Abstract
Cell apoptosis induced by UV irradiation is a highly complex process in which different molecular signaling pathways are involved. p53 up-regulated modulator of apoptosis (PUMA) has been proposed as an important regulator in UV irradiation-induced apoptosis. However, the molecular mechanism through which PUMA regulates apoptosis, especially how PUMA activates Bcl-2-associated X protein (Bax) in response to UV irradiation is still controversial. In this study, by using real-time single-cell analysis and fluorescence resonance energy transfer, we investigated the tripartite nexus among PUMA, Bax, and Bcl-XL in living human lung adenocarcinoma cells (ASTC-a-1) to illustrate how PUMA promotes Bax translocation to initiate apoptosis. Our results show that the interaction between PUMA and Bax increased gradually, with Bax translocating to mitochondria and colocalizing with PUMA after UV irradiation, indicating PUMA promotes Bax translocation directly. Simultaneously, the interaction increased markedly between PUMA and Bcl-XL and decreased significantly between Bcl-XL and Bax after UV treatment, suggesting PUMA competitively binds to Bcl-XL to activate Bax indirectly. The above-mentioned results were further confirmed by coimmunoprecipitation experiments. In addition, pifithrin-α (a p53 inhibitor) and cycloheximide (a protein synthesis inhibitor) could inhibit PUMA-mediated Bax translocation and cell apoptosis. Together, these studies create an important conclusion that PUMA promotes Bax translocation by both by directly interacting with Bax and by competitive binding to Bcl-XL in UV-induced apoptosis.
INTRODUCTION
UV irradiation is a potent carcinogen that can impair cellular functions by directly damaging DNA to induce apoptosis. The cellular response to DNA damage is centered on p53, a transcription factor that exerts its tumor-suppressive function by inducing cell cycle arrest, cell senescence, or apoptosis (Vousden and Lu, 2002). p53 stimulates a wide network of signals to activate the caspases that mediate apoptosis (Strasser et al., 1995). It has been shown that the apoptosis induced by p53 is in large part due to its ability to transcriptionally activate target genes (Chao et al., 2000). Many genes have been identified that are implicated in p53-mediated apoptosis (Vousden and Lu, 2002), such as PUMA and p21. Among the candidate proapoptotic p53 target genes, those that encode mitochondrial proteins such as PUMA stand out because p53-initiated apoptosis seems to proceed through a mitochondrial pathway (Polyak et al., 1997; Li et al., 1999; Soengas et al., 1999; Schuler et al., 2000).
p53 up-regulated modulator of apoptosis (PUMA), a member of the Bcl-2 homology (BH)3-only Bcl-2 family proteins, also known as Bcl-2 binding component 3, is a direct transcriptional target of the p53 (Han et al., 2001; Nakano and Vousden, 2001; Yu et al., 2001; Chipuk et al., 2005). PUMA is localized in the mitochondria and induces apoptosis by activating caspases through mitochondrial dysfunction (Nakano and Vousden, 2001; Yu et al., 2001, 2003). The expression of PUMA is low at normal conditions but increases markedly if cells are exposed to DNA-damaging agents, such as chemotherapeutic drugs or ionizing and UV irradiation. The activation of PUMA by DNA damage is dependent on p53 and is mediated by the direct binding of p53 to the PUMA promoter region (Nakano and Vousden, 2001; Yu et al., 2001; Wang et al., 2007). PUMA plays an essential role in cell apoptosis induced by a variety of stimuli (Yu and Zhang, 2003). Previous studies have shown that PUMA was necessary and sufficient for endoplasmic reticulum stress-induced apoptosis and that it also was activated in cells subjected to proteasome inhibition (Reimertz et al., 2003; Concannon et al., 2007). Cells lacking PUMA are resistant to several death stimuli (Clarke et al., 1993; Strasser et al., 1994; Jeffers et al., 2003; Villunger et al., 2003; Yu et al., 2003). Furthermore, PUMA expression results in potent growth suppression of lung cancer cells, whereas down-regulation of PUMA promotes oncogenic transformation, suggesting PUMA plays an important role in tumor suppression (Hemann et al., 2004; Yu et al., 2006).
Several lines of recent evidence suggest that Bcl-2 family members play a critical role in regulating apoptosis initiation through the mitochondria (Green and Reed, 1998; Vander Heiden and Thompson, 1999; Wang, 2001; Cory et al., 2003; Danial and Korsmeyer, 2004; Yu and Zhang, 2004). Bcl-2 family members can be subdivided into three main groups based on regions of BH domains and function: multidomain antiapoptotic (Bcl-2, Bcl-XL, Bcl-w, Mcl-1, and Bfl-1/A1), multidomain proapoptotic (Bcl-2–associated X protein [Bax] and Bak), and BH3-only proapoptotic (Bid, Bim, Bad, Bik, Noxa, PUMA, Bmf, and Hrk) (Certo et al., 2006). The multidomain antiapoptotic Bcl-2 members, such as Bcl-XL and Bcl-2, inhibit cytochrome release by blocking the activation of the multidomain proapoptotic proteins Bax and Bak (Adams and Cory, 2001). The BH3-only proteins function in two models, either by directly activating proapoptotic proteins (the direct binding model) or by antagonizing antiapoptotic proteins (the displacement model) to induce the oligomerization of Bax and/or Bak, resulting in mitochondrial outer membrane permeabilization (MOMP) (Luo et al., 1998; Desagher et al., 1999; Kuwana et al., 2002; Letai et al., 2002; Cartron et al., 2004; Chen et al., 2005; Kuwana et al., 2005; Willis et al., 2005, 2007; Zhong et al., 2005; Ming et al., 2006). MOMP can trigger a cascade of downstream events to initiate apoptosis, including the release of proapoptotic factors such as second mitochondria-derived activator of caspases, apoptosis-inducing factor, cytochrome c, and the activation of caspase cascade (Wang, 2001; Danial and Korsmeyer, 2004; Dejean et al., 2005).
Our previous studies demonstrate that Bax translocation induced by UV irradiation is a p53 transcription-dependent event (Wu et al., 2007). That means PUMA, as the direct target gene of p53, may play a critical role in Bax translocation and apoptosis induced by UV irradiation. However, the molecular mechanism through which PUMA regulates apoptosis is unclear. To further elucidate it, in this study, based on real-time single-cell analysis, we investigated the tripartite nexus among PUMA, Bax, and Bcl-XL in living cells. We found PUMA promotes Bax translocation both by directly interacting with Bax and by competitive binding to Bcl-XL in UV-induced apoptosis.
MATERIALS AND METHODS
Materials
DMEM was purchased from Invitrogen (Carlsbad, CA). Pifithrin-α (a p53 inhibitor) was purchased from BioVision (Mountain View, CA). Lipofectamine reagent was purchased from Invitrogen. A DNA extraction kit was purchased from QIAGEN (Valencia, CA). Green fluorescent protein expressing plasmid (pGFP)-PUMA and pGFP-PUMA-▵BH3 were kindly supplied by Dr. Jian Yu (Yu et al., 2001). pGFP-Bax was kindly supplied by Richard J. Youle (Nechushtan et al., 1999); pYFP-Bax, pYFP-Bcl-XL, and pCFP-Bcl-XL were kindly supplied by Dr. A. P. Gilmore (Valentijn et al., 2003); and pDsRed-Mit was kindly supplied by Dr. Y. Gotoh (Tsuruta et al., 2002). Other chemicals were mainly from Sigma-Aldrich (St. Louis, MO).
