Abstract
Objective
The main objectives of this study were to explore the preliminary outcomes and assess the feasibility and acceptability of a collaborative care intervention designed to improve treatment and outcomes of depression among youth seen in primary care settings.
Methods
We conducted a pilot intervention study at three clinics in a university affiliated primary care clinic network. The intervention model was designed to support the provision of depression treatment by primary care providers using methods adapted from the IMPACT study developed for the improvement of depression among older adults. Specific components include the provision of regular case management by a nurse depression care manager (DCM), enhanced patient and parent education about depression and its treatment, encouragement of patient self-management with a choice of starting medications or therapy or both, and oversight of the DCM by a mental health specialist. Study participants were assessed regularly by the DCM for 6 months and completed written self-report assessments at baseline, 3, and 6 months after starting the intervention.
Results
40 youth (12-18 years) with major and minor depression enrolled in the intervention. Study participants were predominantly female (90%). The baseline Patient Health Questionnaire (PHQ-9) score was 14.2 (SD = 4.5). Patients were similarly divided among initiating medications (n=12), therapy (n=15), or combination therapy (n=8). Five patients withdrew prior to initiating treatment. The mean number of in person and telephone contacts with the DCM was 9 (range = 5 to 17). Eighty-seven percent of youth completed the 6-month intervention. At 6 month follow-up, 70% of youth had a 50% or more reduction in depressive symptoms as measured by the PHQ-9. Parents, youth and physicians indicated high levels of satisfaction with the intervention on written surveys and in qualitative exit interviews.
Conclusion
The collaborative care model is feasible and highly acceptable to adolescents and parents as demonstrated both by self-report and by engagement in the intervention. It is also associated with improved depressive outcomes at similar levels to adult interventions. Future studies should evaluate these models in a randomized controlled trial.
Introduction
By age 18, 20% of youth have experienced at least one episode of major depression.1 In the short-term, depressed youth are at increased risk for suicide, school failure, substance abuse, nicotine dependence,2 early pregnancy and social isolation.3, 4 In the long-term, they are at risk for low socio-educational attainment, unemployment, recurrent depression and other mental health disorders, and poor health.3, 5-8
Despite significant impairment, many depressed adolescents do not receive treatment.9 Primary care providers (PCPs) are often the only contact that adolescents have with the health care system and have the opportunity to identify depression early in its course and shape future treatment. The new Guidelines for Adolescent Depression in Primary Care 10, 11 outline the steps that are needed to improve outcomes: increasing recognition, educating youth and families, collaborating with youth in families to establish treatment plans, providing evidence-based treatments (antidepressants and/or psychotherapy) for moderate to severe depression, monitoring depressive symptoms regularly, increasing treatment intensity for youth who do not respond to initial treatment, and obtaining mental health specialty consultation for youth with persistent depression.
Unfortunately, studies have shown that the distribution of guidelines alone does not improve depression outcomes without also making health care system changes to address barriers.12-14 One of the most successful models for improving depression treatment and outcomes in primary care is the Collaborative Care Model, a multisystem strategy to reorganizing treatment for depression. In a recent meta-analysis of 37 trials among adults, Collaborative Care Models have been shown to be associated with improved quality of depression care and outcomes.15 There were three features that were associated with the most success: the provision of case management by individuals with mental health treatment experience, regular supervision of case managers by mental health specialists, and increased use and adherence to antidepressant medications. Based on this body of evidence, the collaborative care model has been actively implemented in many health care systems.16
However, only two studies have been conducted testing health systems models to improve primary care treatment of adolescent depression.17, 18 In the first study, the addition of cognitive behavioral psychotherapy to antidepressant treatment in the primary care setting did not significantly improve outcomes compared to antidepressants alone.18 In the second study, case management and treatment with either medication or psychotherapy was associated with a modest decrease in depressive symptoms: at completion of the study the mean Center for Epidemiologic Studies Depression (CES-D) score was 19 in the intervention group versus 21.4 in controls. However, because treatment implementation and supervision protocols were established individually by sites within the study, treatment offered varied across the intervention group. Only 32% of intervention youth received any mental health treatment in the 6 months post-diagnosis, potentially diluting possible effects. An additional valuable finding from this study was that 72% of youth indicated a preference for active treatment over watchful waiting,19 suggesting that the lack of engagement in treatment was not due to a lack of interest.
