Abstract
Atrial fibrillation (AF) is the most common arrhythmia encountered in clinical practice. Its increasing prevalence, particularly among the elderly, renders it one of the most serious current medical epidemics. Several management questions confront the clinician treating a patient with AF: Should the condition be treated? Is the patient at risk of death or serious morbidity as a result of this diagnosis? If treatment is necessary, is rate control or rhythm control superior? Which patients need anticoagulation therapy, and for how long? This review of articles obtained by a search of the PubMed and MEDLINE databases presents the available evidence that can guide the clinician in answering these questions. After discussing the merits of available therapy, including medications aimed at controlling rate, rhythm, or both, we focus on the present status of ablative therapy for AF. Catheter ablation, particularly targeting the pulmonary veins, is being increasingly performed, although the precise indications for this approach and its effectiveness and safety are being actively investigated. We briefly discuss other invasive options that are less frequently used, such as pacemakers, defibrillators, left atrial appendage closure devices, and the surgical maze procedure.
AAD = antiarrhythmic drug; AF = atrial fibrillation; AFFIRM = Atrial Fibrillation Follow-up Investigation of Rhythm Management; CAST = Cardiac Arrhythmia Suppression Trial; CI = confidence interval; INR = international normalized ratio; OR = odds ratio; TIA = transient ischemic attack
Atrial fibrillation (AF) is the most common arrhythmia seen in clinical practice. This condition has been recognized as one of the most serious current medical epidemics. The prevalence of AF increases with the patient's age: it is found in 0.1% of adults younger than 55 years but in more than 9% of those aged 80 years or older. It is important to recognize that prevalence data are reported only for clinically recognized, symptomatic AF. The actual occurrence of asymptomatic AF is probably considerably higher.1 It is estimated that 2.3 million adults in the United States have clinically recognized AF and have sought medical attention for this condition. This number is expected to increase to approximately 6 million by 2050.1 This increasing number brings with it an increasing need for clinicians to counsel patients with AF by addressing several important issues, including a determination of the patient's risk of thromboembolic stroke or death and an understanding of the risks and benefits of the available treatment approaches. This review of the medical literature related to AF (obtained by searching PubMed and MEDLINE) presents the available evidence as a guide to the clinician.
CLASSIFICATION
Atrial fibrillation has been classified in several ways. Lone AF typically affects patients younger than 60 years who have no evidence of structural heart disease. Patients with lone AF are at a lower risk of thromboembolism than other patients with AF. One study found that, after 30 years of follow-up, the all-cause mortality rates of patients with lone AF are similar to those of age- and sex-matched controls older than 30 years who do not have AF.2
Some classification systems distinguish between primary AF and secondary AF. Secondary AF has an acute and possibly reversible cause, such as hyperthyroidism or acute alcohol intoxication. It may also occur after cardiac surgery.
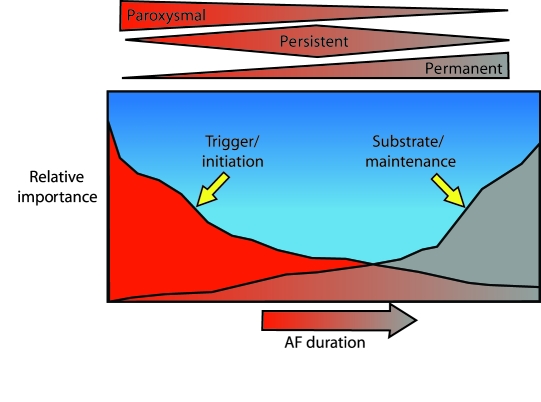
Perhaps the most clinically relevant classification distinguishes between paroxysmal AF and nonparoxysmal AF. In many ways, these conditions are separate entities with marked differences in etiology and management options. Patients with paroxysmal AF experience episodes that terminate spontaneously within 7 days and usually within 24 hours.3 Approximately 40% of all AF cases are paroxysmal. Nonparoxysmal AF is often further classified as persistent or permanent. Both terms describe episodes that last longer than 7 days and require cardioversion for termination. However, permanent AF refers to those cases in which AF has existed for many years and cannot be consistently terminated with cardioversion.4-6
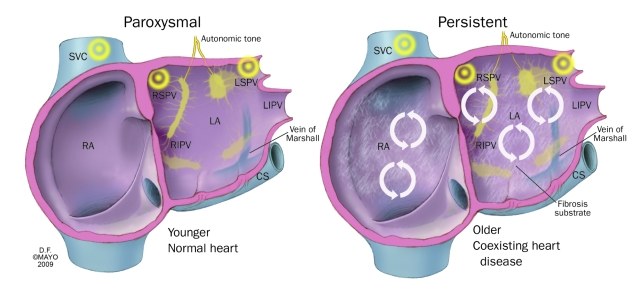
Paroxysmal AF is typically a disease of the thoracic vein and the autonomic nervous system in which pathological triggers repeatedly reinitiate AF. Patients with paroxysmal AF are often younger than those with other types of AF and have no structural heart disease (Figure 1). Persistent AF and permanent AF are primarily diseases of abnormal atrial substrate. They are often characterized histopathologically by marked atrial enlargement with fibrosis and electrophysiologically by multiple reentrant circuits with even minor triggers that can reinitiate and maintain AF.
FIGURE 1.
Important differences between the paroxysmal and persistent forms of atrial fibrillation. These differences have implications for management and for outcome expectations. Circular arrows represent rotors. CS = coronary sinus; LA = left atrium; LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein; RA = right atrium; RIPV = right inferior pulmonary vein; RSPV = right superior pulmonary vein; SVC = superior vena cava.
Nonparoxysmal AF is characterized by abnormal triggers that are often extra-atrial and by abnormal atrial myocardium, often resulting in wide variations in treatment approaches (see Pathophysiology and Disease Association). In as many as 25% of patients, paroxysmal AF will progress to chronic or permanent AF within 5 years.4,5
PATHOPHYSIOLOGY AND DISEASE ASSOCIATION
The exact cause of AF is unknown. A complex interplay exists between triggers that initiate AF and abnormalities in the atrial myocardial substrate that allow perpetuation of the arrhythmia. Although investigators and clinicians have debated for decades whether triggers or substrates (automatic foci or multiple reentrant wavelets) are primarily responsible for AF, newer insights suggest that both mechanisms may be operative for most patients. Recently, 2 important concepts have been elucidated. First, the more frequently AF occurs, the greater the likelihood of a further increase in the frequency and duration of episodes (AF begets AF).7 Second, most triggers that initiate AF appear to arise not from the atria but rather from some anatomic neighbor of the atria, such as a pulmonary vein, the superior vena cava, or the retro-atrial ganglionated plexuses.8
Comorbid Conditions
Of the comorbid conditions associated with AF, hypertension is the most common. Diastolic dysfunction of the ventricles does not allow their normal atrial filling. The resultant back-pressure causes hypertrophy of cardiac myocytes, proliferation of fibrous tissue, and decreases in intercellular coupling.9 Aggressive management of hypertension may also result in clinical improvement of AF.10
Atrial fibrillation is common among patients with coronary artery disease. However, ischemia of the atrium rarely causes AF. Patients with coronary artery disease often exhibit hypertension and left ventricular dysfunction with secondary abnormalities in the atrium.11 Atrial fibrillation also occurs in approximately 8.6% of patients with acute coronary syndromes, but again the phenomenon is probably secondary and is not a direct correlate of ischemia.12
Obesity is another important risk factor for AF. A recent meta-analysis found that obesity increases the risk of AF by 49% in the general population and that the risk escalates in parallel with the body mass index.13 The association between AF and sleep apnea with or without coexisting obesity is also well recognized. Ongoing studies are attempting to discern whether sleep apnea is a causative factor for AF and whether aggressive management of sleep apnea could affect the occurrence of symptomatic AF episodes.14
Overt hyperthyroidism is a well-known cause of AF. Findings show that even subclinical hyperthyroidism may be a risk factor for AF.15,16 A study involving a group of elderly patients with normal thyroid-stimulating hormone levels found that an elevation in the concentration of free thyroxine was independently associated with AF.16
Structural heart disease is strongly associated with AF.17,18 Valvular heart disease has long been recognized as a risk factor for AF. One study found that the prevalence of AF in combination with rheumatic valvular disease varies from 16% (in association with isolated mitral regurgitation) to 70% (in association with mitral stenosis and other valvular disease).19 In patients with degenerative mitral valve regurgitation, AF is a marker of disease progression and is an independent factor that should be considered in the timing of mitral valve surgery.20,21 The existence of AF before mitral valve surgery (particularly mitral valve repair) negatively affects early and late survival rates.22 Approximately 5% of patients with aortic stenosis have poorly tolerated AF, and this condition may be the first sign of clinical decompensation that brings the diagnosis to light.23 Approximately 20% to 25% of patients with hypertrophic cardiomyopathy have AF, which is associated with an increased risk of death due to heart failure.24,25
Postoperative AF
Atrial fibrillation is common after cardiac surgery: postoperative AF occurs in as many as 40% of patients undergoing coronary artery bypass grafting or valve surgery and in as many as 60% of those undergoing combined bypass and valve surgery.25-27 Although the exact causes of postoperative AF are unknown, pericarditis, changes in autonomic tone, and large shifts in fluid volume are thought to contribute to its incidence. Preoperative prophylaxis with β-blockers, amiodarone, corticosteroids, and, more recently, lipid-lowering agents has generally reduced the incidence of postoperative AF.28-31
Autonomic Dysfunction
Intense study is currently being devoted to defining the role of the autonomic nervous system in causing AF. The vagal innervation of the atria is widespread and asymmetrical, and vagal stimulation shortens the refractory period to various extents in different parts of the atrium, thereby increasing the likelihood of AF.32,33 In certain patients, increased sympathetic tone brings on episodes of AF, and it is likely that sympathovagal balance also plays a role in initiating AF.34-36
Alcohol and Caffeine
Binge drinking of alcohol has been associated with AF, producing what is called the holiday heart syndrome. Observational studies have also found an association between sustained heavy use of alcohol and AF.3,37,38 Although patients are typically advised to moderate their caffeine intake, no association between caffeine and AF has yet been found. One observational study involving 47,949 patients found no association between caffeine consumption and AF or flutter.39
Inflammation
Inflammation may play an important role in both triggering and maintaining AF.40 Several studies have documented the association between elevated C-reactive protein concentrations and AF. In addition, left atrial dysfunction may occur with increased C-reactive protein concentrations even without AF.41-43 These findings imply that inflammation alone may affect left atrial function and give rise to electrical derangement.44
Renin-Angiotensin System
Recent findings suggest that the renin-angiotensin system plays an active role in the generation and maintenance of AF.45 Angiotensin II is a potent atrial fibrotic agent that secondarily increases intra-atrial pressures, thereby contributing to atrial stretch.46 An accumulating body of evidence shows that inhibiting the renin-angiotensin system protects atrial remodeling and promotes the maintenance of sinus rhythm.47,48
Genetic Factors
Framingham data show that 30% of patients with AF have a family history of AF.49 Most AF is probably due to polygenic inheritance or a combined effect of the number of genes. The first loci of familial AF was identified on chromosome 10q22-24 in 1997; since then, several loci have been mapped.50 Genes implicated in monogenic inheritance have been recently identified, including the KCNQ1 and SCN5A genes.51
Summary
Atrial fibrillation is best considered to be a symptom of a more widespread process. Common associations include diastolic ventricular dysfunction and aging. Whether aging alone or inflammation and autonomic imbalance are the primary “causes” of AF is currently under investigation.
DIAGNOSIS
Clinical Manifestation
The diagnosis of AF is generally straightforward. Typical symptoms include irregular palpitation of sudden onset, associated with fatigue. The clinical spectrum, however, is highly variable: some patients experience severe discomfort from palpitations, whereas others have absolutely no symptoms and AF is an incidental diagnosis.52 More difficult to evaluate are cases of chronic AF in which patients perceive no palpitation but report either episodic or worsening weakness, shortness of breath, or fatigue. It is difficult to assign a cause even to episodic AF in a patient with nonspecific symptoms because more than 17% of paients undergoing random continuous monitoring exhibit episodes of AF.53
Electrocardiography and Monitoring
Electrocardiography is the mainstay for diagnosing AF. Characteristic irregular fibrillatory waves at 350 to 600 pulses/min in the atrium in conjunction with irregular QRS complexes (90 to 170 beats/min) are diagnostic.54 Atrial flutter, which produces more regular sawtooth-type flutter waves, may paradoxically be associated with higher ventricular rates.
For the diagnosis of paroxysmal AF, some form of long-term monitoring is needed. In patients with relatively frequent or daily episodes, a 24- to 48-hour Holter monitor is sufficient. When palpitations or unexplained symptoms occur only rarely, 30-day event monitors or implantable recording devices can be used.55
WHY TREAT AF?