Cell Culture and Treatments
The human lung adenocarcinoma cell line (ASTC-a-1) was obtained from the Department of Medicine (Jinan University, Guangzhou, Guangdong, People's Republic of China). Cells were cultured in DMEM supplemented with 15% fetal calf serum, penicillin (100 U/ml), and streptomycin (100 mg/ml) in 5% CO2 at 37°C in a humidified incubator. Transfections were performed with Lipofectamine 2000 reagent (Invitrogen), according to the manufacturer's protocol. The medium was replaced with fresh culture medium after 5 h. Cells were examined at 24–48 h after transfection. For UV treatment, medium was removed and saved. The cells were rinsed with phosphate-buffered saline (PBS) and irradiated, and the medium was restored. Unless otherwise specified, cells were exposed to UV irradiation at a fluence of 120 mJ/cm2 and observed at the times indicated.
Time-Lapse Confocal Fluorescence Microscopy
GFP, cyan fluorescent protein (CFP), yellow fluorescent protein (YFP), and red fluorescent protein (DsRed) fluorescence were monitored confocally using a commercial laser scanning microscope (LSM 510/ConfoCor 2) combination system (Carl Zeiss, Jena, Germany) equipped with a Plan-Neofluar 40×/1.3 numerical aperture (NA) oil differential interference contrast (DIC) objective. Excitation wavelength and detection filter settings for each of the fluorescent indicators were as follows: GFP fluorescence was excited at 488 nm with an argon ion laser, and emission was recorded through a 500- to 520-nm band pass filter. CFP fluorescence was excited at 458 nm with an argon ion laser, and emission was recorded through a 470- to 500-nm band pass filter. YFP fluorescence was excited at 514 nm with an argon ion laser, and emission was recorded through a 535- to 545-nm band pass filter. DsRed fluorescence was excited at 543 nm with a helium-neon laser, and emitted light was recorded through a 560-nm long pass filter. MitoTracker fluorescence was excited at 633 nm with a helium-neon laser, and emitted light was recorded through a 650-nm long pass filter.
For time-lapse imaging, culture dishes were mounted onto the microscope stage that was equipped with a temperature-controlled chamber (Carl Zeiss). During control experiments, bleaching of the probe was negligible.
GFP-Bax Translocation Assay
To monitor GFP-Bax translocation in living cells, ASTC-a-1 cells were cotransfected with pGFP-Bax and pDsRed-Mit. Using an LSM 510 confocal microscope (Carl Zeiss), we imaged both the distribution pattern of GFP-Bax and that of DsRed-Mit simultaneously during UV-induced apoptosis. Bax redistribution was assessed by the matching fluorescence of GFP-Bax and DsRed-Mit emission. The cells exhibiting strong punctate staining of GFP, which overlapped with the distribution of DsRed, were counted as the cells with mitochondrially localized Bax.
Fluorescence Resonance Energy Transfer (FRET) Analysis
FRET was performed on a commercial laser scanning microscopes (LSM510/ConfoCor2) combination system (Carl Zeiss). For excitation, the 458-nm line of an argon-ion laser was attenuated with an acousto-optical tunable filter, reflected by a dichroic mirror (main beam splitter HFT458), and focused through a Plan-Neofluar 40×/1.3 NA oil DIC objective (Carl Zeiss) onto the sample. CFP and YFP (FRET acceptor) emission were collected through 470- to 500- and 535- to 545-nm band pass filters, respectively. GFP and YFP (a FRET acceptor) emissions were collected through 500- to 520- and 535- to 545-nm band pass filters, respectively. The quantitative analysis of the fluorescence images was performed using Zeiss Rel3.2 image processing software (Carl Zeiss). After background subtraction, the average fluorescence intensity per pixel was calculated. During control experiments, bleaching of the probe was negligible.
For monitoring the redistribution of YFP-Bax, YFP fluorescence was excited at 514 nm with an argon-ion laser, and the emission was collected through a 535- to 545-nm band bass filter simultaneously.
Cell Viability and Apoptosis Assays
ASTC-a-1 cells were cultured in 96-well microplate at a density of 5 × 103 cells/well for 24 h. The cells were then divided into five groups and exposed to UV irradiation at fluence of 0 (control), 80, 100, 120, and 150 mJ/cm2. Cell viability was assessed with Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan) at 0, 3, 6, 9, and 12 h posttreatment, according to the manufacturer's instructions. OD450, the absorbance value at 450 nm, was read with a 96-well plate reader (DG5032; Hua dong, Nanjing, China) to determine the viability of the cells.
For analysis of apoptosis by nuclear staining, ASTC-a-1 cells were cultured on the coverslip of a chamber, rinsed with PBS, and then 500 ml of DMEM containing 5 μg of Hoechst 33342 was added and the mixture was incubated at 37°C with 5% CO2 for 15 min. Apoptosis was assessed through microscopic visualization of condensed chromatin and micronucleation.
Antibodies and Western Blotting
The antibodies used for Western blotting include antibodies against PUMA (Epitomics, Burlingame, CA), Bax, Bcl-XL (Cell Signaling Technology, Danvers, MA), and GFP (GenScript, Piscataway, NJ). At the indicated times after UV irradiation, cells were harvested and washed twice with ice-cold PBS, pH 7.4, and lysed with ice-cold lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1× Triton X-100, and 100 μg/ml phenylmethylsulfonyl fluoride) for 30 min on ice. The lysates were centrifuged at 12,000 rpm for 5 min at 4°C, and the protein concentration was determined. Equivalent samples (30 μg of protein extract was loaded on each lane) were subjected to SDS-PAGE on 12% gel. The proteins were then transferred onto nitrocellulose membranes and probed with the indicated antibody, followed by IRDye 800 secondary antibody (Rockland Immunochemicals, Gilbertsville, PA). Detection was performed using the Odyssey infrared imaging system (LI-COR, Lincoln, NE).
Immunofluorescence
For Bax activation, MitoTracker Red (0.5 M; Invitrogen) was added into the medium, and cells were incubated at 37°C for 30 min. Slides were fixed in 4% paraformaldehyde for 15 min at room temperature and then washed five times with PBS. Samples were incubated in blocking buffer (10% bovine serum albumin in PBS) for 1 h at room temperature, followed by incubation with anti-Bax antibody 6A7 (5 μg/ml in blocking buffer; Abcam, Cambridge, United Kingdom) at 4°C overnight. Cells were washed five times for 5 min each, after which Alexa Fluor 488-conjugated rabbit anti-mouse secondary antibody (diluted 1:400 in blocking buffer; Invitrogen) was added for 1 h at room temperature. After five additional washes with PBS, slides were mounted and analyzed by confocal microscopy. For PUMA localization, cells were treated in a similar manner, except that samples were incubated with anti-PUMA antibody (diluted 1:200 in blocking buffer) overnight at 4°C, at which point samples were washed and incubated with a mixture of Alexa Fluor 555-conjugated goat anti-rabbit secondary antibody (diluted 1:400 in blocking buffer) for 1 h before mounting and visualization.
