Abstract
Objective
Insulin-like Growth Factor Binding Protein – 3 (IGFBP-3) has been shown to exhibit diverse biological actions, including IGF-independent effects on cell growth and cell death. Here we report that IGFBP-3 sensitizes prostate cancer cells to interferon-gamma (IFN-γ)-induced apoptosis and inhibition of cell proliferation.
Design
The cell growth or cell death of prostate cells in response to the treatments of IGFBPs and/or IFN-γ was measured, and the signaling pathways mediating these actions assessed.
Results
Cell proliferation was minimally affected when M12 prostate cancer cells were treated with exogenous IGFBP-3 (1–5 μg/ml), IGFBP-1 (1–5 μg/ml) or IFN-γ (20 U/ml). However, strong inhibition of cell growth and significant apoptosis were observed when M12 cells were co-treated with IGFBP-3 and IFN-γ, but not with IGFBP-1 and IFN-γ. These effects were IGF-independent and appear not to require intracellular localization of IGFBP-3, as similar results were obtained with mutants of IGFBP-3 that either could not bind IGF or has impaired ability to be internalized. Further analyses revealed that IGFBP-3, but not IGFBP-1, could significantly enhance the weak tyrosine phosphorylation of STAT1 induced by IFN-γ (20 U/ml) alone. The IGFBP-3-promoted apoptosis in the presence of IFN-γ could also be abrogated by blockade of the mTOR pathway with its pharmacological inhibitors, LY294002 or rapamycin.
Conclusions
These results demonstrated that in a cancer cell line not responsive to exogenous IGFBP-3 alone, IGFBP-3 sensitized the cells to the anti-proliferative, proapoptotic actions of IFN-γ through an IGF-independent, STAT1- and mTOR-dependent mechanism.
Keywords: IGFBP-3, Interferon-gamma, STAT1, mTOR, Apoptosis, Prostate Cancer
INTRODUCTION
Insulin-like Growth Factor Binding Proteins (IGFBPs), a family of six secreted proteins that share high structural homology, modulate the bioavailability and mitogenic actions of Insulin-like Growth Factors (IGFs).1,2 A number of studies have also demonstrated that IGFBPs, especially IGFBP-3, can exert IGF-independent actions, including anti-proliferative and pro-apoptotic effects on various cell types.3,4 The mechanism(s) underlying the IGF-independent, growth inhibitory actions of IGFBP-3 still remains to be fully understood, yet it has been proposed that such actions may involve specific IGFBP-3 membrane receptors,5,6 inactivation of the IGFIR pathway,7 activation of the Smad 2/Smad 3 pathway 8 or the STAT1 pathway,9 or nuclear localization of IGFBP-3.10
In addition to exhibiting direct growth inhibitory effects, IGFBP-3 has also been shown to mediate the anti-proliferative actions of cytokines, such as TGF-β, TNF-α and interferon-gamma.11–13 Interferon-gamma (IFN-γ), a type II interferon which can inhibit cell proliferation and induce apoptosis,14,15 functions predominantly through activation of the JAK/STAT1 pathway.16 Other signaling pathways activated by IFN-γ, such as the PI-3K/mTOR pathway and the MAPK/ERK pathways, also significantly contribute to the biological activities exhibited by IFN-γ.15,17 In this report, we demonstrated that in M12 prostate cancer cells, IGFBP-3 strongly promoted apoptosis with an otherwise non-inhibitory dose of IFN-γ through an IGF-independent mechanism(s), which involved the STAT1 pathway and the mTOR pathway.
MATERIALS AND METHODS
Cell Culture
P69 cells is a SV40-T antigen transformed, low tumorigenic human prostate cancer cell line, and M12 cells are a highly metastatic derivative of P69 cells.18 P69 cells or M12 cells were cultured in defined RPMI 1640 medium as described previously.15 For experiments detecting changes in signaling pathways in response to various treatments, M12 cells were grown to 60–70% confluence on 6-well plate and starved in RPMI 1640 overnight before being incubated in RPMI 1640 supplemented with 50 μg/ml bovine serum albumin (BSA) plus human IFN-αA/D (Sigma-Aldrich, St. Louis, MO), human IFN-β (Sigma-Aldrich, St. Louis, MO), human IFN-γ (Roche, Mannheim, Germany), recombinant human IGFBP-3 (rhIGFBP-3), LY294002 (Calbiochem, La Jolla, CA), or rapamycin (Cell Signaling Technology, Beverly, MA) as indicated.
Generation of Recombinant Human IGFBP-3
The KpnI/Not1 digested fragment containing cDNA encoding c-terminal FLAG-tagged wild-type rhIGFBP-3, or the mutant rhIGFBP-3 (G56G80G81) was subcloned from pBSSK plasmids 19 into pShuttle-CMV plasmids (AdenoVator™Vector System, QBiogen, Carlsbad, CA), and the adenovirus carrying cDNA encoding the wild-type or the mutant rhIGFBP-3 was generated following manufacture’s instruction. The rhIGFBP-3 was then purified with M2 anti-FLAG antibody (Sigma-Aldrich, St. Louis, MO) from the conditioned medium of M12 cells infected with the adenovirus for 28 hours. The concentration of the rhIGFBP-3 in the eluted fractions was quantitated as described previously.19 It was estimated that at least 90% of the total protein purified from alpha-M2 affinity column was the 42-45KD IGFBP-3 species.
Cell Proliferation Assay
M12 cells were plated on 96-well plates (2900 cells/well) and grown for 16 hours. The cells were then synchronized in RPMI for 4 hours before treatment with defined RPMI culture medium supplemented with 50 μg/ml BSA plus either IFN-α, IFN-β, IFN-γ, rhIGFBP-3 (Protigen, Inc., Sunnyvale, CA; GroPep, Adelaide, Australia; or self-generated, see above), IGFBP-3 NLS mutant (K228E, R230G ) (Protigen, Inc., Sunnyvale, CA), LY294002, or rapamycin at the concentrations indicated. Cell proliferation was quantitated with CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay kit (MTS/PMS assay) (Promega, Madison, WI) according to the manufacturer’s instructions.
Apoptosis Assay
M12 cells were plated, cultured and treated on 96-well plates as described above. Apoptosis-induced DNA fragmentation was quantitated with a Cell Death Detection ELISAPLUS assay system (Roche Diagnostics, Indianapolis, IN) according to the manufacturer’s protocol. The assay quantitatively measures cytoplasmic histone-associated DNA fragments (mono- and oligo-nucleosomes) generated in the early phase of apoptosis.