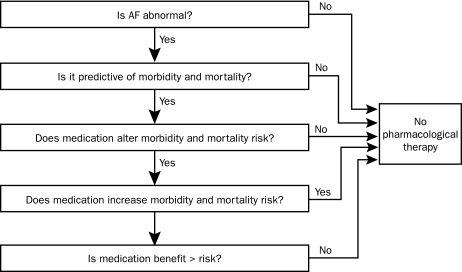
When counseling patients with AF, clinicians should consider such crucial questions as whether AF can be life threatening and what the long-term consequences of untreated AF may be (Figure 2). The longer AF is allowed to exist, the easier it becomes to reinduce and to sustain this arrhythmia. This concept that “AF begets AF” was first demonstrated in 1995 by an important and illustrative mechanistic study using animals.7
FIGURE 2.
Criteria for treating atrial fibrillation (AF). The primary criterion for treating AF is symptom relief. Currently, no conclusive evidence suggests that maintaining sinus rhythm decreases the rates of mortality or severe morbidity. This absence of evidence is probably due to the fact that minimal or no risk of mortality is associated with AF alone, that previously studied methods of maintaining sinus rhythm have been ineffective, and that both pharmacological therapy and invasive therapy are themselves associated with particular risks.
AF and Total Mortality
Despite the abundance of studies, a crucial but difficult question is whether AF is an independent risk factor for mortality in all patients. In the Framingham cohort, the odds ratio (OR) for total mortality (number of deaths per 100,000 population) was 1.5 for men and 1.9 for women with AF, even after the analysis was adjusted for age, overt heart disease, and other risk factors.56
Importantly, no current findings suggest that treating the arrhythmia contributes to a reduction in any potentially enhanced mortality rate. Therefore, patients cannot be counseled to undergo any form of rhythm control with the expectation of improving their chances of survival. The reasons for the absence of such findings may be that mortality rates associated with AF are not enhanced to begin with or that the proarrhythmic potential of antiarrhythmic agents is offset by the procedural risks associated with invasive therapies.
Thromboembolism and Stroke
Thromboembolic stroke may be associated with AF. The overall risk of thromboembolism is approximately 5% per year, although the risk associated with individual risk factors varies widely.57,58 The exact cause of the association between AF and thromboembolism is unknown and may in part be an epiphenomenon: that is, although most emboli probably originate from the left atrium, as many as one-fourth or more may not, such as embolism resulting from the carotid arteries.
Tachycardia-Mediated Cardiomyopathy
A small subset of patients with AF experience dilated cardiomyopathy (tachycardia-mediated cardiomyopathy). Typically, patients have rapid ventricular rates and relatively persistent AF. The prevalence has not been clearly determined, but a study involving a series of 673 patients with dilated cardiomyopathy found that only 1 case of AF was caused by tachycardia.59 Atrial fibrillation may not only cause the cardiomyopathy but may also exacerbate the symptoms of heart failure as a result of diastolic filling abnormalities and tachycardia-induced systolic dysfunction. Various cellular mechanisms have been implicated as causes of tachycardia-mediated cardiomyopathy, including the depletion of myocardial energy stores by chronic tachycardia, which causes oxidative stress and injury and leads to abnormal calcium handling and β-adrenergic responsiveness.54
Quality of Life
The primary reason for treating AF is to improve the patient's quality of life by decreasing the frequency and severity of AF-related symptoms. It has been documented that the quality of life of patients with AF is poor in comparison with that of healthy controls, the general population, and other patients with coronary artery disease.60 Both rate control and rhythm control improve quality of life, and most studies show that controlling both produces a somewhat greater benefit than controlling rate alone. However, the AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) trial found no difference in patients' quality of life, regardless of whether a rate or rhythm control strategy was used.61
It should be noted that younger patients, particularly those with paroxysmal AF and rapid ventricular rates, may exhibit symptoms that are difficult to manage with rate control approaches alone.
MANAGEMENT OF NEW-ONSET AF
Hemodynamic stability must first be assessed. A hemodynamically unstable patient should undergo emergency direct current cardioversion.62 Most patients, however, are relatively stable hemodynamically, and the goal of therapy for them is ventricular rate control with a target heart rate lower than 100 beats/min. Either oral or intravenous atrioventricular nodal blocking agents are used. Calcium channel blockers or β-blockers are the first-line agents, and digoxin is sometimes added. Diltiazem is generally preferable to verapamil because it is associated with fewer negative inotropic effects and less peripheral vasodilation. β-Blockers are more effective for patients with higher adrenergic tones, such as those with postoperative AF.
Some patients with infrequent but highly symptomatic acute episodes of AF can be managed without regular dosing of flecainide or propafenone. This so-called pill-in-the pocket63 is taken by the patient at the first sign of AF. This therapy should be initiated in a monitored setting so that the risk of proarrhythmia can be assessed.
For patients with acute AF that is sustained and continues to be symptomatic despite attempts at rate control, electrical or pharmacological cardioversion should be considered.64
Direct Current Cardioversion
Approximately one-half to two-thirds of patients with acute-onset AF will experience spontaneous conversion to sinus rhythm within 24 hours. If spontaneous conversion to a normal rhythm does not occur within this time period and the AF continues to cause symptoms, cardioversion should be considered. Electrical cardioversion is highly effective (90%) but requires sedation or general anesthesia. If the episode of AF is known to have persisted for less than 48 hours, the risk of stroke after cardioversion is low.65 In these cases, cardioversion can be performed without transesophageal echocardiography or oral anticoagulation. For cases in which the AF lasts longer, the risk of stroke after cardioversion is high (6%-7%), although adequate anticoagulation decreases this risk to less than 1%.65 Although direct current cardioversion with biphasic wave forms is highly effective in the conversion of AF, the AF commonly recurs. Patients should be told that cardioversion itself is not a treatment for AF and that recurrence should be expected at some point.
Pharmacological Cardioversion
Pharmacological cardioversion has the advantage of avoiding anesthesia but is less effective than electrical cardioversion.66 If administered within 24 hours of onset, class I or III antiarrhythmic drugs (AADs) can achieve cardioversion for 47% to 84% of patients. When the AF has persisted for more than 48 hours, drugs are effective for only 15% to 30% of patients.3
LONG-TERM MANAGEMENT OF AF
Although this review briefly discusses the choice of rate control or rhythm control and the guidelines for the use of antiarrhythmic agents, it focuses primarily on the crucial issue of stroke prevention and the increasingly important role of radiofrequency ablation.
Preventing Stroke in Patients With AF
Preventing thromboembolic complications, especially stroke, is a primary goal of AF treatment. Atrial fibrillation is associated with a nearly 5-fold increase in the risk of stroke.67,68 The Framingham study found that the age-adjusted 2-year incidence of symptomatic stroke was 4.8% among patients with AF and 1% among patients without AF.67 For octogenarians, nearly one-fourth of all strokes are attributable to AF.67 Strokes tend to be more severe and disabling for patients with AF than for those without AF.69,70
Mechanisms of Stroke. Multiple pathophysiologic mechanisms contribute to the link between AF and stroke. Atrial fibrillation impairs atrial contraction, and this impairment in turn promotes blood stasis in the left atrium.71 In addition, AF is associated with a hypercoagulable state in which the plasma concentration of fibrinopeptide A is elevated and that of antithrombin III is decreased.72,73 Taken together, stasis and hypercoagulability can lead to atrial thrombus formation, particularly in the left atrial appendage.74 Left atrial thrombus can then break free of the left atrium and embolize to the brain, thereby resulting in an ischemic stroke. The presence of a thrombus in the left atrial appendage is associated with a 3-fold increase in the risk of stroke.74
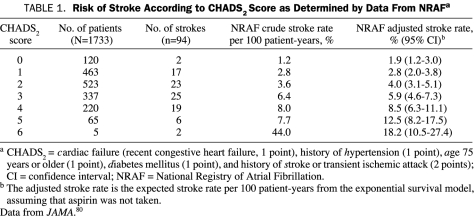
Determining the Clinical Risk of Stroke. The risk of stroke varies widely among patients with AF, depending on the presence or absence of several risk factors.57,75-83 Risk factors that put AF patients at high risk of future stroke include rheumatic mitral valve disease, the presence of a mechanical heart valve, and prior thromboembolism.78,80 Among patients with so-called nonvalvular AF (no rheumatic mitral valve disease and no mechanical heart valve), the risk factors for stroke can be conveniently remembered by using the mnemonic CHADS2, which stands for cardiac failure (recent congestive heart failure), history of hypertension, age 75 years or older, diabetes mellitus, and history of stroke or transient ischemic attack (TIA)80 (Table 1).
TABLE 1.
Risk of Stroke According to CHADS2 Score as Determined by Data From NRAFa
CHADS2 also refers to a scoring system designed to estimate the risk of stroke among patients with AF.80 The CHADS2 scoring system was derived from a study involving 1733 Medicare beneficiaries and was subsequently validated by a larger study involving 11,526 patients enrolled in an integrated health care system.80,81 The CHADS2 score system provides an estimate of a patient's risk of stroke. The system assigns 1 point each for cardiac failure (recent congestive heart failure), history of hypertension, age 75 years or older, and diabetes and assigns 2 points for a history of stroke or TIA (thus the inclusion of the number 2 in the mnemonic). The sum of the points determines the CHADS2 score. The adjusted annual stroke rate increases from 1.9% for patients with a CHADS2 score of 0 to 18.2% for patients with a CHADS2 score of 6.
Pharmacological Prevention of Stroke. Several randomized trials have assessed the ability of warfarin or aspirin to reduce the risk of AF-associated stroke.84-91 Hart et al82 pooled the data from these trials to compare the effectiveness of adjusted-dose warfarin vs placebo, aspirin vs placebo, and adjusted-dose warfarin vs aspirin for the prevention of stroke among patients with AF. This meta-analysis showed that adjusted-dose warfarin is remarkably effective in reducing the risk of stroke for patients with AF.92 Compared with placebo, adjusted-dose warfarin decreased the relative risk of stroke by 62% (95% confidence interval [CI], 48%-72%).82 The absolute reduction of stroke risk by adjusted-dose warfarin vs placebo was 2.7% annually for primary prevention and 8.4% annually for secondary prevention. The rate of cerebral hemorrhage was 0.3% per year for patients receiving adjusted-dose warfarin and 0.1% per year for patients receiving placebo. Adjusted-dose warfarin also decreased all-cause mortality rates by 26% (95% CI, 4%-43%) in relative terms and by 1.6% in absolute terms.
Aspirin is generally less effective than warfarin in reducing the risk of AF-associated stroke.82 An analysis of pooled data from randomized trials comparing antiplatelet therapy (mainly aspirin) and placebo for the prophylaxis of AF-associated stroke showed that aspirin was associated with a relative reduction of the risk of stroke of 22% (95% CI, 2%-38%).82 The absolute reduction of stroke risk by aspirin vs placebo was 1.5% annually for primary prevention and 2.5% annually for secondary prevention. Aspirin use was not associated with a statistically significant reduction in all-cause mortality. The direct comparison of adjusted-dose warfarin and aspirin showed that adjusted-dose warfarin decreased the relative risk of stroke by 36% (95% CI, 14%-52%).82 The use of warfarin, however, was associated with a 2.1-fold higher relative risk of intracranial hemorrhage than the use of aspirin. This analysis found no statistically significant difference in survival rates between patients who received warfarin and those who received aspirin.
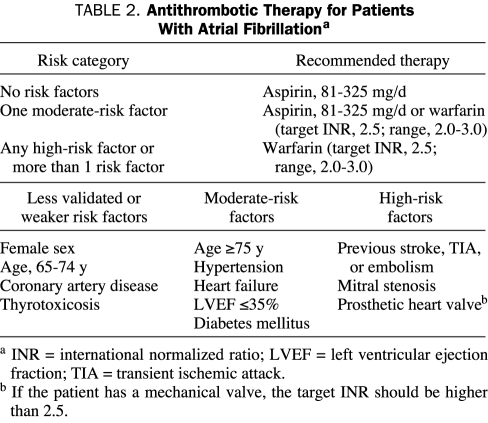
Which patients should receive aspirin and which should receive oral anticoagulation (warfarin) for long-term prophylaxis against AF-associated thromboembolism? The guidelines of the American College of Cardiology, the American Heart Association, and the European Society of Cardiology for administering antithrombotic therapy to patients with AF are summarized in Table 2.3 In general, patients with no risk factors for stroke (a CHADS2 score of 0)80 should take 81 to 325 mg/d of aspirin. Patients with 1 moderate risk factor for stroke (a CHADS2 score of 1) should take either 81 to 325 mg/d of aspirin or adjusted-dose warfarin with a target international normalized ratio (INR) of 2.5 (range, 2.0-3.0). Patients with risk factors that confer a high risk of stroke (a previous stroke or TIA, rheumatic mitral stenosis, or a CHADS2 score of 2 or higher) should take adjusted-dose warfarin with a target INR of 2.5 (range, 2.0-3.0). Because patients with hypertrophic cardiomyopathy and AF have relatively high rates of thromboembolic complications, anticoagulation with adjusted-dose warfarin (target INR, 2.5; range, 2.0-3.0) should also be strongly considered for these patients.3,25 The intensity of anticoagulation for patients with AF and a mechanical heart valve depends on the type of valve, but the target INR is usually at least 2.5.3
TABLE 2.
