Abstract
Background
– While the role of inflammation in the pathophysiology of peripheral arterial disease (PAD) is well established, the contribution of insulin resistance to PAD is less clear. We hypothesized 1) that insulin resistance (IR) is associated with PAD, and 2) that the presence of IR would influence the association between C-reactive protein (CRP) and PAD, an association established predominantly in healthy individuals.
Methods and Results
We analyzed data from 3242 adults in NHANES 1999–2004 who underwent measurement of ankle brachial index (ABI), CRP, and fasting glucose and insulin, enabling calculation of HOMA-IR (homeostasis model of insulin resistance). Odds ratios and 95% confidence intervals were estimated by logistic regression. The mean (SE) prevalence of PAD (defined as an ABI ≤ 0.9) was 5.5% (0.47). HOMA-IR was independently associated with PAD (OR 2.06 [1.1–4.0], p=0.03 for quartile 4, p trend across quartiles = 0.047) after adjustment for age, gender, race/ethnicity, hypertension, hyperlipidemia, smoking, body mass index, chronic kidney disease, and CRP. Elevated CRP (>3mg/L) was also strongly associated with PAD (OR 2.2 [1.3–3.6], p=0.003 vs. CRP<1). Stratifying subjects based on median HOMA-IR, we found that CRP>3 was no longer significantly associated with PAD in subjects with insulin resistance (OR 1.3 [0.8–2.1], p=0.3) (p interaction=0.08).
Conclusion
– These findings demonstrate that insulin resistance is strongly and independently associated with PAD. Furthermore, insulin resistance modifies the association of inflammation with PAD. These data establish a role of insulin resistance in PAD and highlight the relative importance of inflammation in patients with and without insulin resistance.
Keywords: peripheral vascular disease, inflammation, insulin resistance
Introduction
Insulin resistance and inflammation have both been implicated in the development of atherosclerosis. The contribution of inflammation to atherogenesis has been demonstrated both in cellular and molecular investigations and confirmed in epidemiologic studies demonstrating an association of C-reactive protein (CRP, an inflammatory marker) with increased cardiovascular risk.1–3 Similarly, insulin resistance, increasingly appreciated as an important component of atherogenesis, is associated with clinical atherosclerosis in epidemiologic studies and predicts future cardiovascular events.4–8
Peripheral arterial disease (PAD) is an important manifestation of systemic atherosclerosis affecting an estimated 10 million Americans9 and is associated with significant limb morbidity and cardiovascular mortality.10 The role of insulin resistance in PAD is not well established, although several lines of evidence support a linkage. Insulin resistance contributes significantly to the development of diabetes mellitus, a known risk factor for PAD,10 and studies have shown that incident PAD may be associated with the metabolic syndrome11, 12 and glucose intolerance,13 both manifestations of insulin resistance. No prior study has evaluated the relationship between PAD and a direct measurement of insulin resistance. The homeostasis model of insulin resistance (HOMA-IR) is a simple measure of insulin resistance derived from fasting glucose and insulin values and correlates well with insulin sensitivity derived from the glucose clamp technique, the gold standard measure of insulin sensitivity.14 HOMA-IR has been shown to correlate with cardiovascular disease6–8 and cerebrovascular disease,15–18 but the relationship between HOMA-IR and PAD has not previously been established.
In contrast to the limited data on insulin resistance and PAD, several studies have shown that inflammatory markers, including CRP, are directly associated with PAD,19, 20 predict the initial development of PAD21, 22 and portend adverse outcomes in patients with established PAD.23, 24 However, the importance of CRP as a predictor of PAD and cardiovascular disease has been established predominantly in healthy individuals.1, 21 In subjects with diabetes or other established cardiovascular risk factors, the association between CRP and vascular disease is less clear. Indeed, conflicting data exist, with several studies demonstrating a significant attenuation of the association between CRP and myocardial infarction or stroke in subjects with diabetes,25, 26 although the data is not uniform.27 With these inconsistent findings and the accumulating evidence supporting the close inter-relationship of inflammation and insulin resistance,28–30 we hypothesized that the presence of insulin resistance might modify the association between CRP and PAD. Accordingly, we used the National Health and Nutrition Examination Survey (NHANES) from 1999–2004 1) to evaluate the association between HOMA-IR, a direct measure of insulin resistance, and PAD, and 2) to determine the influence of insulin resistance on the association of inflammation and PAD.
Methods
The National Health and Nutrition Examination Survey (NHANES) is a series of surveys of the non-institutionalized civilian population in the United States. Sampling is performed in a complex, stratified, multistage manner to provide nationally representative data in an effort to assess the health and nutritional status of adults and children in the United States. The 1999–2004 NHANES was reviewed and approved by the National Center for Health Statistics (NCHS) Institutional Review Board (IRB). Informed consent was obtained from all subjects.
Ankle Brachial Index Measurements
Beginning in 1999, adults aged 40 years and older were asked to participate in a lower extremity examination, including the ankle-brachial index (ABI), a diagnostic test for PAD with excellent performance characteristics (79–95% sensitivity and 95–100% specificity).10 Systolic pressure was measured in the supine position in the right arm (brachial artery) and in the posterior tibial artery of both ankles using an 8 MHz Doppler probe. In participants aged 40–59, blood pressures were measured twice at each site, whereas only once for participants 60 years and older. The ABI was calculated by dividing the systolic blood pressure in the ankle by the systolic blood pressure in the arm. We assigned a diagnosis of PAD if either leg had an ABI ≤0.90. Patients with ABI values >1.40 were excluded as these values may be falsely elevated due to severe vascular calcification.
