Abstract
Syndecans function as receptors for extracellular matrix (ECM) with integrins in cell spreading. However, the molecular mechanism of their specific involvement in cell migration or in wound healing has not been elucidated yet. Here, we report that a synthetic peptide, PEP75, which contains the syndecan-binding sequence of the laminin α3LG4 module, induces keratinocyte migration in in vitro and in vivo. Soluble PEP75 induced the clustering of syndecan-4 and conformation-modified integrin β1 colocalized with syndecan-4 in soluble PEP75-induced clusters. Treatment of cells in solution with PEP75 resulted in the exposure of the P4G11 antibody epitope of integrin β1 in immunostaining as well as in flow cytometry and augmented integrin β1–dependent cell adhesion to ECM. Pulldown assays demonstrated that PEP75 bound to syndecan-4, but not to integrin β1. A siRNA study revealed a role for syndecan-4 in PEP75-induced up-regulation of P4G11 antibody binding and migration of HaCaT cells. We conclude that binding of soluble PEP75 to syndecan-4 induces the coupling of integrin β1, which is associated with integrin β1-conformational changes and activation, and leads to keratinocyte migration. To activate integrin function through syndecans could be a novel therapeutic approach for chronic wound.
INTRODUCTION
Cutaneous wounding alters cell–cell and cell–extracellular matrix (ECM) interactions, and disrupts the basement membrane. At the wound edge, activated leading keratinocytes are generated from quiescent keratinocytes and participate in the repair of the basement membrane and cell migration into the wound bed (Nguyen et al., 2000). Laminin-5 (LN332) is a heterotrimeric basement membrane-specific glycoprotein composed of α3, β3, and γ2 subunits. Studies of in vivo wound tissue have shown that during repair, leading keratinocytes overexpress (Ryan et al., 1994) and deposit LN332 while migrating over the provisional repair matrix (Kainulainen et al., 1998). These data suggest that LN332 plays a role in facilitating cell migration during wound healing. LN332 undergoes processing of globular repeats 4 and 5 in the carboxy terminus of the α3 subunit (α3LG4-5) to yield the mature form of the protein (Goldfinger et al., 1998). In vivo, unprocessed laminin α3 chain is present in the newly synthesized epidermal basement membrane of wounds, but disappears from the mature basement membrane (Sigle et al., 2004). Recently, it was demonstrated that α3LG4-5 is involved in invasion by squamous cell carcinomas in vivo (Tran et al., 2008). These in vivo studies suggest that the α3LG4 module may contribute to reepithelialization. This hypothesis is reinforced by reports that the unprocessed form of LN332 containing α3LG4-5 is involved in cell migration (Nguyen et al., 2000; Tsubota et al., 2000; Decline et al., 2003).
We previously identified a core sequence within the α3LG4 domain that is recognized by syndecan-2 and -4 (Utani et al., 2001). The recombinant α3LG4 domain, as well as the synthetic peptide PEP75 (formerly termed A3G756), which contains the syndecan-binding sequence, induced cell adhesion (Utani et al., 2001), neurite outgrowth (Kato et al., 2002), and matrix metalloprotease (MMP)-1 secretion via an interleukin 1β autocrine loop involving p38MAPK (mitogen-activated protein kinase) activation (Utani et al., 2003) and MMP-9 secretion (Momota et al., 2005). It has also been reported that syndecan-1 is a keratinocyte adhesive receptor for the LG 4/5 domains of LN332 (Okamoto et al., 2003).
Syndecans (syndecan-1 to -4) comprise a family of cell surface heparan sulfate (HS) proteoglycans that contain a divergent extracellular ectodomain and a conserved cytoplasmic domain (Couchman, 2003; Beauvais et al., 2004; Fears and Woods, 2006). During wound healing of the skin, syndecan-1 and -4 are overexpressed at the leading edge of the wound (Elenius et al., 1991; Oksala et al., 1995; Gallo et al., 1996). Genetic disruption (knockout) experiments have also demonstrated a role for syndecan-1 and -4 in wound repair. Syndecan-1-null mice exhibit delayed skin and corneal wound healing (Stepp et al., 2002), while syndecan-4-null mice exhibit delayed wound healing and angiogenesis in granulation tissue (Echtermeyer et al., 2001). These observations suggest that syndecan family proteins are intimately involved in cutaneous wound healing (Fears and Woods, 2006; Alexopoulou et al., 2007).
The integrin superfamily regulates cell behavior at the ECM by forming adhesive receptors. Integrins are uniquely characterized by their ability to undergo changes in their ligand binding and signaling activities upon stimulation, which is usually associated with conformational changes in these molecules. Integrin-mediated alternations of cell–matrix interactions are essential for cellular processes, including migration and wound healing. Integrin activity is thought to be regulated by “outside-in” and “inside-out” pathways, but its signaling mechanism is still not fully understood. Additionally, it is known that integrin activity is changed by stimuli that directly interact with its extracellular domain, such as Mn2+ (Luo and Springer, 2006), activating antibodies, or adhesion substrates. The functional activation of integrin parallels changes in its conformation, which can be simply revealed by searching for changes in conformation-dependent antibody-binding activity, such as those observed with AG89 (Tsuchida et al., 1997) and 12G10 (Mold et al., 1998, 2002) antibodies.
In the current study, we have demonstrated that the binding of soluble PEP75 to syndecan-4 leads to the up-regulation of integrin β1-ligand interactions and that this up-regulation is associated with conformational changes in integrin β1. These processes may indicate the presence of cross-activation between integrin and syndecan receptors, which are possibly mediated by extracellular interactions between these receptors. Furthermore, these effects were observed with soluble PEP75 and did not require cross-linkers. The unique properties of PEP75 as syndecan-binding ligand enabled us to analyze its effects on syndecan clustering, cell adhesion, cell-surface expression of integrin β1 epitopes, and wound healing in vivo. Using a variety of approaches, we show that the coupling of syndecan-4 and integrin β1 and the activation of integrin β1 in response to soluble PEP75 treatment are required for PEP75- induced cell migration.
MATERIALS AND METHODS
Cultured Cells
Human primary keratinocytes, the human keratinocyte cell line HaCaT (a kind gift of Dr. Fusenig, German Cancer Research Center, Heidelberg, Germany), human neonatal dermal fibroblasts, and HEK293T cells were maintained as described previously(Utani et al., 2001, 2003). Keratinocytes and fibroblasts at the second through fifth passages were used for all experiments.
Reagents
NSC23766, LY294002, SB202190, PD98059, and bisindolylmaleimide I were obtained from Calbiochem (La Jolla, CA) and dissolved in dimethylsulfoxide.
Working concentrations of each inhibitor were based on the manufacturer's instructions. For flow cytometry and augmented cell adhesion assay, the following concentrations were used: 30 μM of the phosphatidylinositol 3′-kinase (PI3K) inhibitor LY294002; 100 μM of the Rac1 inhibitor NSC23766; 30 μM of the p38MAPK inhibitor SB202190; 20 μM of the Erk inhibitor PD98059; 200 nM of the protein kinase Cα (PKCα) inhibitor bisindolylmaleimide I; and 10 μg/ml heparin. Anti-integrin β1 blocking antibody (mAb13) was used at a concentration of 12 μg/ml. The Rac1 inhibitor (NSC23766) was used at concentrations of 100 and 10 μM in augmented cell adhesion assays. For the scratch wound assays, HaCaT cells were incubated in Ca2+-free medium for 12 h. As HaCaT cells exhibited the most sensitivity to inhibitors in this assay, the concentration of LY294002 was reduced to 3.0 μM and SB202190 to 10 μM.
Heparin was from SEIKAGAKU KOGYO, Tokyo, Japan. Acid-soluble porcine tendon-derived collagen I (cell matrix type I-A) was purchased from Nitta Gelatin (Osaka, Japan). Human plasma fibronectin (F0895) was from Sigma (St Louis, MO). PEP75, formerly termed A3G756 (Utani et al., 2003; residues 1411–1429 of human laminin α3 chain, KNSFMALYLSKGRLVFALG) and the control scrambled peptide, PEP con (LVAGAFFKRKLLLMNSSGY), were manually synthesized, as described previously (Utani et al., 2001).