Western Immunoblot
Preparation of the cell lysates and subsequent Western immunoblot analysis were performed as described previously.15 The density of the bands on x-ray film was determined by using IMAGEQUANT 5.1 (Molecular Dynamics, CA). The antibodies used for Western immunoblot analyses in this study were: rabbit polyclonal IgG against human IGFBP-1 (GroPep, Adelaide, Australia), phospho-Tyr701-STAT1, phospho-Thr389 p70 S6K, and p70 S6K from Cell Signaling Technology (Beverly, MA); rabbit polyclonal anti-phospho-Ser727-STAT1 from Biosource International (Camarillo, CA); mouse monoclonal IgG against IGFBP-3 from Diagnostic Systems Laboratories, Inc. (Webster, Texas), and STAT1 (C-136) from Santa Cruz Biotechnology (Santa Cruz, CA). Secondary antibodies (anti-mouse IgG and anti-rabbit IgG) were obtained from Amersham Biosciences (Piscataway, NJ). All immunoblot data shown are representative of at least three independent experiments.
RT-PCR and Quantitative PCR
RNA collection, cDNA synthesis and semi-quantitative PCR were performed as described previously. 20 The18S primers resulted in a 369bp product: (f) 5′-CGGCTACCACATCCAAGGAA -3′, (r) 5′-CCGGCGTCCCTCTTAATC -3′. The IGFBP-3 primers resulted in a 536bp product: (f) 53′-GCCGCCAGCTCCAGGAAAT -3, (r) 5′-GGGCGACACTGCTTTTTCTTA -3′.
Small-interfering RNA Transfection
The transfection of siRNA employed TransIT-TKO® Transfection Reagent (Mirus, Madison, WI) and negative control siRNA (Silencer™ Negative Control #1 siRNA), or STAT1 siRNA (Silencer™ Validated siRNA:STAT1, ID#42860, Ambion, Austin, TX) was performed as described previously.15 The STAT1 siRNA is a mixture containing two siRNAs targeting two coding regions on STAT1 gene.
RESULTS
IGFBP-3 sensitized prostate cells to the anti-proliferative, proapoptotic actions IFN-γ through an IGF-independent mechanism
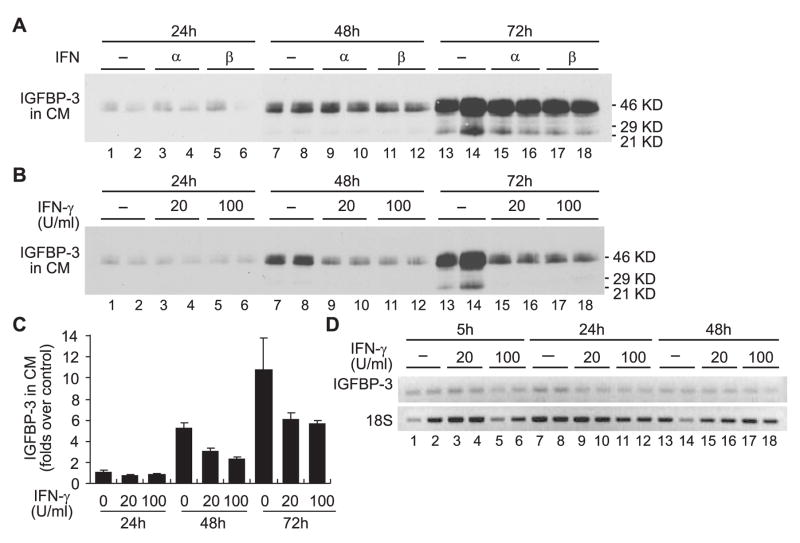
We previously demonstrated that IFN-γ (at 100 U/ml), but not IFN-α or IFN-β, elicited inhibition of cell proliferation and apoptosis in M12 cells, a human metastatic prostate cancer cell line.15 To investigate whether IGFBP-3 mediated the anti-proliferative action of IFN-γ in M12 cells, the expression of IGFBP-3 in the conditioned medium of M12 cells treated with IFN-α, IFN-β, or IFN-γ was examined by Western Immunoblot analyses. Treatment of M12 cells with 1000 U/ml IFN-α or IFN-β, did not significantly alter the secretion of IGFBP-3 into the conditioned media (Fig. 1A). However, treatment with 100 U/ml IFN-γ reduced the concentration IGFBP-3 secreted in the conditioned media, as did treatment with a low-dose IFN-γ (20 U/ml) (Fig. 1, B and C). The reduction of secreted IGFBP-3 upon IFN-γ treatment was likely regulated through a post-transcriptional mechanism(s) since IGFBP-3 mRNA expression was not significantly affected (Fig. 1D). Altogether, these results indicate that, contrary to observations in human salivary gland tumor cells,13 IGFBP-3 did not directly mediate the potent anti-proliferative, proapoptotic action of IFN-γ (100 U/ml) in M12 cells.15
Fig. 1.
IGFBP-3 does not mediate the anti-proliferative, proapoptotic action of IFN-γ in M12 cells. A–B: M12 cells on 6-well plates were starved in RPMI 1640 for 16 hours prior to treatment with BSA (5 ng/ml), IFN-α (1000 U/ml), IFN-β (1000 U/ml) or IFN-γ (20 U/ml or 100 U/ml). Samples, 40 μl out of total 2 ml conditioned medium in each well were collected at the time points indicated, and were subject to Western immunoblot analysis with specific antibodies against proteins indicated at the left to each blot. Molecular weight markers are indicated on the right. C: Relative folds increase of IGFBP-3 secreted in the conditioned medium (CM) of M12 cells treated with interferons, as assessed by densitometry analysis of Western Immunoblots. Quantitation was normalized to the number of cells. The results are expressed relative to control (treated with BSA) 24h post-treatment, which was assigned a value of 1. The data presented here are the mean±SE from two independent experiments performed in duplicates. D: Total RNA samples from M12 cells were collected at the time points indicated, and cDNA was synthesized from 1 μg of total RNA. Semi-quantitative PCR amplification was performed with primers as described in Materials and Methods. Cycling parameters were 95°C 45 seconds, 55°C 45 seconds, and 72 °C 60 seconds, for 40 cycles (IGFBP-3), or 20 cycles (18S). The PCR amplified products were resolved on 1% agarose gel in TAE running buffer and were visualized by ethidium bromide staining.