Coimmunoprecipitation
At the indicated time after UV irradiation, cells were harvested and washed twice with ice-cold PBS, pH 7.4, and lysed with ice-cold lysis buffer [10 mmol of HEPES, pH 7.4, 150 mM NaCl, 1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, and 1% protease inhibitors] for 30 min on ice. For immunoprecipitation (IP), ∼4 μl of IP antibodies was added to 400 μl of cell lysates. The mixtures were mixed on a rocker at ambient temperature for 2 h. The immunocomplexes were captured by the addition of protein G/A-agarose (Roche Applied Sciences, Indianapolis, IN) mixed at 1:10 ratio, followed by incubation at ambient temperature for 1 h. The beads were washed three times by PBS and then collected by centrifugation at 12,000 rpm for 5 s. After the final wash, the beads were mixed with 60 μl of 2× Laemmli sample buffer, heated at 100°C for 5 min, and analyzed by Western blotting.
RESULTS
Cell Apoptosis Induced by UV Irradiation Is Dose and Time Dependent
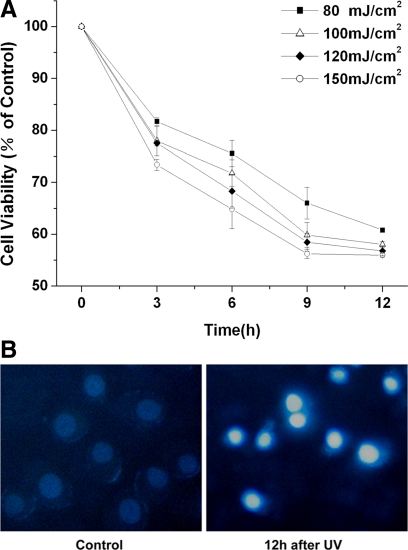
To establish a proper UV irradiation dose to induce apoptosis, ASTC-a-1 cells were treated with various fluences of UV irradiation. Cell viability was analyzed using CCK-8 at 0, 3, 6, 9, and 12 h after UV irradiation. Treated cells showed a decrease in viability with increasing radiation dose, and a correlation was observed with posttreatment period, which indicates that the effect of UV irradiation on cell apoptosis was dose and time dependent (Figure 1A).
Figure 1.
UV irradiation induces apoptosis in ASTC-a-1 cells. (A) Cells viability was analyzed using Cell Counting Kit-8 at 0, 3, 6, 9, and 12 h after various fluences of UV irradiation. Data represent the mean ± SD of four independent experiments. (B) Hoechst 33342 morphological examination of UV irradiation-inducing apoptosis. ASTC-a-1 cells were treated with 120-mJ/cm2 UV irradiation and incubated for 12 h and then stained with Hochest 33342 (right). The fluorescence images were collected via a C-Apochromat objective (40×, 1.3 NA; Carl Zeiss).
To evaluate the status of cell apoptosis directly, morphological examination was performed with Hoechst 33342 staining. As shown in Figure 1B, chromatin condensation was observed 12 h after the cells exposure to 120 J/cm2 UV irradiation, indicating the occurrence of apoptosis. These results (Figure 1, A and B) indicate that 80–150 mJ/cm2 UV irradiation could cause obvious cell apoptosis. Then, we chose the 120 mJ/cm2 UV irradiation for the further experiments.
Real-Time Detection of Bax Translocation Induced by UV Irradiation
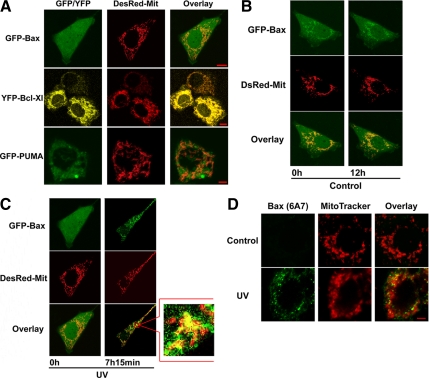
To determine the distribution of PUMA, Bax, and Bcl-XL in cells, ASTC-a-1 cells were transiently transfected with GFP-Bax, YFP-Bcl-XL, and GFP-PUMA, respectively. The DsRed-Mit (a marker for mitochondria) also was transfected into ASTC-a-1 cells to label the mitochondria. The results show that GFP-Bax had a diffuse distribution in the whole cell, whereas GFP-PUMA partly coincided with DsRed-Mit and YFP-Bcl-XL coincided with DsRed-Mit near completely (Figure 2A).
Figure 2.
Spatial and temporal changes in Bax subcellular localization during UV irradiation-induced apoptosis. (A–C) ASTC-a-1 cells were transiently cotransfected with GFP-Bax, YFP-Bcl-XL, or GFP-PUMA and DsRed-Mit (a marker for mitochondria). (A) Distribution of GFP-Bax, YFP-Bcl-XL, and GFP-PUMA in ASTC-a-1 cells. (B) GFP-Bax typically displayed a diffuse, cytoplasmic localization in the untreated cell. (C) GFP-Bax translocated to mitochondria noticeably after UV irradiation. The image in the mark frame was the zoom-in image of the frame indicated region. (D) Mitochondrial localization of conformationally activated Bax in cells treated by UV irradiation. Similar results were obtained from three independent experiments. Bar, 5 μm.
To detect the GFP-Bax redistribution during UV-induced apoptosis in real time, ASTC-a-1 cells were transiently cotransfected with GFP-Bax and DsRed-Mit and then treated with 120-mJ/cm2 UV irradiation. As the result shown in Figure 2C, upon UV stimulation, almost all the GFP-Bax translocated from cytosol to mitochondria, indicating the activation of Bax, at ∼7 h after UV irradiation. By the contrast, GFP-Bax had a diffuse distribution in the whole cell for >12 h in the control group (Figure 2B).
Generally, the activation of Bax is inferred by its translocation from cytosol to mitochondria. To directly detect Bax activation, ASTC-a-1 cells were treated with 120-mJ/cm2 UV irradiation, and then cells were fixed and subjected to immunofluorescence staining for active Bax by using a conformation-specific anti-Bax monoclonal antibody (6A7) that selectively recognizes only the activated/proapoptotic form of Bax, and mitochondria were stained with MitoTracker Red. As shown in Figure 2D, active Bax was not identified in untreated cells (control) but was readily detectable in cells after 7 h of UV irradiation. The yellow color in the overlay indicates the presence of conformationally changed activated Bax colocalized with mitochondria. These results can clearly demonstrate that Bax activation is accompanied by its translocation to mitochondria, suggesting the translocation of Bax means the activation of it.