Antithrombotic Therapy for Patients With Atrial Fibrillationa
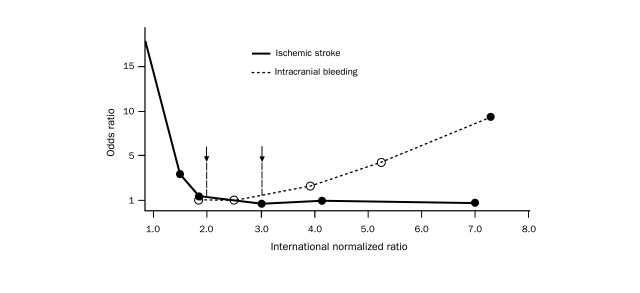
Goal INR. Maintaining INR84 levels within a range of 2.0 to 3.0 minimizes the incidence of both ischemic and hemorrhagic stroke3,93,94 (Figure 3). The crucial relationship between low INR levels (below 2.0) and an increased risk of ischemic stroke was confirmed by the results of the AFFIRM trial: 72% of the 157 patients who experienced ischemic strokes during the trial had INR levels below 2.0 near the time of the strokes.96 For patients with contraindications to warfarin therapy, 81 to 325 mg/d of aspirin should be considered for the prophylaxis of thromboembolism.3 A target INR of 3.0 (range, 2.5-3.5) may be appropriate for patients at very high risk of thromboembolism or for those who experience thromboembolism even with an INR of 2.0 to 3.0.3
FIGURE 3.
Odds ratios for stroke and intracranial bleeding according to the international normalized ratio (INR) for patients with atrial fibrillation. The risk of ischemic stroke is higher for patients with an INR lower than 2, and the risk of intracranial bleeding is higher when the INR is higher than 3 (arrows). Adapted from Ann Intern Med,95 with permission.
Antithrombotic Therapy: Clinical Pearls and Special Circumstances. Several facts about long-term antithrombotic therapy for patients with AF should be kept in mind. First, atrial flutter should be treated in the same manner as AF with regard to antithrombotic therapy.3 This recommendation is based on the recognition that the stroke risks associated with atrial flutter and AF are comparable and that AF commonly develops in patients with atrial flutter.97,98
Second, the pattern of AF (paroxysmal, persistent, or permanent) generally does not influence the method or degree of antithrombotic treatment for AF.3 The Stroke Prevention in Atrial Fibrillation study found that the annual stroke rate for patients with intermittent AF (3.2%) was very similar to that for patients with sustained AF (3.3%).6
Third, AADs do not reduce the need for long-term antithrombotic therapy aimed at preventing stroke. The AFFIRM trial found no statistically significant difference in stroke rate between patients treated with rate control (who were predominantly in AF) and those treated with AADs (who were predominantly in sinus rhythm).96 Most of the ischemic strokes in the rhythm control arm of AFFIRM occurred after warfarin was discontinued.96 Strokes are not unexpected among patients with seemingly successful restoration of sinus rhythm given the fact that asymptomatic AF is common.99,100
Fourth, although left atrial ablation may eliminate symptomatic AF, long-term antithrombotic therapy should still be strongly considered after ablation. An observational study of postablation thromboembolic events raised the possibility that patients with risk factors for stroke may be able to forgo long-term anticoagulation after ablation.101 Nonetheless, asymptomatic AF has been documented in patients who have undergone apparently successful left atrial ablation.99 As a result, we generally base our recommendations regarding the need for postablation long-term antithrombotic therapy on well-validated risk stratification methods such as CHADS2.81
Fifth, the combination of aspirin and clopidogrel should not be considered an adequate substitute for warfarin-based anticoagulation for patients with AF. The risk of stroke is 1.72-fold higher for patients taking aspirin plus clopidogrel than for those taking adjusted-dose warfarin.102
Sixth, the addition of antiplatelet therapy to adjusted-dose warfarin for patients with AF should be approached cautiously. Using aspirin in addition to warfarin has been found to increase elderly patients' absolute risk of serious bleeding by 0.6% during a 90-day observation period and to increase their relative risk of intracranial hemorrhage by a factor of 2.4.92,103 For patients with chronic stable coronary artery disease and AF, adjusted-dose warfarin without aspirin is probably sufficient.3,104 Observational data suggest that the combination of aspirin, clopidogrel, and warfarin may be associated with a high risk of bleeding.105,106 Antithrombotic therapy for patients with a drug-eluting coronary stent and AF should be individualized, with consideration given to the use of adjusted-dose warfarin plus clopidogrel without aspirin.3,106
Anticoagulation and Cardioversion. The risk of thromboembolism is elevated during the first several days after cardioversion from atrial flutter or AF to sinus rhythm. Cardioversion resulting from electric shock, AAD therapy, pacing, or ablation, as well as spontaneous cardioversion, can result in transient atrial stunning or loss of atrial contraction.107-109 This stunning after cardioversion may persist for days or weeks.110,111 Stunning may promote atrial blood stasis and thrombus formation, which, in turn, can lead to stroke.112-114 Cardioversion may also cause a stroke by dislodging a preexisting thrombus in the left atrial appendage. Most strokes and other thromboembolic episodes after cardioversion occur within the first 10 days.114 In the absence of anticoagulation, the rate of cardioversion-associated thromboembolism may exceed 5%.115 Anticoagulation decreases the rate of cardioversion-associated thromboembolism to less than 1%.116,117 As a result, patients with AF that has persisted for more than 48 hours or for an unknown duration should receive anticoagulation therapy (goal INR, 2.0-3.0) for 3 weeks before and 4 weeks after cardioversion.3 Alternatively, anticoagulation with heparin (to an activated partial thromboplastin time 1.5 to 2 times higher than normal) can be initiated at the time of transesophageal echocardiography; if no intracardiac thrombus is identified, cardioversion can be performed with heparin bridging118,119 until an INR of 2.0 has been obtained. At that point, heparin should be discontinued and warfarin therapy should be continued for 4 weeks with a target INR of 2.5 (range, 2.0-3.0).3,118-120 If transesophageal echocardiography shows atrial thrombus, patients should undergo anticoagulation with heparin bridging118,119 to warfarin therapy for 4 weeks with a target INR of 2.0 to 3.0 before cardioversion is attempted.
In general, patients with atrial flutter should undergo treatment similar to that for patients with AF with regard to anticoagulation near the time of cardioversion.3,121
For every patient with AF, the thromboembolic risk should be assessed by the CHADS2 score, and the appropriate antithrombotic approach should be initiated. Importantly, the coexisting risk factors are at least as important as the presence of AF itself in defining stroke risk. Furthermore, rhythm control strategies should be undertaken for symptom management with the understanding that they will have no effect on the risk of stroke.
Rate Control or Rhythm Control for Symptom Management
Historically, it was assumed that maintaining sinus rhythm was better than controlling ventricular rates. This assumption was based on the theory that this practice would lead to more normal hemodynamics and a lower risk of thromboembolism. The landmark AFFIRM study and other key trials have definitively shown that, particularly among older patients, outcomes are not significantly different when rhythm vs rate is controlled.96 We examined mortality data from 6 recent clinical trials involving 3303 patients assigned to rate control and 3312 patients assigned to rhythm control; this analysis included data from the 2007 AF-CHF (Atrial Fibrillation and Congestive Heart Failure) trial.96,122-125 A comparison of the 2 groups demonstrated a relative risk of mortality of 0.95 (95% CI, 0.86-1.05) for the patients assigned to rate control (Figure 4).
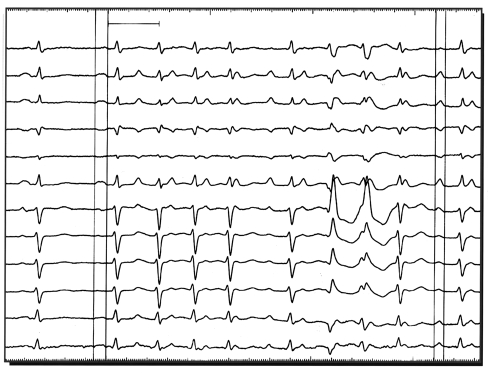
FIGURE 4.
Most antiarrhythmic medications have a proarrhythmic potential. Pictured here is the proarrhythmia of concern when potassium channel blockers such as sotalol are used. Cause-dependent premature ventricular contractions triggering polymorphic ventricular tachycardia are seen.
Subset analysis of the data from the AFFIRM trial has shown that rhythm control yields some benefit for younger patients with symptomatic AF. Although the decision to initiate rhythm control must be individualized for each patient, younger patients with severe symptoms and comorbid conditions generally appear to benefit more from rhythm control than from rate control alone.
Rate control should always be attempted first. Even when rhythm control is preferable, ventricular rates should be controlled because breakthrough AF should be expected. Generally, rate control is safe and effective and is sufficient for most patients. β-Blockers are the typical first-line agents, especially if other comorbid conditions (eg, coronary artery disease) necessitate their use. Calcium channel blockers and digoxin, as previously described, are also used in selected situations. If adequate rate control is obtained but symptoms persist, either from the arrhythmia itself or from the adverse effects of the rate-controlling medication, rhythm control should be attempted.
Pharmacological Therapy for Maintaining Sinus Rhythm
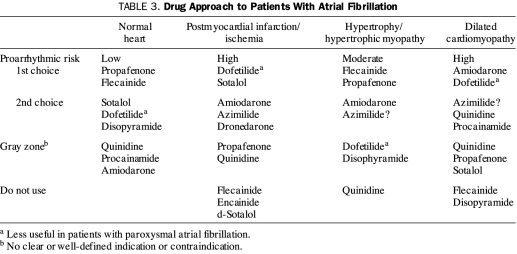
The primary goal of AAD therapy is to improve quality of life of patients with AF that remains symptomatic despite attempts at rate control (Table 3). Currently, the overall effectiveness of available AADs in maintaining long-term sinus rhythm is poor. The CACAF (Catheter Ablation for the Cure of Atrial Fibrillation) trial involved patients with paroxysmal or persistent AF for whom 2 previous AADs had failed. These patients were randomly assigned to catheter ablation or a control group. Of the 69 patients in the control group who had started AAD therapy, 63 (91.3%) experienced a recurrence of AF within 12 months.126 The A4 (Catheter Ablation Versus Antiarrhythmic Drugs for Atrial Fibrillation) study127 and the APAF (Ablation for Paroxysmal Atrial Fibrillation) trial also randomly assigned patients with AF for whom AAD therapy had failed to ablation or another AAD.128 The results were similar to those of the CACAF trial: of the patients treated with AAD, 94% of those in the A4 study and 78% of those in the APAF trial experienced a recurrence of AF within 1 year. A recent systematic review analyzed data from 11,322 patients with AF enrolled in 44 clinical trials of AADs vs placebo or another AAD. The 1-year pooled AF recurrence rate was high: 71% to 84% for control patients and 44% to 67% for treated patients.129 Amiodarone was the most effective agent for preventing recurrent AF (OR, 0.31 compared with all class I drugs; OR, 0.30 compared with sotalol).129 No important differences in efficacy or safety were detected for the other antiarrhythmic agents studied.
TABLE 3.
Drug Approach to Patients With Atrial Fibrillation
In addition to their lack of effectiveness, antiarrhythmic medications may be associated with substantial toxicity. In a recent systematic review, 9% to 23% of patients taking AADs withdrew from clinical trials because of adverse effects.129 Significantly fewer patients taking amiodarone withdrew from trials (OR, 0.52; P=.004), but this agent was associated with a number of toxic effects.