Antibodies
For the detection of integrin β1, 12G10 (Abcam, Cambridge, United Kingdom), JB1A (Chemicon International, Temecula, CA), P4G11 and FITC-conjugated P4G11 (Chemicon), FITC-conjugated K20 (Immunotech, Marseille, France), Cy3-conjugated AG89 (MBL, Nagoya, Japan), mAb13 (a gift from Dr. K. M. Yamada, National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD), and goat anti-human integrin β1 polyclonal antibody (sc-6622, N-20, Santa Cruz Biotechnology, Santa Cruz, CA) were used. The following neutralizing antibodies were used for the inhibition assays: P1E6, directed against integrin α2 (Abcam); P1B5, directed against integrin α3 (Abcam); P1D6, directed against integrin α5 (Chemicon); GoH3, directed against integrin α6 (Abcam); mAb13, directed against integrin β1 and anti-human integrin β3 antibody (MAB1957, Chemicon); and control mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). Anti-heparan sulfate (HS) antibody 10E4 (Seikagaku Kogyo, Tokyo, Japan), anti-syndecan-4 antibody (5G9, Santa Cruz), anti-syndecan-1 mAb (DL-101, Santa Cruz), goat anyi-syndecan-1 antibody (no. 7099, Santa Cruz), goat anti-syndecan-2 (SC-9492, Santa Cruz), rhodamine- or Alexa Fluor 488–conjugated phalloidin (Invitrogen, Carlsbad, CA) for the detection of F-actin, were purchased. FITC-conjugated anti-fibronectin antibody was from Biogenesis (Poole, Dorset, United Kingdom). Alexa Fluor–conjugated secondary antibodies were from Invitrogen. Monoclonal anti-β-actin (AC-74, Sigma) and peroxidase-conjugated anti-goat IgG (Jackson ImmunoResearch Laboratories) were used for Western blotting. For cross-linking, goat anti-human IgG constant fragment (Fc) and goat anti-mouse IgG were used (Jackson ImmunoResearch Laboratories). Fluorescence immunostaining was analyzed by confocal laser scanning microscopy using a Zeiss LSM 510 system (Carl Zeiss Microscopy, Thornwood, NY).
Cell Migration Assay
For the scattering assay, cells were seeded in six-well plates (10,000 cells per well) and allowed to grow and form epithelial islands for 48–72 h in 10% fetal bovine serum (FBS)/DMEM. The cells were incubated with soluble PEP75 (10 μg/ml) in 5% FBS/DMEM for an additional 48 h. Soluble PEP75 (0.2–15 μg/ml) had no stimulatory effect on cell proliferation in 1% or 5% FBS/DMEM. Photographs were obtained using an Olympus CKX41 microscope and a DSE330 microimaging system (Olympus, Tokyo, Japan).
For scratch wound migration assays, HaCaT cells were seeded into 24-well plates (2 × 105 cells per well; Falcon, Becton Dickinson, Franklin Lakes, NJ) in DMEM containing 10% FBS. The cells were grown to confluence for 2 d. Cells were preincubated with Ca2+-free minimum essential medium (EMEM; Bio-Whittaker, Walkersville, MD) supplemented with 1% FBS and antibiotics for 2 h before the scratch wound was made with a 200-μl pipette tip. Two perpendicular wounds were created and loose cells were removed by washing with phosphate-buffered saline (PBS). Cells were allowed to migrate for 24 h in Ca2+-free EMEM/1% FBS in the presence of soluble PEP con or soluble PEP75 at 10 μg/ml with or without inhibitors. The cells were stained with Giemsa's azure eosin methylene blue and photographed using an Olympus microscope and camera.
The colloidal gold phagokinetic track assay using primary keratinocytes was performed as previously described (Momota et al., 2005). Briefly, coverslips (10-mm diameter) were dipped in 1% freshly prepared bovine serum albumin (BSA) in distilled water and then in 100% ethanol before being quickly dried with a hair dryer. The slides were then placed into 24-well tissue culture plates, and 1 volume of a freshly prepared solution containing 10% of 14.5 mM HAuCl4, 58% of H2O, and 32% of 36.5 mM Na2CO3 was added and heated to 100°C. After several seconds, the heat was turned off and one-tenth volume of 0.1% of formaldehyde was immediately added to each well, which resulted in the development of a rusty-iron color. After cooling down to 60°C, the BSA-coated coverslip was covered with 0.3 ml of the solution and left undisturbed for 1–2 h to let the gold salt particles settle. After washing with PBS, the coverslips were kept at 4°C in PBS until use. Primary keratinocytes, harvested with 0.05% trypsin-EDTA according to manufacturer's protocol, were added to the wells (3000/well) and allowed to adhere to the coverslips for 30–60 min, and then the peptide with or without anti-integrin antibody was added. After 12–24-h incubation, cells were fixed in 10% formaldehyde in PBS for 10 min. Three randomly selected and nonoverlapping fields (40× magnification) were photographed using dark-field optics with a CCD camera (Model VB7010, Keyence, Osaka, Japan) and analyzed using the NIH ImageJ 1.60 program (http://rsb.info.nih.gov/ij/). Migration was detected as an area without gold particles. The migration index, representing the area consumed by the cell migration tracks, was expressed as pixels or the ratio of the area consumed by cell migration tracks to the whole area of the field.
Immunostaining
Cells were seeded into the wells of an eight-well chamber plate (Iwaki Glass, Tokyo, Japan). After fixation with 4% paraformaldehyde/PBS for 10 min and blocking with 10% FBS/PBS for 30 min at room temperature, the cells were incubated with primary antibodies for 1 h at 37°C or overnight at 4°C and then with the secondary antibody for 30 min. Images were visualized by confocal laser scanning microscopy using a Zeiss LSM 510 system (Carl Zeiss Microscopy).
Cell Adhesion Assay
We prepared 96-well plates coated with substrate, acid-soluble porcine tendon–derived collagen I, and human plasma fibronectin and cells as described previously (Utani et al., 2001). Cells were prepared with trypsin/EDTA and were allowed to recover in 0.1% BSA/DMEM for 30 min at 37°C after neutralization with 10% FBS. Then, cells were preincubated with soluble PEP75 (7.0 μg/ml) for 10 min in suspension in 0.1% BSA/DMEM. Cells were then plated (40,000 per well) and incubated for 45 min. Adherent cells were stained with crystal violet (0.2%/20% methanol) and then dissolved in 1% SDS in distilled water. Absorbance was measured at 550 nm using a microplate reader (Bio-Rad Laboratories, Hercules, CA).
Flow Cytometry
Monolayer cells were harvested by using trypsin-EDTA. Trypsin-EDTA was then neutralized by adding 10% FBS/DMEM. After a recovery period of 30 min, the cells were resuspended in 0.1% BSA/DMEM at a concentration of 1.0 × 106 cells/0.1 ml and then incubated at 37°C for 30 min in the presence of 0.1 mg/ml soluble PEP con, soluble PEP75, 2 mM Mn2+, or JB1A antibody. Cells were washed with ice-cold PBS and then incubated with antibody for 45 min on ice in 10% FBS/PBS. After washing with ice-cold PBS, cells were analyzed by flow cytometry (Epics XL-MCL; Beckman Coulter, Miami, FL).
Small Interfering RNA
For the down-regulation of syndecan-1 and -4, Sdc1 Stealth Select RNAi (RSS303156, 7, and 8) and Sdc4 Stealth Select RNAi (RSS340728, 9, and 30) were purchased from Invitrogen, respectively. A nonspecific small interfering RNA (siRNA) was used as a negative control (45–2001, Invitrogen). Cells were transfected with siRNA (40 nM) using Lipofectamine RNAiMAX (Invitrogen) in Opti-MEM (Invitrogen). Flow cytometry and the scratch wound assay were performed 48–72 h after transfection. The down-regulation of syndecan-4 and -1 was analyzed by flow cytometry and Western blotting, respectively. Western blotting was performed according to the previous report (McQuade et al., 2006). The cell lysates were prepared in RIPA buffer (Santa Cruz) with protease inhibitor cocktail and 1 mM PMSF (Santa Cruz). Samples were separated on 10% SDS-PAGE gels followed by Western blotting with goat anti-syndecan-1 (no. 7099, Santa Cruz) and peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech).
Pulldown Experiment
HaCaT cell lysate was prepared by incubation on ice for 30 min (vortex briefly each 10 min) in lysate buffer (1 × 107cells/mL) containing, 100 mM n-octyl-β-d-glycoside (Dojindo, Osaka, Japan), 50 mM Tris HCl, pH 7.4, 150 mM NaCl, 1 mM CaCl2,1 mM MgCl2, and Complete-EDTA–free protease inhibitor cocktail (Roche, Alameda, CA).
