Abstract
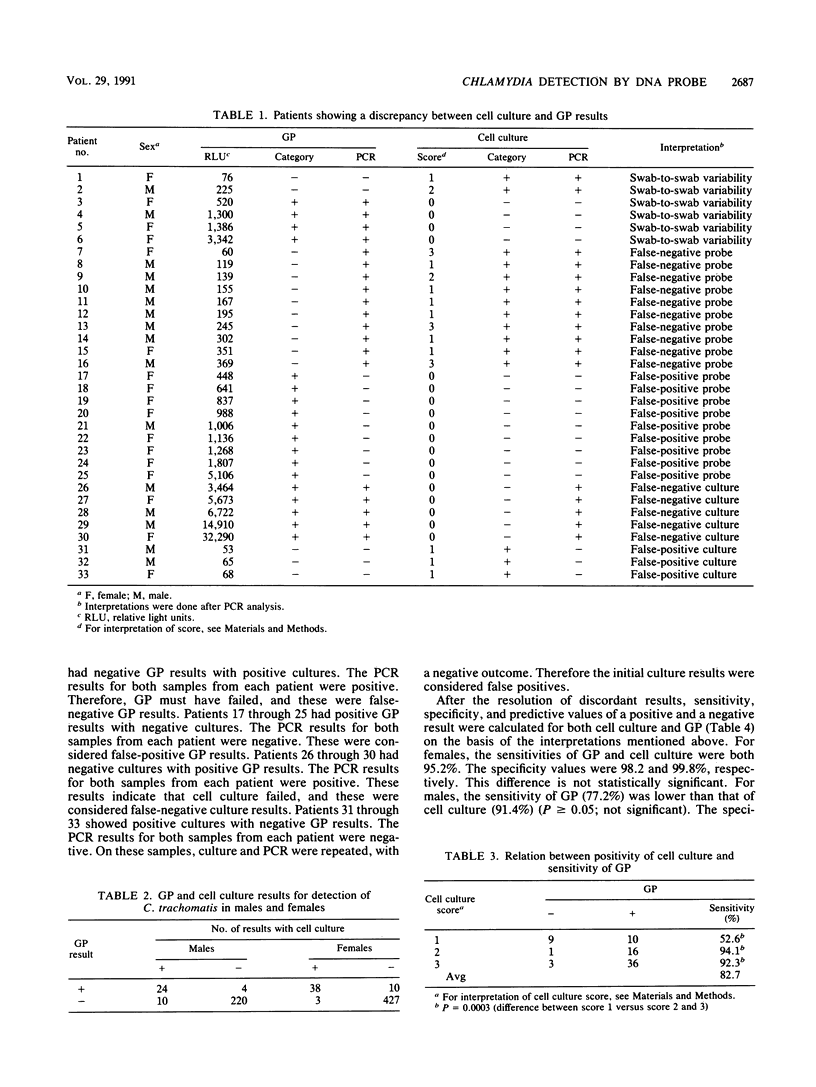
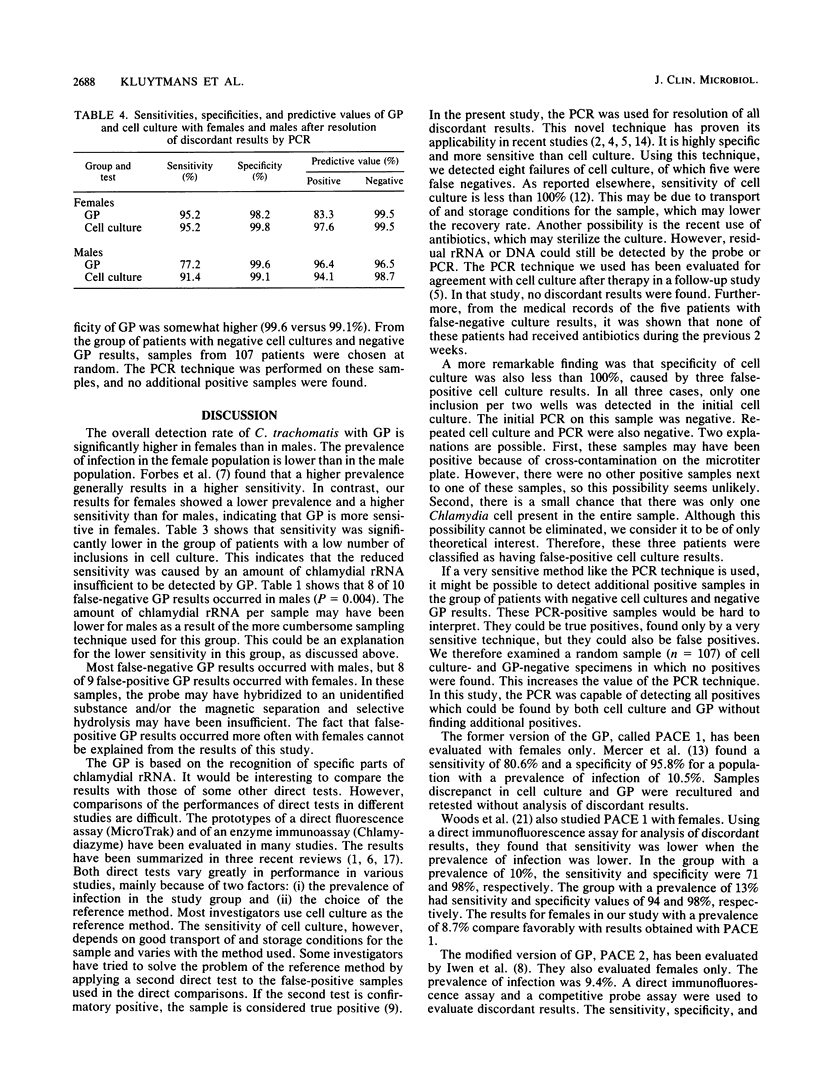
The Gen-Probe PACE 2 assay (GP), a nonisotopic DNA probe, was evaluated by using cell culture as the method of reference. Specimens were collected from 260 men and 482 women visiting the outpatient department for sexually transmitted diseases at the University Hospital in Rotterdam, The Netherlands. The prevalences of Chlamydia culture-positive men and women were 13.2 and 8.6%, respectively. Sensitivity values for the male and female patients were 70.6 and 92.7%, respectively. Specificity values for these groups were 98.2 and 97.7%, respectively. Sensitivity was significantly lower when the number of inclusions in cell culture was low. Samples which showed a discordance between cell culture and GP results were retested by the polymerase chain reaction. If the results of the polymerase chain reaction were considered as the points of reference, the sensitivity and specificity of both GP and cell culture could be calculated. The performance of GP for females was comparable to that of cell culture. In males, the sensitivity of GP was considerably lower than that of cell culture (77.2 versus 91.4%), while specificity was somewhat higher (99.6 versus 99.1%). Compared with cell culture, the GP is a relatively simple and rapid test that is suitable for diagnosing Chlamydia infections in urogenital specimens.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes R. C. Laboratory diagnosis of human chlamydial infections. Clin Microbiol Rev. 1989 Apr;2(2):119–136. doi: 10.1128/cmr.2.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobo L., Coutlee F., Yolken R. H., Quinn T., Viscidi R. P. Diagnosis of Chlamydia trachomatis cervical infection by detection of amplified DNA with an enzyme immunoassay. J Clin Microbiol. 1990 Sep;28(9):1968–1973. doi: 10.1128/jcm.28.9.1968-1973.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claas H. C., Melchers W. J., de Bruijn I. H., de Graaf M., van Dijk W. C., Lindeman J., Quint W. G. Detection of Chlamydia trachomatis in clinical specimens by the polymerase chain reaction. Eur J Clin Microbiol Infect Dis. 1990 Dec;9(12):864–868. doi: 10.1007/BF01967500. [DOI] [PubMed] [Google Scholar]