The primary determinant of the risk of proarrhythmia with AAD therapy is the presence of structural heart disease. Flecainide and propafenone can be used safely to treat patients with normal ventricular function and no coronary disease.130 For those with systolic heart failure, amiodarone or dofetilide is preferred. The landmark Cardiac Arrhythmia Suppression Trial (CAST) showed that the mortality rate associated with flecainide administration was higher for patients with structural heart disease.131 It should be noted that in this trial flecainide was used to suppress ventricular ectopy in patients with coronary artery disease. Nevertheless, on the basis of such findings, class IC agents are used for patients with no coexisting heart disease. Although amiodarone is associated with a lower risk of proarrhythmia than flecainide, its use is limited by serious extracardiac organ toxicity. Dofetilide can be administered to patients with systolic dysfunction, but its use requires inpatient observation, careful dose initiation and titration, and monitoring of the QT interval. Neither dofetilide nor sotalol can be administered to patients with serious renal dysfunction. For patients with left ventricular hypertrophy, dofetilide and sotalol are relatively contraindicated, and amiodarone is preferred. Dofetilide is less useful for patients with paroxysmal AF.
New antiarrhythmic agents are being actively investigated. Dronedarone is a noniodinated amiodarone derivative that produces similar cardiac effects but with less toxicity132 because the iodine moiety of amiodarone is thought to be responsible for many of its adverse effects. Dronedarone has been shown to be more effective than placebo in preventing the recurrence of AF,133 although the Antiarrhythmic Trial With Dronedarone in Moderate-to-Severe Congestive Heart Failure Evaluating Morbidity Decrease (ANDROMEDA) was stopped prematurely because of increased mortality rates among patients with heart failure who were assigned to the dronedarone arm. Tedisamil, another new AAD that was initially developed to treat ischemia by potassium channel blockade, is a class III antiarrhythmic agent that has been shown to be superior to placebo in converting AF to sinus rhythm. The drug may be proarrhythmic: 2 (1.8%) of 175 patients experienced self-terminating ventricular tachycardia.134,135 Blockers of specific atrial channels, such as IKACh and IKur, should produce no ventricular proarrhythmic adverse effects and are being actively investigated.136,137
Several nontraditional (not typically thought of as AADs) medications have been shown to be effective in preventing AF. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers have been found to prevent AF in patients with left ventricular hypertrophy or heart failure.138 Lipid-lowering agents (statins) have also been shown to decrease the incidence of AF for patients with coronary artery disease and left ventricular dysfunction.30 Fish oil may reduce the incidence of AF by altering the composition of the atrial myocyte membrane.139 Another strategy targeting the inflammatory response that seems to be associated with AF is the administration of corticosteroids.29,140
Nonpharmacological Management of AF
Because of the limitations of pharmacological therapy in managing AF, several nonpharmacological approaches have been tried during the past 2 decades. These approaches include pacemakers with specific algorithms and lead placement, atrial cardioverter-defibrillators, the surgical maze procedure, and radiofrequency ablation. Device (pacemaker and cardioverter-defibrillator) therapy for AF is infrequently used because of poor results in improving the symptoms of AF. Although the surgical maze procedure is very effective in preventing the recurrence of AF, the invasive nature of this procedure and some unanswered questions about the normalization of atrial function have rendered this therapy an uncommon choice for the initial nonpharmacological treatment of AF.
This review primarily discusses catheter ablation for AF, which has become an important option for treating patients with drug-refractory symptomatic AF. We have structured the discussion to provide specific information to nonspecialists who are advising or examining a patient with AF.141,142
CARDIAC ABLATION FOR AF
Catheter ablation either heats (radiofrequency) or cools (cryothermy) the atrial myocardium to eliminate the arrhythmogenic tissue and thereby decrease the risk of AF.
During the past decade, the use of radiofrequency ablation to treat patients with symptomatic AF has increased greatly.143 Two reasons account for this important advance. First, AF is not a homogeneous disorder: it includes paroxysmal forms, in which a distinct trigger may be identified, and chronic forms, in which the atrial myocardial substrate is normal. Second, the thoracic veins play an important role in initiating AF (Figure 5).144 For many decades, AF was thought to be a result of meandering wavelets of reentry that themselves result from a diseased atrial myocardium.145,146 Although this is still relevant for persistent AF, in the past decade distinct triggers for AF have been found to arise from the myocardium of the pulmonary veins and other thoracic veins8,147-149 (Figure 6).
FIGURE 5.
Although the electrocardiography of atrial fibrillation is generally straightforward, the onset of arrhythmia (premature atrial contractions or monomorphic atrial tachycardia) should be determined because of the importance of triggers for atrial fibrillation. Such a determination may help guide physicians in counseling patients about atrial fibrillation ablation.
FIGURE 6.
Paroxysmal atrial fibrillation (AF) can be a very different disease from permanent or chronic AF. Triggers are important for initiating paroxysmal AF, whereas substrate abnormalities are necessary for maintaining permanent AF. Although the natural history of paroxysmal AF is not exactly known, many patients will progress to the more permanent form.
Pulmonary Vein Isolation
The most common type of ablation for AF involves electrical isolation of a pulmonary vein (Figure 7). The pulmonary veins are electrically active structures with a sleeve of syncytial myocardium that extends from the atrium into the vein.150 Electrical isolation involves placing a series of circumferential ablation lesions into the left atrium around the ostia of the pulmonary veins. Such circumferential isolation electrically disconnects the pulmonary veins from the rest of the atria. The rationale for this procedure is that electrical isolation of the veins renders the AF triggers that arise from those veins incapable of initiating arrhythmia in the atria.
FIGURE 7.
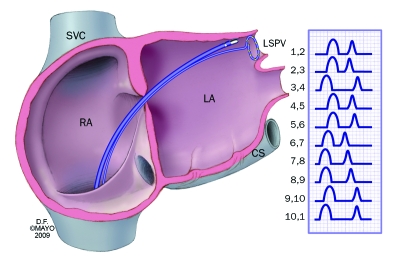
Pulmonary vein isolation is currently the most common ablation technique used for atrial fibrillation. The typical placement of a circular mapping catheter within the pulmonary vein is shown. Importantly, the ablation catheter and the delivery of energy are placed outside the vein within the left atrium. Also pictured are the characteristic intracardiac electrograms from an arrhythmogenic vein. After successful isolation of the vein, these electrographic abnormalities will disappear. CS = coronary sinus; LA = left atrium; LSPV = left superior pulmonary vein; RA = right atrium; SVC = superior vena cava.
During the typical pulmonary vein isolation procedure, a mapping catheter is placed into the pulmonary vein, and the characteristic abnormal signals from an arrhythmogenic vein are documented. The end point for ablation around that vein is the abolition of these abnormal signals, which signifies electrical disconnection of the vein from the atrium.
Nonpulmonary Vein Triggers
Not all initiators of AF arise from the pulmonary veins; any intrathoracic vein can potentially serve this function. The other common trigger sites are the myocardium that extends both into the vein of Marshall and into the superior vena cava. The vein or ligament of Marshall in the adult is a remnant of the left superior vena cava, which is present in the developing fetus but typically regresses before birth.141,142,151 These venous structures can also be electrically isolated using a procedure similar to that described for the pulmonary veins.152
Substrate-Based Ablation Approaches
Ablation targeting the triggers of AF is the most common approach, but it is primarily effective for patients with paroxysmal AF and normal atria (substrate). Ablation is of minimal value for patients with the AF triggers of cardiac disease; diastolic dysfunction; valvular disease; or enlarged and abnormal, possibly fibrotic, atria. For these patients, relief is provided by various approaches aimed at ablating the abnormal substrate.
The most common of these evolving ablation techniques is linear ablation, which anchors the pulmonary vein isolation circle to other ablation sites or to the mitral valve. The goal of this linear ablation is similar to that of the surgical maze procedure: preventing the development of macroreentrant left atrial flutter. In addition, this substrate reduction (again as in the maze procedure) may decrease the likelihood that AF will develop or be sustained.
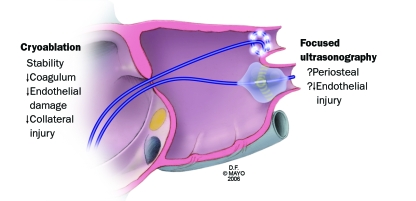
Another ablative approach targets high-frequency left atrial activity that manifests itself by the recording of multiple very rapid fractionated electrograms. Ablation has been performed at these characteristic sites.153 Currently, it is unknown whether this ultrarapid activity results from areas of abnormal conduction, intrinsic rotors, neural activity, or microreentry.154 Newer energy sources, including cryoablation and focused ultrasonography, may potentially facilitate venous isolation along with local substrate modification and transmural ablation of epicardial innervation (Figure 8).
FIGURE 8.
Newer techniques for atrial fibrillation ablation include the use of cryoablation rather than the thermal injury from radiofrequency energy. Balloon catheters and focused ultrasonography are also being investigated to facilitate faster, more effective, and safer left atrial ablation for atrial fibrillation. ↓ = decreased;? = potential.
An exciting new approach targets the action of the atrial parasympathetic and sympathetic nerves.33,155,156 The ganglionated plexuses are located along the posterior and superior portions of the left atrium. Recently, clinicians have adopted the use of ablation at these locations with local stimulation for identifying the presence of these ganglia.33,155 Catheter ablation at these sites alters parasympathetic activity, as reflected by changes in heart rate variability,128,157 and may decrease the ability of the atria to maintain AF.
Success Rates
Early reports describe success rates of 22% to 85% for ablation as a treatment for AF; relatively higher success rates are achieved for patients with paroxysmal AF.158,159 Long-term results are limited because follow-up has generally been no longer than 1 year in randomized trials comparing the effectiveness of pulmonary vein isolation and drug therapy for patients with symptomatic AF. The reported success rates with this limited follow-up period ranged from 31% to 88%; neither strategy was clearly superior.160,161 Generally, patients are informed that the success rate is approximately 60% to 70%, that approximately 10% to 40% of patients require a second procedure, and that 10% to 15% require continued antiarrhythmic therapies. These results are based on average outcome reports from multiple studies.8,147,162-166
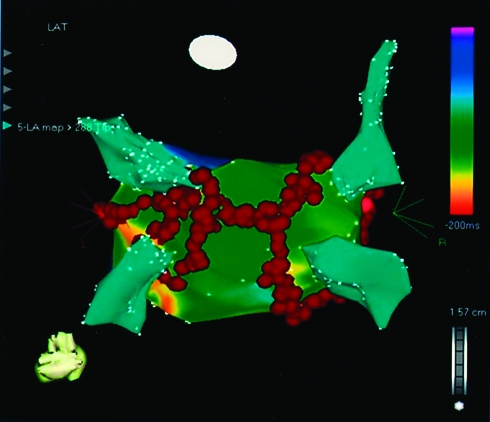
Available findings also indicate that ablation is less effective for chronic AF than for paroxysmal AF. Several studies have reported these lower success rates even with more aggressive ablation paradigms.167-169 Recently, the use of extensive linear ablation aimed at organizing and subsequently eliminating chronic AF achieved slightly better results but with a limited follow-up period168,169 (Figure 9).
FIGURE 9.
Current ablation approaches for patients with persistent atrial fibrillation include pulmonary vein isolation and linear ablation in the atrium, which connects ablation sites from the vein with each other and with the mitral annulus (red dots). Thus, a combined approach aimed at modifying abnormal substrate and eliminating common triggers is used. Linear ablation is primarily performed to prevent macroreentrant atrial tachycardia, including atypical atrial flutter.
Thus, when counseling patients, clinicians must emphasize the fact that long-term cure rates are currently unknown but that approximately 60% to 70% of patients experience marked reduction or elimination of AF. Multiple single-center studies have shown substantial improvement in patients' quality of life after primary ablative intervention.170-172 Several comparative studies found that quality of life after ablation is comparable with that of healthy persons not undergoing ablation.
Although much information is available about the effectiveness of ablation for individual patients, findings about the cost-effectiveness of this intervention are just beginning to emerge. A French study has shown that the cost of ablation is lower than that of pharmacological intervention.173 However, this finding may not apply to the US health care system.
Complications
Serious complications may occur as a direct result of AF ablation procedures. Many of these complications are similar to those associated with other catheter-based interventions, such as infection, bleeding, hematoma, deep venous thrombosis, pneumothorax, and arterial damage.165 Fortunately, more serious complications are rare: early studies show a rate of 0.5% to 1.0% for air emboli, bradycardia, tamponade, and stroke or TIA. These numbers have been confirmed by the more recent International AF Ablation Registry.143
Some complications, however, are relatively specific to AF ablation procedures. These include pulmonary vein stenosis, thromboembolic events, and the formation of atrial esophageal fistula.