After centrifugation, the supernatant was incubated with peptide beads (5 mg beads/100 μl lysate) for 12 h at 4°C. The beads were washed six times with 1 ml of lysate buffer. The beads were treated with heparitinase I (0.1 mU/ml) and chondroitinase ABC (0.5 U/ml; Seikagaku Kogyo, Tokyo, Japan) for 18 h at room temperature in 20 μl of a buffer containing 40 mM Tris-HCl, pH 8.0, 40 mM NaOAc, 5 mM Ca(OAc)2, 0.01% BSA, and complete EDTA-free protease inhibitor cocktail. The samples were separated on 10% SDS-PAGE gels for syndecans followed by Western blotting with anti-syndecan-1 (DL-101), or -4 (5G9) and peroxidase-conjugated secondary antibodies and ECL (Amersham Pharmacia Biotech). For integrin β1, the materials bound to the peptide beads were directly eluted by SDS-buffer and separated by 7% SDS-PAGE, followed by Western blotting with integrin β1 antibody (N20).
Peptide Beads
CN-Br–activated Sepharose 4B (200 mg; Amersham Pharmacia) was activated by washing with cold 1 mM HCl solution and then with 5 ml NaHCO3, H 8.3, after washing the beads with distilled water. The beads were then incubated with 1 ml of peptide solution (5 mg/ml distilled water) and 2 ml of NaHCO3 buffer, pH 8.3 (total 3 ml) for 8 h at room temperature with constant rotation. After washing with distilled water, 1 M ethanolamine, pH 8.0, was added and incubated for 1 h at room temperature with constant rotation. Beads were stored at 4°C in PBS until use.
Animal Cutaneous Wound Experiments
Animals were maintained at the Institute of Laboratory Animals, Graduate School of Medicine, Kyoto University, and experiments were performed in compliance with protocols established by the Animal Research Committee of Kyoto University.
The wounds were made, followed by treatment with peptide, and dressed with an occlusive polyurethane film (Tegaderm; 3M Health Care, St. Paul, MN).
Statistical Analysis
Statistical analysis was carried out using the Student's t test to examine differences between experimental groups. Results represent the means ± SD. Calculated p values were two-sided, and a value of <0.05 was considered statistically significant.
RESULTS
PEP75 Induces Integrin β1–dependent Keratinocyte Migration
We have previously shown that the synthetic peptide PEP75, derived from laminin α3LG4, has adhesive activity via binding to syndecan on the cell surface. In the present study, the mechanisms of PEP75 involvement in cell migration was further studied. We first examined the ability of PEP75 to promote cell migration in a scattering assay. HaCaT cells treated with a control peptide formed epithelial islands (Figure 1a). In contrast, when cells were incubated with soluble PEP75 for 48 h, cells scattered out of the islands and exhibited an altered morphology, including the disruption of stress fibers and the absence of focal adhesions. To determine whether the drastic changes in cell shape induced by soluble PEP75 were accompanied by alterations in integrin function, we performed a scratch wound healing assay in the presence of anti-integrin β1–neutralizing antibody. HaCaT cells migrated from the wound edge in response to soluble PEP75 treatment (Figure 1b), and heparin and anti-integrin β1–neutralizing antibody completely inhibited cell migration.
Figure 1.
PEP75 induces integrin β1–dependent keratinocyte migration. (a) Scattering assay of HaCaT cells in the presence or absence of soluble PEP75 in 5% FBS/DMEM. Cells were exposed to peptide in solution for 48 h. Immunofluorescence revealed the disruption of focal contacts (vinculin staining) and stress fibers (F-actin staining). Scale bars, 20 and 250 μm. (b) Scratch wound migration assays with HaCaT cells in 1%FBS/Ca2+-free EMEM were performed for 24 h in the presence of 10 μg/ml soluble PEP75 or PEP con in the presence of heparin or anti-integrin β1 neutralizing antibodies. Cells that migrated from the starting wound edge were counted in randomly selected and nonoverlapping fields, and the results were expressed as means ± SD of three independent experiments. Scale bar, 250 μm. (c) Colloidal gold phagokinetic assay using primary keratinocytes. Cells were incubated with increasing doses of peptide for 12 h or with 15 μg/ml soluble PEP75 for the indicated periods of time. After incubation, three randomly selected and nonoverlapping fields were analyzed using the NIH ImageJ 1.60 program. The migration index (pixels) is expressed as the area consumed by cell migration tracks. Data represent the means ± SD (n = 3). Scale bar, 200 μm. Experiment was performed two times, and representative data are shown. (d) Colloidal gold phagokinetic assays with primary keratinocytes were carried out using the indicated anti-integrin α chain or β1 chain neutralizing antibodies. The migration index (%) is expressed as a percentage relative to cells treated with control IgG, which was set as 100%. Data represents the means ± SD of three independent experiments performed in triplicate. **p = 0.00068 compared with those of IgG.
In a colloidal gold phagokinetic assay, soluble PEP75 induced the migration of primary keratinocytes in a dose- and time-dependent manner (Figure 1c). Keratinocyte locomotion was augmented a maximum of about fourfold compared with control cells. Neutralizing antibodies to integrin β1 completely inhibited cell migration of primary keratinocytes in the colloidal gold phagokinetic assay with primary keratinocytes, to 9.4 ± 0.3% (Figure 1d). Neutralizing antibodies to integrins α2, α3, and α6 had no effect on PEP75-induced migration, whereas anti-α5 antibodies significantly inhibited cell migration (41 ± 5.5%; Figure 1d). Fibronectin accumulated under the migrating cell bodies of primary keratinocytes (data not shown), which indicated that fibronectin is a major substrate for migration in this assay. These data suggested that PEP75 in solution induces integrin β1–dependent migration.
PEP75 Promotes Wound Healing In Vivo
Because keratinocyte migration is indispensable for normal wound healing in vivo, we next assessed the activity of PEP75 in animal models of wound healing. A cutaneous wound was created on the back of a C57BL/6J mouse, and PEP75 was topically applied on the day of wounding (day 1) and day 3, with occlusive dressing. PEP75 significantly reduced the wound area on day 8 and compared with animals treated with control peptide (Figure 2a). We also examined the effect of PEP75 on wound healing in the concave side of the rabbit earlobe, to exclude the contribution of wound contraction in the healing process. Rabbit earlobe skin wounds were treated with PEP75 once, on the day of wounding (day 1), with occlusive dressing. Microscopic analysis revealed that PEP75 significantly promoted keratinocyte migration on days 4 and 8 compared with the controls (Figure 2b). These results indicated that the application of PEP75 to a cutaneous wound in vivo promotes wound closure by accelerating reepithelialization. The diameter of the original wound was ∼1.0 cm on day 8 after wounding, which indicated that the wound in the earlobe did not contract during that period of time.
Figure 2.
PEP75 promotes wound healing in vivo. (a) A 6-mm full-thickness wound was made on the back of a C57BL/6J mouse, and 15 μl of a 1.0 μg/μl solution of PEP75 was topically applied to the wound twice, on days 1 and 4, with an occlusive dressing. Unclosed areas were photographed and analyzed using the NIH ImageJ 1.60 program. Results are expressed as percent wound healing at day 8 relative to the wound area at day 1, which was set as 100%. Data represents the means ± SD of six animals. **p = 0.014. (b) PEP75 or PEP con (50 μl of a 0.2 μg/μl solution) was applied to a rabbit earlobe skin wound with occlusive dressing once, on day 1. Paraffin-embedded sections were stained with hematoxylin-eosin and then examined by microscopy. Reepithelialization was calculated as the ratio of the diameter of the unclosed (ω) area to that of original wound (W) and is expressed as a percentage of the ratio of the healed wound to that of the original wound, which was set as 100%. Data represents the means ± SD of three animals. **p = 0.0011 at 4 d and 0.0139 at 8 d.