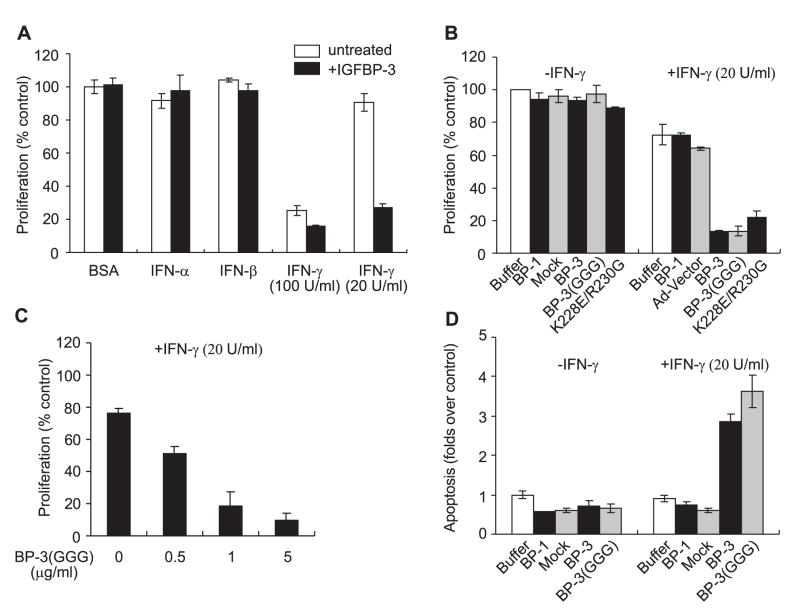
It has been demonstrated that IGFBP-3 is able to inhibit cell proliferation and promote apoptosis a variety of cell lines.3,4 Proliferation of M12 cells, however, was not significantly affected when cells were treated with exogenous rhIGFBP-3 (2.5 μg/ml) for up to 96 hour post-treatment (Fig. 2A). Co-treatment with IGFBP-3 and either IFN-α or IFN-β (1000 U/ml) also did not alter the growth of M12 cells. Co-treatment with IGFBP-3 and IFN-γ (100 U/ml) inhibited growth to a significantly greater level than observed when cells were treated with 100 U/ml IFN-γ alone (Fig. 2A). Strikingly, proliferation of M12 cells was inhibited by more than 70% when the cells were co-treated with IGFBP-3 (2.5 μg/ml) and a low dose of IFN-γ (20 U/ml), which, by itself, only minimally inhibited cell proliferation 96 hours-post treatment (Fig. 2A). A similar observation was made in P69 cells (data not shown), suggesting that the ability of IGFBP-3 to sensitize the cells to the growth inhibitory effect induced by IFN-γ was not cell-line specific.
Fig. 2.
IGFBP-3 inhibited proliferation and induced apoptosis in M12 cells in the presence of low-dose interferon-gamma. A: M12 cells were treated with defined RPMI culture medium supplemented with BSA (50 μg/ml) and 2.5 μg/ml rhIGFBP-3 (Protigen Inc., Sunnyvale, CA) plus 5 ng/ml BSA, 1000 U/ml IFN-α, 1000 U/ml IFN-β, or IFN-γ (20U/ml or 100U/ml). Cell proliferation was measured 96 hour post-treatment as described in Materials and Methods. The results are expressed as the percentages of the proliferation measured in the control (treated with BSA), which was given an arbitrary value of 100%. The data presented here are the mean±SE from a dozen independent experiments performed in triplicates. B: M12 cells were treated with defined RPMI culture medium supplemented with BSA (50 μg/ml) plus either HBSS (saline buffer used for eluting IGFBP-3 protein), 5 μg/ml recombined human IGFBP-1, mock purified from the conditioned medium of M12 cells infected with adenovirus vector (mock), 1 μg/ml flag-tagged rhIGFBP-3 (BP-3) or rhIGFBP-3(GGG) (BP-3(GGG)), or 1 μg/ml rhIGFBP-3 with mutations inside nuclear localization signal (K228E/R230G) in the absence or presence of 20 U/ml IFN-γ as indicated. Cell proliferation at 96 hour post-treatment was measured and the results are expressed as in A. The data presented here are the mean±SE from four independent experiments performed in triplicates. C: The dose dependent effect of proliferation inhibition in M12 cells induced by rhIGFBP-3(GGG) in the presence of 20 U/ml IFN-γ. Cell proliferation at 96 hour post-treatment was measured and the results are expressed as in A. The data presented here are the mean±SE from three independent experiments performed in triplicates. D: M12 cells were treated as described in B and apoptosis was measured 96 hour post-treatment as described in Materials and Methods. The results are expressed relative to control (treated with BSA), which was assigned a value of 1. The data presented here are the mean±SE from three independent experiments performed in triplicates.
The effect of IGFBP-3 was specific, as IGFBP-1 by itself (5 μg/ml) or when added with 20 U/ml IFN-γ, did not inhibit cell growth (Fig. 2B). To determine if the effect of IGFBP-3 was through IGF-dependent or IGF-independent mechanisms, we compared the effect of wild-type IGFBP-3 with a variant of IGFBP-3, IGFBP-3(G56G80G81), which has 1000-fold less affinity for IGF-I.19 IGFBP-3(G56G80G81), like wild-type IGFBP-3, inhibited cell proliferation only in the presence of 20 U/ml IFN-γ. These results suggest that the inhibition of cell proliferation induced by IGFBP-3 in the presence of IFN-γ was via an IGF-independent, IGFBP-3-specific, mechanism.
It has been demonstrated that secreted IGFBP-3 can be endocytosed, translocated to the nucleus where it interacts with nuclear receptor retinoid X receptor-α (RXR-α), and such interactions may account for the IGF-independent, anti-proliferative action of IGFBP-3.10,21 A IGFBP-3 mutant (K228E/R230G), which has significantly reduced ability to be cellular internalized by cells, 21 inhibited cell growth in the presence of 20 U/ml IFN-γ similarly as wild-type IGFBP-3 did (Fig. 2B), suggesting that cytoplasmic presence (and subsequent nuclear localization) may not be required for IGFBP-3 to sensitize M12 cells to the inhibitory effects induced by IFN-γ.
The IGFBP-3-induced inhibitory effect on cell growth in the presence of IFN-γ was dose-dependent. IGFBP-3(G56G80G81), as low as 0.5 μg/ml, inhibited cell proliferation by 51% (Fig. 2C). Increasing the concentration of IGFBP-3(G56G80G81) to 5 μg/ml further inhibited M12 cell proliferation (90%) (Fig. 2C), whereas the same concentration of IGFBP-1 had no effect on cell proliferation (Fig. 2B).
