Many rewards in life require taking risks, and the decision to take such risks often must be made under stressful circumstances—whether one is a stockbroker, a surgeon, or just a commuter in rush-hour traffic. Stress hormones affect prefrontal brain regions and dopaminergic pathways involved in decision making (Moghaddam & Jackson, 2004; Wang et al., 2005). As people age, prefrontal brain regions decline in volume and in dopamine transmission effectiveness (Raz, 2004; Volkow et al., 2000), potentially altering the impact of stress on decision making.
To examine age differences in the effects of stress on risky decisions, we asked younger and older participants to play a computer-based driving game either after a stress challenge or in a control condition.
METHOD
Younger adults (aged 18-33 years; n = 45; 22 males, 23 females) and older adults (aged 65-89 years; n = 40; 21 males, 19 females) played 15 driving-game trials. Participants randomly assigned to the stress condition submerged their nondominant hand in ice water (2.0-4.2°C) for 3 min. In the control condition, participants held their hand in warm water (37.3-38.8°C) for 3 min. They started the driving game 18 min after the stress challenge, during the period of peak cortisol response to acute stress (Dickerson & Kemeny, 2004). Salivary cortisol was measured using an enzyme immunoassay kit (Salimetrics, State College, PA) from saliva samples at three time points.
Each game trial started with a green light that changed to yellow with the first press of the accelerator key. The yellow light lasted for a randomly determined time (2-7 s) before turning red. While participants pressed the accelerator key, the car moved at a constant speed across the screen and the participant’s score increased. Whenever they released the key, the car and the score counter stopped. Participants could start and stop the car as often as they liked until the red light appeared; their behavior did not influence the length of the yellow light. If the car was stopped when the red light appeared, the points from that trial were added to their cumulative total and they heard, “yippie!” However, if the light turned red while they were driving, they lost all their points from that trial and heard a police siren. Time spent driving during yellow lights was our measure of risk taking.
RESULTS
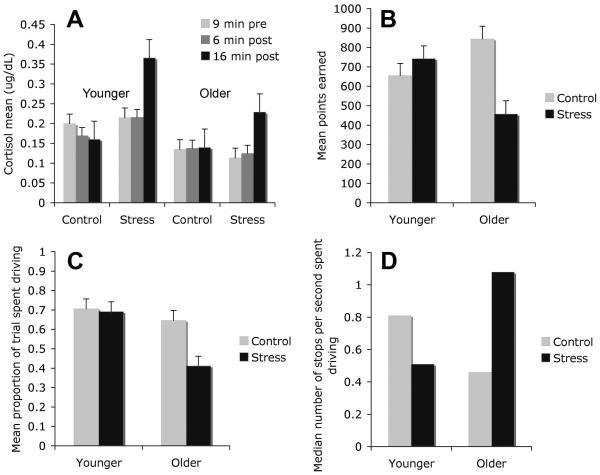
Unlike salivary cortisol levels in the control group, the stress group’s cortisol levels increased by the time of the game (Fig. 1A), for both younger adults, F(1, 43) = 10.64, p < .01, and older adults, F(1, 38) = 8.10, p < .01, with no significant age difference in the increase. The stress condition also resulted in higher postexperiment self-ratings of stress during the water task for both younger adults, t(43) = 8.11, p < .001, and older adults, t(37) = 7.24, p < .001, with no significant age difference in the effect of the cold pressor task.
Fig. 1.
Experimental results: (a) mean cortisol level 9 min before and 6 and 16 min after immersing a hand in water, by age group and whether the water was cold (stress condition) or warm (control condition), (b) mean points earned by older and younger participants in the stress and control conditions, (c) mean proportion of the trial spent driving during yellow lights by age group (older and younger) and condition (stress and control), and (d) median number of stops per second spent driving by age group (older and younger) and condition (stress and control). Medians, rather than means, are shown in (d) because the data were skewed.