In this paper, we describe a pilot intervention study that adapted a collaborative care model to address unique aspects of adolescent care specifically focusing on issues of feasibility and acceptability among participants.
Methods
This pilot study was conducted at three university-affiliated primary care clinics in a large metropolitan area in the Pacific Northwest. Potential study subjects were identified in one of three ways: 1) direct referral from a PCP; 2) monthly screening of electronic medical records to identify youth who had been seen for depression or prescribed an antidepressant who were not yet referred to the study; or 3) direct patient and parent recruitment through a letter about adolescent depression and description of the study that was mailed to a random sample of clinic enrollees. All study procedures were approved by the University of Washington Institutional Review Board.
Youth were eligible for inclusion if they were 12 to 18 years old at time of recruitment and met criteria for major depression (at least 5/9 DSM-IV criteria with 1 cardinal criteria, N = 36 youth) or minor depression (2-4/9 DSM-IV criteria with at least 1 cardinal criteria, N = 4 youth) based on the Patient Health Questionnaire 9-item (PHQ-9) screen and symptoms reported during the intake interview with the depression care manager. All enrolled youth had impairment in at least one area of functioning (home, school, or peer relationships) secondary to depression based on the Columbia Impairment Scale. In addition, youth were required to plan to continue to be enrolled in the clinic for at least 6 months. Youth were excluded from the study if they were judged to be more appropriate for mental health specialty treatment (already engaged in treatment with a psychiatrist, mental health hospitalization within the prior 6 months, pervasive developmental delay, autism, bipolar disorder, psychotic features, active suicidal ideation, or history of suicide attempt). All cases were reviewed with at least one of the mental health specialty oversight committee members (a psychiatrist, psychologist, or a pediatrician) prior to entry.
Intervention Components
The intervention model was adapted from the IMPACT study developed for the improvement of depression among older adults.20 Specific components included from this model include the provision of case management by a depression care manager (DCM), enhanced patient education about depression and its treatment, encouragement of patient self-management, provision of enhanced antidepressant medication care or Problem Solving Treatment – Primary Care (PST-PC) based on patient choice, and case-management supervision of the DCM by child mental health specialists. These components are discussed in further detail below. Table 1 outlines the adaptations made to the IMPACT collaborative care model in order to make it more appropriate for adolescent patients including the development of adolescent-appropriate education materials and an adolescent-specific depression treatment manual, flexibility of appointment times and settings, and oversight by child mental health specialists. Parents were included in the initial session, involved in the choice of treatment and included in follow-up sessions as needed.
Table 1.
Adaptations of the Collaborative Care Model for Adolescent Patients
| CCM Health System Component | Features of the Original Collaborative Care Model | Adaptations for the Adolescent Collaborative Care Intervention |
|---|---|---|
| Self - management support |
|
|
| Delivery system design |
|
|
| Decision support for providers |
|
|
| Clinical information systems |
|
|
The DCM was a registered nurse with experience in working in mental health settings. She was selected based on her experience in working with individuals with mental health concerns, including experience with conducting brief psychotherapy, and her ability to relate to adolescents. Prior to initiating the intervention, she received training from the lead investigators (LR, WK, EM) including review of the intervention manual which specifically pertained to the assessment and treatment of depression in youth, as well as supervised therapy training with an expert in PST-PC. She received weekly case-supervision as described below.
Study Procedures
The parents of potential study participants referred by PCPs or identified via medical records received a letter explaining the study and providing a phone number to call if they did not want to be contacted. This was followed by a phone call to the parents from one of the study investigators to describe the study, review eligibility criteria, and invite their child to participate. If both the parent and child were interested, an intake appointment was scheduled with the DCM at the youth’s primary care clinic. Parents and youth recruited via direct mailing were given a number to contact investigators directly to set up an intake appointment.
During the intake appointment, the DCM used a semi-structured interview adapted from IMPACT to conduct a clinical assessment (a copy of this instrument is provided in Appendix 1). This interview included a review of current DSM-IV depressive symptoms and suicidal ideation using the PHQ-9 as a guide, past depressive history, history of manic symptoms, hallucinations, alcohol use, prior experience with mental health treatment (antidepressants or counseling), functional impairment (in school, family, or peer relationships) due to depression, current stressors, and other mental and physical health problems. The DCM also provided education regarding depression and its treatment and reviewed the patient’s preferences for depression treatment. All youth and parents were given an informational video and pamphlets on adolescent depression. Interviews were first conducted with the youth alone, then with the parent alone, and finally both were seen together for a summary of the plan. Approximately 40 minutes of the initial 1-hour session was spent with the youth alone.