In addition to proliferation inhibition, co-treatment of cells with 20 U/ml IFN-γ and 1 μg/ml IGFBP-3 or mutant IGFBP-3(G56G80G81), but not with 5 μg/ml IGFBP-1, also elicited significant apoptosis (more than 2.8 folds over control) in M12 cells (Fig. 2D).
IGFBP-3 Enhanced tyrosine-phosphorylation of STAT1 Induced by IFN-γ
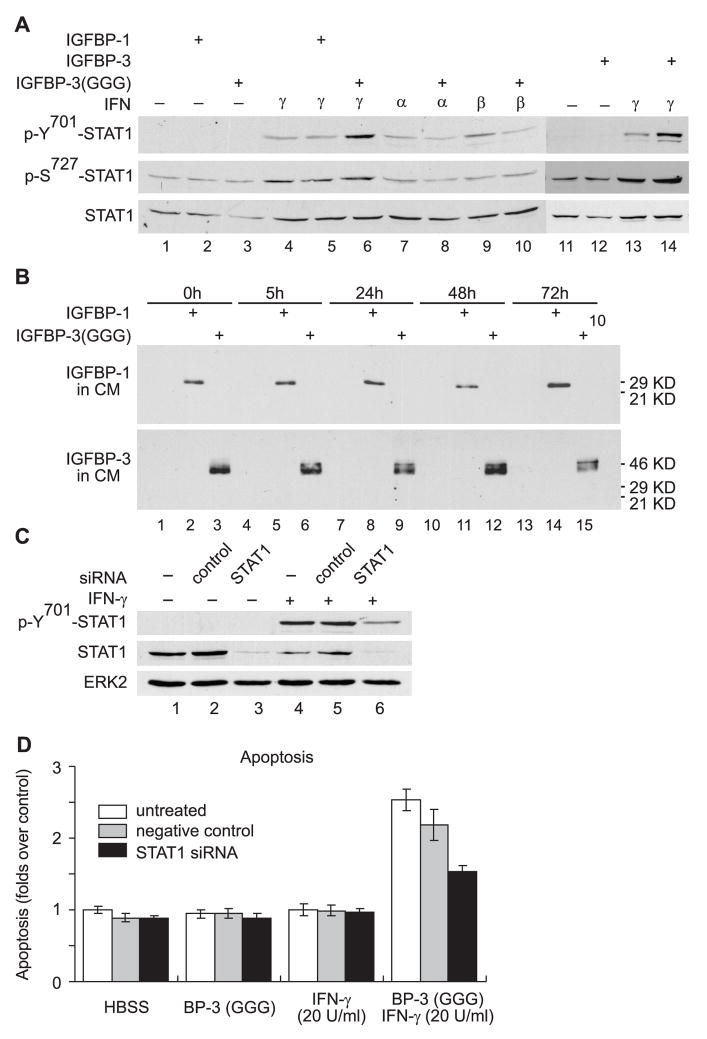
We previously demonstrated that IGFBP-3 induced apoptosis in chondrocytes as a consequence of the activation of the STAT1 pathway.9 In M12 cells, however, IGFBP-3 or IGFBP-3(G56G80G81) at 5 μg/ml, could not activate STAT1 (Fig. 3A, top panel, compare lane 12 with lane 11, or lane 3 with lane 1). 1000 U/ml IFN-α or IFN-β, or 20 U/ml IFN-γ, as expected, induced tyrosine phosphorylation of STAT1 (Fig. 3A, top panel). Remarkably, IGFBP-3 or IGFBP-3(G56G80G81) specifically enhanced IFN-γ-induced STAT1 tyrosine phosphorylation by more than 3 fold (P<0.05) (Fig. 3A, top panel, compare lane 14 with lane 13, or lane 6 with lanes 4), while having no effect on STAT1 tyrosine phosphorylation induced by IFN-α or IFN-β (Fig. 3A, top panel, compare lanes 8 and 7 with lanes 10 and 9). IGFBP-3 did not modulate the STAT1 serine phosphorylation status in M12 cells (Fig. 3A, middle panel). IGFBP-1 at 5 μg/ml, on the contrary, could not enhance IFN-γ-induced STAT1 tyrosine phosphorylation (Fig. 3A, top panel, compare lane 5 with lanes 4). Furthermore, both IGFBP-1 and IGFBP-3(G56G80G81) were stable and not significantly proteolyzed in the conditioned medium of M12 cells up to 72 hours post-treatment (Fig. 3B), suggesting that the inability of IGFBP-1 to enhance IFN-γ-induced STAT1 activation and to promote proliferation inhibition and apoptosis, was not due to the degradation of IGFBP-1 during the treatment.
Fig. 3.
IGFBP-3 enhanced STAT1 activation induced by low-dose IFN-γ. A: M12 cells in defined RPMI culture medium supplemented with BSA (50 μg/ml) were treated with 5 μg/ml flag-tagged rhIGFBP-3, 5 μg/ml flag-tagged rhIGFBP-3(GGG) or 5 μg/ml rhIGFBP-1 in the presence of IFN-α (1000 U/ml), IFN-β (1000 U/ml) or IFN-γ (20 U/ml) as indicated. Cell lysates were collected 5h post-treatment and were subject to Western immunoblot analysis with specific antibodies against proteins indicated at the left to each blot. B: M12 cells were treated with 5 μg/ml rhIGFBP-1, or 5 μg/ml flag-tagged rhIGFBP-3(GGG) in the presence of IFN-γ (20 U/ml). Samples of 5 μl conditioned medium in each well were collected at the time points indicated, and were subject to Western immunoblot analysis with specific antibodies against proteins indicated at the left to each blot. C: M12 cells were seeded at 60% confluency on 12-well plates. siRNA transfection was performed as described in Materials and Methods. Cells were incubated for 44 hours prior to synchronization in RPMI for 6 hours and treated with defined RPMI culture medium supplemented with 50 μg/ml BSA plus IFN-γand/or IGFBP-3 as indicated. Cell lysates were collected at 1h post-treatment with IFN-γ (100 U/ml), and were subjected to Western immunoblot analysis with specific antibodies against proteins indicated at the left to each blot. D: M12 cells were seeded at 25% confluency on 96-well plates and siRNA transfection was performed essentially the same as described in C with some modifications: each milliliter transfection mixture contains 2 μl instead of 4 μl TransIT-TKO® Transfection Reagent, and 30 μM instead of 70 μM siRNA (control or STAT1 siRNA). 84 μl of such mixture was aliquoted into each well on 96-well plates, and an equal volume of fresh culture medium was supplemented into each well at 24 hours post-transfection. Apoptosis in M12 cells at 72 hour post-treatment was measured and the results are expressed as in Fig. 2D. The data presented here are the mean±SE from two independent experiments performed in triplicates.
