Abstract
Evidence suggests that insulin delivery to skeletal muscle interstitium is the rate-limiting step in insulin-stimulated muscle glucose uptake and that this process is impaired by insulin resistance. In this review we examine the basis for the hypothesis that insulin acts on the vasculature at three discrete steps to enhance its own delivery to muscle: (1) relaxation of resistance vessels to increase total blood flow; (2) relaxation of pre-capillary arterioles to increase the microvascular exchange surface perfused within skeletal muscle (microvascular recruitment); and (3) the trans-endothelial transport (TET) of insulin. Insulin can relax resistance vessels and increase blood flow to skeletal muscle. However, there is controversy as to whether this occurs at physiological concentrations of, and exposure times to, insulin. The microvasculature is recruited more quickly and at lower insulin concentrations than are needed to increase total blood flow, a finding consistent with a physiological role for insulin in muscle insulin delivery. Microvascular recruitment is impaired by obesity, diabetes and nitric oxide synthase inhibitors. Insulin TET is a third potential site for regulating insulin delivery. This is underscored by the consistent finding that steady-state insulin concentrations in plasma are approximately twice those in muscle interstitium. Recent in vivo and in vitro findings suggest that insulin traverses the vascular endothelium via a trans-cellular, receptor-mediated pathway, and emerging data indicate that insulin acts on the endothelium to facilitate its own TET. Thus, muscle insulin delivery, which is rate-limiting for its metabolic action, is itself regulated by insulin at multiple steps. These findings highlight the need to further understand the role of the vascular actions of insulin in metabolic regulation.
Keywords: Blood flow, Capillary, Caveolae, Endothelium, Insulin resistance, Insulin transport, Microvascular recruitment, Nitric oxide, Nitric oxide synthase, Skeletal muscle
Introduction
In this review we first examine the evidence that insulin delivery to muscle is rate-limiting for insulin-mediated glucose disposal (IMGD). Classical studies of the kinetics of whole body handling of infused insulin and the kinetic response of glucose disposal to insulin in vivo are considered. We then examine data regarding insulin access to muscle interstitium as estimated by lymphatic sampling, by microdialysis methods, and by estimates of insulin uptake by muscle. Combined, these data make the case that the delivery of insulin to muscle interstitium is rate-limiting for muscle IMGD and that insulin resistance may slow the rate of insulin delivery. We then provide an overview of several steps at which insulin, by acting on vascular tissue, may potentially regulate its own delivery to muscle interstitium. Finally, we consider evidence that one or more of these processes is disturbed in insulin-resistant states.
Insulin delivery to muscle - estimates from whole body insulin kinetics
Compartmental modelling
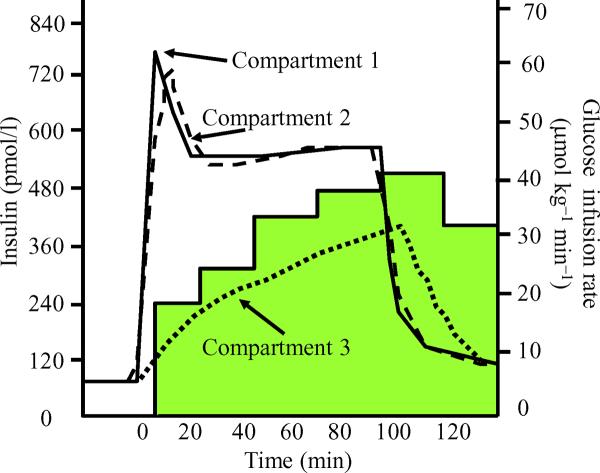
In the 1970s, Andres and colleagues published a series of studies directed at assessing the kinetics of porcine insulin handling in humans [1-3]. Their approach was based on a compartmental model and is an early example of the successful application of computational methods to metabolic studies. Based on measurements of the kinetics of the appearance and disappearance of plasma insulin, they suggested that, in addition to the plasma pool (Fig. 1, compartment 1), there are two other compartments to which insulin is distributed. The first is a small pool of insulin with a rapid turnover (compartment 2), and the second is a larger, more slowly equilibrating, remote insulin pool (compartment 3). The authors postulated that compartment 2 may include the liver and gut, while compartment 3 may correspond to the interstitial fluid compartment within skeletal muscle. They further estimated that the volume of compartment 3 constituted ~ 9.5% of body water and observed that the time-course for insulin equilibration with this pool closely paralleled the glucose infusion rate required to maintain euglycaemia using the insulin clamp method (Fig. 1). During a 1 or 2 mU min-1 kg-1 euglycaemic insulin infusion, 20-25 min were required for the half-maximal stimulation of glucose disposal, and an equivalent half-time (t½) was taken for insulin to fill the slowly equilibrated pool attributed to muscle (see Fig. 1). These pioneering studies of the kinetics of insulin and glucose disposal formed the basis for the development of the insulin clamp methodology that has defined our understanding of insulin action and resistance in vivo [3]. Subsequent studies of the distribution of the non-metabolised polysaccharide inulin confirmed that the interstitial fluid compartment within muscle is approximately 8-12% of muscle volume [4].
Fig. 1.
A euglycaemic-hyperinsulinaemic clamp (1 mU min-1 kg-1 insulin infusion) is initiated at time 0. The three lines illustrate the model-based time-course estimates for insulin concentration in three compartments (compartment 1, plasma; compartment 2, splanchnic bed; compartment 3, muscle). The shaded area illustrates the time-dependent changes in the rate of exogenous glucose infusion required to maintain euglycaemia during steady-state hyperinsulinaemia. Adapted from [1]
Kinetics of insulin-stimulated glucose disposal and suppression of glucose production
Using the euglycaemic-hyperinsulinaemic clamp method, multiple studies have demonstrated that IMGD increases progressively over time in response to a square wave increase in plasma insulin and requires 1-3 h to reach a steady state [5, 6]. Moreover, a more rapid response is observed with higher insulin infusion rates [6] (Table 1). Studies using the leg balance method (arterial-venous concentration difference × blood flow) confirm that muscle accounts for >80% of IMGD during a euglycaemic-hyperinsulinaemic clamp and is the site responsible for the time-dependent increases in whole body glucose disposal [7]. Interestingly, kinetic studies in humans indicate that the insulin-stimulated suppression of endogenous glucose production occurs more rapidly than changes in IMGD. The t½ for the suppression of endogenous glucose production is similar in healthy controls, in obesity [6] and type 2 diabetes [5]. This is not the case for IMGD, which has a t½ that is markedly slowed by insulin resistance (Table 1). In addition, endogenous glucose production appears to be suppressed at insulin concentrations lower than those required to stimulate IMGD. These findings suggest that the rate at which insulin is delivered to its receptor on these metabolically responsive target tissues may differ between liver and muscle, and that, for unexplained reasons, the liver is more sensitive than muscle to insulin. Several factors could contribute to these observations. For example, hepatic blood flow is approximately 100 ml 100g-1 min-1, whereas blood flow in resting muscle is 3-5 ml 100 g-1 min-1. There are also structural differences as regards the vasculature of these tissues. The liver has a highly permeable, fenestrated endothelium, whereas the endothelium of muscle is continuous and considered relatively `tight' [8, 9].
Table 1.
Steady-state glucose disposal rate t½ as a function of insulin infusion rate in lean and obese humans
Temporal dissociation between in vivo and in vitro insulin action on muscle
It is conceivable that the slow onset of insulin action on glucose disposal in vivo could be secondary to a slow response of the cellular machinery within the myocyte, the cell responsible for glucose uptake (e.g. activation of the insulin receptor or recruitment of GLUT4) to the membrane surface). However, when studied in vitro using isolated cells (which eliminates physical barriers to the binding of insulin to its receptor), these processes appear to be fully active in 2-5 min [10, 11]—a much more rapid time-course than that observed in vivo. Thus, the kinetics of insulin delivery determines the in vivo time-course for IMGD and this time dependence is quite slow relative to the time required for IMGD in isolated cell systems.