After the intake appointment, the DCM followed youth every 1-2 weeks (either in-person or via phone depending on the preferences of the youth) to track symptoms using the PHQ-9 and assess any concerns regarding treatment. All new cases and any existing cases that were not improving were discussed during 1-hour weekly supervision meetings with a mental health specialist team (including a psychologist, psychiatrist and a pediatrician). From the time of intake, the DCM worked with the patient, parent, and his/her PCP to establish a treatment plan based on a standardized treatment algorithm. Any differences in treatment preferences were resolved through discussion and consensus with the highest weight placed on the preferences of the youth and his/her family. The DCM followed up with patients every 1-2 weeks to reassess symptoms and any side effects of treatment. These visits were entered in an electronic database to allow for easy tracking and to facilitate discussion during weekly supervision sessions.
The treatment algorithm recommended a first choice of either an antidepressant (primarily a selective serotonin reuptake inhibitor) or a brief psychotherapeutic intervention that was designed for use in primary care settings, Problem Solving Treatment in Primary Care (PST-PC). PST-PC focuses specifically on the behavioral activation components of Cognitive Behavioral Therapy, but with less emphasis on changing cognition and greater emphasis on patient assessment of personal contextual problems and skill-building to enhance self-management skills. It has been shown to be as effective as antidepressant medication in treating adult patients with major depression in primary care.21 PST-PC was chosen because of it’s ease of dissemination in the primary care setting: it has been shown to be effective in primary care when delivered by medical nurses and primary care providers.22 The PST-PC intervention was provided by the DCM in the primary care setting with a recommended course of 6-8 sessions conducted on a weekly basis. The DCM received training in PST-PC from a psychologist with extensive experience. For patients who were already receiving an antidepressant at the time of intake into the study but were still depressed, the algorithm recommended either increasing the dose for those on suboptimal doses or adding PST-PC as an augmentation (for partial responders) or changing to a different medication or to PST-PC (for non-responders).
Study Measures
At each contact with the patient, the DCM completed a PHQ-9 to assess depressive symptoms and impairment. The PHQ-9 provides both a dichotomous diagnosis of major depression and a continuous depression score (0 to 27).23 The PHQ-9 has been found to have high sensitivity (88%) and high specificity (88%) for the diagnosis of major depression among adults when compared to structured interview.23 It has also been shown to be as responsive to improvement with treatment as other commonly used depression scales.24 The PHQ-9 has not been validated among adolescents, but the PHQ-A, an adolescent version that uses the same question structure but includes other diagnostic categories, has been shown to have a sensitivity of 73% and a specificity of 94% for major depressive disorder on clinical interview.25 The PHQ-9 was selected for the current study as PCPs were already familiar with the instrument and were using it for both initial diagnosis and to gauge treatment response for their adult patients and because the PHQ-9 has more response options for each question than the PHQ-A allowing for the calculation of a symptom score. In our work, we found the PHQ-9 to be acceptable to adolescents and feasible with good understanding and consistency with verbal reports of symptoms to the DCM with improvement in scores as symptoms improved. There was a high correlation between the PHQ-9 and the written Moods and Feelings Questionnaire at baseline measurement (corr = 0.60, P<0.001).
Written self-report surveys were completed by youth at baseline, 3 and 6 months. Depressive symptoms were measured using the Mood and Feelings Questionnaire Short Form (MFQ-SF),26 a 13-item questionnaire that codes symptoms on a 3 point scale (“true”, “sometimes true”, “not true”). This scale has been found to have high reliability and validity and the short form is made up of items that best discriminated depressed and non-depressed children in field trials using structured psychiatric interviews.26 In a recent study of 1,375 youth, we found that a cut point of 6 or higher was associated with a sensitivity of 80% and a specificity of 81% for detecting major depression.27
Functional impairment was assessed using the Columbia Impairment Scale (CIS), a psychosocial functional impairment scale with high reliability and validity that is widely used in youth depression studies.28 This 13-item scale has been shown to be highly correlated with other indicators of psychological dysfunction and with the clinician-rated Children’s Global Assessment Scale (CGAS).28, 29 A score of 15 or higher on the CIS has been suggested to be an indicator of need for services.30 In a prior study, we found that the mean CIS score for youth meeting diagnostic criteria for one or more anxiety or depressive disorders on the Diagnostic Interview Schedule for Children was 19 compared to a mean of 9.7 for youth without a disorder.31
Youth were screened for anxiety comorbidity using the brief 5-item Screen for Child Anxiety Related Disorders (SCARED); a score of 3 or more has been shown to have a sensitivity of 74% and a specificity of 73% of distinguishing anxiety from nonanxiety diagnosed by interview with a mental health specialist.32
Satisfaction with care was assessed via a series of Likert scale-based questions regarding the treatment received, an open-ended question regarding study experiences and qualitative exit interviews as described below.
