Abstract
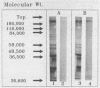
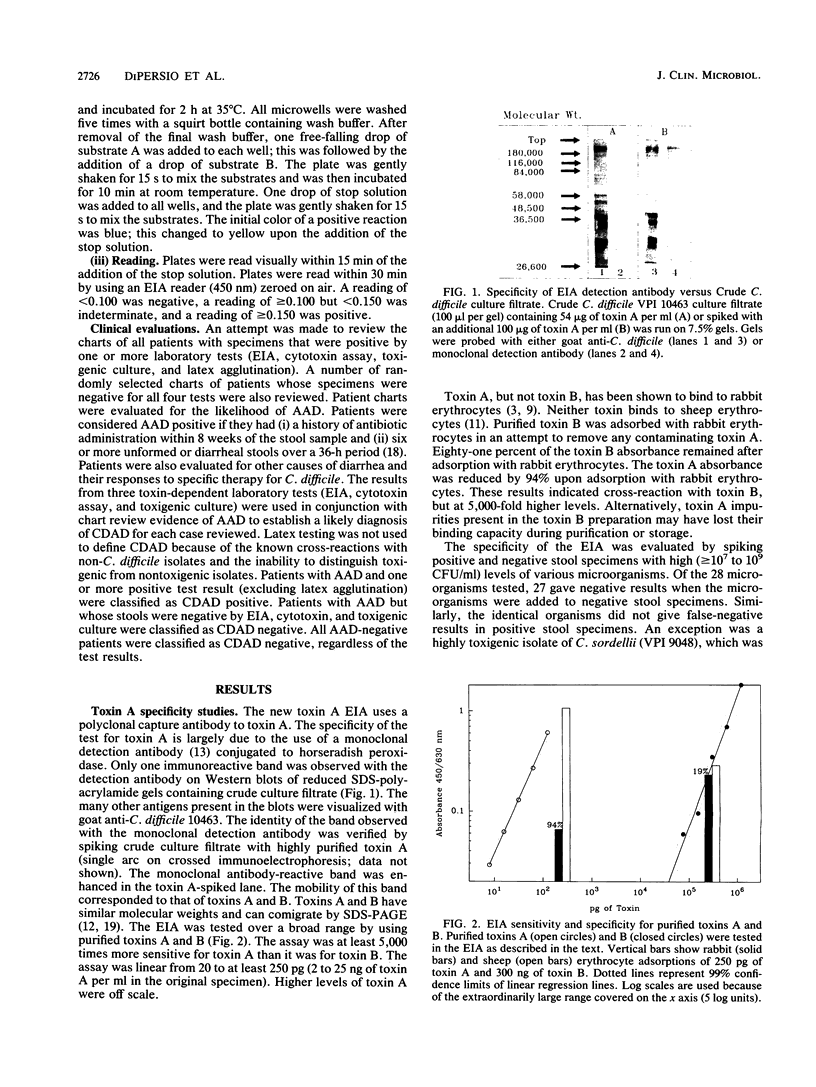
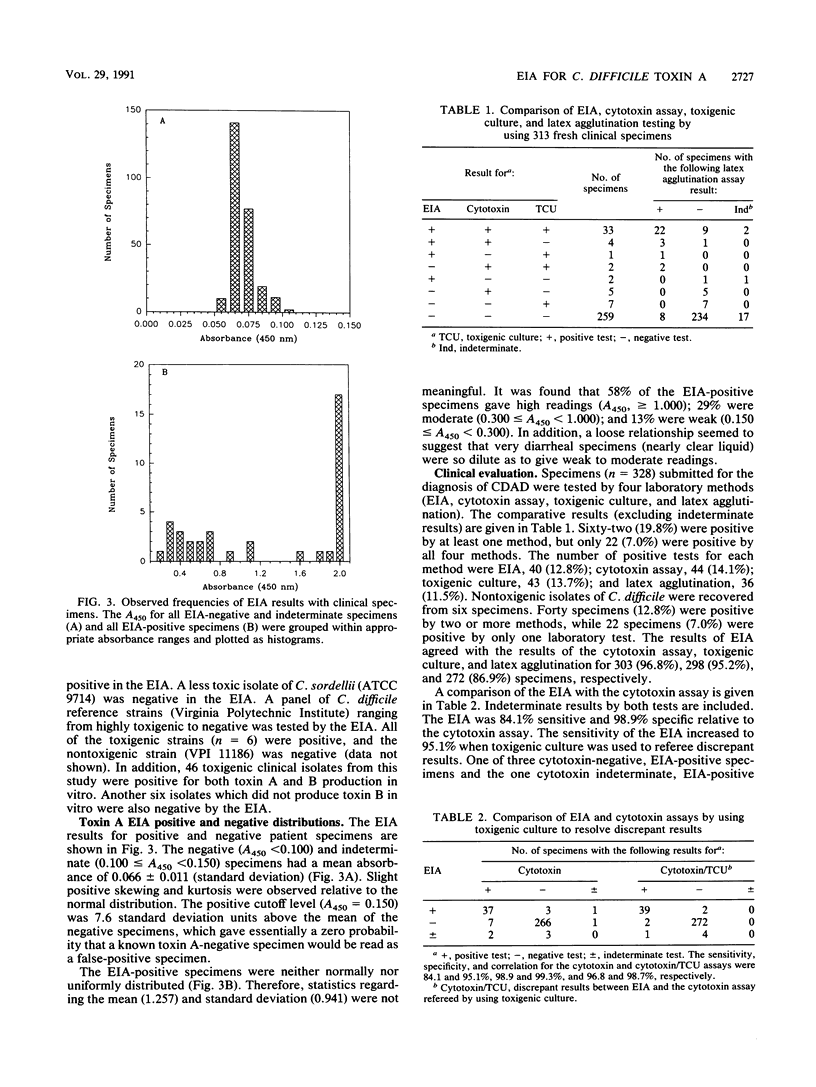
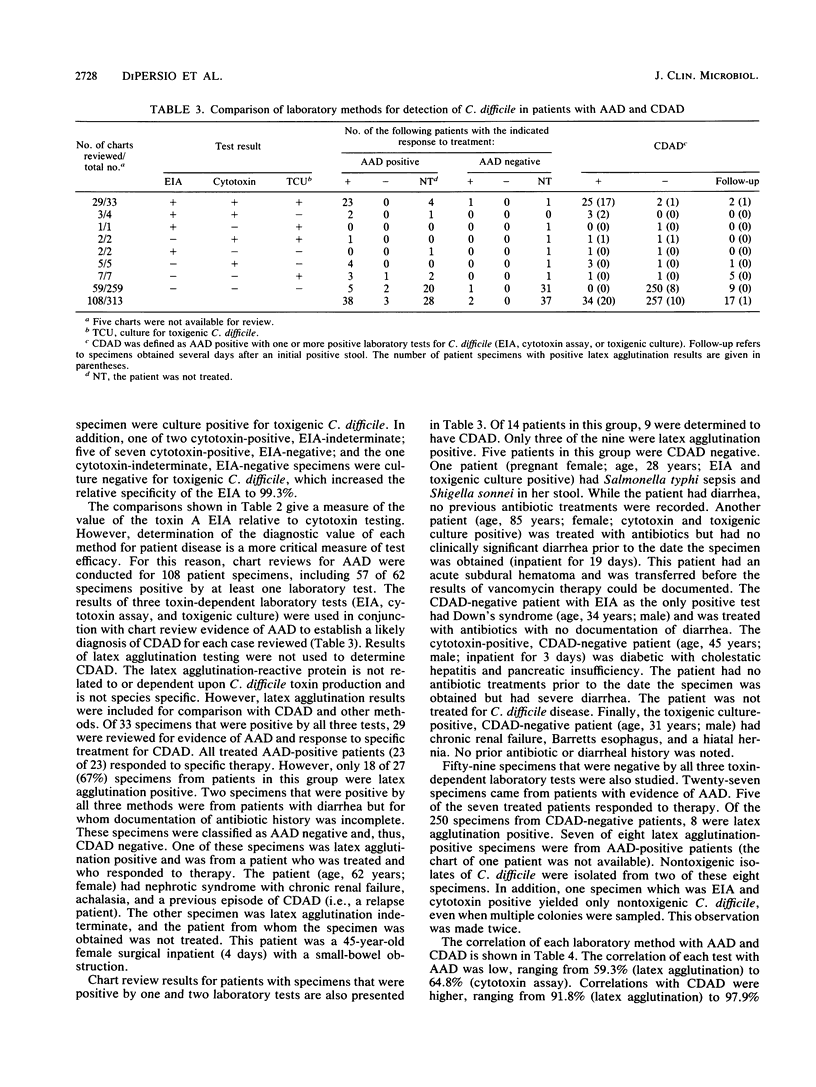
A rapid (2.5 h) direct enzyme immunoassay (EIA) for Clostridium difficile toxin A was developed for clinical use. Specimen centrifugation and filtration were not required. The EIA detected toxin A levels in patient stool as low as 20 pg (2 ng/ml of stool). The test was 5,000 times more sensitive for toxin A than it was for toxin B and did not react with a panel of other bacterial species with the exception of one highly toxigenic strain of Clostridium sordellii. The EIA was compared with the cytotoxin assay, culture of toxigenic C. difficile (toxigenic culture), and latex agglutination by using 313 fresh stool specimens submitted from patients with suspected C. difficile-associated disease. Results read visually and with a plate reader were similar. Sixty-two specimens were positive by one or more tests, but only 22 (35%) were positive by all four laboratory methods. The EIA was 84.1% sensitive and 98.9% specific when it was compared with the cytotoxin assay. The use of toxigenic culture to referee discrepant results (EIA versus cytotoxin assay) showed the EIA sensitivity and specificity to be 95.1 and 99.3%, respectively, with respect to other laboratory methods. Patient charts were reviewed for antibiotic-associated diarrhea on 108 specimens, including all those that were positive by at least one test method. Of 34 patients determined to have C. difficile-associated disease, 29 (85.3%) were positive by EIA, 32 (94.1%) were positive by the cytotoxin assay, 27 (79.4%) were positive by toxigenic culture, and 20 (58.8%) were positive by latex agglutination. Seven patients with antibiotic-associated diarrhea had a positive latex result, but results were negative by EIA, the cytotoxin assay, and toxigenic culture. The EIA demonstrated high specificity and good sensitivity for C. difficile-associated disease cases. The test can be used alone or in combination with the cytotoxin assay or toxigenic culture to provide rapid and sensitive results.