PUMA Interacts and Promotes Bax Translocation Directly during UV-induced Apoptosis
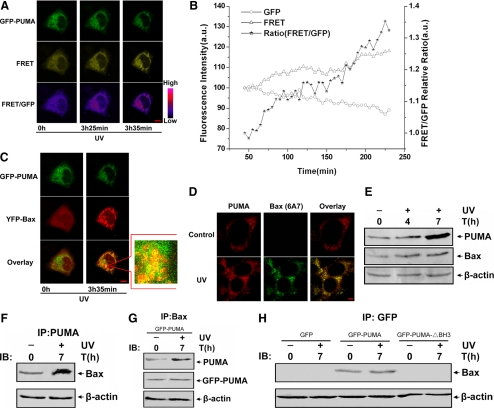
It has been reported that Bax is required for PUMA-mediated apoptosis (Yu et al., 2003). However, how PUMA functions to activate Bax after UV irradiation is unclear. To determine whether PUMA can activate Bax directly, the dynamic interaction between PUMA and Bax was performed in living single cells by using FRET. ASTC-a-1 cells were transiently cotransfected with GFP-PUMA and YFP-Bax and then treated with 120-mJ/cm2 UV irradiation. The typical time course images of GFP-PUMA, FRET, and the ratio of FRET/GFP in cells after UV irradiation are shown in Figure 3A. The fluorescence images show that the emission in the GFP channel decreased, whereas the emission in the FRET channel and the ratio of the FRET/GFP channel increased, indicating that the interaction between PUMA and Bax increased. This result also was confirmed by the quantitative analysis of GFP, FRET fluorescence emission intensities, and the ratio of FRET/GFP in the whole cell (Figure 3B). More interestingly, YFP-Bax translocated and colocalized with GFP-PUMA (Figure 3C). The results of immunofluorescence show that the endogenous activated Bax colocalized with the endogenous PUMA (Figure 3D), indicating that Bax translocated to mitochondria after UV irradiation, because PUMA is largely localized in the mitochondria. Together, these results suggest that PUMA interacted with Bax directly, resulting in Bax conformational modification and subsequent translocation to mitochondria after UV stimulation.
Figure 3.
PUMA interacts with Bax directly and activates its translocation after UV irradiation. (A–C) Real-time monitoring PUMA interacts with Bax and promotes Bax translocation during UV irradiation-induced apoptosis. ASTC-a-1 cells were transiently cotransfected with YFP-Bax and GFP-PUMA and then treated with UV irradiation. (A) Representative fluorescence image series of GFP, FRET, and FRET/GFP ratio of the cells. The images of FRET/GFP ratio were processed with pseudocolor technique. (B) Quantitative analysis of GFP, FRET intensities, and FRET/GFP ratio corresponding to the images in A. The GFP and FRET intensities at the first time point are normalized to 100, and the FRET/GFP ratio is normalized to 1. (C) YFP-Bax translocates and colocalizes with GFP-PUMA after UV irradiation. Fluorescence images show the distribution of GFP-PUMA and YFP-Bax. The two panels of GFP-PUMA and YFP-Bax are shown separately and merged to show the overlay. The fluorescence images of YFP emission are shown in red. The image in the mark frame was the zoom-in image of the frame indicated region. (D) Immunofluorescence analysis for the colocalization of endogenous PUMA and activated Bax. ASTC-a-1 cells were fixed in 4% paraformaldehyde, after washing with PBS, and cells were incubated with rabbit anti-human PUMA and mouse anti-human Bax (6A7) antibodies. Alexa Fluor 555-conjugated goat anti-rabbit and Alexa Fluor 488-conjugated rabbit anti-mouse secondary antibodies were used to visualize PUMA (red) and activated Bax (green) localization patterns. Bar, 5 μm. (E) Expression of PUMA increased after UV treatment. Western blotting was performed to detect the expression of PUMA and Bax at 0, 4, and 7 h after UV irradiation. (F and G) The interaction between PUMA and Bax increased after UV irradiation. Transfecting GFP-PUMA into ASTC-a-1 cells or not, coimmunoprecipitation with an anti-PUMA antibody or anti-Bax antibody was used to pull down PUMA or Bax. (F) Western blotting for Bax shows the amount of Bax binding to PUMA. (G) Western blotting for PUMA or GFP-PUMA shows the amount of PUMA and GFP-PUMA binding to Bax. (H) After transfecting GFP, GFP-PUMA, or GFP-PUMA-▵BH3 into ASTC-a-1 cells, coimmunoprecipitation with an anti-GFP antibody was used to pull down GFP, GFP-PUMA, or GFP-PUMA-▵BH3, respectively, Western blotting was performed to detect Bax in the IP complexes. Similar results were obtained from three independent experiments.
To further confirm the above-mentioned results, Western blotting and coimmunoprecipitation were used to investigate the interaction between PUMA and Bax. Western blotting displays that the expression of both PUMA and Bax increased gradually after UV irradiation (Figure 3E), indicating that UV irradiation could up-regulate the expression of PUMA, probably due to the transcription activation mediated by p53. The results of coimmunoprecipitation show that the amount of Bax binding to PUMA increased markedly after UV irradiation (Figure 3F). In addition, when we transfected GFP-PUMA into ASTC-a-1 cells, we found that both endogenous PUMA and GFP-PUMA have evident interaction with Bax, suggesting that GFP-PUMA has a equivalent function with endogenous PUMA and that they activate Bax together (Figure 3G). However, GFP and GFP-PUMA-▵BH3 (BH3 domain-deleted PUMA) have no interaction with Bax at all (Figure 3H), which indicates that the BH3 domain of PUMA is indispensable for activating Bax. These results were consisted with the former FRET analysis and further revealed that PUMA can activate Bax directly during UV-induced apoptosis.
PUMA Promotes Bax Translocation Indirectly by Competitive Binding to Bcl-XL after UV Irradiation
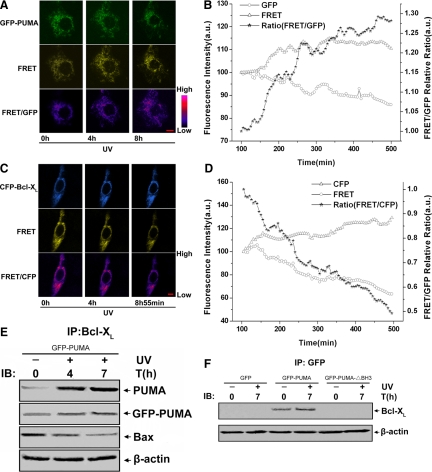
Previous studies have shown that antiapoptotic members of the Bcl-2 family, Bcl-2 and Bcl-XL, can block Bax- and Bak-induced apoptosis (Cheng et al., 2001) and that Bcl-XL interacts with Bax and BH3-only proteins through the same amino acid residues (Wang et al., 1998; Cheng et al., 2001). A recent study reported that PUMA can dissociate Bax and Bcl-XL to induce apoptosis in colon cancer cells under the treatment of adriamycin (Ming et al., 2006), which coincided with our assumption, because we speculated that PUMA could also activate Bax translocation indirectly by modulating the interaction between Bax and Bcl-XL to mediate UV-induced apoptosis. To test this hypothesis, we studied the dynamic interaction among PUMA, Bax, and Bcl-XL in living single cells by using FRET. First, the interaction between PUMA and Bcl-XL was investigated. ASTC-a-1 cells transiently cotransfected with GFP-PUMA and YFP-Bcl-XL were treated with 120-mJ/cm2 UV irradiation, and then the real-time GFP, FRET, and FRET/GFP fluorescence images were collected with LSM. The results show that the emission in the GFP channel decreased, whereas the emission in the FRET channel and the ratio of the FRET/GFP channel increased, (Figure 4A), which also was confirmed by the quantitative analysis of GFP, FRET fluorescence emission intensities, and the ratio of FRET/GFP (Figure 4B). These results indicate that the interaction of PUMA and Bcl-XL increased during UV-induced apoptosis.