Qualitative Exit Interviews
After the completion of the study, youth and parents were re-contacted and invited to participate in a qualitative interview. Consent was obtained from both the parent and the youth. All qualitative interviews were conducted over the phone by the lead study investigator (L.R.) who had no prior contact with youth or parents and was introduced as “a study staff member.” During the interviews, each youth and one parent per youth were asked to answer open-ended questions regarding their experiences with depression treatment prior to the intervention, their experiences with the intervention, how this was different from or similar to prior treatment experiences, and any recommendations for improving the intervention. Interviews were conducted individually with each youth and a parent. Either or both could choose to participate.
Analysis
Quantitative data analysis was performed using STATA 8 statistical software.33 Descriptive analyses were performed to assess the baseline characteristics of the intervention population. T-tests and Chi-squared analyses were then used to assess overall response to the intervention in the following methods: overall change in depressive symptom score from baseline to six month follow-up, percent of youth with a 50% reduction of symptoms between baseline assessment and 6 month follow up, and change in functional impairment score from baseline to 6 month follow up.
Qualitative interview data were transcribed and reviewed by the interviewer for accuracy. Qualitative data analysis software, ATLAS.ti, was used to code text passages and facilitate comparison within and across interviews. The coding scheme was developed using grounded theory methodology in which the analysis strategy is driven by the data collected.34 Coding categories were determined a priori and adapted to accommodate any new themes that arose. Themes were coded, explored in greater depth in later interviews, and then modified to further clarify major themes. Frequently encountered themes were grouped and studied for patterns and discordance to incorporate the richness of data collected into the final model.
Results
Of 115 youth who were identified as possible candidates for the study, 82 met eligibility criteria (Figure 1). The most common reason for exclusion was that the depression was felt to be too severe for primary care treatment either because the patient was already seeing a psychiatrist (n = 11), had been hospitalized in an inpatient mental health facility within the prior year (n = 4) or had psychiatric comorbidity that required mental health specialty care (n = 3). Additional reasons for exclusion were being outside of study age range by the time of initial contact, not meeting criteria for major or minor depression, or having plans to leave the primary care practice. Fifty-eight percent (N = 48) of youth who were contacted and did not meet exclusion criteria agreed to participate in the study. The remainder were either unreachable (N = 11) or refused to participate (N = 23). Most of the families who refused to participate indicated that their child was already receiving the help that he or she needed. Eight of the youth who agreed to participate did not show for intake interviews despite multiple scheduling attempts, resulting in a final enrollment of 40 subjects (49% of eligible).
Figure 1.
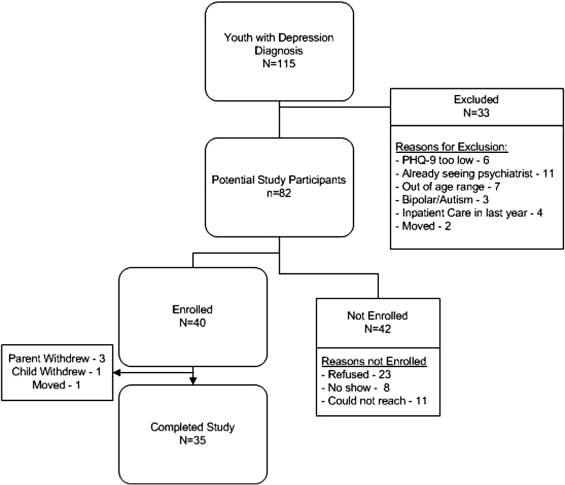
Intervention Enrollment Flowchart
Among the 40 youth enrolled in the study, participants were between 12 and 18 years old with an average age of 15 years (Table 2). The majority of participants were female and White. Fifty-five percent of participants were taking at least one psychotropic medication at study baseline, the most common types being antidepressants and stimulants. The mean PHQ-9 was 14 (SD = 4.5), indicating moderate severity of major depression.