To further investigate the role of STAT1 activation in proliferation inhibition and apoptosis induced by IGFBP-3 in the presence of low-dose IFN-γ (Fig. 2), STAT1 expression was specifically blocked by siRNA. Total STAT1 protein in M12 cells was significantly reduced (80%, P<0.001) by STAT1 siRNA (Fig. 3C, middle panel, compare lane 6 with 4). A nonspecific siRNA (negative control), in contrast, did not alter the level of STAT1 protein (Fig. 3C, middle panel, compare lane 5 with 4). Corresponding to the reduced expression of STAT1, IFN-γ-induced tyrosine phosphorylation of STAT1 was also reduced by 70% (P<0.001) (Fig. 3C, top panel, compare lane 6 with 4). This specific and significant inhibition of STAT1 activation partially suppressed the apoptosis (40%) induced by co-treatment with 1 μg/ml IGFBP-3(G56G80G81) and 20 U/ml IFN-γ (Fig. 3D). Altogether, the results suggest that a fully activated STAT1 pathway is required for IGFBP-3 and IFN-γ to induce apoptosis in M12 cells, and that enhancing STAT1 activation (Fig. 3A) is at least one mechanism by which IGFBP-3 promotes apoptosis in the presence of low-dose IFN-γ.
The Apoptosis Promoted by IGFBP-3 in the Presence of Low-dose IFN-γ is Dependent on the mTOR Pathway
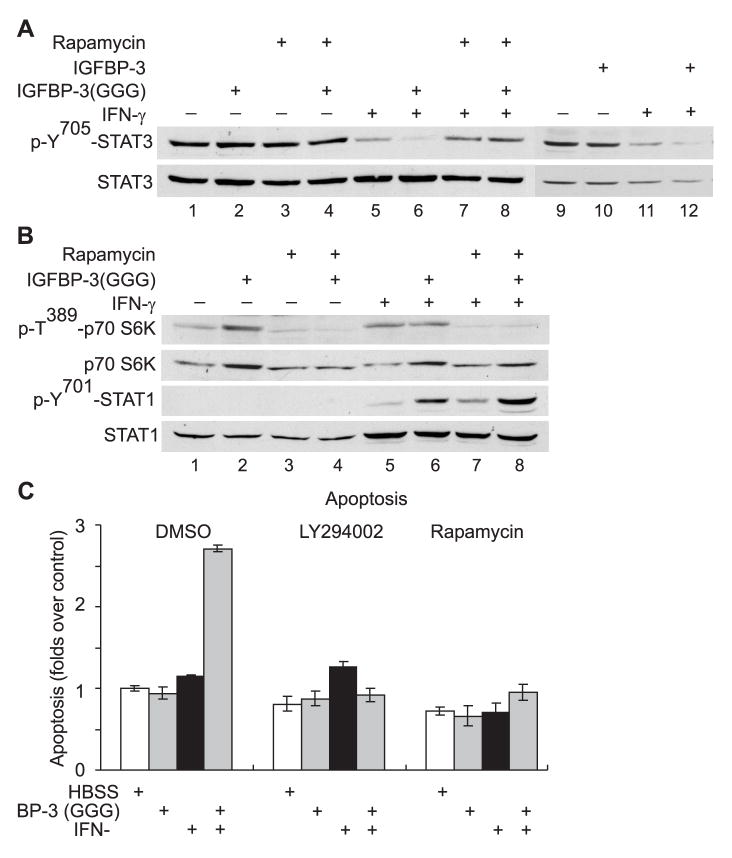
M12 cells, like many other human cancer cell lines, contain constitutively activated STAT3, and we previously demonstrated that the apoptosis induced by high dose IFN-γ (100 U/ml) in M12 cells was associated with persistent dephosphorylation of constitutively tyrosine-phosphorylated STAT3 (pY-STAT3) 24h–48h post treatment, a process which requires an intact mTOR pathway.15 We, therefore, determined if the pro-apoptotic action of IGFBP-3 in the presence of low-dose IFN-γ, was also associated with dephosphorylation of pY-STAT3. A low dose of IFN-γ (20 U/ml) induced significant dephosphorylation of pY-STAT3 (Fig. 4A, lanes 5 and 11), although at this dose, apoptosis of M12 cells was not detected (Fig. 2D). The results suggested that dephosphorylation of p-Y-STAT3, alone, was insufficient to induce apoptosis. Treatment with IGFBP-3 or IGFBP-3(G56G80G81) at 5 μg/ml did not alter pY-STAT3 status in M12 cells (Fig. 4A, upper panel, compare lanes 10 with 9 or lanes 2 with 1). However, IGFBP-3 or IGFBP-3(G56G80G81) enhanced the dephosphorylation of pY-STAT3 induced by 20 U/ml IFN-γ alone (Fig. 4A, upper panel, compare lanes 6 with 5, or lanes 12 with 11). Consistent with our previous report,15 the dephosphorylation of pY-STAT3 was significantly suppressed when cells were treated with rapamycin, the specific inhibitor to mTOR (Fig. 4A, upper panel, compare lanes 7 and 8 with lanes 5 and 6).
Fig. 4.
The proliferation inhibition and apoptosis in M12 cells promoted by IGFBP-3 in the presence of low-dose IFN-γ is dependent on the PI-3K/mTOR pathway. A–B: M12 cells were treated with DMSO, 50 nM rapamycin in the absence or the presence of 20 U/ml IFN-γ and/or 5 μg/ml rhIGFBP-1, 5 μg/ml flag-tagged rhIGFBP-3 or 5 μg/ml flag-tagged rhIGFBP-3(GGG) as indicated. The cell lysates were collected 5h (B) or 36h (A) post-treatment and analyzed as described in Fig. 3A. C: M12 cells on 96-well plates were pretreated for one hour with 0.1% (v/v) DMSO, 5 μM LY294002, or 50 nM rapamycin and then treated with either HBSS, 20 U/ml IFN-γ, or 1 μg/ml rhIGFBP-3(GGG) as indicated. Apoptosis in M12 cells at 96 hour post-treatment was measured and the results are expressed relative to control (treated with HBSS/DMSO), which was assigned a value of 1. The data presented here are the mean±SE from three independent experiments performed in triplicates.