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett J. G. Clostridium difficile: clinical considerations. Rev Infect Dis. 1990 Jan-Feb;12 (Suppl 2):S243–S251. doi: 10.1093/clinids/12.supplement_2.s243. [DOI] [PubMed] [Google Scholar]
- Clark G. F., Krivan H. C., Wilkins T. D., Smith D. F. Toxin A from Clostridium difficile binds to rabbit erythrocyte glycolipids with terminal Gal alpha 1-3Gal beta 1-4GlcNAc sequences. Arch Biochem Biophys. 1987 Aug 15;257(1):217–229. doi: 10.1016/0003-9861(87)90561-3. [DOI] [PubMed] [Google Scholar]
- Corthier G., Muller M. C., Wilkins T. D., Lyerly D., L'Haridon R. Protection against experimental pseudomembranous colitis in gnotobiotic mice by use of monoclonal antibodies against Clostridium difficile toxin A. Infect Immun. 1991 Mar;59(3):1192–1195. doi: 10.1128/iai.59.3.1192-1195.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold S. M. Clinical considerations in the diagnosis of antimicrobial agent-associated gastroenteritis. Diagn Microbiol Infect Dis. 1986 Mar;4(3 Suppl):87S–91S. doi: 10.1016/s0732-8893(86)80046-3. [DOI] [PubMed] [Google Scholar]
- Gerding D. N., Olson M. M., Peterson L. R., Teasley D. G., Gebhard R. L., Schwartz M. L., Lee J. T., Jr Clostridium difficile-associated diarrhea and colitis in adults. A prospective case-controlled epidemiologic study. Arch Intern Med. 1986 Jan;146(1):95–100. [PubMed] [Google Scholar]