Laboratory methods
Standard automated biochemical analysis was used to determine non-fasting serum glucose (Beckman Synchron LX20), and fasting glucose was measured using the enzyme hexokinase method. Fasting insulin was determined using the two-site immunoenzymometric assay (Tosoh AIA-PACK IRI) in years 2003–2004. For earlier years (1999–2002), the Pharmacia Insulin RIA kit was used for insulin measurement (Pharmacia Diagnostics AB, Uppsala, Sweden). Because these methods yield slightly different measurements, insulin values were converted as recommended in the analytic guidelines.31 High-sensitivity CRP was measured by latex-enhanced nephelometry. Serum creatinine and non-fasting cholesterol values were determined by automated biochemical profiling (Beckman Synchron LX20). Because of a change in assay, serum creatinine values from the 1999 and 2000 examinations were corrected as suggested in the laboratory documentation files. Fasting lipids were analyzed using the Hitachi 704 Analyzer (Roche Diagnostics). HOMA-IR was calculated as ([fasting glucose (mmol/L)]*[fasting insulin (μU/mL)])/22.5.32
Covariates
Race/ethnicity, gender and age were assessed by self-report. Race/ethnicity was categorized as non-Hispanic white, non-Hispanic black, Mexican-American, or other. Subjects were considered to have hypertension if they reported a physician diagnosis of hypertension, reported taking prescription medications for hypertension, or if systolic blood pressure was ≥ 140mmHg and/or diastolic blood pressure was ≥90mm Hg. These blood pressures were taken separately from the ABI examination and measured using a mercury sphygmomanometer. A diagnosis of hypercholesterolemia was assigned if the subject reported a physician diagnosis of hypercholesterolemia, reported taking prescription medications for hypercholesterolemia, or if the total cholesterol level was ≥ 6.21mmol/L (240mg/dL). Subjects were considered to have diabetes mellitus if the subject reported a physician diagnosis of diabetes, reported taking prescription medications for diabetes (either insulin or oral agents), if non-fasting plasma glucose was ≥11.1mmol/L (200mg/dL), or if fasting plasma glucose was ≥7mmol/L (126mg/dL). Active smokers were determined based on self-report (a positive answer to the question ‘do you now smoke cigarettes?’). Those who did not meet these criteria were considered former smokers if they answered yes to the question ‘have you smoked at least 100 cigarettes in your life?’ and never smokers if they had never smoked in their lifetime. Body mass index was calculated as weight (kg) divided by height (m2) (n=3198). We used the Modification of Diet in Renal Disease (MDRD) Study equation for estimating glomerular filtration rate (GFR) from serum creatinine (GFR (mL/min/1.73 m2) = 175 × (Scr)−1.154 × (Age)−0.203 × (0.742 if female) × (1.210 if African American).33 Subjects with an estimated GFR <60 mL/min/1.73 m2 were classified as having chronic kidney disease (CKD). Diagnosis of congestive heart failure, angina, heart attack, and stroke was made by self-report. Any cardiovascular disease was defined as prevalent myocardial infarction, heart failure, or stroke, based on a subject’s affirmative response to the question, “Has a doctor or other health professional ever told you that you have had a [heart attack/congestive heart failure/stroke]?” Subjects were categorized as having the metabolic syndrome based the National Cholesterol Education Program (NCEP) criteria if they had 3 of the 5 following criteria: HDL <1.04mmol/L (40mg/dL) (men) and <1.3mmol/L (50mg/dL) (women), waist circumference >102cm (men) or >88cm (women), triglycerides >1.7mmol/L (150mg/dL), fasting glucose >5.6mmol/L (100mg/dL), and elevated blood pressure (systolic >130mmHg or diastolic >85mmHg).
Derivation of Sample Population
Our analysis combined data from the NHANES 1999–2004 examinations. ABI measurements were available for 7571 subjects in NHANES 1999–2004, of which 113 were excluded on the basis of ABI>1.40. One-half of these participants, a representative sub-sample of the US population, were asked to attend a morning session where fasting blood work was collected. Only individuals who had fasted at least 8–24 hours were assigned a non-zero fasting weight and thus were included in our sample population.31 This left 3499 with glucose and insulin values, enabling calculation of HOMA-IR. We excluded subjects who were taking insulin (n=80) given the limitations of HOMA-IR measurements in this subpopulation.34 After excluding subjects who did not have complete information available on other covariates of interest, there were 3242 subjects remaining that constituted the sample population for this analysis.
Statistical Methods
NHANES uses a complex, multistage, probability-sampling design to select participants representative of the civilian, non-institutionalized US population. Given that our analysis was limited to the representative sub-sample of individuals with fasting blood work, we used the fasting sample weights to account for the sampling design. Subjects were included in our analysis only if they had fasted a minimum of 8 hours and had been assigned a non-zero fasting weight. We generated a combined 6-year fasting weight variable by assigning 2/3 of the 4-year weight for 1999–2002 if the subject participated during 1999–2002 or assigning 1/3 of the 2-year weight for 2003–2004 if the subject participated during 2003–2004.
Analyses were performed with SAS version 9.1 (SAS Institute Inc.) callable SUDAAN version 9.01 (Research Triangle Institute) to account for the complex sample design. Age- and gender-adjusted baseline subject characteristics are reported as the weighted mean and standard error (SE) or the weighted percentile and SE. Categorical variables were compared using the chi-squared test or logistic regression. Comparison of mean values across groups and correlations between continuous variables were achieved by linear regression. Odds ratios and 95% confidence intervals were estimated by logistic regression. We divided HOMA-IR values into quartiles and examined the presence of a linear trend across quartiles. We further evaluated the odds of PAD in each quartile compared to the reference group, HOMA-IR quartile 1. In a multivariable model, we then adjusted for age, gender, race/ethnicity, hypertension, hyperlipidemia, smoking, body mass index, chronic kidney disease, and CRP. A p-value of <0.05 was considered statistically significant except for interaction terms for which a p-value of <0.10 was considered statistically significance.
In order to assess whether the presence of insulin resistance influenced the relationship between CRP and PAD, we stratified the population by median HOMA-IR level to indicate the presence or absence of insulin resistance and re-evaluated the relationship between CRP and PAD in these two categories. Given the lack of established threshold values of HOMA-IR to indicate insulin resistance, we used the median HOMA-IR level as a threshold value in our analysis. A formal test for interaction was also performed to evaluate the impact of insulin resistance on the association between CRP and PAD.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Of the 3242 subjects in our sample population, there were 256 participants with an ABI ≤0.90, for an overall unadjusted weighted prevalence (mean (SE)) of PAD of 5.5% (0.47). Age- and gender-adjusted baseline characteristics of participants with and without PAD are summarized in table 1. Subjects with PAD had a significantly higher mean age (66.4 (1.1) years vs. 55.5 (0.3) years, p<0.0001) and a higher prevalence of hypertension, coronary heart disease, and chronic kidney disease. An elevated CRP (>3 mg/L) was noted in a significantly higher proportion of PAD subjects (54.6% (5.0) vs. 40.3% (1.0)) and mean CRP levels were significantly higher in PAD subjects (7.5 (1.3) vs. 4.4 (0.2) mg/dl, p=0.02). There were no significant differences in gender distribution or body mass index.
