment, whereas excessive use of opioids may result in legal action alleging overprescribing or adverse effects (including overdose and death). Another concern, often misunderstood by both clinicians and nonclinicians, is patient habituation vs addiction to long-term opioid use. (Habituation refers to the need for increased drug dose, after long-term use, to achieve a desired effect. Addiction refers to a physiologic and/or psychological response in which the person experiences withdrawal symptoms if opioid is not delivered at an appropriate dose and timing.) Opioid habituation is treated relatively easily by increasing the opioid dose or giving supplemental drugs. Opioid addiction is one of the riskiest of all drug addictions, frequently resulting in death due to overdose, suicide, accidents, or risk-taking behavior (eg, unprotected sex, shared needles).
Perhaps one of cruelest adverse effects of opioid addiction is the toll taken on health care professionals. Anesthesiologists represent one of the highest-risk groups among health care professionals, and this risk is likely shared by nurse anesthetists and sedation nurses (although data for the last 2 groups are somewhat lacking).6,7,20 Berge et al6 expand on earlier writings about opiate and opioid addiction in health care professsionals20,21 and make the case that anesthesiologists are unique among physicians in their susceptibility to opioid abuse. Moreover, once addicted, anesthesiologists have a high rate of relapse and death, even after participating in a formal treatment program.20,21 This forces us to question: If anesthesiologists are at such risk, is it ever appropriate to return once-addicted anesthesiologists to clinical practice where they will have access to opioids and other psychotropic drugs and, if yes, under what conditions? Unfortunately, the available literature on this topic is sufficiently sparse that concerned experts on all sides of the issue are reluctant to change existing policies because of fear of making an incorrect decision. Clearly, more research is needed. In the meantime, Oreskovich and Caldeiro7 provide original information plus other underappreciated information on how anesthesiologists (and presumably related practitioners) have an unacceptable risk of poor outcomes once addicted to opioids. They propose that improvements in and expansion of certain types of treatment programs may remedy many of the current treatment failures.
The many opportunities and challenges related to contemporary opioid use have resulted in the introduction of new drugs and techniques for established indications and the expanded use and retargeting of older drugs for new indications. Methadone is an excellent example, as highlighted by the articles in this month's Proceedings. Methadone maintenance treatment has long been used as a cornerstone of opiate addiction therapy. It is efficacious in preventing or reducing opiate withdrawal symptoms, opioid craving, illicit use of other drugs, and high-risk behaviors; in addition, it provides a vital public health strategy for reducing human immunodeficiency virus infections, AIDS, and hepatitis C infections in opioid addicts. Because of its long half-life and duration of effect, methadone also is a highly effective, and an increasingly used, first-line drug in treating acute, chronic, neuropathic, and cancer pain and as an alternative to morphine and other first-line opioids.3,4 It is cost-effective at less than $0.25 per dose (pharmacy cost) compared to more than $8 per dose for some sustained-release opioids. Unfortunately, coincident with the intensified focus on adequate pain treatment (specifically that involving methadone) and increased administration of pain therapies by primary care professionals, methadone-related deaths have increased considerably. Methadone-related adverse events increased nearly 1800% between 1997 and 2004; fatalities increased 390% from 1999 to 2004 (the most recent national data available), and methadone was the drug with the greatest increase in fatalities22-24; methadone also is the sixth most frequently suspected drug in death and serious nonfatal outcomes.25
How can this be? In part, mishaps with methadone are a striking example that practitioners' basic understanding of opioid pharmacology is simply failing to keep up with the clinical applications of these drugs, a problem compounded by systematic deficiencies in overall knowledge acquisition and dissemination.5,26 The most convincing metric of this failure is the mounting number of patient deaths. As identified by the Substance Abuse and Mental Health Services Administration, “Half of methadone deaths are pain patients who are being mismanaged by physicians who lack sufficient knowledge or skills to use methadone in the treatment of pain.”22 This analysis identifies consequential pharmacological illiteracy among practitioners, perhaps because of the decline in teaching of pharmacology in medical schools (and possibly the coincident increase in pharmaceutical company efforts to educate practitioners, having arisen in part to fill an education vacuum about their drugs).
On the basis of the available information, practitioners can appreciate that, although opiates and opioids have been used therapeutically for millennia, the most exciting, yet sobering, time in opiate use is occurring now. Limitations in our current therapeutic approach include inadequate tailoring of specific drugs for specific indications to optimize efficacy and avoid both short-term (eg, respiratory depression) and long-term (eg, habituation, addiction) adverse effects. Future improvements in drug development (including highly specific agonists and antagonists) and delivery systems will likely provide options for improving opioid use in patient care. None of these exciting new developments will optimally benefit patients unless our understanding of opioid pharmacology keeps up with the expanding use of these drugs, and we adequately disseminate, absorb, and implement the new knowledge. The articles and editorials in this issue of the Proceedings provide a window to better understand the current and future use of the opioids and the opportunities and challenges presented.
Footnotes
Dr Kharasch was supported in part by National Institutes of Health grants R01-DA14211 and K24-DA00417.