Measurements of muscle insulin concentrations: interstitial vs plasma insulin
Lymphatic sampling
The effect the time-dependent access of insulin to muscle interstitial space has on IMGD was clarified by the demonstration that insulin concentrations in thoracic duct lymph were persistently lower than those in plasma throughout a 3 h euglycaemic-hyperinsulinaemic clamp [12]. Furthermore, it was observed that, throughout the study, whole body IMGD correlated strongly with lymphatic, but not with plasma, insulin concentrations [12]. This study also demonstrated that the concentration dependence of insulin-stimulated glucose disposal (presumably occurring in muscle) is similar to that for the suppression of hepatic glucose production by insulin when the muscle lymph insulin concentration is considered [13] Subsequently, these same investigators observed that the temporal relationship of IMGD to hind leg lymphatic insulin concentration was even stronger than that seen for thoracic duct insulin [14]. Additional canine studies have confirmed that insulin activation of hepatic insulin signalling pathways occurs more promptly than insulin activation of muscle insulin signalling pathways [15]. Together, these findings strongly suggest an important role for insulin delivery to interstitium in the regulation of muscle glucose uptake. Support for the relevance of these findings to humans has been provided by a clinical study [16] that involved cannulation of lymphatic ductules in the foot to sample subcutaneous lymph during a euglycaemic-hyperinsulinaemic clamp. In both lean and obese humans, interstitial insulin temporally lagged behind arterial insulin concentrations, and a large arterial-interstitial insulin gradient was present throughout a 150 min insulin clamp. They also observed that the delay in transfer of insulin could not alone account for the decreased IMGD seen in the obese, insulin-resistant participants, indicating that compromised insulin action on the myocyte was also an important contributor to insulin resistance.
Studies comparing the time-course for insulin receptor tyrosine kinase activation with that for changes in interstitial insulin concentration during a euglycaemic-hyperinsulinaemic clamp confirmed that in vivo (in both dogs and humans) kinase activation occurs very promptly following increases in interstitial insulin, but is delayed relative to increases in plasma concentrations [15, 17]. This is in agreement with the extensive in vitro evidence demonstrating that activation of insulin signalling processes in isolated target cells and tissues occurs within a few minutes of the addition of insulin [10, 11].
Measurement of muscle interstitial insulin by microdialysis
A number of laboratories have used microdialysis methods to measure interstitial insulin concentrations in the skeletal muscle of humans [18-21] and experimental animals [18, 22]. It is difficult to obtain such measurements for insulin because of its slow diffusion rate across the dialysis membrane, typically reaching only 3-10% of the actual equilibrium concentration with interstitial media within the sampling times used [18, 20, 22]. Hence, a large `correction factor' is used to estimate interstitial insulin, which introduces significant variance to the measured value. Despite this caveat, in both human and animal studies, interstitial insulin concentrations estimated by microdialysis are consistently reported to be only 40-60% of simultaneously measured plasma concentrations, even after hours of steady-state hyperinsulinaemia (Table 2). As seen with lymphatic sampling, in the few microdialysis studies that have reported time-course data, interstitial insulin concentrations rise slowly over several hours after the initiation of an insulin clamp [19-21]. As the placement of microdialysis catheters increases local flow in subcutaneous tissue [23], it would be expected that the microdialysis catheter is sampling an area where perfusion limitations are, if anything, diminished. Therefore, the observed delayed increases in interstitial and steady-state insulin concentrations may actually overestimate the true interstitial concentrations and underestimate the arterial-interstitial insulin gradient.
Table 2.
Steady-state relationship between plasma and interstitial muscle insulin
| Study | Method | Species | Plasma insulin (pmol/l) | Interstitial insulin (% of plasma value) |
|---|---|---|---|---|
| Sjostrand et al. [18] | Microdialysis | Human | 900 | 42 |
| 2550 | 48 | |||
| Gudbjornsdottir et al. [21] | Microdialysis | Human | 1597 | 40 |
| 3979 | 45 | |||
| Herkner et al. [20] | Microdialysis | Human | 48 | 40 |
| 550 | 12 | |||
| Castillo et al. [16] | Lymph sampling | Human | 300-600 | 34 |
| Yang et al. [13] | Lymph sampling | Canine | 108 | 66 |
| 306 | 63 | |||
| Holmang et al. [22] | Microdialysis | Rat | 6000 | 42 |
Use of the limb balance technique to measure muscle insulin uptake
There are scant data from several early studies in which investigators measured forearm muscle insulin uptake using the limb balance method [24]. These studies reported that there was a positive net balance (uptake) of insulin by muscle, and that this was increased by exercise. As exercise increases muscle blood flow, this finding suggests that `perfusion' may affect insulin delivery. However, these studies were not followed-up in a systematic fashion. As a result, careful measurements of the kinetics of insulin uptake by skeletal muscle tissue are lacking.
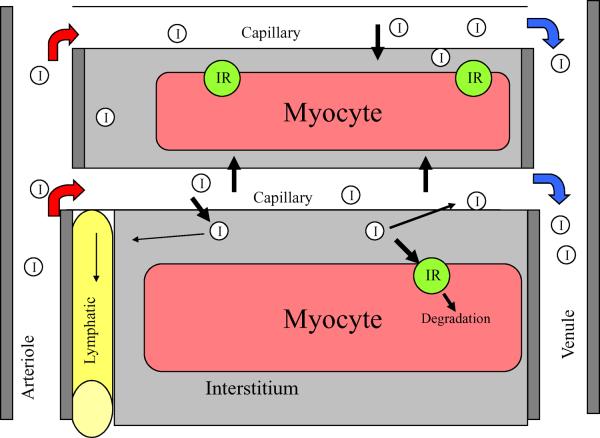
This issue was recently revisited and in healthy fasting human volunteers net forearm insulin uptake was, on average, ~80-120 fmol min-1 [100 ml]-1 during a 1 mU min-1 kg-1 insulin clamp [25]. At this rate, it would take approximately 25 min for the insulin concentration of the interstitium to reach that of plasma. However, this assumes that insulin that enters the interstitium remains there, whereas the fact that interstitial insulin does not reach plasma concentrations (even after several hours of steady-state hyperinsulinaemia) suggests its irreversible disappearance. This may be due to insulin catabolism by the endothelial cell (EC), the myocyte or by insulinases in muscle interstitium. Alternatively, insulin may return to the circulation through the lymphatic system (Fig. 2). Estimates of lymphatic flow from muscle [26] suggest that this route would be a very minor contributor to insulin disappearance (<5%). Though not thoroughly studied, insulin catabolism appears to be a minor fate of insulin taken up by the endothelium [27, 28]. Thus, myocyte uptake or degradation in the interstitium appears to be the major metabolic fate of insulin entering the muscle interstitium. In summary, the results of microdialysis and lymphatic sampling studies provide consistent support for a trans-endothelial insulin concentration gradient under a variety of physiological conditions.
Fig. 2.
A microvascular unit within muscle is composed of a terminal arteriole that feeds 12-20 capillaries and a draining vein. The capillary is the principal site of nutrient/hormone exchange between the muscle interstitium and blood. For insulin (I), the fraction that enters muscle interstitium may either be returned to the systemic circulation, through lymphatic drainage through reverse movement back across the EC (against a concentration gradient), or be taken up by the myocyte. via the insulin receptor (IR) and ultimately degraded
Muscle total blood flow and the `delivery' of insulin to muscle
Effects of insulin on total blood flow to skeletal muscle
As the above studies were building evidence for an important role for muscle interstitial insulin in regulating the onset of insulin action, Baron and colleagues introduced the novel concept that insulin could regulate its own delivery, and that of glucose, by increasing blood flow to muscle [29]. Indeed, they showed a remarkable correlation between the effect of insulin on whole body glucose uptake and the effect of insulin on leg blood flow over a broad range of insulin sensitivities in normal and insulin-resistant states [30-32]. These correlations were typically observed under steady-state conditions, several hours after the initiation of an insulin clamp, and did not specifically address the kinetic properties of insulin delivery described above. The relationship between muscle blood flow and glucose uptake was investigated by a number of other laboratories. Some [33-36], but by no means all [37-39], of these studies supported a regulatory role for insulin as a mediator of increases in total limb blood flow and, consequently, of glucose and insulin delivery to muscle. Additional, and perhaps more compelling, evidence for a role for blood flow in regulating muscle glucose uptake are the observations that increasing muscle blood flow with methacholine infusion during either a high- or low-dose insulin clamp enhances both leg blood flow and leg glucose uptake [40]. The vasodilatory action of insulin is dependent on nitric oxide generation. Inhibiting insulin-induced increases in blood flow with the nitric oxide synthase inhibitor L-NG-monomethyl arginine diminished both blood flow and glucose uptake [41, 42]. These studies have provided the most direct evidence for a role for total limb blood flow in regulating insulin-stimulated muscle glucose uptake.