Table 1.
Age- and Gender-Adjusted Subject Characteristics
| PAD present | PAD absent | p-value | |
|---|---|---|---|
| N | 256 | 2986 | |
| Male gender | 43.1 (3.3) | 48.7 (0.9) | 0.12 |
| Age group | |||
| 40–49 years | 11.7 (3.5) | 38.0 (1.5) | <0.0001 |
| 50–59 years | 16.3 (2.9) | 29.4 (1.2) | |
| 60–69 years | 27.1 (2.9) | 18.6 (0.9) | |
| 70 years or greater | 45.0 (3.8) | 14.5 (0.7) | |
| Race/ethnicity | |||
| White | 77.0 (3.6) | 78.5 (1.8) | 0.02 |
| Black | 12.3 (2.5) | 8.5 (0.9) | |
| Mexican American | 3.1 (1.0) | 4.6 (0.7) | |
| Other | 7.6 (2.9) | 8.4 (1.2) | |
| Smoking status | |||
| Never | 27.1 (3.7) | 46.7 (1.4) | 0.0001 |
| Former | 31.2 (3.0) | 34.3 (1.2) | |
| Current | 41.7 (4.4) | 19.0 (1.3) | |
| Hypertension | 65.1 (4.6) | 48.5 (1.3) | 0.03 |
| Hypercholesterolemia | 54.4 (4.2) | 47.7 (1.3) | 0.3 |
| Diabetes | 26.4 (2.9) | 13.1 (0.9) | 0.06 |
| Coronary heart disease | 8.9 (2.1) | 5.3 (0.4) | 0.007 |
| Stroke | 8.1 (4.0) | 3.5 (0.4) | 0.14 |
| Chronic kidney disease | 18.1 (2.7) | 10.8 (0.7) | 0.007 |
| Body mass index (kg/m2), mean | 29.3 (0.8) | 28.3 (0.2) | 0.8 |
| HOMA-IR, mean | 2.7 (0.1) | 3.4 (0.3) | 0.016 |
| CRP>3 (mg/L) | 54.6 (5.0) | 40.3 (1.0) | 0.03 |
Chi square test was used for statistical comparisons for gender, age and race/ethnicity. All remaining prevalence estimates and mean values are adjusted for age and gender (except for gender, age and race/ethnicity). For categorical variables, data are shown as weighted percent (%) and standard error (SE), and logistic regression adjusting for age and gender was used for statistical comparison. For continuous variables, data are shown as mean and standard error (SE) and statistical comparisons are achieved by linear regression, adjusting for age and gender. HOMA-IR was log-transformed for comparison. PAD=peripheral arterial disease, CRP=C-reactive protein.
Association of HOMA-IR and PAD
Age- and gender-adjusted baseline characteristics of subjects in each HOMA-IR quartile are shown in table 2. Subjects in the higher HOMA-IR quartiles were less likely to be female or of non-Hispanic white race. Cardiovascular risk factors, including hypertension, hyperlipidemia and diabetes, were significantly more prevalent in increasing HOMA-IR quartiles. A history of smoking (current or former) and the presence of CKD were not different in the groups. HOMA-IR correlated significantly with several baseline variables, including BMI (r=0.45, p<0.0001), HDL (r=−0.32, p<0.0001), triglycerides (r=0.22, p<0.0001) and CRP (r=0.12, p=0.0002). There was no correlation between HOMA-IR and LDL (r=0.03, p=0.05) or total cholesterol (r=0.01, p=NS).
Table 2.
Age- and Gender-Adjusted Characteristics by HOMA-IR Quartile
| HOMA-IR Quartile |
|||||
|---|---|---|---|---|---|
| Q1 (<1.08) |
Q2 (1.08–1.86) |
Q3 (1.86–3.34) |
Q4 (>3.34) |
p-value | |
| N | 722 | 802 | 827 | 891 | |
| Age, years | 54.8 (0.6) | 56.1 (0.6) | 57.0 (0.4) | 56.4 (0.6) | 0.04 |
| Female gender | 63.8 (1.7) | 53.0 (2.1) | 48.2 (2.1) | 41.6 (2.0) | <0.0001 |
| White race | 82.8 (1.5) | 78.8 (2.5) | 79.0 (2.2) | 72.9 (2.5) | 0.0006 |
| Diabetes | 2.5 (0.6) | 5.4 (1.0) | 10.8 (1.4) | 33.8 (2.6) | <0.0001 |
| Hypertension | 40.1 (2.1) | 42.1 (2.1) | 53.6 (2.4) | 61.8 (1.9) | <0.0001 |
| Hypercholesterolemia | 36.7 (2.1) | 48.2 (2.1) | 53.9 (2.2) | 53.6 (2.2) | <0.0001 |
| Smoking (current or former) | 54.8 (2.6) | 51.9 (2.3) | 52.9 (2.7) | 54.7 (2.5) | 0.73 |
| Chronic kidney disease | 11.0 (1.2) | 12.2 (1.5) | 13.2 (1.4) | 10.2 (1.1) | 0.63 |
| Coronary heart disease | 3.0 (0.8) | 4.2 (0.8) | 8.6 (0.9) | 5.8 (0.7) | 0.0003 |