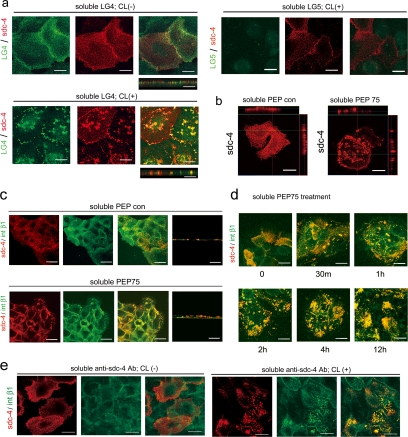
Soluble Ligands Induce Syndecan-4 Clustering and the Colocalization of Syndecan-4 and Integrin β1
To clarify the signaling pathways that were involved in soluble PEP75-induced integrin β1–dependent cell migration, we first analyzed distribution of syndecan-4 and integrin β1 after ligand binding. Because the aggregation of syndecan-1 is induced after cross-linking (Carey et al., 1994), we examined the effect of ligand binding on syndecan-4 distribution. Recombinant α3LG4 is a syndecan ligand and supports cell adhesion and migration (Utani et al., 2001; Momota et al., 2005). Recombinant α3LG4, but not α3LG5, bound to cell surfaces and colocalized with syndecan-4 as fine dots (Figure 3a). The cross-linking of bound recombinant α3LG4 with anti-Fc cross-linking antibody induced ligand-induced syndecan-4 clustering at the apical cell surface and points of cell–cell contact, where syndecan-4 was present as large clusters (Figure 3a). Syndecan-4 distributed as diffuse fine dots at the cell surface in soluble PEP con–treated single HaCaT cells. A side view of a single HaCaT cell treated with soluble PEP75 clearly demonstrated that the clustering of syndecan-4 was located at the apical cell surface (Figure 3b). These results indicated that PEP75 induces syndecan-4 clustering in the absence of cross-linking. Integrin β1 colocalized with syndecan-4 clusters at the apical surface after the addition of soluble PEP75 to the culture medium (Figure 3c). The time-course study indicated that syndecan-4 and integrin β1 clustering began as early as 30 min and reached a maximum at 2–4 h after the start of the assay. This level remained constant for at least 12 h (Figure 3d). To determine whether the colocalization of syndecan-4 and integrin β1 was directly related to syndecan-4 clustering, clustering of syndecan-4 was induced by cross-linking of anti-syndecan-4 antibodies. Syndecan-4 clustering alone induced integrin β1-syndecan-4 colocalization (Figure 3e). These results suggested that PEP75-induced syndecan-4 clustering induces syndecan-4-integrin β1 colocalization.
Figure 3.
Soluble ligands induce syndecan-4 clustering and the colocalization of syndecan-4 and integrin β1. (a) HaCaT cells cultured on glass slides were incubated with soluble recombinant laminin α3LG4- or α3LG5-human Fc chimeric proteins (Momota et al., 2005; 2.0 μg/ml) for 30 min at 4°C, followed by anti-human IgG Fc antibody (20 μg/ml) for 30 min at 37°C for cross-linking (CL+). As a control, anti-human IgG Fc was omitted (CL−). Recombinant α3LG4, but not α3LG5 bound to cell surface syndecan-4. Cross-linking changed the syndecan-4 distribution from fine dots to clusters at cell surface. Side views were also shown. (b) Treatment of a single HaCaT cell with PEP con or PEP75 (10 μg/ml) clearly demonstrated that the clustering of syndecan-4 was located at the apical cell surface. (c) HaCaT cells were treated with soluble PEP con or PEP75 for 2 h in 0.1% BSA/DMEM. Syndecan-4 clustering induced by soluble PEP75 colocalized with integrin β1 (P4G11 antibody). (d) Time-course study of syndecan-4 and integrin β1 clustering that was observed from 30 min to 12 h after the addition of soluble PEP75. (e) Anti-syndecan-4 mAb (2.0 μg/ml) was incubated with HaCaT cells for 30 min at 4°C, followed by cross-linking with anti-mouse IgG (20 μg/ml) for 30 min at 37°C. Antibody-induced syndecan-4 clustering also colocalized with integrin β1. Data are representative of at least two experiments. Scale bar, 20 μm.
Integrin β1 That Colocalizes with Clustered Syndecan-4 Undergoes Conformational Changes
During the course of the experiments, we obtained inconsistent results for integrin β1-syndecan-4 colocalization using different monoclonal antibodies. P4G11 and K20 monoclonal antibodies recognized integrin β1 that colocalized with syndecan-4 clusters (Figure 4). P4G11 showed the most drastic change in the distribution of integrin β1. Unlike P4G11, JB1A, mAb13 and 12G10 failed to detect integrin β1 clusters at PEP75-induced aggregations detected by the anti-HS antibody (Figure 4). The syndecan-4 cluster was located by using anti-HS antibody (data not shown). However, these antibodies mainly detect the integrin β1 that remains at the cell–cell boundary. These results suggested that the integrin that colocalizes with the syndecan-4 cluster has a different set of exposed epitopes than the integrin β1 at cell–cell borders or at the basal cell surface. We were unable to detect integrin α2, α3, α5, or α6 chains in syndecan-4 clusters (data not shown).
Figure 4.
Integrin β1 that colocalizes with clustered syndecan-4 displays conformational changes. HaCaT cells grown on coverslips were treated with soluble PEP75 or PEP con (10 μg/ml), and the codistribution of syndecan-4 or of heparan sulfate (HS) and integrin β1 was examined by immunostaining. Anti-HS antibody (IgM) was used instead of anti-syndecan-4 antibody when no appropriate fluorescence-conjugated anti-integrin β1 antibodies were available. The colocalization of integrin β1 at sites of syndecan-4 clustering was demonstrated with P4G11 and K20 antibodies. However, unlike P4G11, JB1A, mAb13, and 12G10 antibodies failed to detect integrin β1 at sites of HS clustering. The data shown is representative of at least three experiments. Scale bar, 20 μm.
Integrin β1 Conformational Changes Are Induced by PEP75 in Solution
Treatment of cells with PEP75 in suspension resulted in a drastic increase in fluorescence intensity in HaCaT cells, as well as fibroblasts treated with soluble PEP75 using P4G11 mAb as a probe (Figure 5a). When we analyzed several other monoclonal antibodies to integrin β1 by flow cytometry, we did not detect any changes in fluorescence intensity using K20, JB1A, or 12G10 antibodies (Figure 5b). These results indicated that PEP75 induces conformational changes in integrin β1 that can be detected as changes in epitope exposure, in cells in suspension.
Figure 5.
Integrin β1 conformational changes are induced by PEP75 in solution. Cells prepared in suspension (106 cells) were incubated with soluble PEP75 (100 μg/ml) for 30 min at 37°C, followed by incubation with the indicated antibodies on ice. (a) PEP75 significantly increased of the fluorescence intensity (F.I.; x-axis) of HaCaT cells and fibroblasts when P4G11 mAb was used as a probe, and heparin completely blocked this increase. The cells were collected after flow cytometry and examined by confocal laser scanning microscopy. Scale bar, 20 μm. (b) JB1A, K20, and 12G10 antibodies failed to detect changes in the F.I. after treatment with soluble PEP75. The faint gray color represents background fluorescence of JB1A. (c) In the presence of 2 mM Mn2+ for 30 min at 37°C, P4G11 associated F.I. increased, whereas a AG89-associated F.I. was almost completely lost. PEP75 and (Mn2+) showed a similar response pattern. (d) Anti-integrin β1 antibody (JB1A) or control mouse IgG was incubated with cells for 30 min at 0°C, followed by treatment with (CL+) or without cross-linking antibody (CL−) for 30 min at 37°C. Then, P4G11 was incubated with cells on ice for flow cytometry. Antibody-mediated cross-linking of integrin β1 using anti-JB1A antibody increased the P4G11-associated F.I. The data are representative of at least two experiments.
Another anti-integrin β1 mAb AG89 tended to lose its sensitivity to integrin β1 upon PEP75 treatment in solution (Figure 5c). A similar pattern (i.e., increased P4G11 fluorescence and decreased AG89 fluorescence) was seen when we added Mn2+ to the medium, which suppressed the P4G11 and AG89 binding (Figure 5c). Manganese ion is a well-known factor that induces conformational changes and effects the activity of integrins (Ni et al., 1998). These results suggested that PEP75 and Mn2+ share a common mechanism of regulation of integrin structure. Cross-linking of anti-integrin β1 antibody (JB1A) increased P4G11 epitope exposure (Figure 5d).