Physiological relevance of the effect of insulin on total limb blood flow
As noted above, under physiological conditions, insulin has not uniformly been found to increase limb blood flow in humans [33-36, 39, 43-45]. In addition, while there is a report of physiological levels of insulin increasing muscle blood flow within 30 min [42], more typically, 2 h or more are required to see the effects of insulin on limb blood flow. This is not easily reconciled with a significant metabolic role for limb blood flow in regulating muscle up insulin delivery [37]. There is consensus that pharmacological insulin concentrations can more quickly increase limb blood flow and glucose uptake (Table 1). However, the significance of this is uncertain. Interestingly, increasing limb blood flow in insulin-resistant individuals by co-infusion of nitroprusside or bradykinin during a euglycaemic-hyperinsulinaemic clamp did not increase limb glucose uptake [46-48], arguing against a significant contribution of total blood flow to insulin resistance. It is unfortunate that none of the limb balance studies examining the effects of insulin on limb blood flow and muscle glucose metabolism included measurements of muscle insulin uptake or lymphatic or muscle interstitial insulin concentrations. Such data could have provided information on whether insulin resistance, as seen in diabetes or obesity, affects peripheral insulin handling. Nonetheless, on balance, there appears to be evidence that insulin can promote increases in limb blood flow in humans. Whether this occurs sufficiently rapidly at physiologically encountered insulin concentrations to contribute to IMGD in healthy individuals is uncertain. Consequently, whether impairments in the effect of insulin on total muscle blood flow play an important role in the derangements in IMGD seen in obesity and type 2 diabetes remains to be determined. However, these findings, and the controversy that surrounded them, prompted the reexamination of the discrete processes involved in the delivery of insulin to muscle.
Extraction ratios of insulin and glucose across skeletal muscle
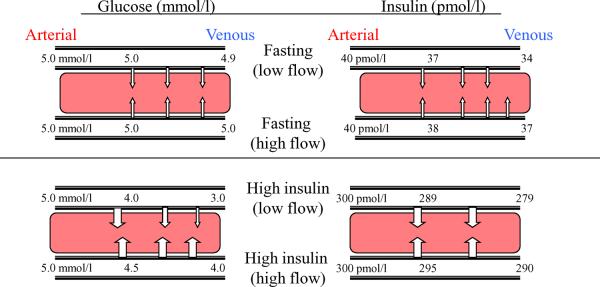
The interaction between the extraction ratios of glucose and insulin across muscle and the delivery of these compounds via changes in total blood flow deserves discussion. In the basal state, the arterial-venous glucose concentration difference across skeletal muscle in humans is only approximately 0.1 mmol/l, or an extraction ratio of 1-2% [25, 31]. It is readily appreciated that increasing blood flow will have little or no impact on total glucose uptake by the tissue in the absence of an appreciable arterial-venous concentration gradient (Fig. 3). However, when insulin concentrations are markedly elevated, the arterial-venous glucose gradient across muscle can approach 2-3 mmol/l under euglycaemic conditions in insulin-responsive individuals [31, 41, 42]. Clearly, increasing blood flow to a given vascular bed will have the effect of increasing the plasma-interstitial glucose gradient at the distal ends of the vascular bed and potentially cause a significant increase in glucose uptake. It is under these conditions that significant correlations between leg or forearm blood flow and whole body glucose disposal have been most consistently demonstrated [49]. It follows that, in insulin-resistant individuals, in whom the arterial-venous glucose concentration difference is reduced for any given insulin concentration, increasing blood flow would increase the plasma-interstitial gradient within muscle and promote glucose uptake to a lesser extent than in controls. This may, in part, explain why the use of vasodilators to increase limb blood flow has generally been ineffective in increasing muscle glucose uptake in insulin-resistant individuals [47, 50].
Fig. 3.
The effect of increasing blood flow on the uptake of glucose and insulin by muscle tissue for post-absorptive and steady-state hyperinsulinaemic conditions. For glucose, after an overnight fast there is a very small concentration gradient between the arterial and venous ends of a capillary bed. Increasing the rate of blood flow will only minimally raise the mean capillary glucose concentration and will have a negligible effect on glucose uptake. In contrast, under high insulin conditions the glucose concentration gradient between arterial and venous plasma can reach 2-3 mmol/l. Increasing delivery of glucose from the arterial system by increasing flow could therefore substantially increase the mean capillary plasma glucose concentration and have a significant impact on trans-endothelial glucose uptake. For insulin, the circumstances that generate a concentration gradient are different. After an overnight fast the concentration of insulin in plasma draining skeletal muscle is 10-15% lower than that in arterial plasma. Increasing flow might be expected to produce a modest increase in mean capillary plasma insulin concentration and enhance insulin TET. When insulin concentrations are raised the total insulin uptake increases. However, the extraction fraction declines to approximately 7% at insulin concentrations of ~250 pmol/L (indicating that delivery alone is not determining insulin uptake) and any increases in flow would be predicted to have a minimally effect insulin TET
Conditions are only slightly more favourable for insulin uptake than for glucose uptake by muscle in the post-absorptive state. In healthy humans the insulin extraction ratio across the human forearm is 10-15% after an overnight fast [24, 25]. This ratio falls to ~7% during a 1 mU min-1 kg-1 insulin clamp [25]. As a result, increasing total muscle blood flow could increase the intravascular-interstitial insulin gradient and thereby increase tissue insulin uptake only modestly. Such observations serve to emphasise the importance of blood flow in circumstances in which the extraction ratio is high (e.g. glucose extraction by muscle can approach 50% under maximally insulinised circumstances) and the relatively minor role of total blood flow changes under circumstances in which the extraction ratio for the observed metabolite or hormone is small.
Insulin action on muscle microvasculature: microvascular recruitment to increase muscle insulin delivery
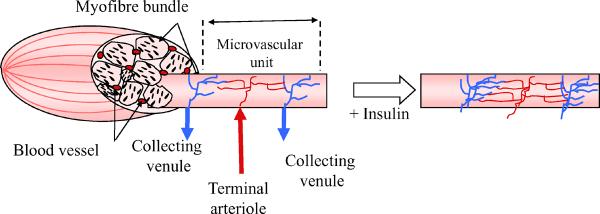
We have previously emphasised that expansion of the endothelial surface area available for exchange of insulin, glucose or other nutrients through the recruitment of additional microvasculature within muscle can enhance nutrient delivery to the tissue, even under circumstances where the extraction ratio is small, provided there is a demonstrable intravascular-interstitial gradient [43]. It follows from simple consideration of Fick's law, F=PS(Cp-Ci), where F is the flux of insulin, Cp and Ci are the plasma and interstitial insulin concentrations, respectively, and PS is the product of the permeability and surface area of the transporting endothelium (Fig. 4), that an increase in endothelial surface area will increase flux when all other variables are held constant. Whether endothelial permeability to insulin may also be regulated is discussed below. Progress in evaluating the potential role of microvascular recruitment, particularly in clinical studies, has been slowed by the lack of available methods to quantify the microvascular volume perfused at a given time. Two methods—one based on measurement of 1-methylxanthine extraction (an index of endothelial surface available for nutrient exchange) [51], and one that uses contrast-enhanced ultrasound (which provides a measure of microvascular volume) [52, 53]—have been developed to assess the effect of insulin [51, 52, 54, 55], feeding [56], muscle contraction [56, 57] and insulin resistance [58, 59] on muscle microvasculature. This process of microvascular recruitment involves the dilation of terminal arterioles, each of which feeds 12-20 capillaries. Under basal conditions, vasomotion appears to determine the extent of vasodilation of terminal arterioles and, by consequence, the extent to which a capillary network is perfused at any time. Insulin may act to simply modulate the dilation or constriction rate constant to extend the period of perfusion. The cellular mechanisms responsible for insulin- or exercise-induced microvascular recruitment cannot be fully addressed using the methods available at present. Efforts to examine this issue using video-microscopic methods have shown that insulin can relax very small muscle arterioles [60]. However, actually visualising the recruitment process has been problematic. Efforts to use video-microscopy of thin muscle preparations (e.g. cremaster, spinotrapezius) have been hampered because the sensitivity of these preparations to the process of surgical exposure and superfusate O2 and CO2 [61] is such that in the `basal state' the microvasculature is extensively recruited, which means that further changes with insulin are not reproducibly observed [62].