| Stroke | 2.3 (0.7) | 3.2 (0.7) | 5.3 (1.0) | 4.1 (0.7) | 0.016 |
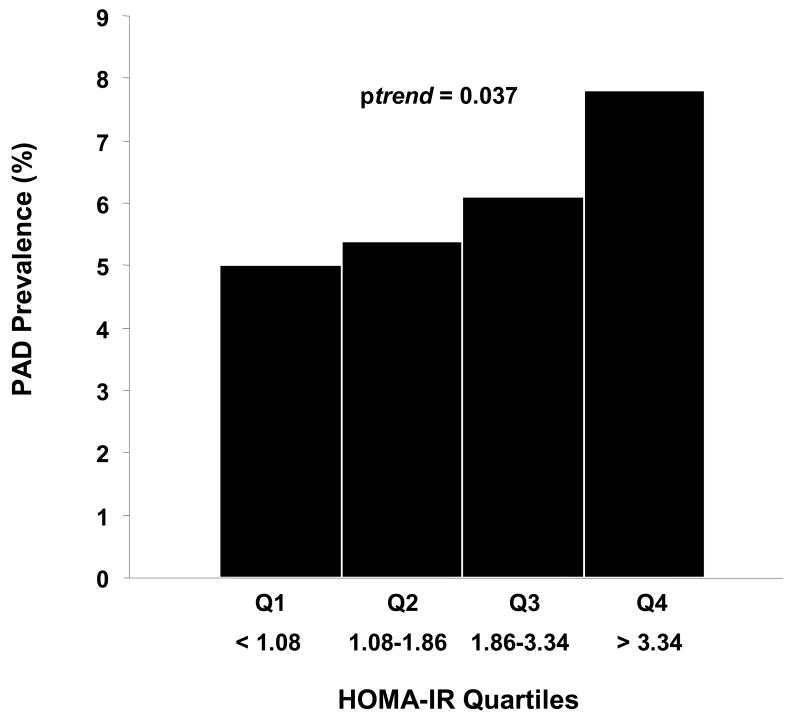
| PAD prevalence | 5.0 (0.8) | 5.4 (0.9) | 6.1 (0.7) | 7.8 (1.1) | 0.037 |
| Any cardiovascular disease | 10.2 (1.3) | 13.3 (1.5) | 18.8 (1.2) | 19.6 (1.4) | <0.0001 |
| Metabolic syndrome | 12.1 (1.2) | 29.6 (2.3) | 58.1 (2.4) | 83.4 (1.5) | <0.0001 |
| C-reactive protein, mg/L | 3.6 (0.3) | 4.0 (0.4) | 4.6 (0.2) | 6.6 (0.5) | 0.0004 |
| Body mass index, kg/m2 | 24.3 (0.2) | 26.9 (0.2) | 29.7 (0.2) | 32.7 (0.3) | <0.0001 |
| HDL cholesterol, mg/dL | 62.2 (0.7) | 55.2 (0.7) | 51.0 (0.6) | 44.7 (0.5) | <0.0001 |
| Triglycerides, mg/dL | 111.8 (2.1) | 141.7 (6.8) | 171.8 (7.4) | 214.6 (8.1) | <0.0001 |
| LDL cholesterol, mg/dL | 121.0 (1.6) | 129.7 (1.5) | 128.6 (1.7) | 125.5 (1.7) | 0.0003 |
| Total cholesterol, mg/dL | 205.1 (1.7) | 212.6 (1.9) | 212.5 (1.9) | 210.1 (2.0) | 0.013 |
| Fasting glucose, mg/dL | 92.2 (0.5) | 98.7 (0.6) | 104.7 (0.9) | 124.0 (1.9) | <0.0001 |
| Fasting insulin, IU/mL | 3.1 (0.1) | 6.1 (0.1) | 9.9 (0.1) | 20.2 (0.5) | <0.0001 |
Prevalence estimates and mean values are standardized to age and gender (except for gender, age and race/ethnicity). For categorical variables, data are shown as weighted percent (%) and standard error (SE), and logistic regression adjusting for age and gender was used for statistical comparison across groups. For continuous variables, data are shown as mean and standard error (SE) and statistical comparisons are achieved by linear regression, adjusting for age and gender. PAD=peripheral arterial disease, HDL=high-density lipoprotein, LDL=low-density lipoprotein
Age- and gender- adjusted PAD prevalence estimates increased in a graded fashion in increasing HOMA-IR quartiles, 5.0% (0.8) in quartile 1, 5.4% (0.9) in quartile 2, 6.1% (0.7) in quartile 3, and 7.8% (1.1) in quartile 4 (chi square p-value 0.037) (Figure 1). Compared with subjects in the lowest HOMA-IR quartile, subjects in increasing quartiles had a graded increase in odds of PAD: OR 1.24 [0.8–2.0] for quartile 2, OR 1.53 [0.96–2.4] for quartile 3, and OR 1.88 [1.2–3.0] for quartile 4 (p trend 0.009) (table 3, unadjusted).
Figure 1.
Age- and gender- standardized PAD prevalence (%) in increasing HOMA-IR quartiles. There is a graded increase in PAD prevalence in increasing HOMA-IR quartiles (p=0.037 by chi-square test). PAD=peripheral arterial disease, HOMA-IR=homeostasis model of insulin resistance.
Table 3.
Multivariable logistic regression analysis of the association of HOMA-IR and PAD
| Quartile 2 |
Quartile 3 |
Quartile 4 |
p trend |
||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Unadjusted | 1.24 | 0.8–2.0 | 1.53 | 0.96–2.4 | 1.88 | 1.2–3.0 | 0.009 |
| Model 1 | 1.20 | 0.8–1.9 | 1.40 | 0.9–2.3 | 1.84 | 1.2–2.9 | 0.015 |
| Model 2 | 1.39 | 0.8–2.3 | 1.59 | 0.8–3.0 | 2.08 | 1.1–3.9 | 0.039 |
| Model 3 | 1.35 | 0.8–2.3 | 1.57 | 0.8–3.0 | 2.06 | 1.1–4.0 | 0.047 |
| Model 3 + diabetes | 1.34 | 0.8–2.3 | 1.55 | 0.8–2.9 | 1.96 | 1.03–3.8 | 0.058 |
| Model 3 + HbA1c | 1.36 | 0.8–2.3 | 1.59 | 0.8–3.0 | 2.14 | 1.06–4.3 | 0.047 |
Odds ratios (OR) and 95% confidence intervals (CI) were estimated by logistic regression using quartile 1 as the reference population. Data were also analyzed for the presence of a linear trend across quartiles (p trend). Model 1 adjusts for age, gender, and race/ethnicity; model 2 additionally adjusts for hypertension, hyperlipidemia, smoking, and BMI; model 3 additionally adjusts for CKD and CRP. HbA1c = Glycosylated hemoglobin.