- Claas H. C., Wagenvoort J. H., Niesters H. G., Tio T. T., Van Rijsoort-Vos J. H., Quint W. G. Diagnostic value of the polymerase chain reaction for Chlamydia detection as determined in a follow-up study. J Clin Microbiol. 1991 Jan;29(1):42–45. doi: 10.1128/jcm.29.1.42-45.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret J. M., Judson F. N. Genital Chlamydia infections. Clin Lab Med. 1989 Sep;9(3):481–500. [PubMed] [Google Scholar]
- Forbes B. A., Bartholoma N., McMillan J., Roefaro M., Weiner L., Welych L. Evaluation of a monoclonal antibody test to detect chlamydia in cervical and urethral specimens. J Clin Microbiol. 1986 Jun;23(6):1136–1137. doi: 10.1128/jcm.23.6.1136-1137.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwen P. C., Blair T. M., Woods G. L. Comparison of the Gen-Probe PACE 2 system, direct fluorescent-antibody, and cell culture for detecting Chlamydia trachomatis in cervical specimens. Am J Clin Pathol. 1991 Apr;95(4):578–582. doi: 10.1093/ajcp/95.4.578. [DOI] [PubMed] [Google Scholar]
- Kluytmans J. A., van der Willigen A. H., van Heyst B. Y., van der Meyden W. I., Stolz E., Wagenvoort J. H. Evaluation of an enzyme immunoassay for detection of Chlamydia trachomatis in urogenital specimens. Int J STD AIDS. 1990 Jan;1(1):49–52. doi: 10.1177/095646249000100112. [DOI] [PubMed] [Google Scholar]
- LeBar W., Herschman B., Jemal C., Pierzchala J. Comparison of DNA probe, monoclonal antibody enzyme immunoassay, and cell culture for the detection of Chlamydia trachomatis. J Clin Microbiol. 1989 May;27(5):826–828. doi: 10.1128/jcm.27.5.826-828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magder L. S., Klontz K. C., Bush L. H., Barnes R. C. Effect of patient characteristics on performance of an enzyme immunoassay for detecting cervical Chlamydia trachomatis infection. J Clin Microbiol. 1990 Apr;28(4):781–784. doi: 10.1128/jcm.28.4.781-784.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahony J. B., Chernesky M. A. Effect of swab type and storage temperature on the isolation of Chlamydia trachomatis from clinical specimens. J Clin Microbiol. 1985 Nov;22(5):865–867. doi: 10.1128/jcm.22.5.865-867.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer L. J., Robinson D. C., Sahm D. F., Lawrie M. J., Hajj S. N. Comparison of chemiluminescent DNA probe to cell culture for the screening of Chlamydia trachomatis in a gynecology clinic population. Obstet Gynecol. 1990 Jul;76(1):114–117. [PubMed] [Google Scholar]