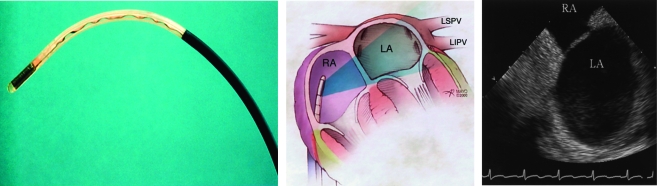
Pulmonary Vein Stenosis. Ablation within a pulmonary vein can lead to fibrosis and narrowing or occlusion of the vessel.174,175 Studies conducted before 2002 documented rates of pulmonary vein stenosis ranging from 4% to 10%.176-178 If the ostium of the pulmonary vein is clearly recognized and ablation within the pulmonary vein is avoided, this complication is rare (Figure 10). Symptoms of stenosis include shortness of breath and, in severe cases, hemoptysis or pulmonary hypertension. Symptoms decrease over time; when they are severe, pulmonary vein dilation or stenting can be performed.174,179
FIGURE 10.
Intracardiac ultrasonography has substantially facilitated radiofrequency ablation procedures. Left, Linear phased array probe. Middle, Placement of the probe in the right atrium (RA) allows visualization of the left atrial pulmonary vein orifices (right) without the need for placing another catheter in the left atrium (LA) itself. LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein.
Thromboembolic Events. Available studies document a rate of stroke ranging from 0.5% to 2.0% with ablative intervention; this rate is similar to that associated with other procedures currently used to treat AF.143,165 Early heparinization, the use of intracardiac ultrasonography, and aggressive anticoagulation regimens appear to have reduced the incidence of this complication.180
Left Atrial-Esophageal Fistula. A potentially catastrophic consequence of AF ablation is the formation of a fistula between the posterior left atrium and the esophagus. This complication was first reported in 2004; since then, a number of similar cases have been reported.181-184 This fistula produces a serious syndrome with fevers, odynophagia, gastrointestinal bleeding, and central nervous system manifestations, which are in part related to air emboli, as well as high fatality rates. Patients occasionally survive when surgery or another invasive intervention is performed promptly.181,185
When Ablation Fails to Control Symptoms
As previously noted, although AF ablation is an important advance in the management of patients with symptomatic AF, success is far from sure: at least 30% to 40% of patients continue to experience symptoms after a single procedure. The current treatment approaches for this patient group include another attempt at AF ablation, the combination of ablation with an antiarrhythmic therapy that may have failed previously, and the adjunctive use of antitachycardia pacing devices to terminate atrial flutter, which may persist after AF ablation.
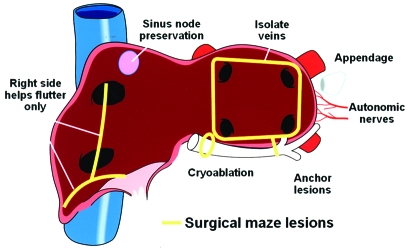
The surgical maze procedure is still the most effective invasive therapy for AF ablation (Figure 11). Because of its invasive nature, and because recently developed techniques involve simpler procedures, it is rarely adopted as a first-line treatment. Patients with symptomatic chronic AF for whom medical and ablation attempts have failed may be considered for this treatment option.
FIGURE 11.
Atrial fibrillation (AF): lessons from the operating room. Current endovascular AF ablation has in many ways benefitted from the surgical experience with managing AF. Isolating the pulmonary veins, anchoring lesions (linear ablation), and understanding the role of eliminating the left atrial appendage or modifying the autonomic nerves are lessons learned directly from surgical experience.
Patient Selection for AF Ablation
For patients who continue to exhibit symptoms after an attempt at controlling ventricular response rates, interventions aimed at maintaining sinus rhythm should be attempted. If at least 1 attempt at AAD therapy has failed (intolerance or inefficacy), AF ablation is typically recommended. The ideal patient is a young and otherwise healthy person without structural heart disease who has paroxysmal AF. The success rates associated with ablation for such patients are substantially higher than those associated with ablation for patients with chronic AF and structural heart disease. For paroxysmal AF, if monitoring has shown a reproducible incitation of AF episodes with a single premature beat, the success rate of pulmonary vein isolation is likely to be high.
Additional trials are necessary to assess the effect of ablation on long-term outcomes. The Radiofrequency Ablation for Atrial Fibrillation Trial (RAAFT), which is currently under way, will better assess the late recurrence rates of AF.127 The effect of ablation on mortality rates, quality of life, and health care costs will probably be better established during the coming years. The CABANA (Ablation vs Drug Therapy for Atrial Fibrillation) pilot study, which is currently under way, will be followed by a 3000-patient CABANA mortality trial, in which 1500 patients older than 65 years or younger than 65 years but with other risk factors for stroke will be randomly assigned to drug therapy, and an additional 1500 such patients will be randomly assigned to ablative intervention. This study will probably require approximately 6 years for completion but should provide important information about the effectiveness, safety, and long-term consequences of current ablative therapies.
CONCLUSION
Atrial fibrillation is the most common arrhythmia seen in clinical practice; therefore, all health care professionals should be familiar with the appropriate management of AF. The past decade has witnessed important changes in the management of symptomatic AF with the advent and increasing adoption of invasive therapies, specifically AF ablation. We have presented an approach that should aid the nonspecialist in recognizing which patients are likely to benefit from newer therapies and in understanding the rationale, techniques, success rates, and limitations of ablation that are pertinent to the appropriate counseling of patients with AF.
Supplementary Material
On completion of this article, you should be able to (1) assess patients with their first onset of atrial fibrillation and manage recurrent episodes, (2) place patients in categories on the basis of their risk of stroke and define the best anticoagulation management option for each patient, and (3) determine which patients may benefit from an invasive ablation-based approach for the management of atrial fibrillation.
This activity was designated for 1 AMA PRA Category 1 Credit(s).™
The contributions to the Symposium on Cardiovascular Diseases are now a CME activity. For CME credit, see the link on our Web site at mayoclinicproceedings.com.
REFERENCES
- 1.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285(18):2370-2375 [DOI] [PubMed] [Google Scholar]
- 2.Jahangir A, Lee V, Friedman PA, et al. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: a 30-year follow-up study. Circulation 2007;115(24):3050-3056 Epub 2007 Jun 4. [DOI] [PubMed] [Google Scholar]
- 3.European Heart Rhythm Association. Heart Rhythm Society. Fuster V, Rydén LE, Cannom DS, et al. American College of Cardiology. American Heart Association Task Force on Practice Guidelines. European Society of Cardiology Committee for Practice Guidelines. Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation) [published correction appears in J Am Coll Cardiol. 2007;50(6):562] J Am Coll Cardiol. 2006;48(4):854-906 [DOI] [PubMed] [Google Scholar]
- 4.Al-Khatib SM, Wilkinson WE, Sanders LL, McCarthy EA, Pritchett EL. Observations on the transition from intermittent to permanent atrial fibrillation. Am Heart J. 2000;140(1):142-145 [DOI] [PubMed] [Google Scholar]
- 5.Kerr CR, Humphries KH, Talajic M, et al. Progression to chronic atrial fibrillation after the initial diagnosis of paroxysmal atrial fibrillation: results from the Canadian Registry of Atrial Fibrillation. Am Heart J. 2005;149(3):489-496 [DOI] [PubMed] [Google Scholar]
- 6.Hart RG, Pearce LA, Rothbart RM, McAnulty JH, Asinger RW, Halperin JL, Stroke Prevention in Atrial Fibrillation Investigators Stroke with intermittent atrial fibrillation: incidence and predictors during aspirin therapy. J Am Coll Cardiol. 2000;35(1):183-187 [DOI] [PubMed] [Google Scholar]
- 7.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation: a study in awake chronically instrumented goats. Circulation 1995;92(7):1954-1968 [DOI] [PubMed] [Google Scholar]
- 8.Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339(10):659-666 [DOI] [PubMed] [Google Scholar]
- 9.Aidietis A, Laucevicius A, Marinskis G. Hypertension and cardiac arrhythmias. Curr Pharm Des. 2007;13(25):2545-2555 [DOI] [PubMed] [Google Scholar]
- 10.Hennersdorf MG, Schueller PO, Steiner S, Strauer BE. Prevalence of paroxysmal atrial fibrillation depending on the regression of left ventricular hypertrophy in arterial hypertension. Hypertens Res. 2007;30(6):535-540 [DOI] [PubMed] [Google Scholar]
- 11.McCarthy PM, Kruse J. Atrial fibrillation in patients with coronary disease. J Interv Card Electrophysiol. 2007;20(30):113-117 [DOI] [PubMed] [Google Scholar]
- 12.Budaj A, Flasinska K, Gore JM, et al. GRACE Investigators Magnitude of and risk factors for in-hospital and postdischarge stroke in patients with acute coronary syndromes: findings from a Global Registry of Acute Coronary Events. Circulation 2005;111(24):3242-3247 Epub 2005 Jun 13. [DOI] [PubMed] [Google Scholar]
- 13.Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity—results of a meta-analysis. Am Heart J. 2008;155(2):310-315 Epub 2007 Dec 19 [DOI] [PubMed] [Google Scholar]
- 14.Orban M, Bruce CJ, Pressman GS, et al. Dynamic changes of left ventricular performance and left atrial volume induced by the mueller maneuver in healthy young adults and implications for obstructive sleep apnea, atrial fibrillation, and heart failure. Am J Cardiol. 2008;102(11):1557-1561 Epub 2008 Sep 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auer J, Scheibner P, Mische T, Langsteger W, Eber O, Eber B. Subclinical hyperthyroidism as a risk factor for atrial fibrillation. Am Heart J. 2001;142(5):838-842 [DOI] [PubMed] [Google Scholar]
- 16.Gammage MD, Parle JV, Holder RL, et al. Association between serum free thyroxine concentration and atrial fibrillation. Arch Intern Med. 2007;167(9):928-934 [DOI] [PubMed] [Google Scholar]
- 17.Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials: Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1998;32(3):695-703 [DOI] [PubMed] [Google Scholar]
- 18.Olsson LG, Swedberg K, Ducharme A, et al. CHARM Investigators. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program. J Am Coll Cardiol. 2006;47(10):1997-2004 Epub 2006 Apr 27 [DOI] [PubMed] [Google Scholar]
- 19.Diker E, Aydogdu S, Ozdemir M, et al. Prevalence and predictors of atrial fibrillation in rheumatic valvular heart disease. Am J Cardiol. 1996;77(1):96-98 [DOI] [PubMed] [Google Scholar]
- 20.American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Society of Cardiovascular Anesthesiologists. Society for Cardiovascular Angiography and Interventions. Society of Thoracic Surgeons. Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease) [published correction appears in Circulation. 2007;115(15):e409] Circulation 2006;114(5):e84-e231 [DOI] [PubMed] [Google Scholar]
- 21.Grigioni F, Avierinos JF, Ling LH, et al. Atrial fibrillation complicating the course of degenerative mitral regurgitation: determinants and long-term outcome. J Am Coll Cardiol. 2002;40(1):84-92 [DOI] [PubMed] [Google Scholar]
- 22.Alexiou C, Doukas G, Oc M, et al. The effect of preoperative atrial fibrillation on survival following mitral valve repair for degenerative mitral regurgitation. Eur J Cardiothorac Surg. 200April;31(4):586-591 Epub 2007 Feb 5 [DOI] [PubMed] [Google Scholar]