In a multivariable model adjusting for age, gender, and race/ethnicity (table 3, model 1), HOMA-IR remained associated with PAD with increasing odds of PAD across HOMA-IR quartiles: OR 1.19 [0.8–1.9] for quartile 2, OR 1.4 [0.9–2.3] for quartile 3, and OR 1.83 [1.2–2.9] for quartile 4 compared to subjects in HOMA-IR quartile 1 (p trend 0.015). In further analyses also adjusting for traditional atherosclerotic risk factors (hypertension, hyperlipidemia and smoking) and BMI (table 3, model 2), the linear association between HOMA-IR quartiles and PAD persisted (p trend 0.039) and quartile 4 had a more than 2-fold increased odds of PAD (OR 2.07 [1.1–3.9]) compared to quartile 1. Finally, the magnitude and direction of the association between HOMA-IR and PAD persisted after additionally adjusting for non-traditional factors related to PAD, including chronic kidney disease and CRP (p trend 0.047) and subjects in quartile 4 had an OR 2.06 [1.1–4.0] compared to those in quartile 1 (table 3, model 3). Additional adjustment for diabetes resulted in a marginal attenuation of the association of insulin resistance with PAD (p trend 0.058), though subjects in the highest HOMA-IR quartile continued to have an approximately 2-fold increase in the odds of PAD (OR 1.96 [1.03–3.8]). Adjusting for hemoglobin A1c as a measure of recent glycemic control instead of diabetes, the relationship remained statistically significant (p trend 0.047) (table 3).
These findings were unchanged when HOMA-IR was used as a continuous variable (after log transformation to improve normality). Using a multivariable model accounting for age, gender, race/ethnicity, hypertension, hyperlipidemia, smoking, BMI, CKD, and CRP, we found that a 1 unit increase in log-HOMA conferred a 33% increased odds of PAD (OR 1.33 [1.03–1.70], p=0.029). This relationship persisted even after additional adjustment for diabetes (OR 1.30 [1.0–1.67], p=0.047) or hemoglobin A1c (OR 1.36 [1.02–1.8], p=0.036).
Given the recognized relationship between insulin resistance and diabetes, a well-established risk factor for PAD, we repeated our analyses excluding subjects with diabetes in order to evaluate the association of HOMA-IR with PAD independent of the effect of diabetes. We excluded all subjects with diabetes (n=485, including those with self-report of diabetes, those taking insulin or oral agents, and subjects with a fasting blood glucose level >126 mg/dL or non-fasting levels >200 mg/dL), leaving 2757 subjects remaining for analysis. In this non-diabetic sample population, there remained a graded increase in PAD prevalence across increasing HOMA-IR quartiles, with prevalence estimates (and SE) of 3.8% (0.69), 4.7% (0.94), 5.7% (0.79), and 6.1% (1.38) across the four quartiles, respectively (chi-square p-value=0.24). In univariate logistic regression analysis, a one-quartile increase in HOMA-IR was associated with an 18% increased odds of PAD (OR 1.18 [0.98–1.42]). Additionally, compared to subjects in the lowest HOMA-IR quartile (in an unadjusted model), subjects in increasing quartiles maintained a graded increase in odds of PAD, OR 1.24 [0.8–1.97] for quartile 2, OR 1.52 [0.92–2.5] for quartile 3, and OR 1.62 [0.9–2.90] for quartile 4. As expected, exclusion of nearly 500 individuals from our sample population attenuated the associations between HOMA-IR and PAD; nonetheless a graded increase in PAD prevalence and graded increases in the odds of PAD by increasing HOMA-IR quartiles was still observed.
Association of CRP and PAD
In order to evaluate the relationship between CRP and PAD, CRP was first categorized as low (<1mg/L), intermediate (1–3mg/L), or high (>3mg/L) according to published American Heart Association/Centers for Disease Control guidelines.35 There was a significant graded increase in PAD prevalence according to CRP category, with prevalence rates of 3.3% (0.7), 5.5% (0.8), and 6.8% (0.8) for CRP levels of <1, 1–3, and >3mg/L, respectively (chi squared p=0.003). Compared to subjects with a low CRP (<1mg/L), subjects with a high CRP (>3mg/L) had a significantly increased odds of PAD with an OR 2.2 [1.3–3.6], p=0.003. Adjusting for age, gender, race/ethnicity, hypertension, hyperlipidemia, smoking status, and CKD, we found a significant association between CRP and PAD (OR 1.02 [1.0–1.03], p=0.014, for a 1 mg/L change).
Influence of insulin resistance on the association of CRP and PAD
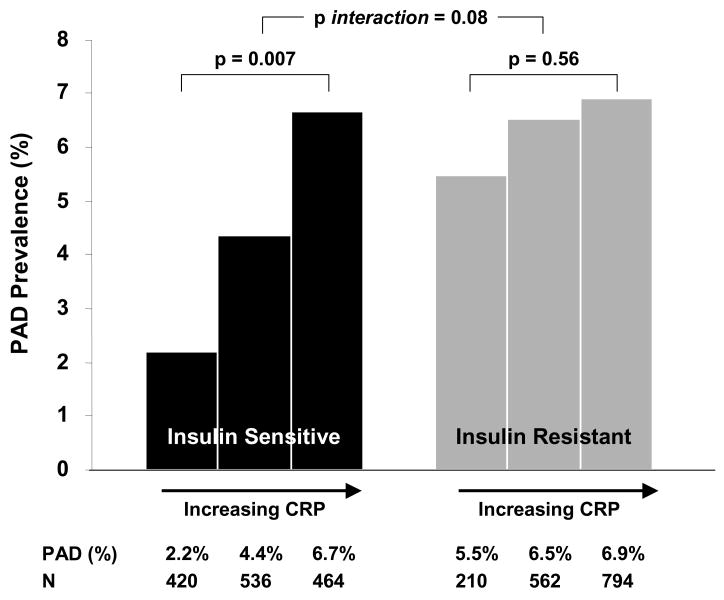
Given previous conflicting data suggesting that the association of inflammation with atherosclerosis may be altered in states of insulin resistance,25, 26 we examined whether the association of CRP with PAD is modified by the presence of insulin resistance. We analyzed the association of CRP with PAD in analyses stratified by median HOMA-IR level (1.86). Among subjects without insulin resistance, there was a graded increase in PAD prevalence among increasing CRP categories, with prevalence estimates of 2.2% (1.0), 4.4% (0.9), and 6.7% (1.1) for CRP levels of <1, 1–3, and >3mg/L, respectively (chi squared p=0.007) (figure 2). Expressed as an odds ratio, CRP>3 significantly increased the likelihood of PAD compared to a CRP<1 (OR 3.2, 95% CI 1.2–8.4, p=0.02) in subjects without insulin resistance. However, the association was markedly blunted in subjects with insulin resistance. Subjects with insulin resistance but with low CRP (<1) had a notably higher prevalence of PAD compared to subjects with low CRP without insulin resistance (5.5% (0.8) vs. 2.2% (1.0), p=0.019). In the insulin resistant group, PAD prevalence rates increased slightly but not significantly so, from 5.5% (0.8) to 6.5% (1.1) and 6.9% (1.0) in increasing CRP categories (chi squared p=0.56) (figure 2). CRP was no longer significantly associated with PAD in subjects with insulin resistance (OR 1.3, 95% CI 0.8–2.1, p=0.31). We further confirmed that the association of CRP and PAD was significantly different in insulin sensitive and insulin resistant subjects (p-value for interaction term = 0.08).