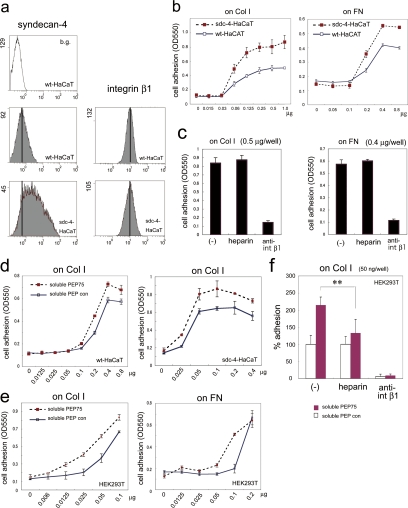
Overexpression of Syndecan-4 Enhances Integrin β1–dependent Cell Adhesion to ECM Proteins and PEP75 Treatment in Solution Augments Integrin β1–dependent Cell Adhesion
To determine whether the conformational changes in integrin β1 affected its function, we analyzed cell adhesion to the ECM, which is mediated by integrin β1. Because syndecan-1 overexpression has been reported to up-regulate integrin αvβ3 function (Beauvais et al., 2004), we first generated syndecan-4–overexpressing HaCaT cells (sdc-4-HaCaT; Figure 6a). The level of integrin β1 was not up-regulated in sdc4-overexpressing HaCaT cells compared with wild-type HaCaT cells. We examined their ability to adhere to the ECM. Sdc-4-HaCaT cells attached to collagen I and fibronectin more efficiently than parental cells (wt-HaCaT; Figure 6b).
Figure 6.
Overexpression of syndecan-4 enhances integrin β1–dependent cell adhesion to ECM proteins and PEP75 treatment in solution augments integrin β1–dependent cell adhesion. (a) Syndecan-4 and integrin β1 expression in stable HaCaT transformants that overexpressed syndecan-4 (sdc-4-HaCaT) and parental HaCaT cells (wt-HaCaT) was analyzed by flow cytometry. JB1A antibody was used for integrin β1. b.g., background. (b) Adhesion assay of sdc-4-HaCaT and wt-HaCaT cells on 96-well plates coated with various amounts of collagen I (Col I) and fibronectin (FN). y-axis, cell number shown as OD550; x-axis, coated substrates: μg/well. (c) Sdc-4-HaCaT adhesion to Col I- or FN-coated dishes was dependent on integrin β1 and was not affected by heparin. Cells were seeded onto Col 1 (0.5 μg/well) or FN (0.4 μg/well). Cells were allowed to adhere for 45 min. For inhibition assays, cells were preincubated with heparin (10 μg/ml) or anti-integrin β1 antibody (mAb13, 12 μg/ml) for 15 min before seeding. Heparin and mAb13 were also present during the assay. (d) Incubation with soluble PEP75 (10 μg/ml) for 30 min in solution enhanced cell adhesion to collagen I in both wt-HaCaT and sdc-4-HaCaT cells. (e) Adhesion of HEK293T cells to Col I and FN was also enhanced by soluble PEP75 treatment in solution. (f) The soluble PEP75-induced adhesion of HEK293T cells to Col I was reduced by heparin and was entirely dependent on integrin β1. (b–e) Data represents the means ± SD of at least three experiments performed in triplicate. For the inhibition experiments in panel f, the percentage of adherent cells is expressed relative to cells treated with PEP con in the absence of inhibitor, which was set as 100%. **p = 0.0069.
This augmented adhesion to ECM proteins was inhibited by anti-integrin β1 antibody, but not by heparin (Figure 6c). To examine the effect of soluble PEP75 on cell adhesion, cells were seeded on collagen I after incubation with a control peptide, soluble PEP con, or soluble PEP75 (10 μg/ml) in suspension for 30 min. This protocol was identical to the protocol used in the flow cytometry experiments that resulted in exposure of the P4G11 epitope. Soluble PEP75 treatment augmented both wt-HaCaT and sdc4-HaCaT cell adhesion to collagen I and to fibronectin (Figure 6d). The presence of soluble PEP75 also enhanced HEK293T cell adhesion to collagen I and fibronectin (Figure 6e). The increased adhesion of soluble PEP75 (10 μg/ml) of HEK293T cells to collagen I was reduced by the presence of heparin and was entirely dependent on integrin β1 (Figure 6f). These results indicated that PEP75-syndecan binding augments ECM receptor activity of integrin β1. Although the ligand–syndecan interaction was blocked by the presence of heparin in HEK293T cells, Sdc4-HaCaT cell binding to substrates such as collagen I and fibronectin was independent of heparin as shown in Figure 6c. The failure to observe inhibition of enhanced Sdc4-HaCaT cell adhesion by heparin may be explained by the hypothesis that integrin β1 of Sdc4-HaCaT cells had already been moderately activated by a significantly high level of overexpressed syndecan-4 (Figure 6a) at the cell surface, even in the absence of ligands.
Specific Involvement of Syndecan-4 in Integrin β1 Conformational Changes and Cell Migration
Syndecan-1 in HaCaT cells did not form clusters in response to soluble PEP75 treatment during a 2-h incubation. Soluble PEP75 did not induce syndecan-2 clustering in fibroblasts, even though strong syndecan-4 clustering was observed (data not shown). To confirm syndecan binding to PEP75, we prepared peptide-conjugated Sepharose 4B beads. HaCaT cell lysates and peptide-conjugated beads were mixed together, and bound materials were eluted and digested with heparitinase I and chondroitinase ABC. Western blotting showed that syndecan-4 bound to PEP75, but not to PEP con (Figure 7a). Syndecan-1 failed to bind to PEP75 beads, and integrin β1 did not bind to PEP75 (Figure 7a). To examine the specific role of syndecan-4 in integrin β1 conformational changes and cell migration, we examined cells that expressed syndecan-specific siRNAs (Figure 7b). The expression of syndecan-4–specific siRNA resulted in the reduced expression of syndecan-4, and a significant inhibition of integrin β1 conformational changes in response to soluble PEP75 treatment. In contrast, syndecan-1 siRNA showed that syndecan-1 did not involve up-regulation of P4G11 binding to integrin β1 (Figure 7b). In a scratch wound healing assay, HaCaT cells that expressed syndecan-4–specific siRNA failed to migrate in response to soluble PEP75 (Figure 7b). However, reduced syndecan-1 expression did not affect cell migration in response to soluble PEP75 (Figure 7b).
Figure 7.
Specific involvement of syndecan-4 in integrin β1 conformational changes and cell migration. (a) Pulldown assay with peptide-conjugated Sepharose beads. HaCaT cell lysates were incubated with peptide beads. For syndecans, bound materials were digested with heparitinase I/chondrotinase ABC and were then subjected to 10% SDS-PAGE and Western blotting. Syndecan-4, but not -1 specifically bound to PEP75. For integrin β1, bound materials were directly eluted by boiling in SDS-buffer and separated by 7% SDS-PAGE. Immunoprecipitation was also done to indicate the input amount of integrin β1 (input). **Note that syndecan-1 (∼90 kDa) bound only at a background level to both peptide-beads. (b) siRNA-mediated knockdown is shown by flow cytomeric analysis for syndecan-4 (siRNA sdc-4 RSS340730) or by Western blotting for syndecan-1 (siRNA sdc-1 RSS303157). Flow cytomeric analysis revealed that integrin β1 conformational changes after soluble PEP75 treatment were dependent on stndecan-4, but not on syndecan-1. The total fluorescence intensity is indicated in parenthesis. In Western blotting of syndecan-1, optical densities of the bands were obtained by NIH image and are shown in parentheses. The siRNA assays were performed using three different siRNAs. The data are representative of at least two independent experiments with each siRNA. Knockdown of syndecan-4, but not -1 resulted in severely decreased cell mobility in a scratch wound assay of HaCaT cells in 1%FBS/Ca2+-free EMEM. Data are representative of at least two experiments. The number of cells that migrated from the starting wound edge was counted in randomly selected and nonoverlapping fields. Data are shown as means ± SD of two independent experiments. The results obtained with syndecan-4 siRNA (RSS340730) and syndecan-1 siRNA (RSS303157) are shown for both flow cytometry analysis and scratch wound assay.
These results indicated that PEP75-induced changes in integrin β1 conformation and migration activity are mediated predominantly by syndecan-4 in HaCaT cells.