- Kelly M. T., Champagne S. G., Sherlock C. H., Noble M. A., Freeman H. J., Smith J. A. Commercial latex agglutination test for detection of Clostridium difficile-associated diarrhea. J Clin Microbiol. 1987 Jul;25(7):1244–1247. doi: 10.1128/jcm.25.7.1244-1247.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan C. Detection of Clostridium difficile toxins by enzyme immunoassay. J Hyg (Lond) 1986 Feb;96(1):5–12. doi: 10.1017/s0022172400062471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivan H. C., Clark G. F., Smith D. F., Wilkins T. D. Cell surface binding site for Clostridium difficile enterotoxin: evidence for a glycoconjugate containing the sequence Gal alpha 1-3Gal beta 1-4GlcNAc. Infect Immun. 1986 Sep;53(3):573–581. doi: 10.1128/iai.53.3.573-581.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughon B. E., Viscidi R. P., Gdovin S. L., Yolken R. H., Bartlett J. G. Enzyme immunoassays for detection of Clostridium difficile toxins A and B in fecal specimens. J Infect Dis. 1984 May;149(5):781–788. doi: 10.1093/infdis/149.5.781. [DOI] [PubMed] [Google Scholar]
- Lyerly D. M., Krivan H. C., Wilkins T. D. Clostridium difficile: its disease and toxins. Clin Microbiol Rev. 1988 Jan;1(1):1–18. doi: 10.1128/cmr.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Lockwood D. E., Richardson S. H., Wilkins T. D. Biological activities of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1147–1150. doi: 10.1128/iai.35.3.1147-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Phelps C. J., Wilkins T. D. Monoclonal and specific polyclonal antibodies for immunoassay of Clostridium difficile toxin A. J Clin Microbiol. 1985 Jan;21(1):12–14. doi: 10.1128/jcm.21.1.12-14.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland L. V., Mulligan M. E., Kwok R. Y., Stamm W. E. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989 Jan 26;320(4):204–210. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- Peterson L. R., Holter J. J., Shanholtzer C. J., Garrett C. R., Gerding D. N. Detection of Clostridium difficile toxins A (enterotoxin) and B (cytotoxin) in clinical specimens. Evaluation of a latex agglutination test. Am J Clin Pathol. 1986 Aug;86(2):208–211. doi: 10.1093/ajcp/86.2.208. [DOI] [PubMed] [Google Scholar]