Being stressed reduced older adults’ final scores by nearly half, t(38) = -3.50, p < .01, but did not significantly affect younger adults’ scores, t(43) = 1.03, p = .31 (Fig. 1B), leading to a significant interaction of age group and stress condition, F(1, 81) = 12.10, p < .001. Stressed older adults risked driving for a smaller proportion of the yellow-light intervals than did control older adults, t(38) = -3.64, p < .01, whereas younger adults showed little effect of stress on risk taking, t(43) = -.38, p = .71 (Fig. 1C), F(1, 81) = 9.09, p < .01. In general, stopping and restarting more often was negatively correlated with points scored (r = -.61, p < .001). Stress increased older adults’ stopping rate, t(38) = 3.21, p < .01, but did not significantly affect younger adults’ stopping rate, t(43) = 1.41, p = .17, leading to a significant interaction, F(1, 81) = 11.12, p < .01 (Fig. 1D; stops per second driven yielded a skewed distribution, thus we analyzed rank-transformed data). Overall, older adults had fewer losing trials (M = 27%) than younger adults (M = 41%), F(1, 81) = 17.74, p < .001, but there were no effects of stress on losing rates.
Partitioning results into three phases of five trials each and including phase as a factor revealed consistent effects of stress on driving time, stopping rate, and points throughout the game (all ps > .3 for the stress-condition-by-phase and stress-condition-by-age-by-phase interactions). Furthermore, the age-by-stress-condition interactions were significant for each phase analyzed separately (all nine ps < .05).
All the age-by-stress-condition interactions remained significant (p ≤ .01) when years of education, intelligence (as assessed by a Wechsler Test of Adult Reading; Wechsler, 2001), mood (assessed by a Positive and Negative Mood Scale; Watson Clark, & Tellegen, 1988), depression (assessed by a Geriatric Depression Scale; Yesavage, 1988), or health self-ratings were entered separately as covariates into the analyses of variance examining each driving-game dependent measure. In addition, all the age-by-stress-condition interactions remained significant (p < .05) when male and female data were analyzed separately.
DISCUSSION
This study reveals that stress can change older adults’ decision strategies. In the control condition, older adults risked driving during yellow lights for only a little less time than younger adults and performed better than younger adults in terms of final scores. This result is consistent with previous lab studies showing little or no decline in decision-making abilities with age (for a review, see Mather, 2006). In contrast, older adults in the stress condition not only risked significantly less driving time, but also stopped and restarted more frequently than those in the control condition. The age differences in the effects of stress were robust, occurring even when each subphase of the game was analyzed separately and when male and female data were analyzed separately. These findings highlight the need for more research on age differences in the impact of stress. In particular, further research is needed using different measures of risky decision making to see whether these age differences are specific to risky decisions made under time pressure (as in the current task) or also extend to other decision tasks.
Acknowledgments
We thank Corey Wasson for data collection assistance. This work was supported by National Institute on Aging Grant R21AG030758.
REFERENCES
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Mather M. A review of decision-making processes: Weighing the risks and benefits of aging. In: Carstensen LL, Hartel CR, editors. When I’m 64: Committee on aging frontiers in social psychology, personality, and adult developmental psychology. The National Academies Press; Washington, DC: 2006. pp. 145–173. [Google Scholar]
- Moghaddam B, Jackson M. Effect of stress on prefrontal cortex function. Neurotoxicity Research. 2004;6:73–78. doi: 10.1007/BF03033299. [DOI] [PubMed] [Google Scholar]
- Raz N. The aging brain: Structural changes and their implications for cognitive aging. In: Dixon RA, Backman L, Nilsson L-G, editors. New frontiers in cognitive aging. Oxford University Press; New York: 2004. pp. 115–133. [Google Scholar]
- Volkow ND, Logan J, Fowler JS, Wang G-J, Gur RC, Wong C, et al. Association between age-related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. American Journal of Psychiatry. 2000;157:75–80. doi: 10.1176/ajp.157.1.75. [DOI] [PubMed] [Google Scholar]
- Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, Detre JA. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proceedings of the National Academy of Sciences, USA. 2005;102:17804–17809. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler test of adult reading. Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Yesavage JA. Geriatric depression scale. Psychopharmacology Bulletin. 1988;24:709–711. [PubMed] [Google Scholar]