Figure 4.
PUMA activates Bax indirectly by competitive binding to Bcl-XL after UV irradiation. (A and B) Single-cell imaging analysis of PUMA interacts with Bcl-XL during UV irradiation-induced apoptosis. ASTC-a-1 cells were transiently cotransfected with YFP-Bcl-XL and GFP-PUMA and then treated with UV irradiation. (A) Representative fluorescence image series of GFP, FRET, and FRET/GFP ratio of the cells. (B) Quantitative analysis of GFP, FRET intensities, and FRET/GFP ratio corresponding to the images in A. The GFP and FRET intensities at the first time point are normalized to 100, and the FRET/GFP ratio is normalized to 1. (C and D) Real-time monitoring Bcl-XL interacted with Bax during UV irradiation-induced apoptosis. ASTC-a-1 cells were transiently cotransfected with CFP-Bcl-XL and YFP-Bax, followed by UV irradiation. (C) Representative fluorescence image series of GFP, FRET, and FRET/GFP ratio of the cells. The images of FRET/CFP ratio were processed with pseudocolor technique. (D) Quantitative analysis of GFP, FRET intensities, and FRET/GFP ratio corresponding to the images in A. The CFP and FRET intensities at the first time point are normalized to 100, and the FRET/CFP ratio is normalized to 1. Bar, 5 μm. (E) Interaction between PUMA and Bcl-XL increased, whereas the interaction between Bcl-XL and Bax decreased. After transfecting GFP-PUMA into ASTC-a-1 cells, coimmunoprecipitation with an anti-Bcl-XL antibody was used to pull down Bcl-XL, and Western blotting was performed to detect PUMA, GFP-PUMA, and Bax. (F) After transfecting GFP, GFP-PUMA, or GFP-PUMA-▵BH3 into ASTC-a-1 cells, coimmunoprecipitation with an anti-GFP antibody was used to pull down GFP, GFP-PUMA, or GFP-PUMA-▵BH3, respectively, Western blotting was performed to detect Bcl-XL in the IP complexes. Similar results were obtained from three independent experiments.
Second, we investigated the dynamic interaction between Bcl-XL and Bax. ASTC-a-1 cells were transiently cotransfected with CFP-Bcl-XL and YFP-Bax. After UV irradiation, the emission in the CFP channel increased, whereas the emission in the FRET channel and the ratio of FRET/CFP channel decreased (Figure 4C), which was confirmed by quantitative analysis of CFP, FRET fluorescence emission intensities, and the ratio of FRET/CFP in the whole cell (Figure 4D), indicating the interaction between Bcl-XL and Bax decreased after UV treatment. These results (Figure 4, A–D) demonstrate that PUMA releases Bax and promotes its translocation by competitive binding to Bcl-XL during UV-induced apoptosis.
In parallel, coimmunoprecipitation was performed to analyze the effect of PUMA (both exogenous and endogenous) on the interaction between Bcl-XL and Bax. The results display that the amount of PUMA binding to Bcl-XL increased markedly, whereas the amount of Bax binding to Bcl-XL reduced evidently after UV irradiation. In addition, except for the endogenous PUMA, the GFP-PUMA also could interact with Bcl-XL (Figure 4E). When transfecting GFP, GFP-PUMA, or GFP-PUMA-▵BH3 into ASTC-a-1 cells, we found that only GFP-PUMA has interaction with Bcl-XL, whereas GFP or GFP-PUMA-▵BH3 does not (Figure 4F), indicating the BH3 domain is essential for PUMA binding to Bcl-XL. These results were consistent with our FRET analysis and further confirmed that PUMA can neutralize Bcl-XL to promote Bax translocation indirectly.
PUMA-mediated Bax Translocation and Cell Apoptosis Induced by UV Irradiation Are Inhibited by Pifithrin-α and Cycloheximide (CHX)
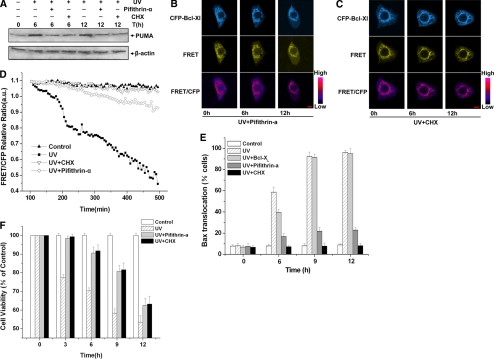
The expression of endogenous PUMA was up-regulated by UV irradiation, and increased PUMA competitive binding to Bcl-XL, resulting in the decrease of the interaction between Bcl-XL and Bax (Figures 3E and 4). To further confirm this conclusion, pifithrin-α (a p53 inhibitor) and CHX (an inhibitor of protein biosynthesis) were used in our experimental model, and we investigated whether the interaction between Bcl-XL and Bax changed after UV treatment. First, Western blotting was performed to detect the expression of PUMA in the presence of pifithrin-α or CHX. As a result, the expression of PUMA increased markedly when cells were treated with UV irradiation only, whereas in the presence of pifithrin-α or CHX, the expression of PUMA did not have an obvious increase up to 12 h after UV irradiation (Figure 5A).
Figure 5.
Bax translocation and cells apoptosis induced by UV irradiation were inhibited by pifithrin-α and CHX. (A) Expression of PUMA decreased in the presence of pifithrin-α or CHX. Western blotting was performed to detect the expression of PUMA at 0, 6, and 12 h after UV irradiation in the presence or absence of pifithrin-α or CHX. (B–D) Real-time monitoring Bcl-XL interacts with Bax in the presence of CHX or pifithrin-α after UV irradiation. ASTC-a-1 cells were transiently cotransfected with CFP-Bcl-XL and YFP-Bax, added with pifithrin-α or CHX 1 h before UV irradiation. (B) Representative fluorescence images of CFP, FRET, and FRET/CFP ratio of the cells treated with pifithrin-α. (C) Representative fluorescence images of CFP, FRET, and FRET/CFP ratio of the cells treated with CHX. The images of FRET/CFP ratio were processed with pseudocolor technique. Bar, 5 μm. (D) Quantitative analysis of FRET/GFP ratios in various conditions. (E) Bax translocation induced by UV irradiation was inhibited by pifithrin-α or CHX. Statistical analysis of Bax translocation was conducted under different conditions. At the indicated time points, the percentage of cells showing Bax translocation to mitochondria was assessed by counting the number of cells. (F) Cell apoptosis induced by UV irradiation was inhibited by pifithrin-α or CHX. Cell viability was assessed by the CCK-8 assay at 0, 3, 6, 9, and 12 h after 120-mJ/cm2 UV irradiation in the presence or absence of pifithrin-α or CHX. Data represent the mean ± SD of four independent experiments.