Analysis of the Signaling Molecules Involving in Soluble PEP75-induced Biological Activities
To identify the signaling molecules that were involved in mediating PEP75-induced effects on cells, we carried out several assays, including scratch wound assay, flow cytometric analysis of P4G11 binding, and augmented cell adhesion, in the absence and presence of chemical inhibitors of p38MAPK (Utani et al., 2003), PKCα (Couchman and Woods, 1999), PI3K (Park et al., 2005), and Rac1 (Bass et al., 2007), all of which are known to mediate syndecan signaling (Figure 8).
Figure 8.
Analysis of the signaling molecules involving in PEP75-induced biological activities. Chemical inhibitors to signal molecules were examined in PEP75-induced activities by scratch wound healing assays (a), flow cytometry analysis of P4G11 binding (b), and augmented cell adhesion assay (c). (a) HaCaT cells were incubated with soluble PEP75 or PEP con (10 μg/ml) in 1%FBS/Ca2+-free EMEM in the presence or absence of chemical inhibitor. Scale bar, 250 μm. The number of cells that migrated from the starting wound edge was counted in randomly selected and nonoverlapping fields and the data are shown as means ± SD of two independent experiments. (b) HaCaT cells in suspension were incubated with chemical inhibitors for 15 min, and followed by addition of peptide (100 μg/ml) for 30 min at 37°C. None of chemical inhibitors blocked the increase of P4G11 binding induced by PEP75 treatment. The data are representative of two independent experiments. (c) Soluble PEP75 (10 μg/ml augmentation of cell adhesion of HEK293T cells on collagen I was examined in the presence or absence of chemical inhibitors. The assay was performed in sextuplicate, and the data represents the means ± SD. Data are representative of at least two experiments. The number of attached cells in the presence of PEP con was set as 100%. **p = 0.00022. For inhibition studies, cells were preincubated for 15 min with the indicated chemical inhibitors and then incubated with soluble peptide for 30 min at 37°C. The Rac1 inhibitor (NSC23766) was used at concentrations of 100 μM in scratch assay and flow cytometric analysis or 100 and 10 μM in augmented cell adhesion assays.
In HaCaT cells, PI3K, Rac1, and p38MAPK, but not PKCα were required for cell migration after scratch wounding (Figure 8a). None of the inhibitors examined blocked integrin β1 conformational changes in flow cytometry analysis (Figure 8b). The p38MAPK inhibitor partially blocked PEP75-induced 293T cell adhesion to collagen I, whereas PI3K and Erk (extracellular signal–regulated kinase) inhibitors had no effect (Figure 8c). The Rac1 inhibitor, NSC23766, increased cell adhesion to collagen I at a concentration of 100 μM (Figure 8c), and completely inhibited cell adhesion to collagen I at a concentration of 10 μM, independently of soluble PEP75 treatment. Thus, we were unable to determine whether Rac1 was involved in PEP75-induced cell adhesion. These results suggested that p38MAPK, PKCα, PI3K, and Rac1 are not involved in integrin β1 conformational changes induced by soluble PEP75, although p38MAPK, PI3K, and Rac1 are necessary for migration in scratch wound assay and p38MAPK is partially involved in PEP75-induced 293T cell adhesion to collagen I. Thus, syndecan-4 clustering and integrin β1 conformational changes may precede the recruitment of these signaling molecules in PEP75-induced cell migration.
DISCUSSION
Syndecan-4 responds to adhesion signals by promoting the formation of focal adhesions and modulating migratory behavior (Couchman, 2003; Morgan et al., 2007). For example, syndecan-4 functions as a coreceptor in focal contacts with α5β1 integrin to mediate attachment to fibronectin and with αvβ3 integrin to mediate attachment to vitronectin (Woods and Couchman, 2001; Mostafavi-Pour et al., 2003). The attachment of CHO cells to recombinant ADAM12 cysteine-rich domain through syndecan-4 promotes subsequent integrin β1–dependent cell spreading (Thodeti et al., 2003). Syndecan-1 can activate αvβ3 integrins and lead to cell adhesion and spreading, a phenomenon that has been termed “functional coupling” (Couchman, 2003; Beauvais et al., 2004). However, it is not clear whether syndecan-integrin functional coupling is through a direct interaction, or indirectly through an intermediate signaling pathway, with the exception of syndecan-1–mediated regulation of integrin β5 activity, which has been shown to occur through a direct interaction (McQuade et al., 2006).
Integrin β1 function is regulated through changes in its ligand affinity. It has been suggested that integrin activation involves an allosteric mechanism, as well as the existence of multiple intermediate conformations in the transition to an adhesion-competent conformation (Mold and Humphries, 2004).
The interdependency of activation and conformation of integrin β1 has been revealed by utilizing different monoclonal antibodies that bind to different conformation-dependent epitopes. JB1A (epitope: 82–87aa of integrin β1; Ni and Wilkins, 1998) and K20 (epitope: 426–587aa) antibodies recognize integrin β1 independently of its functional status or conformation, which provides a way to estimate the total amount of integrin β1. In flow cytometry, the binding of these two monoclonal antibodies was not altered by soluble PEP75 (Figure 5), indicating that the total amount of cell surface integrin β1 does not change under these conditions. Unlike JB1A, K20 binds to integrin β1 during PEP75-induced clustering (Figure 4). It is suggested that the JB1A epitope (82–87aa of integrin β1) in clustered integrin β1 may be masked by the PEP75-induced syndecan cluster during immunostaining and flow cytometry analysis.
On the other hand, P4G11 (Wayner et al., 1993), mAb13 (Mold et al., 1996), AG89 (Tsuchida et al., 1997), and 12G10 (Mold et al., 1998, 2002) recognize conformation-dependent epitopes. Conformational changes parallel changes in the functional activation of integrin β1. Conformation and activity are affected by the presence of Mn2+/Mg2+, α integrin coupling, binding substrates, and interactions with stimulating anti-integrin β1 antibody.
The 12G10 antibody binds to an epitope of integrin β1 that comprises the residues K218, R154, and R155 (Mold et al., 2002). After PEP75-induced syndecan clustering, 12G10 binding to integrin β1 did not increase in the flow cytometry assay (Figure 5). It is possible that the 12G10 epitope, including the positively charged amino residues, was enveloped by the negatively charged HS of syndecans that aggregate around integrin β1 after the addition of soluble PEP75. The epitopes of mAb13 (Mold et al., 1998) contains a 12-amino acid sequence (207 K–218 K of integrin β1) that is located close to the 12G10 epitope (Mold et al., 2002). Therefore, the unresponsiveness to soluble PEP75 of these two monoclonal antibodies could be caused by the masking of the region close to the 12-amino acid sequence by the HS of the clustered syndecans, or alternatively, it could be caused by a steric change that does not modify the conformations of the epitopes recognized by these monoclonal antibodies.
Mn2+ binding to the extracellular domain of integrin β1 activates integrin β1 (Luo and Springer, 2006) by inducing a conformational change that mimics the state of the ligand occupied receptor, thereby permitting ligand access to the integrin-binding region (Ni et al., 1998). Interestingly, the PEP75–syndecan-4 interaction induced a pattern of integrin β1 conformational changes that were similar to those seen after Mn2+ treatment, as assessed by P4G11 and AG89 antibodies (Figure 5c). These results suggest that PEP75–syndecan-4 binding may induce integrin β1 conformational changes in a manner that is similar to that of Mn2+ binding to the metal ion-dependent adhesion site region of integrin β1. However, unlike Mn2+, which is known to increase 12G10 binding to integrin β1 and its activity (Mold et al., 2002), addition of PEP75 induced no change in 12G10 binding to integrin β1 (Figure 5b). Taken these data together, the conformational changes of integrin β1 induced by PEP75–syndecan-4 complex is not completely the same as those induced by Mn2+.
The expression of the AG89 epitope was recently reported to be up-regulated in WI38VA13 and K562 cells by treatment with tenascin C–derived syndecan-binding peptide (Saito et al., 2007), which is in contrast to our results showing that AG89 expression is down-regulated in response to soluble PEP75 (Figure 5c). This difference may reflect the different cell types used in the two studies.
The signaling molecules p38MAPK (Utani et al., 2003), PKCα (Couchman and Woods, 1999), PI3K (Park et al., 2005), and Rac1 (Bass et al., 2007) have been reported to mediate downstream signaling from syndecans. None of these factors were significantly involved in P4G11 binding to activate integrin β1 (Figure 8b). However, p38MAPK, PI3K, and Rac1 blocked cell migration in the scratch assay. A simple explanation for these results would be that p38MAPK, PI3K, and Rac1 are involved in a migration signaling pathway that is stimulated by soluble PEP75 but only after integrin β1 activation has been completed.