Table 2.
Sample Demographics
| N (%) | |
|---|---|
| Sex | |
| Male | 4 (10%) |
| Female | 36 (90%) |
| Age (mean (SD)) | 15 (1.45) |
| Race | |
| White | 34 (87%) |
| Black | 1 (2%) |
| Asian | 2 (5%) |
| Native American | 2 (5%) |
| Highest Level of Parental Education | |
| High school or less | 5 (12%) |
| College or higher | 28 (70%) |
| Missing | 7 (17%) |
| Comorbidity | |
| History of ADHD diagnosis | 10 (26%) |
| Learning Problems | 11 (27%) |
| Anxiety on the Brief SCARED screen | 23 (58%) |
| Health Risk Behaviors | |
| Has Ever Smoked | 6 (15%) |
| Has Ever Been Drunk | 8 (20%) |
| Has Used Drugs | 7 (17%) |
| Medications | |
| Any Psychotropic Medication | 22(55%) |
| SSRI | 14 (35%) |
| Other antidepressant | 6 (15%) |
| Stimulant | 6 (15%) |
Ten youth received medication treatment only, 12 received psychotherapy only, and 16 received both types of treatment during the study (8 youth who were already receiving one type of therapy, including 4 on antidepressant medications, and 4 in psychotherapy were provided a second treatment at the start of the study; 8 others had a second treatment added during the study due to persistent depressive symptoms). Two youth withdrew prior to selecting or starting any treatment. Over the 6 month treatment period, youth had an average of 9 contacts with the DCM (SD=3.2, Range=2 to 17). Most of these contacts were in person (mean = 6.8 per youth (SD=3.5, Range=1 to 16)) with fewer by phone (mean = 3.1 per youth (SD=2.3, Range= 0 to 9)).
There was a significant improvement in depression scores and functional impairment from baseline to six month follow-up (Table 3). Seventy-four percent of youth with an assessment at 6 months (n=35) had a greater than 50% decrease in depressive symptoms on the PHQ-9 between their baseline to their final follow-up assessment with the DCM. On written MFQ-SF assessments at baseline and six months, 52% of youth who completed the 6 month survey (n=31) demonstrated a 50% or greater reduction in depressive symptoms. There was a significant decrease in the mean CIS score from 22.5 (SD = 7.76) at baseline to 15.3 (SD = 8.99) at 6-month follow-up (p<0.001), which represents a 32% decrease in functional impairment symptoms. At baseline, 82.5% of youth had CIS scores of 15 or higher, indicating a “need for services”, compared to 50% at the 6-month assessment.
Table 3.
Pre and Post Intervention Depression and Functional Impairment
| Baseline (mean (SD)) | Final (mean (SD)) | P value | |
|---|---|---|---|
| PHQ-9 | 14.0 (4.5) | 5.7 (4.1) | <0.001 |
| MFQ-SF | 16.0 (6.3) | 9.0 (6.4) | <0.001 |
| CIS | 22.5 (7.8) | 15.3 (9.0) | <0.001 |
Both parents and youth reported high levels of satisfaction with the intervention. On a scale of 1 to 7, 81% of youth completing satisfaction surveys (N=31) and 81% of parents who completed satisfaction surveys (n=26) reported that they were satisfied or very satisfied with the intervention.
Semi-structured exit interviews were completed with 16 youth and 21 parents. In exit interviews, youth and parents again expressed high levels of satisfaction with the intervention. Youth particularly appreciated that the DCM was “non-judgmental”, “cared about” them, was “available” and took the time to listen to their concerns, communicated concern by calling to check in with them and remembering what they had discussed, and made practical suggestions that they felt they could apply in their every day lives. As one youth stated in comparing the DCM to her doctor:
“She was more I felt a counselor, like someone I could cry to or… kind of more personal and I could ask her anything and told her my drug history and everything… It was just really open. And she didn’t judge me - she just gave me honest facts. She was more like a friend.”
In addition to meetings with the DCM, about two-thirds of youth interviewed also felt that group sessions with other teens would be helpful so that they did not “feel so alone”.