- 23.Otto CM. Valvular aortic stenosis: disease severity and timing of intervention. J Am Coll Cardiol. 2006;47(11):2141-2151 Epub 2006 May 15 [DOI] [PubMed] [Google Scholar]
- 24.Maron BJ, McKenna WJ, Danielson GK, et al. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol. 2003;42(9):1687-1713 [DOI] [PubMed] [Google Scholar]
- 25.Olivotto I, Cecchi F, Casey SA, Dolara A, Traverse JH, Maron BJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation 2001;104(21):2517-2524 [DOI] [PubMed] [Google Scholar]
- 26.Hravnak M, Hoffman LA, Saul MI, Zullo TG, Whitman GR, Griffith BP. Predictors and impact of atrial fibrillation after isolated coronary artery bypass grafting. Crit Care Med. 2002;30(2):330-337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jongnarangsin K, Oral H. Postoperative atrial fibrillation. Med Clin North Am. 2008;92(1):87-99, x-x1. [DOI] [PubMed] [Google Scholar]
- 28.Burgess DC, Kilborn MJ, Keech AC. Interventions for prevention of post-operative atrial fibrillation and its complications after cardiac surgery: a meta-analysis. Eur Heart J. 2006;27(23):2846-2857 Epub 2006 Oct 2 [DOI] [PubMed] [Google Scholar]
- 29.Halonen J, Halonen P, Järvinen O, et al. Corticosteroids for the prevention of atrial fibrillation after cardiac surgery: a randomized controlled trial. JAMA 2007;297(14):1562-1567 [DOI] [PubMed] [Google Scholar]
- 30.Liu T, Li L, Korantzopoulos P, Liu E, Li G. Statin use and development of atrial fibrillation: a systematic review and meta-analysis of randomized clinical trials and observational studies. Int J Cardiol. 2008;126(2):160-170 Epub 2007 Nov 26 [DOI] [PubMed] [Google Scholar]
- 31.Yared JP, Bakri MH, Erzurum SC, et al. Effect of dexamethasone on atrial fibrillation after cardiac surgery: prospective, randomized, double-blind, placebo-controlled trial. J Cardiothorac Vasc Anesth. 2007;21(1):68-75 Epub 2006 Feb 14 [DOI] [PubMed] [Google Scholar]
- 32.Chen PS, Tan AY. Autonomic nerve activity and atrial fibrillation. Heart Rhythm. 2007;4:S61-S64 Epub 2006 Dec 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scherlag BJ, Nakagawa H, Jackman WM, et al. Electrical stimulation to identify neural elements on the heart: their role in atrial fibrillation. J Interv Card Electrophysiol. 2005;13(suppl 1):37-42 [DOI] [PubMed] [Google Scholar]
- 34.Olson TM, Alekseev AE, Moreau C, et al. KATP channel mutation confers risk for vein of Marshall adrenergic atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2007;4(2):110-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Segerson NM, Sharma N, Smith ML, et al. The effects of rate and irregularity on sympathetic nerve activity in human subjects. Heart Rhythm. 2007;4(1):20-26 Epub 2006 Sep 16 [DOI] [PubMed] [Google Scholar]
- 36.Wasmund SL, Li JM, Page RL, et al. Effect of atrial fibrillation and an irregular ventricular response on sympathetic nerve activity in human subjects. Circulation 2003;107(15):2011-2015 Epub 2003 Apr 7 [DOI] [PubMed] [Google Scholar]
- 37.Djoussé L, Levy D, Benjamin EJ, et al. Long-term alcohol consumption and the risk of atrial fibrillation in the Framingham Study. Am J Cardiol. 2004;93(6):710-713 [DOI] [PubMed] [Google Scholar]
- 38.Mukamal KJ, Psaty BM, Rautaharju PM, et al. Alcohol consumption and risk and prognosis of atrial fibrillation among older adults: the Cardiovascular Health Study. Am Heart J. 2007;153(2):260-266 [DOI] [PubMed] [Google Scholar]
- 39.Frost L, Vestergaard P. Caffeine and risk of atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Clin Nutr. 2005;81(3):578-582 [DOI] [PubMed] [Google Scholar]
- 40.Issac TT, Dokainish H, Lakkis NM. Role of inflammation in initiation and perpetuation of atrial fibrillation: a systematic review of the published data. J Am Coll Cardiol. 2007;50(21):2021-2028 Epub 2007 Nov 5 [DOI] [PubMed] [Google Scholar]
- 41.Anderson JL, Allen Maycock CA, Lappé DL, et al. Frequency of elevation of C-reactive protein in atrial fibrillation. Am J Cardiol. 2004;94(10):1255-1259 [DOI] [PubMed] [Google Scholar]
- 42.Asselbergs FW, van den Berg MP, Diercks GF, van Gilst WH, van Veldhuisen DJ. C-reactive protein and microalbuminuria are associated with atrial fibrillation. Int J Cardiol. 2005;98(1):73-77 [DOI] [PubMed] [Google Scholar]
- 43.Aviles RJ, Martin DO, Apperson-Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation 2003;108(24):3006-3010 Epub 2003 Nov 17 [DOI] [PubMed] [Google Scholar]
- 44.Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001;104(24):2886-2891 [DOI] [PubMed] [Google Scholar]
- 45.Ehrlich JR, Hohnloser SH, Nattel S. Role of angiotensin system and effects of its inhibition in atrial fibrillation: clinical and experimental evidence. Eur Heart J. 2006;27(5):512-518 Epub 2005 Nov 25 [DOI] [PubMed] [Google Scholar]
- 46.Nakashima H, Kumagai K, Urata H, Gondo N, Ideishi M, Arakawa K. Angiotensin II antagonist prevents electrical remodeling in atrial fibrillation. Circulation 2000;101(22):2612-2617 [DOI] [PubMed] [Google Scholar]
- 47.Kumagai K, Nakashima H, Urata H, Gondo N, Arakawa K, Saku K. Effects of angiotensin II type 1 receptor antagonist on electrical and structural remodeling in atrial fibrillation. J Am Coll Cardiol. 2003;41(12):2197-2204 [DOI] [PubMed] [Google Scholar]
- 48.Kawamura M, Ito H, Onuki T, et al. Combination therapy of renin angiotensin system inhibitors and bepridil is useful for maintaining sinus rhythm in patients with atrial fibrillation. J Cardiol. 2007;50(6):343-350 [PubMed] [Google Scholar]
- 49.Fox CS, Parise H, D'Agostino RB, Sr, et al. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA 2004;291(23):2851-2855 [DOI] [PubMed] [Google Scholar]
- 50.Brugada R. Is atrial fibrillation a genetic disease? J Cardiovasc Electrophysiol. 2005;16(5):553-556 [DOI] [PubMed] [Google Scholar]
- 51.Roberts R. Mechanisms of disease: genetic mechanisms of atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2006;3(5):276-282 [DOI] [PubMed] [Google Scholar]
- 52.Rho RW, Page RL. Asymptomatic atrial fibrillation. Prog Cardiovasc Dis. 2005;48(2):79-87 [DOI] [PubMed] [Google Scholar]
- 53.Israel CW, Grönefeld G, Ehrlich JR, Li YG, Hohnloser SH. Long-term risk of recurrent atrial fibrillation as documented by an implantable monitoring device: implications for optimal patient care. J Am Coll Cardiol. 2004;43(1):47-52 [DOI] [PubMed] [Google Scholar]
- 54.Calò L, De Ruvo E, Sette A, et al. Tachycardia-induced cardiomyopathy: mechanisms of heart failure and clinical implications. J Cardiovasc Med (Hagerstown) 2007;8(3):138-143 [DOI] [PubMed] [Google Scholar]
- 55.Rothman SA, Laughlin JC, Seltzer J, et al. The diagnosis of cardiac arrhythmias: a prospective multi-center randomized study comparing mobile cardiac outpatient telemetry versus standard loop event monitoring. J Cardiovasc Electrophysiol. 2007;18(3):241-247 [DOI] [PubMed] [Google Scholar]
- 56.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98(10):946-952 [DOI] [PubMed] [Google Scholar]
- 57.Frost L, Engholm G, Johnsen S, Møller H, Husted S. Incident stroke after discharge from the hospital with a diagnosis of atrial fibrillation. Am J Med. 2000;108(1):36-40 [DOI] [PubMed] [Google Scholar]
- 58.Lip GY, Lim HS. Atrial fibrillation and stroke prevention. Lancet Neurol. 2007;6(11):981-993 [DOI] [PubMed] [Google Scholar]
- 59.Kasper EK, Agema WR, Hutchins GM, Deckers JW, Hare JM, Baughman KL. The causes of dilated cardiomyopathy: a clinicopathologic review of 673 consecutive patients. J Am Coll Cardiol. 1994;23(3):586-590 [DOI] [PubMed] [Google Scholar]
- 60.Thrall G, Lane D, Carroll D, Lip GY. Quality of life in patients with atrial fibrillation: a systematic review. Am J Med. 2006;119(5):448.e1-448.e19 [DOI] [PubMed] [Google Scholar]
- 61.Jenkins LS, Brodsky M, Schron E, et al. Quality of life in atrial fibrillation: the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am Heart J. 2005;149(1):112-120 [DOI] [PubMed] [Google Scholar]
- 62.Raghavan AV, Decker WW, Meloy TD. Management of atrial fibrillation in the emergency department. Emerg Med Clin North Am. 2005;23(4):1127-1139 [DOI] [PubMed] [Google Scholar]
- 63.Alboni P, Botto GL, Baldi N, et al. Outpatient treatment of recent-onset atrial fibrillation with the “pill-in-the-pocket” approach. N Engl J Med. 2004;351(23):2384-2391 [DOI] [PubMed] [Google Scholar]
- 64.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation 2004;110(4):364-367 Epub 2004 Jul 12 [DOI] [PubMed] [Google Scholar]
- 65.Boriani G, Diemberger I, Biffi M, et al. Electrical cardioversion for persistent atrial fibrillation or atrial flutter in clinical practice: predictors of long-term outcome. Int J Clin Pract. 2007;61(5):748-756 [DOI] [PubMed] [Google Scholar]
- 66.Kim SS, Knight BP. Electrical and pharmacologic cardioversion for atrial fibrillation. Med Clin North Am. 2008;92(1):101-120 [DOI] [PubMed] [Google Scholar]
- 67.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22(8):983-988 [DOI] [PubMed] [Google Scholar]
- 68.Brand FN, Abbott RD, Kannel WB, Wolf PA. Characteristics and prognosis of lone atrial fibrillation: 30-year follow-up in the Framingham Study. JAMA 1985;254(24):3449-3453 [PubMed] [Google Scholar]
- 69.Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation: the Framingham Study. Stroke 1996;27(10):1760-1764 [DOI] [PubMed] [Google Scholar]
- 70.Anderson DC, Kappelle LJ, Eliasziw M, Babikian VL, Pearce LA, Barnett HJ. Occurrence of hemispheric and retinal ischemia in atrial fibrillation compared with carotid stenosis. Stroke 2002;33(8):1963-1967 [DOI] [PubMed] [Google Scholar]
- 71.Atrial Fibrillation Investigators. Atrial Fibrillation, Aspirin, Anticoagulation Study. European Atrial Fibrillation Study. Stroke Prevention in Atrial Fibrillation Study. Boston Area Anticoagulation Trial for Atrial Fibrillation Study. Canadian Atrial Fibrillation Study. Veterans Affairs Prevention in Atrial Fibrillation Study Echocardiographic predictors of stroke in patients with atrial fibrillation: a prospective study of 1066 patients from 3 clinical trials. Arch Intern Med. 1998;158(12):1316-1320 [DOI] [PubMed] [Google Scholar]
- 72.Mitusch R, Siemens HJ, Garbe M, Wagner T, Sheikhzadeh A, Diederich KW. Detection of a hypercoagulable state in nonvalvular atrial fibrillation and the effect of anticoagulant therapy. Thromb Haemost. 1996;75(2):219-223 [PubMed] [Google Scholar]
- 73.Uno M, Tsuji H, Sawada S, Toyoda T, Nakagawa M. Fibrinopeptide A (FPA) levels in atrial fibrillation and the effects of heparin administration. Jpn Circ J. 1988;52(1):9-12 [DOI] [PubMed] [Google Scholar]
- 74.Stroke Prevention in Atrial Fibrillation Investigators Committee on Echocardiography Transesophageal echocardiographic correlates of thromboembolism in high-risk patients with nonvalvular atrial fibrillation. Ann Intern Med. 1998;128(8):639-647 [DOI] [PubMed] [Google Scholar]
- 75.Atrial Fibrillation Investigators. Atrial Fibrillation, Aspirin, Anticoagulation Study. Boston Area Anticoagulation Trial for Atrial Fibrillation Study. Canadian Atrial Fibrillation Anticoagulation Study. Stroke Prevention in Atrial Fibrillation Study ; Veterans Affairs Prevention in Nonrheumatic Atrial Fibrillation Study Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation: analysis of pooled data from five randomized controlled trials [published correction appears in Arch Intern Med. 1994;154(19):2254] Arch Intern Med. 1994;154(13):1449-1457 [PubMed] [Google Scholar]
- 76.Stroke Prevention in Atrial Fibrillation Investigators Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation III randomised clinical trial. Lancet 1996;348(9028):633-638 [PubMed] [Google Scholar]