- Peterson L. R., Olson M. M., Shanholtzer C. J., Gerding D. N. Results of a prospective, 18-month clinical evaluation of culture, cytotoxin testing, and culturette brand (CDT) latex testing in the diagnosis of Clostridium difficile-associated diarrhea. Diagn Microbiol Infect Dis. 1988 Jun;10(2):85–91. doi: 10.1016/0732-8893(88)90045-4. [DOI] [PubMed] [Google Scholar]
- Sullivan N. M., Pellett S., Wilkins T. D. Purification and characterization of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1032–1040. doi: 10.1128/iai.35.3.1032-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscidi R., Willey S., Bartlett J. G. Isolation rates and toxigenic potential of Clostridium difficile isolates from various patient populations. Gastroenterology. 1981 Jul;81(1):5–9. [PubMed] [Google Scholar]
- Walker R. C., Ruane P. J., Rosenblatt J. E., Lyerly D. M., Gleaves C. A., Smith T. F., Pierce P. F., Jr, Wilkins T. D. Comparison of culture, cytotoxicity assays, and enzyme-linked immunosorbent assay for toxin A and toxin B in the diagnosis of Clostridium difficile-related enteric disease. Diagn Microbiol Infect Dis. 1986 May;5(1):61–69. doi: 10.1016/0732-8893(86)90092-1. [DOI] [PubMed] [Google Scholar]
- Woods G. L., Iwen P. C. Comparison of a dot immunobinding assay, latex agglutination, and cytotoxin assay for laboratory diagnosis of Clostridium difficile-associated diarrhea. J Clin Microbiol. 1990 May;28(5):855–857. doi: 10.1128/jcm.28.5.855-857.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. C., Gersch S. M. Evaluation of a commercial kit for the routine detection of Clostridium difficile cytotoxin by tissue culture. J Clin Microbiol. 1986 Apr;23(4):792–793. doi: 10.1128/jcm.23.4.792-793.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]