Figure 2.
Unadjusted PAD prevalence (%) in increasing CRP categories (<1, 1–3, and >3mg/L) in groups stratified by absence or presence of insulin resistance (defined as having a HOMA-IR below or above the median value of 1.86, respectively). In the absence of insulin resistance, there is a robust relationship between increasing CRP category and prevalence of PAD, with a PAD prevalence of 2.2%, 4.4% and 6.7% in increasing CRP categories (p=0.007). However, in the presence of insulin resistance, the relationship between CRP and PAD is significantly blunted with PAD prevalence rates of 5.5%, 6.5% and 6.9% (p=0.56). IR=insulin resistance; CRP=C-reactive protein. P-values are generated by chi-square test.
Discussion
We examined the interrelationships between inflammation, insulin resistance and PAD in this nationally representative sample population and found 1) that insulin resistance, indicated by HOMA-IR, is strongly associated with PAD and 2) that the presence of insulin resistance attenuates the association between CRP and PAD. Furthermore, this is the first study to illustrate the association of PAD and a direct measure of insulin resistance, HOMA-IR, a simple method that uses plasma insulin and glucose in a single fasting blood sample. HOMA-IR has previously only been reported in relation to cardiovascular6–8 and cerebrovascular disease.15–18 Indeed, we found a roughly 25% increased odds of PAD for each one-quartile increase in HOMA-IR, a finding that remained consistent despite adjustment for typical atherosclerotic risk factors, factors related to insulin resistance (i.e., body mass index), and glycemic control (hemoglobin A1c). In addition, although the trend across quartiles was marginally no longer statistically significant after adjusting for diabetes, the point estimates were essentially unchanged and subjects in the highest HOMA-IR quartile continued to have a nearly 2-fold increased odds of PAD. Using HOMA-IR as a continuous variable, we found a strong relationship between PAD and insulin resistance that persisted even after additional adjustment for diabetes or hemoglobin A1c. These data suggest that insulin resistance may have an association with peripheral arterial disease along the entire spectrum of insulin resistance and distinct from the impact of diabetes. Even after excluding subjects with diabetes, there were graded increases in PAD prevalence with increasing HOMA-IR quartiles, although the associations between HOMA-IR and PAD were no longer statistically significant. Our data support prior observations that PAD is associated with the metabolic syndrome11, 12, 36 and glucose intolerance13, both surrogate markers of insulin resistance.
In contrast to the limited data on the association of insulin resistance and PAD, the link between inflammation and PAD has been well established. Our demonstration of a strong association between CRP and PAD is consistent with prior epidemiologic data and prospective studies linking inflammation with atherosclerosis in the coronary and peripheral arterial beds1–3, 19–23, 37. However, much of the data on inflammation and atherosclerosis has been generated in healthy individuals. Less clear is whether this relationship persists in individuals with established cardiovascular risk factors, such as diabetes. Sakkinen et al. studied the relationship between CRP and myocardial infarction (MI) over a 20 year period and although they found a positive relationship between CRP and MI in the overall population, the association was abolished in individuals with diabetes.26 The same group also showed no significant relationship between CRP and stroke among patients with diabetes or hypertension.25 Prospective data from the Strong Heart Study in an American Indian population found no predictive value of CRP for incident cardiovascular events in diabetic individuals.38 In contrast, the Women’s Health Study2 found that CRP added prognostic information regarding cardiovascular events even in subjects with the metabolic syndrome, and a study by Schulze et al.27 found a strong relationship between CRP and cardiovascular events among men with diabetes. However, none of these studies focused specifically on peripheral arterial disease, and none directly examined the role of insulin resistance.
Given the conflicting data on the relationship of CRP and vascular disease among subjects with diabetes and metabolic syndrome, we explored the possibility that insulin resistance in particular might influence the relationship between CRP and PAD. Indeed, our analysis found a strong association between CRP and PAD among individuals who were insulin sensitive. However, this association was no longer evident in subjects with insulin resistance.
The attenuation of the relationship between CRP and PAD in the presence of insulin resistance may be explained in part by the close inter-relationship of inflammation and insulin resistance in vascular disease.28–30, 39 Extensive experimental data demonstrate that inflammation leads to impaired insulin metabolic signaling resulting in insulin resistance.39 Insulin resistance may in turn exacerbate inflammation via increased cytokine and adipo-chemokine expression (including tumor necrosis factor alpha (TNF-α), interleukin-6, leptin, monocyte chemoattractant protein-1 (MCP-1), plasminogen activator inhibitor-1 (PAI-1) and others), elevation of free fatty acid levels, and impaired endothelial nitric oxide synthase activity.39, 40 The reciprocal interaction of inflammation and insulin resistance creates a cycle of further increasing cardiovascular risk.