Fig. 4.
Effect of increasing microvascular volume on the potential uptake of insulin or glucose by muscle tissue based on Fick's law. The architecture of the microvascular unit is shown within the myofibre bundle to the left of the white arrow. The terminal arteriole supplies a small network of capillaries that run longitudinally along the myofibre. The principal surface area available for nutrient exchange is within the endothelial surface of the capillary bed. In the presence of insulin the terminal arteriole relaxes and additional capillaries within the microvascular unit are more richly perfused. Using the contrast ultrasound method this is seen as an increase in microvascular volume
At present, successful efforts to visually examine the microvascular action of insulin have come from work examining skin microvasculature. Using capillary video-microscopy and laser Doppler flowmetry, Stehouwer and colleagues have reported that, in healthy individuals, systemic hyperinsulinaemia increases skin microvascular perfusion and increases the number of nailbed capillaries carrying erythrocytes [63]. This provides direct evidence for the regulation of capillary perfusion by insulin in humans. The same investigators have demonstrated that capillary recruitment in both the nailbed and skin is blunted by the insulin resistance seen in hypertension [64], obesity [65, 66], elevated plasma NEFA concentrations [67] and the metabolic syndrome [68]. These last two observations are particularly interesting as they suggest that altered microvascular responses may be an early dysfunction in individuals at risk of diabetes.
There is concern as to whether the vascular responses observed in skin reflect those in muscle, as the vasculature of skin is highly specialised for processes such as heat exchange. Nevertheless, these studies provide direct visual evidence that insulin can regulate the microvasculature and, specifically, can expand the surface area available for nutrient exchange.
Relevance of microvascular recruitment to muscle insulin resistance
Impaired microvascular recruitment leading to a decreased surface area available for nutrient exchange may be important to skeletal muscle insulin resistance. Muscle insulin resistance is considered to involve an impairment in the recruitment of GLUT4 glucose transporters to the myocyte sarcolemma [69, 70]. This appears to be secondary to alterations early in the insulin signalling cascade, involving the phosphorylation of serine residues of insulin receptor substrates [71]. Available data in humans and animals suggest that these myocyte processes occur in parallel with impairments in insulin action within skeletal muscle microvasculature. While studies have shown that diminished IMGD is accompanied by functional changes in the microvasculature, data demonstrating biochemical correlates of this within microvascular cells in vivo are lacking. For example, the infusion of TNFα [72] or NEFA [73] into rodents inhibits insulin-stimulated microvascular recruitment and interferes with insulin signalling to nitric oxide synthase in cultured ECs [74, 75]. Their effects on NO generation in situ have not been defined, but in isolated vessels, TNFα [76] and NEFA [77] are known to block NO-dependent vasodilation. Clearly, there may be interrelated aspects to these observations, in that impaired insulin transport into muscle interstitium, through diminished total blood flow, decreased capillary recruitment or impaired movement of insulin across the endothelial barrier (see below), would affect muscle interstitial insulin concentrations and therefore insulin action at the myocyte.
Relevance to hypertension
The EC responds to insulin by increasing the phosphorylation of endothelial nitric oxide synthase (eNOS) on serine 1177, and this increases Ca2+-independent nitric oxide synthase activity [78, 79]. Interestingly, insulin also activates the mitogen-activated protein kinase pathway in ECs, which enhances the generation of the vasoconstrictor endothelin-1 [80]. This can lead to insulin-stimulated vasoconstriction if signalling from the insulin receptor to eNOS is inhibited pharmacologically or downregulated by insulin resistance [62, 68, 76]. In this manner, endothelial insulin resistance may contribute to the development of hypertension and account in part for the epidemiological relationship between hypertension and insulin resistance [68].
Movement of insulin across the endothelial lining of the microvasculature within muscle
Beyond the relaxing effect of insulin on resistance vessels (increasing flow) and terminal arterioles (recruiting the microvasculature), insulin may exert a third highly significant endothelial action to promote its own movement across the EC barrier. As with studies on the effects of insulin on total limb blood flow, studies on the trans-endothelial transport (TET) of insulin have at times yielded seemingly conflicting findings. For example, early work by King & Johnson using cultured ECs reported that the transendothelial movement of insulin was saturable and blocked by antibodies to the exofacial domain of the insulin receptor [81], and several additional studies supported this finding [27, 82-84]. However, two other studies, both of which reported observations in vivo, suggested that the TET of insulin was not saturable and involved a passive diffusional process, perhaps similar to the TET of inulin [85, 86]. Indeed, whether insulin crosses the endothelium by a cellular or a paracellular pathway has been a source of some controversy. The continuous endothelium of muscle forms a relatively tight barrier that could prevent insulin from freely diffusing and thus sustain the significant plasma-interstitial insulin gradient in muscle, discussed above.
ECs express substantially more insulin-like growth factor-I (IGF-I) receptors than insulin receptors [87]. Experiments using fluorochrome-labelled insulin and cultured bovine aortic ECs grown on transwell plates suggested that insulin TET is inhibited by insulin, IGF-I and antibody to the IGF-I receptor [84]. Early studies demonstrated that ECs accumulate and only slowly metabolise 125I-labelled insulin [27, 28]. Such observations certainly suggest that a receptor-mediated trans-cellular insulin transport process may mediate insulin TET.
Intravenously infused fluorescein isothiocyanate (FITC)-labelled insulin was shown by confocal microscopy to be rapidly localised within the vascular endothelium of skeletal muscle in vivo [84]. There was no evidence for movement of the labelled-insulin between ECs, and the intensity of the EC labelling was substantial, suggesting that the uptake process may even concentrate insulin within the EC.
Role of caveolin-1
Further microscopic studies suggested the co-localisation of insulin with the insulin receptor and the caveolar structural protein caveolin-1 in ECs. Furthermore, the insulin receptor co-immunoprecipitated with caveolin-1, and disruption of the caveolae using filipin, cyclodextrin [84] or, more recently, small interfering RNA directed against caveolin-1 [88] inhibited insulin uptake by cultured ECs. In an early electron microscopic investigation, exposure of the eel rete mirabili to 0.4 mmol/l immunogold-labelled insulin showed that insulin associates with caveolae-like structures within the EC [89]. Whether this reflects pathways used at physiological insulin concentrations is unclear.
Evidence for a saturable insulin TET pathway
If insulin associates with its receptor for TET, it would be expected to display saturation kinetics in response to stepwise increments in insulin concentration. Early in vitro studies by King and Johnson [81] and others [90] suggested this was the case. However, in the canine hindlimb [85], the appearance of insulin in lymphatic drainage did not reach saturation point when plasma insulin was raised from high physiological to pharmacological concentrations. This finding was interpreted as evidence against a receptor-mediated process for insulin uptake. However, as the endothelium contains an abundance of IGF-I receptors (that are also capable of transporting insulin [84]), the observed insulin TET in vivo might not follow simple saturation kinetics, particularly at very high insulin concentrations, when the IGF-I receptor contribution may dominate. A recent study examined `saturability' of insulin TET in vivo using insulin concentrations below those associated with IGF-I receptor activation [25]. In this human forearm study, insulin clearance decreased when plasma insulin concentrations were raised within the physiological range (approximately 30-300 pmol/l), implicating the involvement of a saturable transport process in the movement of insulin out of the vasculature. This observation, together with findings from studies of insulin transport across EC monolayers, strongly points to the EC insulin receptor as a mediator of insulin entry into muscle (Fig. 5). The possibility that insulin TET involves caveolae shows remarkable similarities with current theories for albumin TET [91, 92].
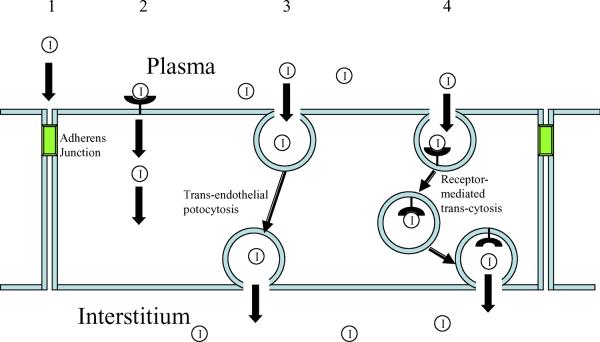
Fig. 5.