Interestingly, exogenous IGFBP-3(G56G80G81) appears to positively regulate the activity of the mTOR pathway, as shown by enhanced phosphorylation of p70 S6K at Thr 389 and increased amount of total p70 S6K, the downstream target of mTOR (Fig. 4B, top panel, compare lanes 1 with 2). Enhanced phosphorylation of p70 S6K was also observed when the cells were treated with 20 U/ml IFN-γ or together with IGFBP-3(G56G80G81) (Fig. 4B, top panel, compare lanes 5 and 6). Treatment with 50 nM rapamycin, completely blocked the mTOR pathway (Fig. 4B, upper two panels), but did not interfere with the enhanced STAT1 activation induced by IGFBP-3 in the presence of IFN-γ (Fig. 4B, lower two panels). As demonstrated previously 15, blocking mTOR pathway did not inhibit STAT1 up-regulation induced by IFN-γ (Fig. 4B, lower two panels).
Strikingly, the apoptosis induced by IGFBP-3 in the presence of 20 U/ml IFN-γ, was abolished when the mTOR pathway was blocked by 5 μM LY294002, the dual inhibitor for PI3K and mTOR, 22 or with 50 nM rapamycin (Fig. 4C). Taken together, the results shown in Fig. 4 suggest that in addition to the STAT1 pathway, the mTOR pathway is also involved in the apoptosis promoted by IGFBP-3 with low-dose IFN-γ.
DISCUSSION
In this study, we have demonstrated that exogenous IGFBP-3 sensitized M12 prostate cancer cells to apoptosis induced by an otherwise non-effective dose of IFN-γ through an IGF-independent, STAT1- and mTOR-dependent mechanism. IGFBP-3 neither directly induced significant inhibition of cell proliferation or apoptosis, nor mediated the potent growth inhibitory effects elicited by high-dose IFN-γ.
A number of studies have demonstrated that IGFBP-3 alone can inhibit cell growth and induce apoptosis.8,23–31 In a previous report, we showed that in M12 cells, stable transfection with IGFBP-3 cDNA, also induced apoptosis.32 Addition of exogenous IGFBP-3 to the conditional medium of M12 cells, however, did not induce significant apoptosis. One possible explanation for these contrary observations is that ectopic expression of IGFBP-3 in M12 cells may have other unknown effects on normal physiological processes of the cells; for example, the morphology of M12 cells was significantly altered when expressing ectopic IGFBP-3,32 but was not altered upon treatment with exogenous IGFBP-3 (data not shown). These observations thus argue that ectopically expressed IGFBP-3 may inhibit M12 cell growth and induce apoptosis through different mechanisms(s) to exogenously added IGFBP-3.
IGFBP-3 has been show to be capable of promoting apoptosis through IGF-independent mechanisms.3,4 In M12 cells, wild-type IGFBP-3 and a mutant form of IGFBP-3 that has a dramatically reduced affinity for IGF-I, both promoted apoptosis when co-treated low-dose IFN-γ. On the contrary, IGFBP-1 has no effect on apoptosis. In addition, treatment with IFN-γ at 20 U/ml or 100 U/ml did not significantly alter expression of IGF-I mRNA (data not shown), and proliferation of M12 cells was not affected when the culture medium was supplemented with IGF-I (data not shown). Altogether, these data suggest that the inhibition of cell proliferation and apoptosis induced by IGFBP-3 in the presence of IFN-γ is via an IGF-independent mechanism(s).
The STAT1 pathway has been shown to be essential for growth inhibition and apoptosis induced by IFN-γ.33 IGFBP-3, in primary rat chondrocytes, also induced apoptosis through activation of the STAT1 pathway.9 In M12 cells, IGFBP-3, by itself, neither activated STAT1 pathway nor induced apoptosis; whereas IFN-γ at 20 U/ml, as expected, activated STAT1, but activation was not associated with apoptosis. Remarkably, IGFBP-3, but not IGFBP-1, significantly enhanced the STAT1 activation induced by the low-dose IFN-γ, with subsequent apoptosis of M12 cells observed. This association thus suggests that IGFBP-3 can modulate specific cytokine-activated signaling pathway(s) to control cell growth and death. The detailed mechanism(s) by which IGFBP-3 enhances STAT1 activation induced by IFN-γ remains to be established.
In addition to the STAT1 pathway, IGFBP-3 also appears to activate the mTOR/p70 S6K pathway, a traditional pro-survival pathway (Fig. 4B). Enhancing the mTOR pathway by IGFBP-3 alone did not have a significant effect on cell proliferation or cell death (Fig. 2). However, blocking the mTOR pathway abolished the apoptosis induced by co-treatment of IGFBP-3 and low-dose IFN-γ (Fig. 4C). Furthermore, the mTOR/p70 S6K pathway was shown to be required for the apoptosis induced by high-dose IFN-γ.15 Taken together, these results suggest that IGFBP-3 may positively regulate the mTOR pathway to synergistically promote growth inhibition and apoptosis with low-dose IFN-γ.
The ability of IGFBP-3 to sensitize the cells to the anti-proliferative, proapoptotic agent is not limited to IFN-γ, as a number of previous studies have demonstrated that although IGFBP-3 could only induce minimal growth inhibitory effect in various cell types, IGFBP-3 significantly potentiate the cellular responses to a number of proapoptotic agents, such as ceramide,34 radiation,35,36 paclitaxel,37 TGF-β,38 butyrate,39 retinoid,40 TRAIL,41 and Hercepcin.42 Consistent with these other studies, our study further expands the scope of the pleiotropic and potent cellular effects of IGFBP-3, and support the concept that IGFBP-3 could be exploited as an enhancing agent to other anti-cancer drugs in cancer therapy.
In summary, our study implicated a possible cross-talk between the signaling pathways activated by IFN-γ and IGFBP-3, thus uncovering a novel mechanism by which IGFBP-3 participates in the control of cell growth and cell death, and suggests that IGFBP-3 may have potential clinical applications in combination with IFN-γ.
Acknowledgments
This work was supported by NIH Grant CA58110 (to RGR), and DOD Grant W81XWH-04-1-0107 (to PF).
Grant sponsor: NIH Grant CA58110 (to RGR), and DOD Grant W81XWH-04-1-0107 (to PF).