Saito et al. (2007) showed that the intracellular domains of syndecan-4 and PKCα were not involved in AG89 binding of activated integrin β1 after attaching the tenascin C–derived peptide to syndecan-4. Accordingly, the fact that PKCα did not mediate cell migration suggests that integrin β1 activation via syndecan clustering does not need the intracytoplasmic domain of syndecan-4 for cell migration stimulated by soluble PEP75.
Pulldown assays showed that syndecan-4 bound to PEP75, but syndecan-1 failed to bind to PEP75-beads (Figure 7a). This is similar to what was seen with tenascin C–derived peptide beads, in which syndecan-4, but not syndecan-1 bound to the tenascin C–derived peptide (Saito et al., 2007). Furthermore, syndecan-1 clusters were not observed in the presence of PEP75 (data not shown). In addition, the reduced expression of syndecan-1 in cells treated with siRNA did not result in inhibition of PEP75 induced activation of β1 integrin and cell migration (Figure 7b). These data are consistent with the absence of an involvement of syndecan-1 in these processes.
Taken all these data together, we speculate that syndecan-4 cluster formation and integrin β1 colocalization with syndecan is an immediate early event after exposure to soluble PEP75, followed by conformational changes in integrin β1 and the activation of integrin β1, an event that is essential for PEP75-induced cell migration. Alternatively, integrin β1 activation by PEP75-syndecan binding could be mediated by the cross-activation of integrin and syndecan receptors via extracellular interactions between these two receptors, i.e., “the syndecan-clustering mechanism,” rather than by outside-in or inside-out mechanisms.
More than 50 cytoplasmic proteins are present in cell-adhesion structures (Berrier and Yamada, 2007), and there are many signaling pathways that can regulate integrin activation. Inhibitor to p38MAPK partially blocked the augmentation of adhesion activity induced by soluble PEP75 (Figure 8c), suggesting that P38 MAPK activation induced by soluble PEP75 binding to syndecan (Utani et al., 2003) mediates downstream signal from syndecan-4 clustering and integrin β1 activation to cell adhesion. Because skin wounding activated p38MAPK in the leading keratinocytes (Harper et al., 2005), it is intriguing to think that α3LG4 binding to syndecan-4–activated p38MAPK, leading to integrin β1 functional up-regulation at the wound. Although the detail mechanism remains to be elucidated, we have identified a regulatory pathway of cell migration that involves the PEP75-induced clustering of syndecan-4, followed by cross-activation of integrin β1. This novel pathway underscores the complexity of syndecan-4-integrin β1 cross-interactions in cell migration. PEP75 itself could induce syndecan-4 clustering without cross-linking. It is possible that PEP75 binding to HS of syndecan-4 creates a high enough local concentration of PEP75 to induce PEP75 multimer formation, leading to syndecan-4 clustering. This unique characteristic of PEP75 underscores the great potential of PEP75 as a reagent for promoting wound healing.
ACKNOWLEDGMENTS
This work was supported by Grant-in-aid for Scientific Research from the Ministry of Education, Science, Culture, and Sports of Japan and grants from the Takeda-Science Foundation, Cosmetology Foundation, Fugaku Foundation, and Rehabilitation Foundation (A.U.).
Abbreviations used:
- ECM
extracellular matrix.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-09-0977) on April 29, 2009.
REFERENCES
- Alexopoulou A. N., Multhaupt H. A., Couchman J. R. Syndecans in wound healing, inflammation and vascular biology. Int. J. Biochem. Cell Biol. 2007;39:505–528. doi: 10.1016/j.biocel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Bass M. D., Roach K. A., Morgan M. R., Mostafavi-Pour Z., Schoen T., Muramatsu T., Mayer U., Ballestrem C., Spatz J. P., Humphries M. J. Syndecan-4-dependent Rac1 regulation determines directional migration in response to the extracellular matrix. J. Cell Biol. 2007;177:527–538. doi: 10.1083/jcb.200610076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvais D. M., Burbach B. J., Rapraeger A. C. The syndecan-1 ectodomain regulates alphavbeta3 integrin activity in human mammary carcinoma cells. J. Cell Biol. 2004;167:171–181. doi: 10.1083/jcb.200404171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrier A. L., Yamada K. M. Cell-matrix adhesion. J. Cell Physiol. 2007;213:565–573. doi: 10.1002/jcp.21237. [DOI] [PubMed] [Google Scholar]
- Carey D. J., Stahl R. C., Tucker B., Bendt K. A., Cizmeci-Smith G. Aggregation-induced association of syndecan-1 with microfilaments mediated by the cytoplasmic domain. Exp. Cell Res. 1994;214:12–21. doi: 10.1006/excr.1994.1228. [DOI] [PubMed] [Google Scholar]
- Couchman J. R. Syndecans: proteoglycan regulators of cell-surface microdomains? Nat. Rev. Mol. Cell Biol. 2003;4:926–937. doi: 10.1038/nrm1257. [DOI] [PubMed] [Google Scholar]
- Couchman J. R., Woods A. Syndecan-4 and integrins: combinatorial signaling in cell adhesion. J. Cell Sci. 1999;112(Pt 20):3415–3420. doi: 10.1242/jcs.112.20.3415. [DOI] [PubMed] [Google Scholar]
- Decline F., Okamoto O., Mallein-Gerin F., Helbert B., Bernaud J., Rigal D., Rousselle P. Keratinocyte motility induced by TGF-beta1 is accompanied by dramatic changes in cellular interactions with laminin 5. Cell Motil. Cytoskelet. 2003;54:64–80. doi: 10.1002/cm.10086. [DOI] [PubMed] [Google Scholar]
- Echtermeyer F., Streit M., Wilcox-Adelman S., Saoncella S., Denhez F., Detmar M., Goetinck P. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J. Clin. Invest. 2001;107:R9–R14. doi: 10.1172/JCI10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenius K., Vainio S., Laato M., Salmivirta M., Thesleff I., Jalkanen M. Induced expression of syndecan in healing wounds. J. Cell Biol. 1991;114:585–595. doi: 10.1083/jcb.114.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fears C. Y., Woods A. The role of syndecans in disease and wound healing. Matrix Biol. 2006;25:443–456. doi: 10.1016/j.matbio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Gallo R., Kim C., Kokenyesi R., Adzick N. S., Bernfield M. Syndecans-1 and -4 are induced during wound repair of neonatal but not fetal skin. J. Invest. Dermatol. 1996;107:676–683. doi: 10.1111/1523-1747.ep12365571. [DOI] [PubMed] [Google Scholar]
- Goldfinger L. E., Stack M. S., Jones J. C. Processing of laminin-5 and its functional consequences: role of plasmin and tissue-type plasminogen activator. J. Cell Biol. 1998;141:255–265. doi: 10.1083/jcb.141.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper E. G., Alvares S. M., Carter W. G. Wounding activates p38 map kinase and activation transcription factor 3 in leading keratinocytes. J. Cell Sci. 2005;118:3471–3485. doi: 10.1242/jcs.02475. [DOI] [PubMed] [Google Scholar]
- Kainulainen T., Hakkinen L., Hamidi S., Larjava K., Kallioinen M., Peltonen J., Salo T., Larjava H., Oikarinen A. Laminin-5 expression is independent of the injury and the microenvironment during reepithelialization of wounds. J. Histochem. Cytochem. 1998;46:353–360. doi: 10.1177/002215549804600309. [DOI] [PubMed] [Google Scholar]