These findings also support the notion that atherogenesis results from the complex interplay of multiple atherosclerotic risk factors, each of variable influence in different vascular beds. In individuals with few risk factors, inflammation may have a relatively large contribution to the development of atherosclerosis. In contrast, in the presence of a potent cardiovascular risk factor, insulin resistance, the relative contribution of inflammation to the pathogenesis of atherosclerosis may be diminished. In our study, subjects with insulin resistance but without inflammation (CRP <1 mg/L) already had a high prevalence of PAD (5.5%) compared to those who were insulin sensitive and without inflammation (2.2%). Among insulin resistant subjects, the presence of inflammation then resulted in only a marginal increase in PAD prevalence (to 6.9% in the highest CRP category) that was not statistically significant. Whether these data imply that insulin resistance reduces the association of inflammation and PAD by a distinct effect on vascular function or simply by increasing inflammation, these data highlight the complexity of the interaction of these risk factors in PAD and suggest that the role of inflammation in PAD may be modified in individuals with insulin resistance.
Additionally, although there are common risk factors for the development of vascular disease, the impact of specific risk factors on the development of disease is not the same in the peripheral vascular bed as in the coronary or cerebrovascular circulations.41 Indeed, cigarette smoking, one of the strongest risk factors for the development of PAD, was shown in the Edinburgh Artery Study42 to have a greater impact on PAD (OR 1.8–5.6 for the risk of PAD in smokers vs. non-smokers) than on coronary artery disease (OR 1.1–1.6). In contrast, hypertension and hyperlipidemia, potent risk factors for coronary atherosclerosis, appear to have less impact on the development of PAD with a 10 mg/dL increase in total cholesterol conferring only a 10% increased risk of PAD.41 These findings suggest that individual risk factors may have varying impact in different vascular beds, and as such, a focused analysis of the impact of insulin resistance on PAD is warranted and may shed light on the differential pathophysiology of PAD compared to CAD and cerebrovascular disease.
These pathophysiologic differences may in part explain the differences observed between our results and the findings of the Women’s Health Study and a study by Schulze et al. which suggest that CRP adds prognostic information regarding incident cardiovascular disease in subjects with the metabolic syndrome2 or diabetes27 at baseline. Our results also stand in contrast to a cross-sectional study from Vu et al.43 in a smaller NHANES cohort suggesting that CRP remains associated with PAD even in patients with metabolic syndrome or diabetes, although not in those with established cardiovascular disease. The discrepancy is explained in part by differences in statistical methodology (the authors used the group with low CRP and no established disease as the reference population for all comparisons), and use of metabolic syndrome as opposed to a direct measure of insulin resistance.
These data are derived from a nationally representative cohort and therefore are generalizable to the US adult population. The ABI method has been demonstrated to have excellent sensitivity and specificity for the diagnosis of PAD.10 Despite these strengths, potential limitations of the present study merit consideration. In NHANES, the ABI was calculated using the blood pressure in only one arm, raising the potential for misclassification by not excluding the possibility of subclavian stenosis. Additionally, our analysis was limited to the subset of participants with ABI measurements who had a fasting blood sample drawn, reducing our sample size and potentially limiting power. However, our final sample population was nonetheless substantial, consisting of more than 3200 individuals in whom there were 256 cases of PAD. We are also limited by the lack of established threshold values of HOMA-IR for indicating presence or absence of insulin resistance. Prior studies have used quartiles8 or quintiles,6 and given the lack established guidelines, we elected to use standard median and quartile cut-points in our analysis.
Additionally, there are notable differences in clinical variables by HOMA-IR quartile; even with multivariable models, there may be confounding that may not be adequately accounted for in our statistical models. Finally, the cross-sectional nature of our study does not allow us to draw conclusions regarding causality, and prospective studies would be required to clarify any temporal relationship and to delineate the true causal relationships between inflammation, insulin resistance, and PAD.
In summary, insulin resistance is strongly and independently associated with PAD. The presence of insulin resistance attenuates the association of inflammation with PAD. These data establish a role of insulin resistance in PAD and suggest that future studies are warranted to better understand the complex interplay of inflammation and insulin resistance in peripheral arterial disease.
Acknowledgments
Sources of Funding
Dr. Creager is the Simon C. Fireman Scholar in Cardiovascular Medicine. Dr. Pande was supported by a Research Career Development Award (K12 HL083786) from the National Heart, Lung, and Blood Institute (NHLBI) and by NHLBI training grant T32 HL07604. Dr. Perlstein was supported by an American College of Cardiology Foundation / Merck Research Fellowship Award training grant and by NHLBI training grant T32 HL07604. Dr. Beckman was supported by American Diabetes Association career development award 1-06-CD-01. This work was also supported by a grant from the NHLBI (R01 HL075771).
Footnotes
Disclaimer: The manuscript and its contents are confidential, intended for journal review purposes only, and not to be further disclosed.