The four potential pathways by which insulin may move from the vascular lumen to the interstitium of muscle. The possibility of movement via a paracellular pathway (1) through the junctional structure appears to be unlikely in either the arteriole or capillary bed within skeletal muscle, which has a continuous endothelium. This pathway may be open in small venules, or under circumstances where the endothelium is rendered `leaky'. Insulin could bind to a receptor anywhere on the plasma membrane, leading to internalisation, diffusion to the anti-luminal membrane of the EC and subsequent release into the muscle interstitium (2). Caveolae may allow the passage of insulin as part of a bulk fluid movement in the setting of vesicular trafficking (3). Because of the low plasma concentration of insulin, movement in this manner would be a relatively rare event. Caveolae may also function as areas of insulin receptor localisation. This would facilitate receptor-mediated association of insulin with transporting caveolae and increase the probability of insulin transiting the endothelium as part of a vesicular trafficking process (4)
From the dimensions of caveolae (~800 Å diameter), one can estimate that passive EC insulin uptake by caveolar potocytosis would provide only one insulin molecule per 5000 caveolae at physiological insulin concentrations. The localisation of high-affinity insulin receptors within caveolae would allow efficient insulin trapping and could greatly increase the likelihood of insulin moving across the endothelium. Beyond simply trapping insulin, which can then shuttle between the luminal and ablumenal face of the EC, an intriguing question is whether insulin acts on the cell to stimulate caveolar-mediated insulin transport. By parallel, albumin stimulates a Src kinase-mediated pathway to enhance caveolar-mediated albumin uptake. Multiple studies show that insulin can stimulate classical insulin signalling pathways in ECs [74, 75, 93, 94]. As mentioned above, eNOS is a substrate for Akt in the EC [93]. Nearly all studies that have examined EC insulin signalling have used insulin concentrations (10-100 nmol/l) that activate both insulin and IGF-1 receptors. In a dose-response study, 100 pmol/l insulin enhanced the phosphorylation of Akt, eNOS and ERK [95], and this was not blocked by pre-incubation of the cells with an antibody to the exofacial domain of the IGF-I receptor, arguing for a role for physiological insulin concentrations. Recent data indicate that inhibition of either phosphatidylinositol 3-kinase (wortmannin), ERK (PD 98059) or tyrosine kinase activity (genistein) diminishes endothelial uptake of FITC-labelled insulin [96], suggesting that insulin, like albumin, can promote its own movement into ECs [96]. Based on the data presented, it is reasonable to hypothesise that insulin exerts yet a third action on endothelium—facilitation of TET by binding to its receptor. Indeed, beyond binding, it may also act on the EC to stimulate its own uptake and transport across the vascular endothelium. In this line, inflammatory factors (e.g. TNFα) and oxidative stress, which provoke insulin resistance both in vivo and in vitro, have been observed to diminish both insulin-induced activation of Akt and eNOS and insulin uptake by cultured bovine aortic ECs [96].
A seeming difficulty with hypothesising a major role for EC insulin action in the overall effects of insulin in muscle is the that EC-specific insulin receptor deletion in mice did not alter insulin sensitivity as assessed by the insulin clamp [97]. It was not addressed whether mechanisms (e.g. greater endothelial permeability, increased IGF-1 receptor concentration) compensated for the loss of insulin receptors. Recent observations that EC-specific knockout of IRS-2 [98] produced insulin resistance (insulin clamp) and decreased both the transport of insulin to muscle interstitium and insulin-induced changes in muscle blood flow suggest an important role for EC insulin action. This important adapter/signalling protein is downstream of both the insulin and IGF-1 receptors.
Summary
Over the past two decades, it has been increasingly recognised that vascular tissue, particularly the EC, is a potentially important physiological target for insulin. It appears that insulin can act on ECs at multiple levels of the vasculature. In addition to the vascular actions explicitly addressed in this review, insulin has been shown to acutely relax conduit vessels in healthy, but not in insulin-resistant, individuals [99]. This decreased compliance is unlikely to influence insulin or glucose delivery to muscle, but may, over time, contribute to vascular injury within the walls of conduit vessels. At the level of resistance arterioles, terminal arterioles and capillaries within muscle, resistance to the actions of insulin may impede insulin delivery to skeletal muscle interstitium and thereby contribute significantly to insulin resistance. Postprandially, the resulting compensatory hyperinsulinaemia will promote hepatic triacylglycerol synthesis as an alternative fate for ingested carbohydrate. This could set up a positive feedback loop, whereby elevated triacylglycerol or NEFA levels provoke further increases in vascular insulin resistance [73]. The quantitative contribution of slowed muscle insulin delivery to overall insulin and glucose homeostasis in states of insulin resistance will require considerable further study. Fortunately, the tools necessary to probe this important aspect of insulin action are increasingly becoming available.
Acknowledgements
This work was supported by National Institutes of Health grants DK-057878, DK-073759, DK063609 (to E. J. Barrett) and RR00847 (to the University of Virginia General Clinical Research Center). We thank M. Thorner (University of Virginia, Charlottesville, VA, USA), J. Yudkin (University College London, London, UK) and E. Eringa (VU University Medical Center, Amsterdam, the Netherlands) for thoughtful suggestions for the manuscript.
Abbreviations
- EC
Endothelial cell
- enos
Endothelial nitric oxide synthase
- ERK
Extracellular receptor kinase
- FITC
Fluorescein isothiocyanate
- IGF-I
insulin-like growth factor-I
- IMGD
Insulin-mediated glucose disposal
- TET
Trans-endothelial transport
- t½
Half-time
Footnotes
Duality of interest The authors declare that there is no duality of interest associated with this manuscript.