Then, we used FRET to investigate the dynamic interaction between Bcl-XL and Bax in the presence of pifithrin-α or CHX. ASTC-a-1 cells were transiently cotransfected with CFP-Bcl-XL and YFP-Bax, added with pifithrin-α or CHX 1 h before UV irradiation. The typical time course images of CFP-Bcl-XL, FRET, and the ratio of FRET/CFP channel in ASTC-a-1 cells are shown in Figure 5, B and C. The fluorescence images show that the emission in CFP, FRET, and the ratio of FRET/CFP channel did not change significantly. The quantification of FRET/CFP fluorescence emission intensities after UV treatment show that the ratio decreased markedly when cells were treated with UV irradiation only, whereas in the presence of pifithrin-α or CHX, the ratio remained nearly unchanged in the whole course (Figure 5D). Together, these data suggest that pifithrin-α and CHX could inhibit the expression of endogenous PUMA, so the amount of PUMA was insufficient to neutralize Bcl-XL, which inhibited the decrease of the interaction between Bcl-XL and Bax after UV irradiation.
Because PUMA could promote Bax translocation directly and indirectly to mediate apoptosis, we expected to know how important PUMA is in this process. To further investigate whether PUMA is indispensable in UV-induced Bax translocation and cell apoptosis, we next analyzed Bax translocation in the presence of pifithrin-α or CHX. ASTC-a-1 cells were transiently transfected with YFP-Bax or cotransfected with CFP-Bcl-XL and YFP-Bax, and DsRed-Mit was also transfected to label the mitochondria. Then, cells were treated with UV irradiation in the absence or presence of pifithrin-α or CHX and incubated for 0, 3, 6, 9, and 12 h. The percentage of cells showing Bax translocation to mitochondria was assessed by counting the number of cells. As shown in Figure 5E, pifithrin-α and CHX could significantly inhibit UV-induced Bax translocation at all the time points we tested, whereas overexpression of Bcl-XL could not inhibit this process but only delay it.
Finally, we observed the effects of pifithrin-α and CHX on UV-induced apoptosis. Pifithrin-α or CHX was added into cultured cells 1 h before UV irradiation, and then cell viability was analyzed by using Cell Counting Kit-8 at 0 (control), 3, 6, 9, and 12 h. As shown in Figure 5F, cell viability increased obviously in the presence of pifithrin-α or CHX after UV irradiation compared with that of UV treatment only, indicating that pifithrin-α or CHX could inhibit UV-induced cell apoptosis.
DISCUSSION
Bcl-2 family members are evolutionarily conserved and essential mediators in the apoptosis of the mitochondrial pathway. The BH3-only protein PUMA was initially identified as a downstream target of p53 and subsequently shown to play a critical role in apoptosis (Oda et al., 2000; Yu and Zhang, 2003). PUMA is induced at the transcriptional level in response to DNA damage and other stimuli. Previous studies have shown that PUMA plays an essential role in apoptosis induced by a variety of stimuli in many kinds of tissues and cell types (Yu and Zhang, 2003). Bax is also an executor of the mitochondrial pathway of apoptosis whose activation can be prevented by antiapoptotic Bcl-2 family proteins such as Bcl-XL. Bax translocation from cytosol to mitochondria is a critical step in many drug-mediated apoptoses (Valentijn et al., 2003). It is reported that Bax is necessary for UV-induced apoptosis (Locksley et al., 2001), and our previous studies have demonstrated that Bax translocation from cytosol to mitochondria after UV irradiation is a p53 transcription-dependent event (Wu et al., 2007).
Although the role of PUMA in apoptosis has been well established, unfortunately, the mechanisms through which PUMA regulates apoptosis, especially how PUMA activates Bax in response to UV irradiation, are still controversial. The previous studies have engendered two models of BH3-only protein action. One model (the direct binding model) proposes that the BH3-only proteins can directly bind and activate Bax/Bak (Kuwana et al., 2002; Cartron et al., 2004; Kuwana et al., 2005; Certo et al., 2006). The second model (the displacement model) proposes that Bax/Bak can autoactivate once the inhibition by Bcl-2–like proteins has been removed by BH3-only proteins but that no direct interaction between BH3-only proteins and Bax/Bak is required (Chen et al., 2005; Willis et al., 2005, 2007). However, the two views about how BH3-only proteins activate Bax/Bak are under hot debate, and very little research elaborates the interaction between BH3-only proteins and Bax or Bcl-XL in intact living cells. Here, based on the real-time single-cell analysis, we investigated the dynamic interaction among PUMA, Bax, and Bcl-XL in living single cells, and these results contributed to the general idea that the BH3-only protein PUMA promoted Bax translocation via both direct and indirect pathways during UV-induced apoptosis.
The first question is how PUMA functions to activate Bax translocation directly? To answer this question, the FRET technique and coimmunoprecipitation were used to investigate the interaction between PUMA and Bax. The typical time course images show that the interaction between PUMA and Bax increased markedly (Figure 3, A and B), with Bax translocating and colocalizing with PUMA after UV irradiation (Figure 3, C and D). In parallel, the same results were obtained from the experiment of coimmunoprecipitation (Figure 3, F–H). Because PUMA is largely localized in the mitochondria (Nakano and Vousden, 2001; Yu et al., 2001, 2003) and interacts with Bax directly (Figure 3, A and B, and F–H), it may activate Bax translocation directly through the induction of Bax conformational modification (Figure 3D). It has been reported that the BH3-only protein Bid and its BH3 peptides interact with Bax directly in a “hit-and-run” transient manner, resulting in conformational change, oligomerization, and activation of Bax to permeabilize the membranes (Kuwana et al., 2002, 2005). In addition, the BH3 domains of PUMA and Bid or their BH3 peptides specifically interact with the first helix of Bax (Cartron et al., 2004). So, PUMA may have the similar features and play its function to active Bax translocation directly in the same way as does Bid. However, which domain of Bax is modified and has a conformational change should be further studied.
Another question is how PUMA dissociates Bcl-XL and Bax to promote Bax translocation. To answer this question, we used FRET to investigate the dynamic interaction among PUMA, Bax, and Bcl-XL in living cells. The results show that the interaction between PUMA and Bcl-XL increased noticeably, whereas the interaction between Bcl-XL and Bax decreased markedly after UV treatment (Figure 4, A–D). This means PUMA can release Bax by competitive binding to Bcl-XL and modulate the interaction between Bcl-XL and Bax. In parallel, coimmunoprecipitation experiments show the similar results (Figure 4, E and F) and further confirmed our conclusion. It has been reported that PUMA displaces p53 from Bcl-XL to activate its mitochondrial apoptotic activity through antagonizing Bcl-XL (Chipuk et al., 2005). In our study, we found that PUMA also could displace Bax from Bcl-XL in the same way. It is exciting to find that overexpression Bcl-XL was unable to block Bax translocation to mitochondria completely (Figure 5E). The reason may be that endogenous PUMA expression is up-regulated by UV irradiation (Figures 3E and 5A), and subsequently, the increasing PUMA neutralizes Bcl-XL to release Bax and promote its translocation.