- Kato K., Utani A., Suzuki N., Mochizuki M., Yamada M., Nishi N., Matsuura H., Shinkai H., Nomizu M. Identification of neurite outgrowth promoting sites on the laminin alpha 3 chain G domain. Biochemistry. 2002;41:10747–10753. doi: 10.1021/bi020180k. [DOI] [PubMed] [Google Scholar]
- Luo B. H., Springer T. A. Integrin structures and conformational signaling. Curr. Opin. Cell Biol. 2006;18:579–586. doi: 10.1016/j.ceb.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuade K. J., Beauvais D. M., Burbach B. J., Rapraeger A. C. Syndecan-1 regulates alphavbeta5 integrin activity in B82L fibroblasts. J. Cell Sci. 2006;119:2445–2456. doi: 10.1242/jcs.02970. [DOI] [PubMed] [Google Scholar]
- Momota Y., Suzuki N., Kasuya Y., Kobayashi T., Mizoguchi M., Yokoyama F., Nomizu M., Shinkai H., Iwasaki T., Utani A. Laminin alpha3 LG4 module induces keratinocyte migration: involvement of matrix metalloproteinase-9. J. Recept. Signal Transduct. Res. 2005;25:1–17. doi: 10.1081/rrs-200047870. [DOI] [PubMed] [Google Scholar]
- Morgan M. R., Humphries M. J., Bass M. D. Synergistic control of cell adhesion by integrins and syndecans. Nat. Rev. Mol. Cell Biol. 2007;8:957–969. doi: 10.1038/nrm2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafavi-Pour Z., Askari J. A., Parkinson S. J., Parker P. J., Ng T. T., Humphries M. J. Integrin-specific signaling pathways controlling focal adhesion formation and cell migration. J. Cell Biol. 2003;161:155–167. doi: 10.1083/jcb.200210176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould A. P., Akiyama S. K., Humphries M. J. The inhibitory anti-beta1 integrin monoclonal antibody 13 recognizes an epitope that is attenuated by ligand occupancy. Evidence for allosteric inhibition of integrin function. J. Biol. Chem. 1996;271:20365–20374. doi: 10.1074/jbc.271.34.20365. [DOI] [PubMed] [Google Scholar]
- Mould A. P., Askari J. A., Barton S., Kline A. D., McEwan P. A., Craig S. E., Humphries M. J. Integrin activation involves a conformational change in the alpha 1 helix of the beta subunit A-domain. J. Biol. Chem. 2002;277:19800–19805. doi: 10.1074/jbc.M201571200. [DOI] [PubMed] [Google Scholar]
- Mould A. P., Garratt A. N., Puzon-McLaughlin W., Takada Y., Humphries M. J. Regulation of integrin function: evidence that bivalent-cation-induced conformational changes lead to the unmasking of ligand-binding sites within integrin alpha5 beta1. Biochem. J. 1998;331(Pt 3):821–828. doi: 10.1042/bj3310821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould A. P., Humphries M. J. Regulation of integrin function through conformational complexity: not simply a knee-jerk reaction? Curr. Opin. Cell Biol. 2004;16:544–551. doi: 10.1016/j.ceb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Nguyen B. P., Ryan M. C., Gil S. G., Carter W. G. Deposition of laminin 5 in epidermal wounds regulates integrin signaling and adhesion. Curr. Opin. Cell Biol. 2000;12:554–562. doi: 10.1016/s0955-0674(00)00131-9. [DOI] [PubMed] [Google Scholar]
- Ni H., Li A., Simonsen N., Wilkins J. A. Integrin activation by dithiothreitol or Mn2+ induces a ligand-occupied conformation and exposure of a novel NH2-terminal regulatory site on the beta1 integrin chain. J. Biol. Chem. 1998;273:7981–7987. doi: 10.1074/jbc.273.14.7981. [DOI] [PubMed] [Google Scholar]
- Ni H., Wilkins J. A. Localisation of a novel adhesion blocking epitope on the human beta 1 integrin chain. Cell Adhes. Commun. 1998;5:257–271. doi: 10.3109/15419069809040296. [DOI] [PubMed] [Google Scholar]
- Okamoto O., Bachy S., Odenthal U., Bernaud J., Rigal D., Lortat-Jacob H., Smyth N., Rousselle P. Normal human keratinocytes bind to the alpha3LG4/5 domain of unprocessed laminin-5 through the receptor syndecan-1. J. Biol. Chem. 2003;278:44168–44177. doi: 10.1074/jbc.M300726200. [DOI] [PubMed] [Google Scholar]
- Oksala O., Salo T., Tammi R., Hakkinen L., Jalkanen M., Inki P., Larjava H. Expression of proteoglycans and hyaluronan during wound healing. J. Histochem. Cytochem. 1995;43:125–135. doi: 10.1177/43.2.7529785. [DOI] [PubMed] [Google Scholar]
- Park H., Han I., Kwon H. J., Oh E. S. Focal adhesion kinase regulates syndecan-2-mediated tumorigenic activity of HT1080 fibrosarcoma cells. Cancer Res. 2005;65:9899–9905. doi: 10.1158/0008-5472.CAN-05-1386. [DOI] [PubMed] [Google Scholar]
- Ryan M. C., Tizard R., VanDevanter D. R., Carter W. G. Cloning of the LamA3 gene encoding the alpha 3 chain of the adhesive ligand epiligrin. Expression in wound repair. J. Biol. Chem. 1994;269:22779–22787. [PubMed] [Google Scholar]
- Saito Y., et al. A peptide derived from tenascin-C induces beta1 integrin activation through syndecan-4. J. Biol. Chem. 2007;282:34929–34937. doi: 10.1074/jbc.M705608200. [DOI] [PubMed] [Google Scholar]
- Sigle R. O., et al. Globular domains 4/5 of the laminin alpha3 chain mediate deposition of precursor laminin 5. J. Cell Sci. 2004;117:4481–4494. doi: 10.1242/jcs.01310. [DOI] [PubMed] [Google Scholar]
- Stepp M. A., et al. Defects in keratinocyte activation during wound healing in the syndecan-1-deficient mouse. J. Cell Sci. 2002;115:4517–4531. doi: 10.1242/jcs.00128. [DOI] [PubMed] [Google Scholar]
- Thodeti C. K., Albrechtsen R., Grauslund M., Asmar M., Larsson C., Takada Y., Mercurio A. M., Couchman J. R., Wewer U. M. ADAM12/syndecan-4 signaling promotes beta 1 integrin-dependent cell spreading through protein kinase Calpha and RhoA. J. Biol. Chem. 2003;278:9576–9584. doi: 10.1074/jbc.M208937200. [DOI] [PubMed] [Google Scholar]
- Tran M., Rousselle P., Nokelainen P., Tallapragada S., Nguyen N. T., Fincher E. F., Marinkovich M. P. Targeting a tumor-specific laminin domain critical for human carcinogenesis. Cancer Res. 2008;68:2885–2894. doi: 10.1158/0008-5472.CAN-07-6160. [DOI] [PubMed] [Google Scholar]
- Tsubota Y., Mizushima H., Hirosaki T., Higashi S., Yasumitsu H., Miyazaki K. Isolation and activity of proteolytic fragment of laminin-5 alpha3 chain. Biochem. Biophys. Res. Commun. 2000;278:614–620. doi: 10.1006/bbrc.2000.3851. [DOI] [PubMed] [Google Scholar]
- Tsuchida J., Ueki S., Saito Y., Takagi J. Classification of ‘activation’ antibodies against integrin beta1 chain. FEBS Lett. 1997;416:212–216. doi: 10.1016/s0014-5793(97)01206-4. [DOI] [PubMed] [Google Scholar]
- Utani A., Momota Y., Endo H., Kasuya Y., Beck K., Suzuki N., Nomizu M., Shinkai H. Laminin alpha 3 LG4 module induces matrix metalloproteinase-1 through mitogen-activated protein kinase signaling. J. Biol. Chem. 2003;278:34483–34490. doi: 10.1074/jbc.M304827200. [DOI] [PubMed] [Google Scholar]
- Utani A., Nomizu M., Matsuura H., Kato K., Kobayashi T., Takeda U., Aota S., Nielsen P. K., Shinkai H. A unique sequence of the laminin alpha 3 G domain binds to heparin and promotes cell adhesion through syndecan-2 and -4. J. Biol. Chem. 2001;276:28779–28788. doi: 10.1074/jbc.M101420200. [DOI] [PubMed] [Google Scholar]
- Wayner E. A., Gil S. G., Murphy G. F., Wilke M. S., Carter W. G. Epiligrin, a component of epithelial basement membranes, is an adhesive ligand for alpha 3 beta 1 positive T lymphocytes. J. Cell Biol. 1993;121:1141–1152. doi: 10.1083/jcb.121.5.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A., Couchman J. R. Syndecan-4 and focal adhesion function. Curr. Opin. Cell Biol. 2001;13:578–583. doi: 10.1016/s0955-0674(00)00254-4. [DOI] [PubMed] [Google Scholar]