References
- [1].Sherwin RS, Kramer KJ, Tobin JD, et al. A model of the kinetics of insulin in man. J Clin Invest. 1974;53:1481–1492. doi: 10.1172/JCI107697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].McGuire EA, Tobin JD, Berman M, Andres R. Kinetics of native insulin in diabetic, obese, and aged men. Diabetes. 1979;28:110–120. doi: 10.2337/diab.28.2.110. [DOI] [PubMed] [Google Scholar]
- [3].DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique, a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- [4].Holmang A, Bjorntorp P, Rippe B. Tissue uptake of insulin and inulin in red and white skeletal muscle in vivo. Am J Physiol. 1992;263:H1170–H1176. doi: 10.1152/ajpheart.1992.263.4.H1170. [DOI] [PubMed] [Google Scholar]
- [5].Turk D, Alzaid A, Dinneen S, Nair KS, Rizza R. The effects of noninsulin-dependent diabetes mellitus on the kinetics of onset of insulin action in hepatic and extrahepatic tissues. J Clin Invest. 1995;95:755–762. doi: 10.1172/JCI117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Prager R, Wallace P, Olefsky JM. In vivo kinetics of insulin action on peripheral glucose disposal and hepatic glucose output in normal and obese subjects. J Clin Invest. 1986;78:472–481. doi: 10.1172/JCI112599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].DeFronzo R, Gunnarsson R, Ojorkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985;76:149–155. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- [9].Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- [10].Ogihara T, Shin B-C, Anai M, et al. Insulin receptor substrate (IRS)-2 is dephosphorylated more rapidly than IRS-1 via its association with phosphatidylinositol 3-kinase in skeletal muscle cells. J Biol Chem. 1997;272:12868–12873. doi: 10.1074/jbc.272.19.12868. [DOI] [PubMed] [Google Scholar]
- [11].Karnieli E, Zarnowski MJ, Hissin PJ, Simpson IA, Salans LB, Cushman SW. Insulin-stimulated translocation of glucose transport systems in the isolated rat adipose cell. Time course, reversal, insulin concentration dependency, and relationship to glucose transport activity. J Biol Chem. 1981;256:4772–4777. [PubMed] [Google Scholar]
- [12].Yang YJ, Hope ID, Ader M, Bergman RN. Insulin transport across capillaries is rate limiting for insulin action in dogs. J Clin Invest. 1989;84:1620–1628. doi: 10.1172/JCI114339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yang YJ, Hope I, Ader M, Poulin RA, Bergman RN. Dose-response relationship between lymph insulin and glucose uptake reveals enhanced insulin sensitivity of peripheral tissues. Diabetes. 1992;41:241–253. doi: 10.2337/diabetes.41.2.241. [DOI] [PubMed] [Google Scholar]
- [14].Poulin RA, Steil GM, Moore DM, Ader M, Bergman RN. Dynamics of glucose production and uptake are more closely related to insulin in hindlimb lymph than in thoracic duct lymph. Diabetes. 1994;43:180–190. doi: 10.2337/diab.43.2.180. [DOI] [PubMed] [Google Scholar]
- [15].Miles PD, Levisetti M, Reichart D, Khoursheed M, Moossa AR, Olefsky JM. Kinetics of insulin action in vivo. Identification of rate-limiting steps. Diabetes. 1995;44:947–953. doi: 10.2337/diab.44.8.947. [DOI] [PubMed] [Google Scholar]
- [16].Castillo C, Bogardus C, Bergman R, Thuillez P, Lillioja S. Interstitial insulin concentrations determine glucose uptake rates but not insulin resistance in lean and obese men. J Clin Invest. 1994;93:10–16. doi: 10.1172/JCI116932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Freidenberg GR, Suter S, Henry RR, Nolan J, Reichart D, Olefsky JM. Delayed onset of insulin activation of the insulin receptor kinase in vivo in human skeletal muscle. Diabetes. 1994;43:118–126. doi: 10.2337/diab.43.1.118. [DOI] [PubMed] [Google Scholar]
- [18].Sjostrand M, Holmang A, Lonnroth P. Measurement of interstitial insulin in human muscle. Am J Physiol. 1999;276:E151–E154. doi: 10.1152/ajpendo.1999.276.1.E151. [DOI] [PubMed] [Google Scholar]
- [19].Sjostrand M, Gudbjornsdottir S, Holmang A, Lonn L, Strindberg L, Lonnroth P. Delayed transcapillary transport of insulin to muscle interstitial fluid in obese subjects. Diabetes. 2002;51:2742–2748. doi: 10.2337/diabetes.51.9.2742. [DOI] [PubMed] [Google Scholar]
- [20].Herkner H, Klein N, Joukhadar C, et al. Transcapillary insulin transfer in human skeletal muscle. Eur J Clin Invest. 2003;33:141–146. doi: 10.1046/j.1365-2362.2003.01106.x. [DOI] [PubMed] [Google Scholar]
- [21].Gudbjornsdottir S, Sjostrand M, Strindberg L, Wahren J, Lonnroth P. Direct measurements of the permeability surface area for insulin and glucose in human skeletal muscle. J Clin Endocrinol Metab. 2003;88:4559–4564. doi: 10.1210/jc.2003-030434. [DOI] [PubMed] [Google Scholar]
- [22].Holmang A, Mimura K, Bjorntorp P, Lonnroth P. Interstitial muscle insulin and glucose levels in normal and insulin-resistant Zucker rats. Diabetes. 1997;46:1799–1804. doi: 10.2337/diab.46.11.1799. [DOI] [PubMed] [Google Scholar]
- [23].Anderson C, Andersson T, Wardell K. Changes in skin circulation after insertion of a microdialysis probe visualized by laser Doppler perfusion imaging. J Invest Dermatol. 1994;102:807–811. doi: 10.1111/1523-1747.ep12378630. [DOI] [PubMed] [Google Scholar]
- [24].Kalant N, Leibovici T, Rohan I, Ozaki S. Interrelationships of glucose and insulin uptake by muscle of normal and diabetic man. Evidence of a difference in metabolism of endogenous and exogenous insulin. Diabetologia. 1979;16:365–372. doi: 10.1007/BF01223156. [DOI] [PubMed] [Google Scholar]
- [25].Eggleston EM, Jahn LA, Barrett EJ. Hyperinsulinaemia rapidly increases human muscle microvascular perfusion but fails to increase muscle insulin clearance: evidence that a saturable process mediates muscle insulin uptake. Diabetes. 2007;56:2958–2963. doi: 10.2337/db07-0670. [DOI] [PubMed] [Google Scholar]
- [26].Renkin EM, Wiig H. Limits to steady-state lymph flow rates derived from plasma-to-tissue uptake measurements. Microvasc Res. 1994;47:318–328. doi: 10.1006/mvre.1994.1025. [DOI] [PubMed] [Google Scholar]
- [27].Dernovsek KD, Bar RS. Processing of cell-bound insulin by capillary and macrovascular endothelial cells in culture. Am J Physiol. 1985;248:E244–E251. doi: 10.1152/ajpendo.1985.248.2.E244. [DOI] [PubMed] [Google Scholar]
- [28].Jialal I, King GL, Buchwald S, Kahn CR, Crettaz M. Processing of insulin by bovine endothelial cells in culture. Internalization without degradation. Diabetes. 1984;33:794–800. doi: 10.2337/diab.33.8.794. [DOI] [PubMed] [Google Scholar]
- [29].Baron A. Hemodynamic actions of insulin. Am J Physiol. 1994;267:E187–E202. doi: 10.1152/ajpendo.1994.267.2.E187. [DOI] [PubMed] [Google Scholar]
- [30].Baron AD, Laakso M, Brechtel G, Edelman SV. Mechanism of insulin resistance in insulin-dependent diabetes mellitus: a major role for reduced skeletal muscle blood flow. J Clin Endocrinol Metab. 1991;73:637–643. doi: 10.1210/jcem-73-3-637. [DOI] [PubMed] [Google Scholar]
- [31].Laakso M, Edelman SV, Brechtel G, Baron AD. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. J Clin Invest. 1990;85:1844–1852. doi: 10.1172/JCI114644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Laakso M, Edelman SV, Brechtel G, Baron AD. Impaired insulin-mediated skeletal muscle blood flow in patients with NIDDM. Diabetes. 1992;41:1076–1083. doi: 10.2337/diab.41.9.1076. [DOI] [PubMed] [Google Scholar]
- [33].Vollenweider P, Tappy L, Randin D, et al. Differential effects of hyperinsulinaemia and carbohydrate metabolism on sympathetic nerve activity and muscle blood flow in humans. J Clin Invest. 1993;92:147–154. doi: 10.1172/JCI116542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Raitakari M, Knuuti MJ, Ruotsalainen U, et al. Insulin increases blood volume in human skeletal muscle: studies using [15O]CO and positron emission tomography. Am J Physiol. 1995;269:E1000–E1005. doi: 10.1152/ajpendo.1995.269.6.E1000. [DOI] [PubMed] [Google Scholar]