- 77.SPAF III Writing Committee for the Stroke Prevention in Atrial Fibrillation Investigators Patients with nonvalvular atrial fibrillation at low risk of stroke during treatment with aspirin: Stroke Prevention in Atrial Fibrillation III Study. JAMA 1998;279(16):1273-1277 [PubMed] [Google Scholar]
- 78.Casella L, Abelmann WH, Ellis LB. Patients with mitral stenosis and systemic emboli: hemodynamic and clinical observations. Arch Intern Med. 1964;114:773-781 [DOI] [PubMed] [Google Scholar]
- 79.Daley R, Mattingly TW, Holt CL, Bland EF, White PD. Systemic arterial embolism in rheumatic heart disease. Am Heart J. 1951;42(4):566-581 [DOI] [PubMed] [Google Scholar]
- 80.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001;285(22):2864-2870 [DOI] [PubMed] [Google Scholar]
- 81.Go AS, Hylek EM, Chang Y, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA 2003;290(20):2685-2692 [DOI] [PubMed] [Google Scholar]
- 82.Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999;131(7):492-501 [DOI] [PubMed] [Google Scholar]
- 83.Polanczyk CA, Goldman L, Marcantonio ER, Orav EJ, Lee TH. Supraventricular arrhythmia in patients having noncardiac surgery: clinical correlates and effect on length of stay. Ann Intern Med. 1998;129(4):279-285 [DOI] [PubMed] [Google Scholar]
- 84.Ezekowitz MD, Bridgers SL, James KE, et al. Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation Investigators Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation [published correction appears in N Engl J Med. 1993;328(2):148] N Engl J Med. 1992;327(20):1406-1412 [DOI] [PubMed] [Google Scholar]
- 85.Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators The effect of low-dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. N Engl J Med. 1990;323(22):1505-1511 [DOI] [PubMed] [Google Scholar]
- 86.Stroke Prevention in Atrial Fibrillation Investigators Stroke prevention in atrial fibrillation study: final results. Circulation 1991;84(2):527-539 [DOI] [PubMed] [Google Scholar]
- 87.EAFT (European Atrial Fibrillation Trial) Study Group Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet 1993;342(8882):1255-1262 [PubMed] [Google Scholar]
- 88.Stroke Prevention in Atrial Fibrillation Investigators Warfarin versus aspirin for prevention of thromboembolism in atrial fibrillation: Stroke Prevention in Atrial Fibrillation II Study. Lancet 1994;343(8899):687-691 [PubMed] [Google Scholar]
- 89.Diener HC. Antiplatelet agents and randomized trials. Rev Neurol Dis. 2007;4(4):177-183 [PubMed] [Google Scholar]
- 90.Petersen P, Boysen G, Godtfredsen J, Andersen ED, Andersen B. Placebo-controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation: the Copenhagen AFASAK study. Lancet 1989;1(8631):175-179 [DOI] [PubMed] [Google Scholar]
- 91.Posada IS, Barriales V, LASAF Pilot Study Group Alternate-day dosing of aspirin in atrial fibrillation. Am Heart J. 1999;138(1, pt 1):137-143 [DOI] [PubMed] [Google Scholar]
- 92.Hart RG, Benavente O, Pearce LA. Increased risk of intracranial hemorrhage when aspirin is combined with warfarin: a meta-analysis and hypothesis. Cerebrovasc Dis. 1999;9(4):215-217 [DOI] [PubMed] [Google Scholar]
- 93.Hylek EM, Go AS, Chang Y, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003;349(11):1019-1026 [DOI] [PubMed] [Google Scholar]
- 94.Hylek EM, Skates SJ, Sheehan MA, Singer DE. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med. 1996;335(8):540-546 [DOI] [PubMed] [Google Scholar]
- 95.Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med. 1994;120(11):897-902 [DOI] [PubMed] [Google Scholar]
- 96.Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825-1833 [DOI] [PubMed] [Google Scholar]
- 97.Biblo LA, Yuan Z, Quan KJ, Mackall JA, Rimm AA. Risk of stroke in patients with atrial flutter. Am J Cardiol. 2001;87(3):346-349, A9. [DOI] [PubMed] [Google Scholar]
- 98.Halligan SC, Gersh BJ, Brown RD, Jr, et al. The natural history of lone atrial flutter. Ann Intern Med. 2004;140(4):265-268 [DOI] [PubMed] [Google Scholar]
- 99.Neumann T, Erdogan A, Dill T, et al. Asymptomatic recurrences of atrial fibrillation after pulmonary vein isolation. Europace 2006;8(7):495-498 [DOI] [PubMed] [Google Scholar]
- 100.Page RL, Wilkinson WE, Clair WK, McCarthy EA, Pritchett EL. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation 1994;89(1):224-227 [DOI] [PubMed] [Google Scholar]
- 101.Oral H, Chugh A, Ozaydin M, et al. Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation 2006;114(8):759-765 Epub 2006 Aug 14 [DOI] [PubMed] [Google Scholar]
- 102.ACTIVE Writing Group of the ACTIVE Investigators Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 2006;367(9526):1903-1912 [DOI] [PubMed] [Google Scholar]
- 103.Shireman TI, Howard PA, Kresowik TF, Ellerbeck EF. Combined anticoagulant-antiplatelet use and major bleeding events in elderly atrial fibrillation patients. Stroke 2004;35(10):2362-2367 Epub 2004 Aug 26 [DOI] [PubMed] [Google Scholar]
- 104.Anand SS, Yusuf S. Oral anticoagulant therapy in patients with coronary artery disease: a meta-analysis [published correction appears in JAMA. 2000;284(1):45] JAMA 1999;282(21):2058-2067 [DOI] [PubMed] [Google Scholar]
- 105.Ruiz-Nodar JM, Marín F, Hurtado JA, et al. Anticoagulant and antiplatelet therapy use in 426 patients with atrial fibrillation undergoing percutaneous coronary intervention and stent implantation implications for bleeding risk and prognosis. J Am Coll Cardiol. 2008;51(8):818-825 [DOI] [PubMed] [Google Scholar]
- 106.Francescone S, Halperin JL. “Triple therapy” or triple threat? Balancing the risks of antithrombotic therapy for patients with atrial fibrillation and coronary stents [editorial]. J Am Coll Cardiol. 2008;51(8):826-827 [DOI] [PubMed] [Google Scholar]
- 107.Khan IA. Atrial stunning: determinants and cellular mechanisms. Am Heart J. 2003;145(5):787-794 [DOI] [PubMed] [Google Scholar]
- 108.Falcone RA, Morady F, Armstrong WF. Transesophageal echocardiographic evaluation of left atrial appendage function and spontaneous contrast formation after chemical or electrical cardioversion of atrial fibrillation. Am J Cardiol. 1996;78(4):435-439 [DOI] [PubMed] [Google Scholar]
- 109.Antonielli E, Pizzuti A, Bassignana A, et al. Transesophageal echocardiographic evidence of more pronounced left atrial stunning after chemical (propafenone) rather than electrical attempts at cardioversion from atrial fibrillation. Am J Cardiol. 1999;84(9):1092-1096, A9-A10. [DOI] [PubMed] [Google Scholar]
- 110.Manning WJ, Silverman DI, Katz SE, et al. Temporal dependence of the return of atrial mechanical function on the mode of cardioversion of atrial fibrillation to sinus rhythm. Am J Cardiol. 1995;75(8):624-626 [DOI] [PubMed] [Google Scholar]
- 111.Grimm RA, Leung DY, Black IW, Stewart WJ, Thomas JD, Klein AL. Left atrial appendage “stunning” after spontaneous conversion of atrial fibrillation demonstrated by transesophageal Doppler echocardiography. Am Heart J. 1995;130(1):174-176 [DOI] [PubMed] [Google Scholar]
- 112.Zabalgoitia M, Halperin JL, Pearce LA, Blackshear JL, Asinger RW, Hart RG, Stroke Prevention in Atrial Fibrillation III Investigators Transesophageal echocardiographic correlates of clinical risk of thromboembolism in nonvalvular atrial fibrillation. J Am Coll Cardiol. 1998;31(7):1622-1626 [DOI] [PubMed] [Google Scholar]
- 113.Fatkin D, Kuchar DL, Thorburn CW, Feneley MP. Transesophageal echocardiography before and during direct current cardioversion of atrial fibrillation: evidence for “atrial stunning” as a mechanism of thromboembolic complications. J Am Coll Cardiol. 1994;23(2):307-316 [DOI] [PubMed] [Google Scholar]
- 114.Berger M, Schweitzer P. Timing of thromboembolic events after electrical cardioversion of atrial fibrillation or flutter: a retrospective analysis. Am J Cardiol. 1998;82(12):1545-1547, A8. [DOI] [PubMed] [Google Scholar]
- 115.Bjerkelund CJ, Orning OM. The efficacy of anticoagulant therapy in preventing embolism related to D.C. electrical conversion of atrial fibrillation. Am J Cardiol. 1969;23(2):208-216 [DOI] [PubMed] [Google Scholar]
- 116.Kinch JW, Davidoff R. Prevention of embolic events after cardioversion of atrial fibrillation: current and evolving strategies. Arch Intern Med. 1995;155(13):1353-1360 [PubMed] [Google Scholar]
- 117.Collins LJ, Silverman DI, Douglas PS, Manning WJ. Cardioversion of nonrheumatic atrial fibrillation: reduced thromboembolic complications with 4 weeks of precardioversion anticoagulation are related to atrial thrombus resolution. Circulation 1995;92(2):160-163 [DOI] [PubMed] [Google Scholar]
- 118.Stellbrink C, Nixdorff U, Hofmann T, et al. ACE (Anticoagulation in Cardioversion using Enoxaparin) Study Group Safety and efficacy of enoxaparin compared with unfractionated heparin and oral anticoagulants for prevention of thromboembolic complications in cardioversion of nonvalvular atrial fibrillation: the Anticoagulation in Cardioversion using Enoxaparin (ACE) trial. Circulation 2004;109(8):997-1003 Epub 2004 Feb 16 [DOI] [PubMed] [Google Scholar]
- 119.Klein AL, Jasper SE, Katz WE, et al. ACUTE II Steering and Publications Committe for the ACUTE II Investigator The use of enoxaparin compared with unfractionated heparin for short-term antithrombotic therapy in atrial fibrillation patients undergoing transoesophageal echocardiography-guided cardioversion: assessment of Cardioversion Using Transoesophageal Echocardiography (ACUTE) II randomized multicentre study. Eur Heart J. 2006;27(23):2858-2865 Epub 2006 Nov 10. [DOI] [PubMed] [Google Scholar]
- 120.Klein AL, Grimm RA, Murray RD, et al. Assessment of Cardioversion Using Transesophageal Echocardiography Investigators Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med. 2001;344(19):1411-1420 [DOI] [PubMed] [Google Scholar]
- 121.Mehta D, Baruch L. Thromboembolism following cardioversion of “common” atrial flutter: risk factors and limitations of transesophageal echocardiography. Chest 1996;110(4):1001-1003 [DOI] [PubMed] [Google Scholar]
- 122.Carlsson J, Miketic S, Windeler J, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol. 2003;41(10):1690-1696 [DOI] [PubMed] [Google Scholar]
- 123.Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation—Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomised trial. Lancet 2000;356(9244):1789-1794 [DOI] [PubMed] [Google Scholar]
- 124.Opolski G, Torbicki A, Kosior DA, et al. Investigators of the Polish HOT CAFE Trial Rate control vs rhythm control in patients with nonvalvular persistent atrial fibrillation: the results of the Polish How to Treat Chronic Atrial Fibrillation (HOT CAFE) Study. Chest 2004;126(2):476-486 [DOI] [PubMed] [Google Scholar]
- 125.Van Gelder IC, Hagens VE, Bosker HA, et al. Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834-1840 [DOI] [PubMed] [Google Scholar]
- 126.Stabile G, Bertaglia E, Senatore G, et al. Catheter ablation treatment in patients with drug-refractory atrial fibrillation: a prospective, multi-centre, randomized, controlled study (Catheter Ablation For The Cure Of Atrial Fibrillation Study). Eur Heart J. 2006;27(2):216-221 Epub 2005 Oct 7 [DOI] [PubMed] [Google Scholar]
- 127.Jaïs P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 Study. Circulation 2008;118(24):2498-2505 Epub 2008 Nov 24 [DOI] [PubMed] [Google Scholar]
- 128.Pappone C, Augello G, Sala S, et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J Am Coll Cardiol. 2006;48(11):2340-2347 Epub 2006 Oct 16 [DOI] [PubMed] [Google Scholar]
- 129.Lafuente-Lafuente C, Mouly S, Longás-Tejero MA, Mahé I, ergmann JF. Antiarrhythmic drugs for maintaining sinus rhythm after cardioversion of atrial fibrillation: a systematic review of randomized controlled trials. Arch Intern Med. 2006;166(7):719-728 [DOI] [PubMed] [Google Scholar]
- 130.Lafuente-Lafuente C, Mouly S, Longas-Tejero MA, Bergmann JF. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2007;(4):CD005049 [DOI] [PubMed] [Google Scholar]