The above-mentioned results demonstrate that PUMA promotes Bax translocation via both direct and indirect pathways. However, which pathway is the main mechanism, the direct or the indirect pathway? This question is worth thinking about seriously and should be further studied. We speculated that the direct and indirect pathways work simultaneously. After UV irradiation, PUMA expressed much more than the normal level, localizing in the mitochondria to interact with the nearby Bax monomer and activate its translocation to mitochondria, which we named the direct activation pathway. Meanwhile, PUMA also interacted with the complex of Bcl-XL and Bax, dissociating them to free Bax by competitive binding to Bcl-XL, which we called the indirect activation pathway. The two processes might work in dynamic balance to mediate UV-induced apoptosis.
Finally, we wanted to know whether PUMA is indispensable in Bax translocation and UV-induced apoptosis. To clarify this, pifithrin-α and CHX were used in our experiment model because they could inhibit the expression of endogenous PUMA (Figure 5A), and then we investigated whether Bax translocation and cell apoptosis changed. As a result, the interaction between Bcl-XL and Bax decreased when cells were treated with UV irradiation only, whereas this interaction remained nearly unchanged in the whole course in the presence of pifithrin-α and CHX, indicating pifithrin-α and CHX did inhibit the decrease of interaction between Bcl-XL and Bax (Figure 5, B–D). In addition, that pifithrin-α had the same effect as CHX on inhibiting PUMA expression (Figure 5A) indicates the expression of PUMA could be inhibited by blocking the activation of p53; thus, this process was a p53-dependent event. Furthermore, Bax translocation was almost completely inhibited by pifithrin-α and CHX, and cell apoptosis was inhibited, too (Figure 5, E and F). In conclusion, pifithrin-α or CHX inhibited the expression of endogenous PUMA, resulting in insufficient amount of PUMA to activate Bax translocation, which strongly indicates that PUMA is indispensable in UV-induced Bax translocation and apoptosis.
More interestingly, pifithrin-α and CHX had the same effect on inhibiting PUMA expression, but CHX had a more evident effect than pifithrin-α on blocking Bax translocation (Figure 5, D and E). The reason may be that pifithrin-α specially inhibited the expression of PUMA through inhibiting the activation of p53, which was the direct transcriptional factor of PUMA, whereas CHX could inhibit all of the endogenous proteins' expressions, including PUMA and other BH3-only proteins such as Bid and Bim. Previous studies have shown that the expression of Bim is up-regulated by c-Jun NH2-terminal kinase (JNK) and that UV irradiation has some effects on JNK activation (Lei and Davis, 2003; Putcha et al., 2003). So, it was possible that Bim plays a minor role in Bax translocation during the later stage of UV-induced apoptosis.
For the first time, we demonstrate that PUMA promotes Bax translocation by both directly interacting with Bax and by competitive binding to Bcl-XL, and that PUMA is indispensable in UV-induced Bax translocation and apoptosis. Our results also indicate that PUMA mediates the UV-induced apoptosis through a p53-dependent pathway (Figures 5A and 6). However, a recent study reported that FOXO3a, a member of the FOXO family of transcription factors, can up-regulate PUMA expression in response to cytokine or growth factor deprivation in a p53-independent manner (You et al., 2006). Further studies are in progress to illustrate the mechanisms of PUMA up-regulation and how PUMA functions in response to p53-independent apoptotic stimuli.
Figure 6.
Schematic representation of UV irradiation-induced apoptotic pathway.
ACKNOWLEDGMENTS
We thank Dr. Yu (University of Pittsburgh, Pittsburgh, PA) for kindly providing the pGFP-PUMA and pGFP-PUMA-▵BH3 plasmids; Dr. Y. Gotoh (University of Yokyo, Yayoi, Tokyo, Japan) for kindly providing the pDsRed-Mit plasmid; Richard J. Youle (National institutes of Health, Bethesda, MD) for kindly providing the pGFP-Bax plasmid; and Dr. A. P. Gilmore (University of Manchester, Manchester, United Kingdom) for kindly providing the pYFP-Bcl-XL, pYFP-Bax, and pCFP-Bcl-XL plasmids. This research is supported by the Program for Changjiang Scholars and Innovative Research Team in University (IRT0829), the National Natural Science Foundation of China (30870676, 30870658), and the Natural Science Foundation of Guangdong Province (7117865).
Abbreviations used:
- Bax
Bcl-2-associated X protein
- BH
Bcl-2 homology domain
- CCK-8
Cell Counting Kit-8
- CFP
cyan fluorescent protein
- DsRed
red fluorescent protein
- GFP
green fluorescent protein
- MOMP
mitochondrial outer membrane permeabilization
- PUMA
p53 up-regulated modulator of apoptosis.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-11-1109) on May 13, 2009.
REFERENCES
- Adams J. M., Cory S. Life-or-death decisions by the Bcl-2 protein family. Trends Biochem. Sci. 2001;26:61–66. doi: 10.1016/s0968-0004(00)01740-0. [DOI] [PubMed] [Google Scholar]
- Cartron P. F., Gallenne T., Bougras G., Gautier F., Manero F., Vusio P., Meflah K., Vallette F. M., Juin P. The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol. Cell. 2004;16:807–818. doi: 10.1016/j.molcel.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Certo M., Del Gaizo Moore V., Nishino M., Wei G., Korsmeyer S., Armstrong S. A., Letai A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Chao C., Saito S., Kang J., Anderson C. W., Appella E., Xu Y. p53 transcriptional activity is essential for p53-dependent apoptosis following DNA damage. EMBO J. 2000;19:4967–4975. doi: 10.1093/emboj/19.18.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Willis S. N., Wei A., Smith B. J., Fletcher J. I., Hinds M. G., Colman P. M., Day C. L., Adams J. M., Huang D. C. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Cheng E. H., Wei M. C., Weiler S., Flavell R. A., Mak T. W., Lindsten T., Korsmeyer S. J. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- Chipuk J. E., Bouchier-Hayes L., Kuwana T., Newmeyer D. D., Green D. R. PUMA couples the nuclear and cytoplasmic proapoptotic function of p53. Science. 2005;309:1732–1735. doi: 10.1126/science.1114297. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Purdie C. A., Harrison D. J., Morris R. G., Bird C. C., Hooper M. L., Wyllie A. H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993;362:849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- Concannon C. G., et al. Apoptosis induced by proteasome inhibition in cancer cells: predominant role of the p53/PUMA pathway. Oncogene. 2007;26:1681–1692. doi: 10.1038/sj.onc.1209974. [DOI] [PubMed] [Google Scholar]