- [35].Raitakari M, Nuutila P, Knuuti J, et al. Effects of insulin on blood flow and volume in skeletal muscle of patients with IDDM: studies using [15O]H2O, [15O]CO, and positron emission tomography. Diabetes. 1997;46:2017–2021. doi: 10.2337/diab.46.12.2017. [DOI] [PubMed] [Google Scholar]
- [36].Tack CJJ, Ong MKE, Lutterman JA, Smits P. Insulin-induced vasodilatation and endothelial function in obesity/insulin resistance—effects of troglitazone. Diabetologia. 1998;41:569–576. doi: 10.1007/s001250050948. [DOI] [PubMed] [Google Scholar]
- [37].Yki-Jarvinen H, Utriainen T. Insulin-induced vasodilatation: physiology or pharmacology? Diabetologia. 1998;41:369–379. doi: 10.1007/s001250050919. [DOI] [PubMed] [Google Scholar]
- [38].Taddei S, Virdis A, Mattei P, Natali A, Ferrannini E, Salvetti A. Effect of insulin on acetylcholine-induced vasodilation in normotensive subjects and patients with essential hypertension. Circulation. 1995;92:2911–2918. doi: 10.1161/01.cir.92.10.2911. [DOI] [PubMed] [Google Scholar]
- [39].Bonadonna RC, Saccomani MP, Delprato S, Bonora E, Defronzo RA, Cobelli C. Role of tissue-specific blood flow and tissue recruitment in insulin-mediated glucose uptake of human skeletal muscle. Circulation. 1998;98:234–241. doi: 10.1161/01.cir.98.3.234. [DOI] [PubMed] [Google Scholar]
- [40].Baron AD, Steinberg H, Brechtel G, Johnson A. Skeletal muscle blood flow independently modulates insulin-mediated glucose uptake. Am J Physiol. 1994;266:E248–E253. doi: 10.1152/ajpendo.1994.266.2.E248. [DOI] [PubMed] [Google Scholar]
- [41].Steinberg HO, Brechtel G, Johnson A, Fineberg F, Baron AD. Insulinmediated skeletal muscle vasodilation is nitric oxide dependent: a novel action of insulin to increase nitric oxide release. J Clin Invest. 1994;94:1172–1179. doi: 10.1172/JCI117433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Baron AD, Brechtel-Hook G, Johnson A, Cronin J, Leaming R, Steinberg HO. Effect of perfusion rate on the time course of insulin-mediated skeletal muscle glucose uptake. Am J Physiol. 1996;271:E1067–E1072. doi: 10.1152/ajpendo.1996.271.6.E1067. [DOI] [PubMed] [Google Scholar]
- [43].Vincent MA, Clerk LH, Rattigan S, Clark MG, Barrett EJ. Active role for the vasculature in the delivery of insulin to skeletal muscle. Clin Exp Pharmacol Physiol. 2005;32:302–307. doi: 10.1111/j.1440-1681.2005.04188.x. [DOI] [PubMed] [Google Scholar]
- [44].Cleland SJ, Petrie JR, Ueda S, Elliott HL, Connell JMC. Insulin-mediated vasodilation and glucose uptake are functionally linked in humans. Hypertension. 1999;33:554–558. doi: 10.1161/01.hyp.33.1.554. [DOI] [PubMed] [Google Scholar]
- [45].Natali A, Buzzigoli G, Taddei S, et al. Effects of insulin on hemodynamics and metabolism in human forearm. Diabetes. 1990;39:490–500. doi: 10.2337/diab.39.4.490. [DOI] [PubMed] [Google Scholar]
- [46].Natali A, Quinones Galvan A, Pecori N, Sanna G, Toschi E, Ferrannini E. Vasodilation with sodium nitroprusside does not improve insulin action in essential hypertension. Hypertension. 1998;31:632–636. doi: 10.1161/01.hyp.31.2.632. [DOI] [PubMed] [Google Scholar]
- [47].Nuutila P, Raitakari M, Laine H, et al. Role of blood flow in regulating insulin-stimulated glucose uptake in humans: Studies using bradykinin, [15O]water, and [18F]fluoro-deoxy-glucose and positron emission tomography. J Clin Invest. 1996;97:1741–1747. doi: 10.1172/JCI118601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Laine M, Ykijarvinen H, Kirvela O, et al. Insulin resistance of glucose uptake in skeletal muscle cannot be ameliorated by enhancing endothelium-dependent blood flow in obesity. J Clin Invest. 1998;101:1156–1162. doi: 10.1172/JCI1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Baron AD, Steinberg HO, Chaker H, Learning R, Johnson A, Brechtel G. Insulin-mediated skeletal muscle vasodilation contributes to both insulin sensitivity and responsiveness in lean humans. J Clin Invest. 1995;96:786–792. doi: 10.1172/JCI118124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Natali A, Galvan AQ, Pecori N, Sanna G, Toschi E, Ferrannini E. vasodilation with sodium nitroprusside does not improve insulin action in essential hypertension. Hypertension. 1998;31:632–636. doi: 10.1161/01.hyp.31.2.632. [DOI] [PubMed] [Google Scholar]
- [51].Rattigan S, Clark MG, Barrett EJ. Hemodynamic actions of insulin in rat skeletal muscle: Evidence for capillary recruitment. Diabetes. 1997;46:1381–1388. doi: 10.2337/diab.46.9.1381. [DOI] [PubMed] [Google Scholar]
- [52].Coggins MP, Lindner J, Rattigan S, et al. Physiologic hyperinsulinaemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes. 2001;50:2682–2690. doi: 10.2337/diabetes.50.12.2682. [DOI] [PubMed] [Google Scholar]
- [53].Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab. 2003;285:E123–E129. doi: 10.1152/ajpendo.00021.2003. [DOI] [PubMed] [Google Scholar]
- [54].Vincent MA, Clerk LH, Lindner JR, et al. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes. 2004;53:1418–1423. doi: 10.2337/diabetes.53.6.1418. [DOI] [PubMed] [Google Scholar]
- [55].Zhang L, Vincent MA, Richards SM, et al. Insulin sensitivity of muscle capillary recruitment in vivo. Diabetes. 2004;53:447–453. doi: 10.2337/diabetes.53.2.447. [DOI] [PubMed] [Google Scholar]
- [56].Vincent MA, Clerk LH, Lindner JR, et al. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab. 2006;290:E1191–E1197. doi: 10.1152/ajpendo.00497.2005. [DOI] [PubMed] [Google Scholar]
- [57].Wheatley CM, Rattigan S, Richards SM, Barrett EJ, Clark MG. Skeletal muscle contraction stimulates capillary recruitment and glucose uptake in insulin-resistant obese Zucker rats. Am J Physiol Endocrinol Metab. 2004;287:E804–E809. doi: 10.1152/ajpendo.00077.2004. [DOI] [PubMed] [Google Scholar]
- [58].Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes. 2006;55:1436–1442. doi: 10.2337/db05-1373. [DOI] [PubMed] [Google Scholar]
- [59].Rattigan S, Clark MG, Barrett EJ. Acute insulin resistance in rat skeletal muscle in vivo induced by vasoconstriction. Diabetes. 1999;48:564–569. doi: 10.2337/diabetes.48.3.564. [DOI] [PubMed] [Google Scholar]
- [60].Chen YL, Messina EJ. Dilation of isolated skeletal muscle arterioles by insulin is endothelium dependent and nitric oxide mediated. Am J Physiol. 1996;270:H2120–2124. doi: 10.1152/ajpheart.1996.270.6.H2120. [DOI] [PubMed] [Google Scholar]
- [61].Delashaw JB, Duling BR. A study of the functional elements regulating capillary perfusion in striated muscle. Microvasc Res. 1988;36:162–171. doi: 10.1016/0026-2862(88)90016-7. [DOI] [PubMed] [Google Scholar]
- [62].Eringa EC, Stehouwer CD, Merlijn T, Westerhof N, Sipkema P. Physiological concentrations of insulin induce endothelin-mediated vasoconstriction during inhibition of NOS or PI3-kinase in skeletal muscle arterioles. Cardiovasc Res. 2002;56:464–471. doi: 10.1016/s0008-6363(02)00593-x. [DOI] [PubMed] [Google Scholar]
- [63].Serne EH, IJzerman RG, Gans ROB, et al. Direct evidence for insulin-induced capillary recruitment in skin of healthy subjects during physiological hyperinsulinemia. Diabetes. 2002;51:1515–1522. doi: 10.2337/diabetes.51.5.1515. [DOI] [PubMed] [Google Scholar]
- [64].Serne EH, Stehouwer CD, ter Maaten JC, et al. Microvascular function relates to insulin sensitivity and blood pressure in normal subjects. Circulation. 1999;99:896–902. doi: 10.1161/01.cir.99.7.896. [DOI] [PubMed] [Google Scholar]
- [65].de Jongh RT, Serne EH, IJzerman RG, de Vries G, Stehouwer CD. Impaired microvascular function in obesity: implications for obesity-associated microangiopathy, hypertension, and insulin resistance. Circulation. 2004;109:2529–2535. doi: 10.1161/01.CIR.0000129772.26647.6F. [DOI] [PubMed] [Google Scholar]