Youth and parents appreciated that the care took place in their primary care physician’s office and felt that it was easy to arrange appointments. Parents felt that their children benefited from having “another adult” to relate to and appreciated that regular visits with the DCM encouraged them to keep underlying issues “on the table” and helped their children to “learn to take care of themselves.” Parents’ main request was that they would have liked more information about what tasks their children were working on with the DCM. Although a few youth indicated that they would be upset if their parents were more involved, most thought that it would be alright if their parents learned more about what tasks they were working on as long as confidentiality regarding specific details of what the youth was sharing were preserved.
A final theme that arose related to concern about the timing of the intervention. For a few youth the intervention ended at a particularly bad time - during a relationship break-up or at the beginning or end of the school year. These youth had particularly difficult transitions off of the study and reported an increase in their depressive symptoms in the months following study completion.
Discussion
In this study of collaborative care among youth with depression, we found significant improvements in depressive symptoms and functional impairment. Although it is difficult to assess active intervention effects versus regression to the mean or placebo effects without a control group, these results are similar to results observed in collaborative care trials in adults15 and demonstrate greater improvement than what has been seen in either the treatment or control groups for the only prior multimodal Collaborative Care trial among adolescents.17 We also found that this model of care was feasible and was associated with high rates of satisfaction for both teens and parents. Based on these results, collaborative care seems promising for improving depression treatment and outcomes among youth.
Findings from the exit interviews highlight adaptations to the model needed to reach adolescent populations. First, models must consider the role of parents and incorporate them in treatment as appropriate. In our program, parents were provided with education and were expected to participate in decisions regarding treatment and in ongoing support of the child. In exit interviews, we found that most parents wanted more information on the skills their teens were learning to make sure that they were responding appropriately at home. Although they valued their confidentiality, most teens thought it would be helpful to have their parents learn more about the treatment they were receiving. Future interventions should find ways to further include parents in recovery in a way that does not compromise confidentiality and is sensitive to the developmental needs of the adolescent.
Second, during adolescence, youth develop a sense of individual self-identity outside of the family environment. Peer interactions and personal relationships are very important in helping to develop identity during this time. It is not surprising then that most teens in the study requested opportunities to meet other depressed youth. We did provide a video regarding teen depression but teens desired more personal interactions. This focus on interpersonal interaction also carried over to relationships with the DCM. In qualitative interviews, teens reported that they valued the approachability and non-judgmental nature of the DCM and many commented on how helpful it was to meet the DCM in person as opposed to meeting by telephone.
A third consideration when planning an intervention for adolescents is the timing of study completion. A few of the teens and families in this study provided feedback that the intervention ended at a particularly bad time for the teen such as at the end or start of the school year when stress was high. Interventions for youth may need some flexibility so that they do not end at a time when a teen is feeling most vulnerable to the loss of support or structure.
A final consideration is the method of recruitment for interventions. In the two previously published health services intervention studies with primary care populations, the recruitment rates were 40% and 27% of potentially eligible youth.30,31 Although our recruitment rate of 49% was higher than in either of the prior trials, future interventions may need to further explore the reason for low levels of engagement in these trials and develop more innovative strategies to engage youth in treatment.
The main limitations of this study include the small sample size and lack of control group. In addition, the population was mostly white and female and further study may be required to adapt this model for more diverse populations. Finally, the structure of this study, including the incorporation of patient choice and increasing intensity of treatment for youth who are not improving, precludes comparisons of treatment effects of antidepressants versus psychotherapy. The strengths or the study include the high level of study adherence and completion and the collection of exit interview data that provides insights into potential adaptations of the collaborative care model that may further improve engagement in treatment and outcomes for these youth.
In summary, collaborative care has been shown to be effective among adults,15 but the evidence for its effectiveness in youth is limited. This study shows that the intervention is feasible and is associated with high levels of adherence and satisfaction. Depressive symptom responses are also very encouraging and are higher than has been seen in prior trials. This suggests that collaborative care models hold promise for enhancing quality of depression care and improving depressive outcomes in youth. Based on the results of the exit interviews, there is still room for improvement to make this intervention even more meaningful and helpful for youth and their families.
Acknowledgments
This work was supported by a Leadership Award from the Robert Wood Johnson Depression in Primary Care Initiative and a K23 award from the National Institute of Mental Health (3K23 MH069814-01A1).