The abbreviations used are
- IGF
insulin-like growth factor
- IGFBP-3
insulin-like growth factor binding protein-3
- rhIGFBP-3
recombinant human IGFBP-3
- IFN-γ
interferon-gamma
- IFN-α
interferon-alpha
- IFN-β
interferon-beta
- JAK
Janus kinase
- STAT
signal transducers and activators of transcription
- PI-3K
phosphatidylinositol 3-kinase
- mTOR
mammalian target of rapamycin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 2.Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999;20:761–787. doi: 10.1210/edrv.20.6.0382. [DOI] [PubMed] [Google Scholar]
- 3.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 4.Mohan S, Baylink DJ. IGF-binding proteins are multifunctional and act via IGF-dependent and -independent mechanisms. J Endocrinol. 2002;175:19–31. doi: 10.1677/joe.0.1750019. [DOI] [PubMed] [Google Scholar]
- 5.Oh Y, Muller HL, Lee DY, Fielder PJ, Rosenfeld RG. Characterization of the affinities of insulin-like growth factor (IGF)- binding proteins 1-4 for IGF-I, IGF-II, IGF-I/insulin hybrid, and IGF-I analogs. Endocrinology. 1993;132:1337–1344. doi: 10.1210/endo.132.3.7679979. [DOI] [PubMed] [Google Scholar]
- 6.Leal SM, Liu Q, Huang SS, Huang JS. The type V transforming growth factor beta receptor is the putative insulin-like growth factor-binding protein 3 receptor. J Biol Chem. 1997;272:20572–20576. doi: 10.1074/jbc.272.33.20572. [DOI] [PubMed] [Google Scholar]
- 7.Ricort JM, Binoux M. Insulin-like growth factor-binding protein-3 activates a phosphotyrosine phosphatase. Effects on the insulin-like growth factor signaling pathway. J Biol Chem. 2002;277:19448–19454. doi: 10.1074/jbc.M200439200. [DOI] [PubMed] [Google Scholar]
- 8.Fanayan S, Firth SM, Baxter RC. Signaling through the Smad pathway by insulin-like growth factor- binding protein-3 in breast cancer cells. Relationship to transforming growth factor-beta 1 signaling. J Biol Chem. 2002;277:7255–7261. doi: 10.1074/jbc.M108038200. [DOI] [PubMed] [Google Scholar]
- 9.Spagnoli A, Torello M, Nagalla SR, et al. Identification of STAT-1 as a molecular target of IGFBP-3 in the process of chondrogenesis. J Biol Chem. 2002;277:18860–18867. doi: 10.1074/jbc.M200218200. [DOI] [PubMed] [Google Scholar]
- 10.Liu B, Lee HY, Weinzimer SA, et al. Direct functional interactions between insulin-like growth factor- binding protein-3 and retinoid X receptor-alpha regulate transcriptional signaling and apoptosis. J Biol Chem. 2000;275:33607–33613. doi: 10.1074/jbc.M002547200. [DOI] [PubMed] [Google Scholar]
- 11.Gucev ZS, Oh Y, Kelley KM, Rosenfeld RG. Insulin-like growth factor binding protein 3 mediates retinoic acid- and transforming growth factor beta2-induced growth inhibition in human breast cancer cells. Cancer Res. 1996;56:1545–1550. [PubMed] [Google Scholar]
- 12.Rozen F, Zhang J, Pollak M. Antiproliferative action of tumor necrosis factor-alpha on MCF-7 breastcancer cells is associated with increased insulin-like growth factor binding protein-3 accumulation. Int J Oncol. 1998;13:865–869. doi: 10.3892/ijo.13.4.865. [DOI] [PubMed] [Google Scholar]
- 13.Katz J, Nasatzky E, Werner H, Le Roith D, Shemer J. Tumor necrosis factor alpha and interferon gamma--induced cell growth arrest is mediated via insulin-like growth factor binding protein-3. Growth Horm IGF Res. 1999;9:174–178. doi: 10.1054/ghir.1999.0101. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 15.Fang P, Hwa V, Rosenfeld RG. Interferon-gamma-induced dephosphorylation of STAT3 and apoptosis are dependent on the mTOR pathway. Exp Cell Res. 2006;312:1229–1239. doi: 10.1016/j.yexcr.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 17.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 18.Bae VL, Jackson-Cook CK, Maygarden SJ, Plymate SR, Chen J, Ware JL. Metastatic sublines of an SV40 large T antigen immortalized human prostate epithelial cell line. Prostate. 1998;34:275–282. doi: 10.1002/(sici)1097-0045(19980301)34:4<275::aid-pros5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 19.Buckway CK, Wilson EM, Ahlsen M, Bang P, Oh Y, Rosenfeld RG. Mutation of three critical amino acids of the N-terminal domain of IGF- binding protein-3 essential for high affinity IGF binding. J Clin Endocrinol Metab. 2001;86:4943–4950. doi: 10.1210/jcem.86.10.7936. [DOI] [PubMed] [Google Scholar]
- 20.Hwa V, Little B, Kofoed EM, Rosenfeld RG. Transcriptional regulation of insulin-like growth factor-I by interferon-gamma requires STAT-5b. J Biol Chem. 2004;279:2728–2736. doi: 10.1074/jbc.M310495200. [DOI] [PubMed] [Google Scholar]
- 21.Lee KW, Liu B, Ma L, et al. Cellular internalization of insulin-like growth factor binding protein-3: distinct endocytic pathways facilitate re-uptake and nuclear localization. J Biol Chem. 2004;279:469–476. doi: 10.1074/jbc.M307316200. [DOI] [PubMed] [Google Scholar]
- 22.Brunn GJ, Williams J, Sabers C, Wiederrecht G, Lawrence JC, Jr, Abraham RT. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. Embo J. 1996;15:5256–5267. [PMC free article] [PubMed] [Google Scholar]
- 23.Oh Y, Muller HL, Lamson G, Rosenfeld RG. Insulin-like growth factor (IGF)-independent action of IGF-binding protein-3 in Hs578T human breast 20 cancer cells. Cell surface binding and growth inhibition. J Biol Chem. 1993;268:14964–14971. [PubMed] [Google Scholar]
- 24.Pratt SE, Pollak MN. Insulin-like growth factor binding protein 3 (IGF-BP3) inhibits estrogen-stimulated breast cancer cell proliferation. Biochem Biophys Res Commun. 1994;198:292–297. doi: 10.1006/bbrc.1994.1041. [DOI] [PubMed] [Google Scholar]