- Cory S., Huang D. C., Adams J. M. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- Danial N. N., Korsmeyer S. J. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Dejean L. M., et al. Oligomeric Bax is a component of the putative cytochrome c release channel MAC, mitochondrial apoptosis-induced channel. Mol. Biol. Cell. 2005;16:2424–2432. doi: 10.1091/mbc.E04-12-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desagher S., Osen-Sand A., Nichols A., Eskes R., Montessuit S., Lauper S., Maundrell K., Antonsson B., Martinou J. C. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. R., Reed J. C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Han J., Flemington C., Houghton A. B., Gu Z., Zambetti G. P., Lutz R. J., Zhu L., Chittenden T. Expression of bbc3, a pro-apoptotic BH3-only gene, is regulated by diverse cell death and survival signals. Proc. Natl. Acad. Sci. USA. 2001;98:11318–11323. doi: 10.1073/pnas.201208798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemann M. T., Zilfou J. T., Zhao Z., Burgess D. J., Hannon G. J., Lowe S. W. Suppression of tumorigenesis by the p53 target PUMA. Proc. Natl. Acad. Sci. USA. 2004;101:9333–9338. doi: 10.1073/pnas.0403286101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers J. R., et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321–328. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- Kuwana T., Bouchier-Hayes L., Chipuk J. E., Bonzon C., Sullivan B. A., Green D. R., Newmeyer D. D. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Kuwana T., Mackey M. R., Perkins G., Ellisman M. H., Latterich M., Schneiter R., Green D. R., Newmeyer D. D. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- Lei K., Davis R. J. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc. Natl. Acad. Sci. USA. 2003;100:2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letai A., Bassik M. C., Walensky L. D., Sorcinelli M. D., Weiler S., Korsmeyer S. J. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- Li P. F., Dietz R., von Harsdorf R. p53 regulates mitochondrial membrane potential through reactive oxygen species and induces cytochrome c-independent apoptosis blocked by Bcl-2. EMBO J. 1999;18:6027–6036. doi: 10.1093/emboj/18.21.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley R. M., Killeen N., Lenardo M. J. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Luo X., Budihardjo I., Zou H., Slaughter C., Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- Ming L., Wang P., Bank A., Yu J., Zhang L. PUMA dissociates Bax and Bcl-X(L) to induce apoptosis in colon cancer cells. J. Biol. Chem. 2006;281:16034–16042. doi: 10.1074/jbc.M513587200. [DOI] [PubMed] [Google Scholar]
- Nakano K., Vousden K. H. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- Nechushtan A., Smith C. L., Hsu Y. T., Youle R. J. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda E., Ohki R., Murasawa H., Nemoto J., Shibue T., Yamashita T., Tokino T., Taniguchi T., Tanaka N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- Polyak K., Xia Y., Zweier J. L., Kinzler K. W., Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- Putcha G. V., et al. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron. 2003;38:899–914. doi: 10.1016/s0896-6273(03)00355-6. [DOI] [PubMed] [Google Scholar]
- Reimertz C., Kogel D., Rami A., Chittenden T., Prehn J. H. Gene expression during ER stress-induced apoptosis in neurons: induction of the BH3-only protein Bbc3/PUMA and activation of the mitochondrial apoptosis pathway. J. Cell Biol. 2003;162:587–597. doi: 10.1083/jcb.200305149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler M., Bossy-Wetzel E., Goldstein J. C., Fitzgerald P., Green D. R. p53 induces apoptosis by caspase activation through mitochondrial cytochrome c release. J. Biol. Chem. 2000;275:7337–7342. doi: 10.1074/jbc.275.10.7337. [DOI] [PubMed] [Google Scholar]
- Soengas M. S., Alarcon R. M., Yoshida H., Giaccia A. J., Hakem R., Mak T. W., Lowe S. W. Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science. 1999;284:156–159. doi: 10.1126/science.284.5411.156. [DOI] [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Huang D. C., Krammer P. H., Cory S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J. 1995;14:6136–6147. doi: 10.1002/j.1460-2075.1995.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Jacks T., Cory S. DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell. 1994;79:329–339. doi: 10.1016/0092-8674(94)90201-1. [DOI] [PubMed] [Google Scholar]
- Tsuruta F., Masuyama N., Gotoh Y. The phosphatidylinositol 3-kinase (PI3K)-Akt pathway suppresses Bax translocation to mitochondria. J. Biol. Chem. 2002;277:14040–14047. doi: 10.1074/jbc.M108975200. [DOI] [PubMed] [Google Scholar]
- Valentijn A. J., Metcalfe A. D., Kott J., Streuli C. H., Gilmore A. P. Spatial and temporal changes in Bax subcellular localization during anoikis. J. Cell Biol. 2003;162:599–612. doi: 10.1083/jcb.200302154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden M. G., Thompson C. B. Bcl-2 proteins: regulators of apoptosis or of mitochondrial homeostasis? Nat. Cell Biol. 1999;1:E209–E216. doi: 10.1038/70237. [DOI] [PubMed] [Google Scholar]
- Villunger A., Michalak E. M., Coultas L., Mullauer F., Bock G., Ausserlechner M. J., Adams J. M., Strasser A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- Vousden K. H., Lu X. Live or let die: the cell's response to p53. Nat. Rev. Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- Wang K., Gross A., Waksman G., Korsmeyer S. J. Mutagenesis of the BH3 domain of BAX identifies residues critical for dimerization and killing. Mol. Cell. Biol. 1998;18:6083–6089. doi: 10.1128/mcb.18.10.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Yu J., Zhang L. The nuclear function of p53 is required for PUMA-mediated apoptosis induced by DNA damage. Proc. Natl. Acad. Sci. USA. 2007;104:4054–4059. doi: 10.1073/pnas.0700020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- Willis S. N., Chen L., Dewson G., Wei A., Naik E., Fletcher J. I., Adams J. M., Huang D. C. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis S. N., et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- Wu Y., Xing D., Chen W. R., Wang X. Bid is not required for Bax translocation during UV-induced apoptosis. Cell Signal. 2007;19:2468–2478. doi: 10.1016/j.cellsig.2007.07.024. [DOI] [PubMed] [Google Scholar]
- You H., Pellegrini M., Tsuchihara K., Yamamoto K., Hacker G., Erlacher M., Villunger A., Mak T. W. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J. Exp. Med. 2006;203:1657–1663. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Wang Z., Kinzler K. W., Vogelstein B., Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc. Natl. Acad. Sci. USA. 2003;100:1931–1936. doi: 10.1073/pnas.2627984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Yue W., Wu B., Zhang L. PUMA sensitizes lung cancer cells to chemotherapeutic agents and irradiation. Clin. Cancer Res. 2006;12:2928–2936. doi: 10.1158/1078-0432.CCR-05-2429. [DOI] [PubMed] [Google Scholar]
- Yu J., Zhang L. No PUMA, no death: implications for p53-dependent apoptosis. Cancer Cell. 2003;4:248–249. doi: 10.1016/s1535-6108(03)00249-6. [DOI] [PubMed] [Google Scholar]
- Yu J., Zhang L. Apoptosis in human cancer cells. Curr. Opin. Oncol. 2004;16:19–24. doi: 10.1097/00001622-200401000-00005. [DOI] [PubMed] [Google Scholar]
- Yu J., Zhang L., Hwang P. M., Kinzler K. W., Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol. Cell. 2001;7:673–682. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- Zhong Q., Gao W., Du F., Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121:1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]