Appendix 1 - Assessment Interviw Form


Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Lewinsohn PM, Hops H, Roberts RE, Seeley JR, Andrews JA. Adolescent psychopathology: I. Prevalence and incidence of depression and other DSM-III-R disorders in high school students. J Abnorm Psychol. 1993;102(1):133–144. doi: 10.1037//0021-843x.102.1.133. [DOI] [PubMed] [Google Scholar]
- 2.Brown RA, Lewinsohn PM, Seeley JR, Wagner EF. Cigarette smoking, major depression, and other psychiatric disorders among adolescents. J Am Acad Child Adolesc Psychiatry. 1996;35(12):1602–1610. doi: 10.1097/00004583-199612000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Birmaher B, Ryan ND, Williamson DE, et al. Childhood and adolescent depression: a review of the past 10 years. Part I. J Am Acad Child Adolesc Psychiatry. 1996 Nov;35(11):1427–1439. doi: 10.1097/00004583-199611000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Kovacs M. Presentation and course of major depressive disorder during childhood and later years of the life span. J Am Acad Child Adolesc Psychiatry. 1996;35(6):705–715. doi: 10.1097/00004583-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Bardone AM, Moffitt T, Caspi A, Dickson N. Adult mental health and social outcomes of adolescent girls with depression and conduct disorder. Development and Psychopathology. 1996;8(4):811–829. [Google Scholar]
- 6.Lewinsohn PM, Rohde P, Seeley JR, Klein DN, Gotlib IH. Natural course of adolescent major depressive disorder in a community sample: predictors of recurrence in young adults. Am J Psychiatry. 2000;157(10):1584–1591. doi: 10.1176/appi.ajp.157.10.1584. [DOI] [PubMed] [Google Scholar]
- 7.Weissman MM, Wolk S, Goldstein RB, et al. Depressed adolescents grown up. Jama. 1999;281(18):1707–1713. doi: 10.1001/jama.281.18.1707. [DOI] [PubMed] [Google Scholar]
- 8.Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry. 2002;59(3):225–231. doi: 10.1001/archpsyc.59.3.225. [DOI] [PubMed] [Google Scholar]
- 9.Wu P, Hoven CW, Bird HR, et al. Depressive and disruptive disorders and mental health service utilization in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1999;38(9):1081–1090. doi: 10.1097/00004583-199909000-00010. discussion 1090-1082. [DOI] [PubMed] [Google Scholar]
- 10.Zuckerbrot RA, Cheung AH, Jensen PS, Stein RE, Laraque D. Guidelines for Adolescent Depression in Primary Care (GLAD-PC): I. Identification, assessment, and initial management. Pediatrics. 2007 Nov;120(5):e1299–1312. doi: 10.1542/peds.2007-1144. [DOI] [PubMed] [Google Scholar]
- 11.Cheung AH, Zuckerbrot RA, Jensen PS, Ghalib K, Laraque D, Stein RE. Guidelines for Adolescent Depression in Primary Care (GLAD-PC): II. Treatment and ongoing management. Pediatrics. 2007 Nov;120(5):e1313–1326. doi: 10.1542/peds.2006-1395. [DOI] [PubMed] [Google Scholar]
- 12.Callahan CM, Hendrie HC, Dittus RS, Brater DC, Hui SL, Tierney WM. Improving treatment of late life depression in primary care: a randomized clinical trial. J Am Geriatr Soc. 1994;42(8):839–846. doi: 10.1111/j.1532-5415.1994.tb06555.x. [DOI] [PubMed] [Google Scholar]
- 13.Dowrick C, Buchan I. Twelve month outcome of depression in general practice: does detection or disclosure make a difference? Bmj. 1995;311(7015):1274–1276. doi: 10.1136/bmj.311.7015.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson C, Kinmonth AL, Stevens L, et al. Effects of a clinical-practice guideline and practice-based education on detection and outcome of depression in primary care: Hampshire Depression Project randomised controlled trial. Lancet. 2000;355(9199):185–191. doi: 10.1016/s0140-6736(99)03171-2. [DOI] [PubMed] [Google Scholar]
- 15.Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med. 2006 Nov 27;166(21):2314–2321. doi: 10.1001/archinte.166.21.2314. [DOI] [PubMed] [Google Scholar]