- 25.Rajah R, Valentinis B, Cohen P. Insulin-like growth factor (IGF)-binding protein-3 induces apoptosis and mediates the effects of transforming growth factor-beta1 on programmed cell death through a p53- and IGF-independent mechanism. J Biol Chem. 1997;272:12181–12188. doi: 10.1074/jbc.272.18.12181. [DOI] [PubMed] [Google Scholar]
- 26.Spagnoli A, Hwa V, Horton WA, et al. Antiproliferative effects of insulin-like growth factor-binding protein-3 in mesenchymal chondrogenic cell line RCJ3.1C5.18. relationship to differentiation stage. J Biol Chem. 2001;276:5533–5540. doi: 10.1074/jbc.M005088200. [DOI] [PubMed] [Google Scholar]
- 27.Hong J, Zhang G, Dong F, Rechler MM. Insulin-like growth factor (IGF)-binding protein-3 mutants that do not bind IGF-I or IGF-II stimulate apoptosis in human prostate cancer cells. J Biol Chem. 2002;277:10489–10497. doi: 10.1074/jbc.M109604200. [DOI] [PubMed] [Google Scholar]
- 28.Lee HY, Chun KH, Liu B, et al. Insulin-like growth factor binding protein-3 inhibits the growth of non-small cell lung cancer. Cancer Res. 2002;62:3530–3537. [PubMed] [Google Scholar]
- 29.Butt AJ, Fraley KA, Firth SM, Baxter RC. IGF-binding protein-3-induced growth inhibition and apoptosis do not require cell surface binding and nuclear translocation in human breast cancer cells. Endocrinology. 2002;143:2693–2699. doi: 10.1210/endo.143.7.8876. [DOI] [PubMed] [Google Scholar]
- 30.Kim HS, Ingermann AR, Tsubaki J, Twigg SM, Walker GE, Oh Y. Insulin-like growth factor-binding protein 3 induces caspase-dependent apoptosis through a death receptor-mediated pathway in MCF-7 human breast cancer cells. Cancer Res. 2004;64:2229–2237. doi: 10.1158/0008-5472.can-03-1675. [DOI] [PubMed] [Google Scholar]
- 31.Silha JV, Sheppard PC, Mishra S, et al. Insulin-like growth factor (IGF) binding protein-3 attenuates prostate tumor growth by IGF-dependent and IGF-independent mechanisms. Endocrinology. 2006;147:2112–2121. doi: 10.1210/en.2005-1270. [DOI] [PubMed] [Google Scholar]
- 32.Devi GR, Sprenger CC, Plymate SR, Rosenfeld RG. Insulin-like growth factor binding protein-3 induces early apoptosis in malignant prostate cancer cells and inhibits tumor formation in vivo. Prostate. 2002;51:141–152. doi: 10.1002/pros.10068. [DOI] [PubMed] [Google Scholar]
- 33.Bromberg JF, Horvath CM, Wen Z, Schreiber RD, Darnell JE., Jr Transcriptionally active Stat1 is required for the antiproliferative effects of both interferon alpha and interferon gamma. Proc Natl Acad Sci U S A. 1996;93:7673–7678. doi: 10.1073/pnas.93.15.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gill ZP, Perks CM, Newcomb PV, Holly JM. Insulin-like growth factor-binding protein (IGFBP-3) predisposes breast cancer cells to programmed cell death in a non-IGF-dependent manner. J Biol Chem. 1997;272:25602–25607. doi: 10.1074/jbc.272.41.25602. [DOI] [PubMed] [Google Scholar]
- 35.Williams AC, Collard TJ, Perks CM, et al. Increased p53-dependent apoptosis by the insulin-like growth factor binding protein IGFBP-3 in human colonic adenoma-derived cells. Cancer Res. 2000;60:22–27. [PubMed] [Google Scholar]
- 36.Butt AJ, Firth SM, King MA, Baxter RC. Insulin-like growth factor-binding protein-3 modulates expression of Bax and Bcl-2 and potentiates p53-independent radiation-induced apoptosis in human breast cancer cells. J Biol Chem. 2000;275:39174–39181. doi: 10.1074/jbc.M908888199. [DOI] [PubMed] [Google Scholar]
- 37.Fowler CA, Perks CM, Newcomb PV, Savage PB, Farndon JR, Holly JM. Insulin-like growth factor binding protein-3 (IGFBP-3) potentiates paclitaxel-induced apoptosis in human breast cancer cells. Int J Cancer. 2000;88:448–453. [PubMed] [Google Scholar]
- 38.Fanayan S, Firth SM, Butt AJ, Baxter RC. Growth inhibition by insulin-like growth factor-binding protein-3 in T47D breast cancer cells requires transforming growth factor-beta (TGF- beta ) and the type II TGF-beta receptor. J Biol Chem. 2000;275:39146–39151. doi: 10.1074/jbc.M006964200. [DOI] [PubMed] [Google Scholar]
- 39.Collard TJ, Guy M, Butt AJ, et al. Transcriptional upregulation of the insulin-like growth factor binding protein IGFBP-3 by sodium butyrate increases IGF-independent apoptosis in human colonic adenoma-derived epithelial cells. Carcinogenesis. 2003;24:393–401. doi: 10.1093/carcin/24.3.393. [DOI] [PubMed] [Google Scholar]
- 40.Liu B, Lee KW, Li H, et al. Combination therapy of insulin-like growth factor binding protein-3 and retinoid X receptor ligands synergize on prostate cancer cell apoptosis in vitro and in vivo. Clin Cancer Res. 2005;11:4851–4856. doi: 10.1158/1078-0432.CCR-04-2160. [DOI] [PubMed] [Google Scholar]
- 41.Williams AC, Smartt H, AM HZ, Macfarlane M, Paraskeva C, Collard TJ. Insulin-like growth factor binding protein 3 (IGFBP-3) potentiates TRAIL-induced apoptosis of human colorectal carcinoma cells through inhibition of NF-kappaB. Cell Death Differ. 2007;14:137–145. doi: 10.1038/sj.cdd.4401919. [DOI] [PubMed] [Google Scholar]
- 42.Jerome L, Alami N, Belanger S, et al. Recombinant Human Insulin-like Growth Factor Binding Protein 3 Inhibits Growth of Human Epidermal Growth Factor Receptor-2-Overexpressing Breast Tumors and Potentiates Herceptin Activity In vivo. Cancer Res. 2006;66:7245–7252. doi: 10.1158/0008-5472.CAN-05-3555. [DOI] [PubMed] [Google Scholar]