- Ostergaard L., Birkelund S., Christiansen G. Use of polymerase chain reaction for detection of Chlamydia trachomatis. J Clin Microbiol. 1990 Jun;28(6):1254–1260. doi: 10.1128/jcm.28.6.1254-1260.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., Oda R., Alexander R., Greenwood J. R., de la Maza L. M. Molecular techniques for the detection of Chlamydia trachomatis. J Clin Microbiol. 1989 Oct;27(10):2359–2363. doi: 10.1128/jcm.27.10.2359-2363.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriprakash K. S., Macavoy E. S. Characterization and sequence of a plasmid from the trachoma biovar of Chlamydia trachomatis. Plasmid. 1987 Nov;18(3):205–214. doi: 10.1016/0147-619x(87)90063-1. [DOI] [PubMed] [Google Scholar]
- Stamm W. E. Diagnosis of Chlamydia trachomatis genitourinary infections. Ann Intern Med. 1988 May;108(5):710–717. doi: 10.7326/0003-4819-108-5-710. [DOI] [PubMed] [Google Scholar]
- Thewessen E. A., Freundt I., van Rijsoort-Vos J. H., Stolz E., Michel M. F., Wagenvoort J. H. Comparison of HeLa 229 and McCoy cell cultures for detection of Chlamydia trachomatis in clinical specimens. J Clin Microbiol. 1989 Jun;27(6):1399–1400. doi: 10.1128/jcm.27.6.1399-1400.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenvoort J. T., van Rijsoort-Vos T., Overkleeft-van de Ree A., Stolz E. Enhancement of yield of Chlamydia trachomatis Hela 229 cell culture. Eur J Clin Microbiol Infect Dis. 1988 Dec;7(6):822–822. doi: 10.1007/BF01975064. [DOI] [PubMed] [Google Scholar]
- Weisburg W. G., Hatch T. P., Woese C. R. Eubacterial origin of chlamydiae. J Bacteriol. 1986 Aug;167(2):570–574. doi: 10.1128/jb.167.2.570-574.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods G. L., Young A., Scott J. C., Jr, Blair T. M., Johnson A. M. Evaluation of a nonisotopic probe for detection of Chlamydia trachomatis in endocervical specimens. J Clin Microbiol. 1990 Feb;28(2):370–372. doi: 10.1128/jcm.28.2.370-372.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