- 131.Cardiac Arrhythmia Suppression Trial (CAST) Investigators Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321(6):406-412 [DOI] [PubMed] [Google Scholar]
- 132.Dale KM, White CM. Dronedarone: an amiodarone analog for the treatment of atrial fibrillation and atrial flutter. Ann Pharmacother. 2007;41(4):599-605 Epub 2007 Mar 27 [DOI] [PubMed] [Google Scholar]
- 133.Touboul P, Brugada J, Capucci A, Crijns HJ, Edvardsson N, Hohnloser SH. Dronedarone for prevention of atrial fibrillation: a dose-ranging study. Eur Heart J. 2003;24(16):1481-1487 [DOI] [PubMed] [Google Scholar]
- 134.Hohnloser SH, Dorian P, Straub M, Beckmann K, Kowey P. Safety and efficacy of intravenously administered tedisamil for rapid conversion of recent-onset atrial fibrillation or atrial flutter. J Am Coll Cardiol. 2004;44(1):99-104 [DOI] [PubMed] [Google Scholar]
- 135.Freestone B, Lip GY. Tedisamil: a new novel antiarrhythmic. Expert Opin Investig Drugs 2004;13(2):151-160 [DOI] [PubMed] [Google Scholar]
- 136.Blaauw Y, Crijns HJ. Atrial fibrillation: insights from clinical trials and novel treatment options. J Intern Med. 2007;262(6):593-614 [DOI] [PubMed] [Google Scholar]
- 137.Lévy S. Do we need pharmacological therapy for atrial fibrillation in the ablation era? J Interv Card Electrophysiol. 2006;17(3):189-194 [DOI] [PubMed] [Google Scholar]
- 138.Jibrini MB, Molnar J, Arora RR. Prevention of atrial fibrillation by way of abrogation of the renin-angiotensin system: a systematic review and meta-analysis. Am J Ther. 2008;15(1):36-43 [DOI] [PubMed] [Google Scholar]
- 139.Sakabe M, Shiroshita-Takeshita A, Maguy A, et al. Omega-3 poly-unsaturated fatty acids prevent atrial fibrillation associated with heart failure but not atrial tachycardia remodeling. Circulation 2007;116(19):2101-2109 Epub 2007 Oct 22 [DOI] [PubMed] [Google Scholar]
- 140.Howard PA, Barnes BJ. Potential use of statins to prevent atrial fibrillation after coronary artery bypass surgery. Ann Pharmacother. 2008;42(2):253-258 Epub 2008 Jan 15 [DOI] [PubMed] [Google Scholar]
- 141.Asirvatham SJ. Pulmonary vein-related manuevers: part I. Heart Rhythm. 2007;4(4):538-544 Epub 2007 Jan 12 [DOI] [PubMed] [Google Scholar]
- 142.Asirvatham SJ. Pacing manuevers for nonpulmonary vein sources: part II. Heart Rhythm. 2007;4(5):681-685 Epub 2007 Jan 12 [DOI] [PubMed] [Google Scholar]
- 143.Cappato R, Calkins H, Chen SA, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation 2005;111(9):1100-1105 Epub 2005 Feb 21 [DOI] [PubMed] [Google Scholar]
- 144.Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation: analysis and implications. Arch Intern Med. 1995;155(5):469-473 [PubMed] [Google Scholar]
- 145.Jalife J, Berenfeld O, Mansour M. Mother rotors and fibrillatory conduction: a mechanism of atrial fibrillation. Cardiovasc Res. 2002;54(2):204-216 [DOI] [PubMed] [Google Scholar]
- 146.Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. Am Heart J. 1964;67:200-220 [DOI] [PubMed] [Google Scholar]
- 147.Chen SA, Hsieh MH, Tai CT, et al. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation 1999;100(18):1879-1886 [DOI] [PubMed] [Google Scholar]
- 148.Haïssaguerre M, Hocini M, Sanders P, et al. Localized sources maintaining atrial fibrillation organized by prior ablation. Circulation 2006;113(5):616-625 [DOI] [PubMed] [Google Scholar]
- 149.Jaïs P, Haïssaguerre M, Shah DC, et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation 1997;95(3):572-576 [DOI] [PubMed] [Google Scholar]
- 150.Nathan H, Eliakim M. The junction between the left atrium and the pulmonary veins: an anatomic study of human hearts. Circulation 1966;34(3):412-422 [DOI] [PubMed] [Google Scholar]
- 151.Asirvatham SJ. Anatomy of the coronary sinus. In: Yu CM, Hayes DL, Auricchio A, eds. Cardiac Resynchronization Therapy Malden, MA: Blackwell/Futura; 2006:211-238 [Google Scholar]
- 152.Asirvatham SJ. Supraventricular tachycardia: diagnosis and treatment. In: Murphy JG, Lloyd MA, eds. Mayo Clinic Cardiology: Concise Textbook 3rd ed.Rochester, MN: Mayo Clinic Scientific Press; 2007:379-387 [Google Scholar]
- 153.Nademanee K, McKenzie J, Kosar E, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43(11):2044-2053 [DOI] [PubMed] [Google Scholar]
- 154.Oral H, Chugh A, Good E, et al. A tailored approach to catheter ablation of paroxysmal atrial fibrillation. Circulation 2006;113(15):1824-1831 Epub 2006 Apr 10 [DOI] [PubMed] [Google Scholar]
- 155.Nakagawa H, Scherlag BJ, Lockwood D, et al. Localization of left atrial autonomic ganglionated plexuses using endocardial and epicardial high frequency stimulation in patients with atrial fibrillation [abstract AB6-1]. Heart Rhythm. 2005;2(5)(suppl 1):S10-S11 [Google Scholar]
- 156.Schauerte P, Scherlag BJ, Pitha J, et al. Catheter ablation of cardiac autonomic nerves for prevention of vagal atrial fibrillation. Circulation 2000;102(22):2774-2780 [DOI] [PubMed] [Google Scholar]
- 157.Hsieh MH, Chiou CW, Wen ZC, et al. Alterations of heart rate variability after radiofrequency catheter ablation of focal atrial fibrillation originating from pulmonary veins. Circulation 1999;100(22):2237-2243 [DOI] [PubMed] [Google Scholar]
- 158.Karch MR, Zrenner B, Deisenhofer I, et al. Freedom from atrial tachyarrhythmias after catheter ablation of atrial fibrillation: a randomized comparison between 2 current ablation strategies. Circulation 2005;111(22):2875-2880 Epub 2005 May 31 [DOI] [PubMed] [Google Scholar]
- 159.Nilsson B, Chen X, Pehrson S, Køber L, Hilden J, Svendsen JH. Recurrence of pulmonary vein conduction and atrial fibrillation after pulmonary vein isolation for atrial fibrillation: a randomized trial of the ostial versus the extraostial ablation strategy. Am Heart J. 2006;152(3):537.e1-537.e8 [DOI] [PubMed] [Google Scholar]
- 160.Calkins H, Brugada J, Packer DL, et al. HRS/EHRA/ECAS Expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up: a report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation [published correction appears in Heart Rhythm. 2009;6(1):148] Heart Rhythm. 2007;4(6):816-861 Epub 2007 Apr 30 [DOI] [PubMed] [Google Scholar]
- 161.Oral H, Veerareddy S, Good E, et al. Prevalence of asymptomatic recurrences of atrial fibrillation after successful radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 2004;15(8):920-924 [DOI] [PubMed] [Google Scholar]
- 162.Haïssaguerre M, Jaïs P, Shah DC, et al. Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation 2000;101(12):1409-1417 [DOI] [PubMed] [Google Scholar]
- 163.Marrouche NF, Dresing T, Cole C, et al. Circular mapping and ablation of the pulmonary vein for treatment of atrial fibrillation: impact of different catheter technologies. J Am Coll Cardiol. 2002;40(3):464-474 [DOI] [PubMed] [Google Scholar]
- 164.Oral H, Knight BP, Tada H, et al. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation 2002;105(9):1077-1081 [DOI] [PubMed] [Google Scholar]
- 165.Packer DL, Asirvatham S, Munger TM. Progress in nonpharmacologic therapy of atrial fibrillation. J Cardiovasc Electrophysiol. 2003;14(12) (suppl):S296-S309 [DOI] [PubMed] [Google Scholar]
- 166.Pappone C, Oreto G, Rosanio S, et al. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation 2001;104(21):2539-2544 [DOI] [PubMed] [Google Scholar]
- 167.Earley MJ, Abrams DJ, Staniforth AD, Sporton SC, Schilling RJ. Catheter ablation of permanent atrial fibrillation: medium term results. Heart 2006;92(2):233-238 Epub 2005 Aug 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Haïssaguerre M, Hocini M, Sanders P, et al. Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J Cardiovasc Electrophysiol. 2005;16(11):1138-1147 [DOI] [PubMed] [Google Scholar]
- 169.Haïssaguerre M, Jaïs P, Shah D, et al. Catheter ablation of chronic atrial fibrillation targeting the reinitiating triggers. J Cardiovasc Electrophysiol. 2000;11(1):2-10 [DOI] [PubMed] [Google Scholar]
- 170.Berkowitsch A, Greiss H, Vukajlovic D, et al. Usefulness of atrial fibrillation burden as a predictor for success of pulmonary vein isolation. Pacing Clin Electrophysiol. 2005;28(12):1292-1301 [DOI] [PubMed] [Google Scholar]
- 171.Pappone C, Santinelli V, Manguso F, et al. A randomized study of prophylactic catheter ablation in asymptomatic patients with the Wolff-Parkinson-White syndrome. N Engl J Med. 2003;349(19):1803-1811 [DOI] [PubMed] [Google Scholar]
- 172.Weerasooriya R, Jaïs P, Hocini M, et al. Effect of catheter ablation on quality of life of patients with paroxysmal atrial fibrillation. Heart Rhythm. 2005;2(6):619-623 [DOI] [PubMed] [Google Scholar]
- 173.Hocini M, Jaïs P, Haïssaguerre M, Garrigue S, le Metayer P, Clementy J. Radiofrequency ablation of atrial fibrillation [in French]. Ann Cardiol Angeiol (Paris) 2003;52(4):258-263 [DOI] [PubMed] [Google Scholar]
- 174.Packer DL, Keelan P, Munger TM, et al. Clinical presentation, investigation, and management of pulmonary vein stenosis complicating ablation for atrial fibrillation. Circulation 2005;111(5):546-554 [DOI] [PubMed] [Google Scholar]
- 175.Tada H, Naito S, Kurosaki K, et al. Segmental pulmonary vein isolation for paroxysmal atrial fibrillation improves quality of life and clinical outcomes. Circ J. 2003;67(10):861-865 [DOI] [PubMed] [Google Scholar]
- 176.Ahmed J, Sohal S, Malchano ZJ, Holmvang G, Ruskin JN, Reddy VY. Three-dimensional analysis of pulmonary venous ostial and antral anatomy: implications for balloon catheter-based pulmonary vein isolation. J Cardiovasc Electrophysiol. 2006;17(3):251-255 [DOI] [PubMed] [Google Scholar]
- 177.Arentz T, Jander N, von Rosenthal J, et al. Incidence of pulmonary vein stenosis 2 years after radiofrequency catheter ablation of refractory atrial fibrillation. Eur Heart J. 2003;24(10):963-969 [DOI] [PubMed] [Google Scholar]
- 178.Robbins IM, Colvin EV, Doyle TP, et al. Pulmonary vein stenosis after catheter ablation of atrial fibrillation. Circulation 1998;98(17):1769-1775 [DOI] [PubMed] [Google Scholar]
- 179.Saad EB, Rossillo A, Saad CP, et al. Pulmonary vein stenosis after radiofrequency ablation of atrial fibrillation: functional characterization, evolution, and influence of the ablation strategy. Circulation 2003;108(25):3102-3107 Epub 2003 Nov 17 [DOI] [PubMed] [Google Scholar]
- 180.Bruce CJ, Friedman PA, Narayan O, et al. Early heparinization decreases the incidence of left atrial thrombi detected by intracardiac echocardiography during radiofrequency ablation for atrial fibrillation. J Interv Card Electrophysiol. 2008;22(3):211-219 Epub 2008 Jun 21 [DOI] [PubMed] [Google Scholar]
- 181.Cummings JE, Schweikert RA, Saliba WI, et al. Brief communication: atrial-esophageal fistulas after radiofrequency ablation. Ann Intern Med. 2006;144(8):572-574 [DOI] [PubMed] [Google Scholar]
- 182.Pappone C, Oral H, Santinelli V, et al. Atrio-esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation 2004;109(22):2724-2726 Epub 2004 May 24 [DOI] [PubMed] [Google Scholar]
- 183.Schley P, Gülker H, Horlitz M. Atrio-oesophageal fistula following circumferential pulmonary vein ablation: verification of diagnosis with multislice computed tomography. Europace 2006;8(3):189-190 Epub 2006 Feb 7 [DOI] [PubMed] [Google Scholar]
- 184.Sosa E, Scanavacca M. Left atrial-esophageal fistula complicating radiofrequency catheter ablation of atrial fibrillation [editorial]. J Cardiovasc Electrophysiol. 2005;16(3):249-250 [DOI] [PubMed] [Google Scholar]
- 185.Bunch TJ, Nelson J, Foley T, et al. Temporary esophageal stenting allows healing of esophageal perforations following atrial fibrillation ablation procedures. J Cardiovasc Electrophysiol. 2006;17(4):435-439 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.