References
- 1.American Pain Society Quality of Care Committee Quality improvement guidelines for the treatment of acute pain and cancer. JAMA 1995;274(23):1874-1880 [DOI] [PubMed] [Google Scholar]
- 2.Brennan F, Carr DB, Cousins M. Pain management: a fundamental human right. Anesth Analg. 2007;105(1):205-221 [DOI] [PubMed] [Google Scholar]
- 3.Passik SD. Issues in long-term opioid therapy: unmet needs, risks, and solutions. Mayo Clin Proc. 2009;84(7):593-601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Argoff CE, Silvershein DI. A comparison of long- and short-acting opioids for the treatment of chronic noncancer pain: tailoring therapy to meet patient needs. Mayo Clin Proc. 2009;84(7):602-612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith HS. Opioid metabolism. Mayo Clin Proc. 2009;84(7):613-624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berge KH, Seppala MD, Schipper AM. Chemical dependency and the physician. Mayo Clin Proc. 2009;84(7):625-631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oreskovich MR, Caldeiro RM. Anesthesiologists recovering from chemical dependency: can they safely return to the operating room [editorial]? Mayo Clin Proc. 2009;84(7):576-580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janssen PAJ. The development of new synthetic narcotics. In: Estafanous FG, ed. Opioids in Anesthesia Stoneham, MA: Buttorworth Publishers; 1984:37-44 [Google Scholar]
- 9.Volavka J, Resnick RB, Kestenbaum RS, Freedman AM. Short-term effects of naltrexone in 155 heroin ex-addicts. Biol Psychiatry. 1976;11(6):679-685 [PubMed] [Google Scholar]
- 10.Moss J, Rosow CE. Development of peripheral opioid antagonists' new insights into opioid effects. Mayo Clin Proc. 2008;83(10):1116-1130 [DOI] [PubMed] [Google Scholar]
- 11.Yaksh TL, Rudy TA. Analgesia mediated by a direct spinal action of narcotics. Science 1976;192(4246):1357-1358 [DOI] [PubMed] [Google Scholar]
- 12.Wang JK, Nauss LA, Thomas JE. Pain relief by intrathecally applied morphine in man. Anesthesiology 1979;50(2):149-151 [DOI] [PubMed] [Google Scholar]
- 13.Stanley TH, Philbin DM, Coggins CH. Fentanyl-oxygen anaesthesia for coronary artery surgery: cardiovascular and antidiuretic hormone responses. Can Anaesth Soc J. 1979;26(3):168-172 [DOI] [PubMed] [Google Scholar]
- 14.Lowenstein E. Morphine “anesthesia”—a perspective. Anesthesiology 1971;35(6):563-565 [DOI] [PubMed] [Google Scholar]
- 15.Howard LL, Kearns KS, Clippinger TL, Larsen RS, Morris PJ. Chemical immobilization of rhebok (Pelea capreolus) with carfentanil-xylazine or etorphine-xylazine. J Zoo Wildl Med. 2004;35(3):312-319 [DOI] [PubMed] [Google Scholar]
- 16.Wax PM, Becker CE, Curry SC. Unexpected “gas” casualties in Moscow: a medical toxicology perspective [published correction appears in Ann Emerg Med. 2003;42(2):285]Ann Emerg Med. 2003;41(5):700-705 [DOI] [PubMed] [Google Scholar]
- 17.IMS Health http://www.fda.gov/OHRMS/DOCKETS/ac/03/slides/3978S1_05_Rigoni.ppt. National Prescription Audit Plus™, Year 1998 to 2002, Excluding Long-Term Care & Mail Order Channels, Data Extracted August 2003. Rigoni GC. Drug utilization for immediate- and modified release opioids in the U.S. Accessed June 11, 2009.
- 18.National Survey on Drug Use and Health http://www.oas.samhsa.gov/2k4/pain/pain.htm. Nonmedical use of prescription pain relievers. doi: 10.1016/j.jpain.2008.03.001. Published May 21, 2004. Accessed June 3, 2009. [DOI] [PubMed]
- 19.Centers for Disease Control and Prevention, National Drug Intelligence Center Web site http://www.usdoj.gov/ndic/pubs33/33775/execsum.htm#Figure1. National prescription drug threat assessment 2009: executive summary. Accessed June 3, 2009.
- 20.Berge KH, Seppala MD, Lanier WL. The anesthesiology community's approach to opioid- and anesthetic-abusing personnel: time to change course. Anesthesiology 2008;109(5):762-764 [DOI] [PubMed] [Google Scholar]
- 21.Berge KH, Seppala MD, Lanier WL. One strike, you're out: one size fits none [reply]. Anesthesiology 2009;110(6):1425-1428 [DOI] [PubMed] [Google Scholar]
- 22.US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration http://www.dpt.samhsa.gov/pdf/MethadoneBackgroundPaper_72007_2_.pdf. Background information for methadone mortality—a reassessment. Published July 20, 2007. Accessed June 1, 2009.
- 23.US Department of Health and Human Services, Substance Abuse and Mental Health Services http://www.dpt.samhsa.gov/pdf/Methadone_Report_10%2018%2007_Brief%20w%20attch.pdf. Summary report of the meeting: methadone mortality —a reassessment. Published July 20, 2007. Accessed June 1, 2009.
- 24.Wysowski DK. Surveillance of prescription drug-related mortality using death certificate data. Drug Saf. 2007;30(6):533-540 [DOI] [PubMed] [Google Scholar]
- 25.Moore TJ, Cohen MR, Furberg CD. Serious adverse drug events reported to the Food and Drug Administration, 1998-2005. Arch Intern Med. 2007;167(16):1752-1759 [DOI] [PubMed] [Google Scholar]
- 26.Kharasch ED, Bedynek PS, Park S, Whittington D, Walker A, Hoffer C. Mechanism of ritonavir changes in methadone pharmacokinetics and pharmacodynamics: I, evidence against CYP3A mediation of methadone clearance. Clin Pharmacol Ther. 2008;84(4):497-505 [DOI] [PMC free article] [PubMed] [Google Scholar]