- [66].de Jongh RT, Serne EH, IJzerman RG, Jorstad HT, Stehouwer CD. Impaired local microvascular vasodilatory effects of insulin and reduced skin microvascular vasomotion in obese women. Microvasc Res. 2007 doi: 10.1016/j.mvr.2007.08.001. [DOI] [PubMed] [Google Scholar]
- [67].de Jongh RT, Serne EH, IJzerman RG, de Vries G, Stehouwer CD. Free fatty acid levels modulate microvascular function: relevance for obesity-associated insulin resistance, hypertension, and microangiopathy. Diabetes. 2004;53:2873–2882. doi: 10.2337/diabetes.53.11.2873. [DOI] [PubMed] [Google Scholar]
- [68].Serne EH, de Jongh RT, Eringa EC, IJzerman RG, Stehouwer CD. Microvascular dysfunction: a potential pathophysiological role in the metabolic syndrome. Hypertension. 2007;50:204–211. doi: 10.1161/HYPERTENSIONAHA.107.089680. [DOI] [PubMed] [Google Scholar]
- [69].Guma A, Zierath JR, Wallberg-Henriksson H, Klip A. Insulin induces translocation of GLUT-4 glucose transporters in human skeletal muscle. Am J Physiol. 1995;268:E613–E622. doi: 10.1152/ajpendo.1995.268.4.E613. [DOI] [PubMed] [Google Scholar]
- [70].Garvey WT, Maianu L, Zhu JH, Brechtel-Hook G, Wallace P, Baron AD. Evidence for defects in the trafficking and translocation of GLUT4 glucose transporters in skeletal muscle as a cause of human insulin resistance. J Clin Invest. 1998;101:2377–2386. doi: 10.1172/JCI1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- [72].Youd JM, Rattigan S, Clark MG. Acute impairment of insulin-mediated capillary recruitment and glucose uptake in rat skeletal muscle in vivo by TNF-α. Diabetes. 2000;49:1904–1909. doi: 10.2337/diabetes.49.11.1904. [DOI] [PubMed] [Google Scholar]
- [73].Clerk LH, Rattigan S, Clark MG. Lipid infusion impairs physiologic insulin-mediated capillary recruitment and muscle glucose uptake in vivo. Diabetes. 2002;51:1138–1145. doi: 10.2337/diabetes.51.4.1138. [DOI] [PubMed] [Google Scholar]
- [74].Kim F, Gallis B, Corson MA. TNF-α inhibits flow and insulin signaling leading to NO production in aortic endothelial cells. Am J Physiol Cell Physiol. 2001;280:C1057–C1065. doi: 10.1152/ajpcell.2001.280.5.C1057. [DOI] [PubMed] [Google Scholar]
- [75].Kim F, Tysseling KA, Rice J, et al. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKβ. Arterioscler Thromb Vasc Biol. 2005;25:989–994. doi: 10.1161/01.ATV.0000160549.60980.a8. [DOI] [PubMed] [Google Scholar]
- [76].Eringa EC, Stehouwer CD, Walburg K, et al. Physiological concentrations of insulin induce endothelin-dependent vasoconstriction of skeletal muscle resistance arteries in the presence of tumor necrosis factor-α. Dependence on c-Jun N-terminal kinase. Arterioscler Thromb Vasc Biol. 2006;26:274–280. doi: 10.1161/01.ATV.0000198248.19391.3e. [DOI] [PubMed] [Google Scholar]
- [77].Bakker W, Sipkema P, Stehouwer CD, et al. Protein kinase C θ activation induces insulin-mediated constriction of muscle resistance arteries. Diabetes. 2008;57:706–713. doi: 10.2337/db07-0792. [DOI] [PubMed] [Google Scholar]
- [78].Zeng G, Nystrom FH, Ravichandran LV, et al. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation. 2000;101:1539–1545. doi: 10.1161/01.cir.101.13.1539. [DOI] [PubMed] [Google Scholar]
- [79].Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser1179. J Biol Chem. 2001;276:30392–30398. doi: 10.1074/jbc.M103702200. [DOI] [PubMed] [Google Scholar]
- [80].Jiang ZY, He Z, King BL, et al. Characterization of multiple signaling pathways of insulin in the regulation of vascular endothelial growth factor expression in vascular cells and angiogenesis. J Biol Chem. 2003;278:31964–31971. doi: 10.1074/jbc.M303314200. [DOI] [PubMed] [Google Scholar]
- [81].King GL, Johnson SM. Receptor-mediated transport of insulin across endothelial cells. Science. 1985;227:1583–1586. doi: 10.1126/science.3883490. [DOI] [PubMed] [Google Scholar]
- [82].Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994;127:1217–1232. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Bar RS, Boes M, Sandra A. Vascular transport of insulin to rat cardiac muscle. Central role of the capillary endothelium. J Clin Invest. 1988;81:1225–1233. doi: 10.1172/JCI113439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Wang H, Liu Z, Li G, Barrett EJ. The vascular endothelial cell mediates insulin transport into skeletal muscle. Am J Physiol Endocrinol Metab. 2006;291:E323–E332. doi: 10.1152/ajpendo.00047.2006. [DOI] [PubMed] [Google Scholar]
- [85].Steil GM, Ader M, Moore DM, Rebrin K, Bergman RN. Transendothelial insulin transport is not saturable in vivo. No evidence for a receptor-mediated process. J Clin Invest. 1996;97:1497–1503. doi: 10.1172/JCI118572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Hamilton-Wessler M, Ader M, Dea MK, et al. Mode of transcapillary transport of insulin and insulin analog NN304 in dog hindlimb: evidence for passive diffusion. Diabetes. 2002;51:574–582. doi: 10.2337/diabetes.51.3.574. [DOI] [PubMed] [Google Scholar]
- [87].Chisalita SI, Arnqvist HJ. Insulin-like growth factor I receptors are more abundant than insulin receptors in human micro- and macrovascular endothelial cells. Am J Physiol Endocrinol Metab. 2004;286:E896–E901. doi: 10.1152/ajpendo.00327.2003. [DOI] [PubMed] [Google Scholar]
- [88].Wang H, Wang AX, Barrett EJ. siRNA-mediated silencing of caveolin-1 inhibits insulin transport but enhances insulin-stimulated nitric oxide production. Diabetes. 2008;57:A383. (Abstract) [Google Scholar]
- [89].Bendayan M, Rasio EA. Transport of insulin and albumin by the microvascular endothelium of the rete mirabile. J Cell Sci. 1996;109:1857–1864. doi: 10.1242/jcs.109.7.1857. [DOI] [PubMed] [Google Scholar]
- [90].Kaiser N, Vlodavsky I, Tur-Sinai A, Fuks Z, Cerasi E. Binding, internalization, and degradation of insulin in vascular endothelial cells. Diabetes. 1982;31:1077–1083. doi: 10.2337/diacare.31.12.1077. [DOI] [PubMed] [Google Scholar]
- [91].Tiruppathi C, Song W, Bergenfeldt M, Sass P, Malik AB. Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathway. J Biol Chem. 1997;272:25968–25975. doi: 10.1074/jbc.272.41.25968. [DOI] [PubMed] [Google Scholar]
- [92].Predescu SA, Predescu DN, Malik AB. Molecular determinants of endothelial transcytosis and their role in endothelial permeability. Am J Physiol Lung Cell Mol Physiol. 2007;293:L823–L842. doi: 10.1152/ajplung.00436.2006. [DOI] [PubMed] [Google Scholar]
- [93].Zeng G, Quon MJ. Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J Clin Invest. 1996;98:894–898. doi: 10.1172/JCI118871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Jiang ZY, Lin YW, Clemont A, et al. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest. 1999;104:447–457. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Li G, Barrett EJ, Wang H, Chai W, Liu Z. Insulin at physiological concentrations selectively activates insulin but not insulin-like growth factor I (IGF-I) or insulin/IGF-I hybrid receptors in endothelial cells. Endocrinology. 2005;146:4690–4696. doi: 10.1210/en.2005-0505. [DOI] [PubMed] [Google Scholar]
- [96].Wang H, Wang AX, Liu Z, Barrett EJ. Insulin signaling stimulates insulin transport by bovine aortic endothelial cells. Diabetes. 2008;57:540–547. doi: 10.2337/db07-0967. [DOI] [PubMed] [Google Scholar]
- [97].Vicent D, Ilany J, Kondo T, et al. The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J Clin Invest. 2003;111:1373–1380. doi: 10.1172/JCI15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Kubota T, Hubota N, Kozono H, et al. Insulin signaling in endothelial cells participates in the regulation of skeletal muscle insulin sensitivity. Diabetes. 2008;57:A369. (Abstract) [Google Scholar]
- [99].Westerbacka J, Seppala-Lindroos A, Yki-Jarvinen H. Resistance to acute insulin induced decreases in large artery stiffness accompanies the insulin resistance syndrome. J Clin Endocrinol Metab. 2001;86:5262–5268. doi: 10.1210/jcem.86.11.8047. [DOI] [PubMed] [Google Scholar]