- 16.Katon WJ, Unutzer J. Pebbles in a pond: NIMH grants stimulate improvements in primary care treatment of depression. Gen Hosp Psychiatry. 2006 May-Jun;28(3):185–188. doi: 10.1016/j.genhosppsych.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Asarnow JR, Jaycox LH, Duan N, et al. Effectiveness of a quality improvement intervention for adolescent depression in primary care clinics: a randomized controlled trial. Jama. 2005 Jan 19;293(3):311–319. doi: 10.1001/jama.293.3.311. [DOI] [PubMed] [Google Scholar]
- 18.Clarke G, Debar L, Lynch F, et al. A randomized effectiveness trial of brief cognitive-behavioral therapy for depressed adolescents receiving antidepressant medication. J Am Acad Child Adolesc Psychiatry. 2005 Sep;44(9):888–898. [PubMed] [Google Scholar]
- 19.Jaycox LH, Asarnow JR, Sherbourne CD, Rea MM, LaBorde AP, Wells KB. Adolescent primary care patients’ preferences for depression treatment. Adm Policy Ment Health. 2006 Mar;33(2):198–207. doi: 10.1007/s10488-006-0033-7. [DOI] [PubMed] [Google Scholar]
- 20.Unutzer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. Jama. 2002;288(22):2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 21.Mynors-Wallis LM, Gath DH, Day A, Baker F. Randomised controlled trial of problem solving treatment, antidepressant medication, and combined treatment for major depression in primary care. Bmj. 2000;320(7226):26–30. doi: 10.1136/bmj.320.7226.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mynors-Wallis L. Problem-solving treatment: evidence for effectiveness and feasibility in primary care. Int J Psychiatry Med. 1996;26(3):249–262. doi: 10.2190/0HVY-CD2F-0KC7-FVTB. [DOI] [PubMed] [Google Scholar]
- 23.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowe B, Unutzer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the patient health questionnaire-9. Med Care. 2004 Dec;42(12):1194–1201. doi: 10.1097/00005650-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Johnson JG, Harris ES, Spitzer RL, Williams JB. The patient health questionnaire for adolescents: validation of an instrument for the assessment of mental disorders among adolescent primary care patients. J Adolesc Health. 2002;30(3):196–204. doi: 10.1016/s1054-139x(01)00333-0. [DOI] [PubMed] [Google Scholar]
- 26.Costello EJ, Angold A. Scales to assess child and adolescent depression: checklists, screens, and nets. J Am Acad Child Adolesc Psychiatry. 1988;27(6):726–737. doi: 10.1097/00004583-198811000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Katon W, Russo J, Richardson L, McCauley E, Lozano P. Anxiety and depression screening for youth in a primary care population. Ambul Pediatr. 2008 May-Jun;8(3):182–188. doi: 10.1016/j.ambp.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bird HR, Andrews H, Schwab-Stone M, et al. Columbia Impairment Scale (CIS). Global measures of impairment for epidemiologic and clinical use with children and adolescents. International Journal of Methods in Psychiatric Research. 1996;6(4):295–307. [Google Scholar]
- 29.Bird HR, Canino G, Rubio-Stipec M, et al. Further measures of the psychometric properties of the children’s global assessment scale (CGAS) Arch Gen Psychiatry. 1987;44:821–824. doi: 10.1001/archpsyc.1987.01800210069011. [DOI] [PubMed] [Google Scholar]
- 30.Bird HR, Shaffer D, Fisher P, et al. The Columbia Impairment Scale: Pilot finding on a measure of global impairment for children and adolescents. International Journal of Methods in Psychiatric Research. 1993;3:167–176. [Google Scholar]
- 31.McCauley E, Katon W, Russo J, Richardson L, Lozano P. Impact of anxiety and depression on functional impairment in adolescents with asthma. Gen Hosp Psychiatry. 2007 May-Jun;29(3):214–222. doi: 10.1016/j.genhosppsych.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, Baugher M. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. J Am Acad Child Adolesc Psychiatry. 1999 Oct;38(10):1230–1236. doi: 10.1097/00004583-199910000-00011. [DOI] [PubMed] [Google Scholar]
- 33.STATA 7 Statistical Software. College Station, TX: computer program. [Google Scholar]
- 34.Denzin DK, Lincoln YS. Handbook of Qualitative Research. Thousand Oaks, CA: SAGE publications; 1994. [Google Scholar]