Author Disclosures
Reena L. Pande: No disclosures
Todd S. Perlstein: No disclosures
Joshua A. Beckman: No disclosures
Mark A. Creager: No disclosures
Journal Subject Head: [17] Peripheral vascular disease, [8] Epidemiology, [135] Atherosclerosis Risk Factors
Disclosures:
None
References
- 1.Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000 Mar 23;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Buring JE, Cook NR, et al. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003 Jan 28;107(3):391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 3.Tracy RP, Lemaitre RN, Psaty BM, et al. Relationship of C-reactive protein to risk of cardiovascular disease in the elderly. Results from the Cardiovascular Health Study and the Rural Health Promotion Project. Arterioscler Thromb Vasc Biol. 1997 Jun;17(6):1121–1127. doi: 10.1161/01.atv.17.6.1121. [DOI] [PubMed] [Google Scholar]
- 4.Howard G, O’Leary DH, Zaccaro D, et al. Insulin sensitivity and atherosclerosis. The Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Circulation. 1996 May 15;93(10):1809–1817. doi: 10.1161/01.cir.93.10.1809. [DOI] [PubMed] [Google Scholar]
- 5.Hedblad B, Nilsson P, Engstrom G, et al. Insulin resistance in non-diabetic subjects is associated with increased incidence of myocardial infarction and death. Diabet Med. 2002 Jun;19(6):470–475. doi: 10.1046/j.1464-5491.2002.00719.x. [DOI] [PubMed] [Google Scholar]
- 6.Hanley AJ, Williams K, Stern MP, et al. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes Care. 2002 Jul;25(7):1177–1184. doi: 10.2337/diacare.25.7.1177. [DOI] [PubMed] [Google Scholar]
- 7.Bonora E, Formentini G, Calcaterra F, et al. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabetes Care. 2002 Jul;25(7):1135–1141. doi: 10.2337/diacare.25.7.1135. [DOI] [PubMed] [Google Scholar]
- 8.Bonora E, Kiechl S, Willeit J, et al. Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in caucasian subjects from the general population: the Bruneck study. Diabetes Care. 2007 Feb;30(2):318–324. doi: 10.2337/dc06-0919. [DOI] [PubMed] [Google Scholar]
- 9.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007 Jan;45(1 Suppl):S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic) Circulation. 2006 Mar 21;113(11):e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 11.Brevetti G, Schiano V, Sirico G, et al. Metabolic syndrome in peripheral arterial disease: relationship with severity of peripheral circulatory insufficiency, inflammatory status, and cardiovascular comorbidity. J Vasc Surg. 2006 Jul;44(1):101–107. doi: 10.1016/j.jvs.2006.02.048. discussion 107. [DOI] [PubMed] [Google Scholar]
- 12.Rana JS, Jansen AC, Zwinderman AH, et al. Metabolic syndrome and risk of coronary, cerebral, and peripheral vascular disease in a large Dutch population with familial hypercholesterolemia. Diabetes Care. 2006 May;29(5):1125–1127. doi: 10.2337/diacare.2951125. [DOI] [PubMed] [Google Scholar]
- 13.Tapp RJ, Balkau B, Shaw JE, et al. Association of glucose metabolism, smoking and cardiovascular risk factors with incident peripheral arterial disease: the DESIR study. Atherosclerosis. 2007 Jan;190(1):84–89. doi: 10.1016/j.atherosclerosis.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000 Jan;23(1):57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad J, Ahmed F, Siddiqui MA, et al. Inflammation, insulin resistance and carotid IMT in first degree relatives of north Indian type 2 diabetic subjects. Diabetes Res Clin Pract. 2006 Aug;73(2):205–210. doi: 10.1016/j.diabres.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Atabek ME, Pirgon O, Kivrak AS. Evidence for association between insulin resistance and premature carotid atherosclerosis in childhood obesity. Pediatr Res. 2007 Mar;61(3):345–349. doi: 10.1203/pdr.0b013e318030d206. [DOI] [PubMed] [Google Scholar]
- 17.Hidvegi T, Szatmari F, Hetyesi K, et al. Intima-media thickness of the carotid arteries in subjects with hyperinsulinaemia (insulin resistance) Diabetes Nutr Metab. 2003 Jun;16(3):139–144. [PubMed] [Google Scholar]
- 18.Yang B, Li TD, Wang JS, et al. Insulin resistance and carotid atherosclerosis in 221 patients with potential hyperglycemia. Chin Med Sci J. 2005 Jun;20(2):108–111. [PubMed] [Google Scholar]
- 19.Wildman RP, Muntner P, Chen J, et al. Relation of inflammation to peripheral arterial disease in the national health and nutrition examination survey, 1999–2002. Am J Cardiol. 2005 Dec 1;96(11):1579–1583. doi: 10.1016/j.amjcard.2005.07.067. [DOI] [PubMed] [Google Scholar]
- 20.McDermott MM, Guralnik JM, Corsi A, et al. Patterns of inflammation associated with peripheral arterial disease: the InCHIANTI study. Am Heart J. 2005 Aug;150(2):276–281. doi: 10.1016/j.ahj.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM, Cushman M, Stampfer MJ, et al. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998 Feb 10;97(5):425–428. doi: 10.1161/01.cir.97.5.425. [DOI] [PubMed] [Google Scholar]
- 22.Pradhan AD, Rifai N, Ridker PM. Soluble intercellular adhesion molecule-1, soluble vascular adhesion molecule-1, and the development of symptomatic peripheral arterial disease in men. Circulation. 2002 Aug 13;106(7):820–825. doi: 10.1161/01.cir.0000025636.03561.ee. [DOI] [PubMed] [Google Scholar]
- 23.Owens CD, Ridker PM, Belkin M, et al. Elevated C-reactive protein levels are associated with postoperative events in patients undergoing lower extremity vein bypass surgery. J Vasc Surg. 2007 Jan;45(1):2–9. doi: 10.1016/j.jvs.2006.08.048. discussion 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beckman JA, Preis O, Ridker PM, et al. Comparison of usefulness of inflammatory markers in patients with versus without peripheral arterial disease in predicting adverse cardiovascular outcomes (myocardial infarction, stroke, and death) Am J Cardiol. 2005 Nov 15;96(10):1374–1378. doi: 10.1016/j.amjcard.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 25.Curb JD, Abbott RD, Rodriguez BL, et al. C-reactive protein and the future risk of thromboembolic stroke in healthy men. Circulation. 2003 Apr 22;107(15):2016–2020. doi: 10.1161/01.CIR.0000065228.20100.F7. [DOI] [PubMed] [Google Scholar]
- 26.Sakkinen P, Abbott RD, Curb JD, et al. C-reactive protein and myocardial infarction. J Clin Epidemiol. 2002 May;55(5):445–451. doi: 10.1016/s0895-4356(01)00502-9. [DOI] [PubMed] [Google Scholar]
- 27.Schulze MB, Rimm EB, Li T, et al. C-reactive protein and incident cardiovascular events among men with diabetes. Diabetes Care. 2004 Apr;27(4):889–894. doi: 10.2337/diacare.27.4.889. [DOI] [PubMed] [Google Scholar]
- 28.Festa A, D’Agostino R, Jr, Howard G, et al. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000 Jul 4;102(1):42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Wildman RP, Hamm LL, et al. Association between inflammation and insulin resistance in U.S. nondiabetic adults: results from the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004 Dec;27(12):2960–2965. doi: 10.2337/diacare.27.12.2960. [DOI] [PubMed] [Google Scholar]
- 30.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006 Dec 14;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 31. [Accessed December 26, 2007]; Available at: < http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l10am_c.pdf>.
- 32.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985 Jul;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 33.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006 Aug 15;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 34.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004 Jun;27(6):1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 35.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003 Jan 28;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 36.Lahoz C, Vicente I, Laguna F, et al. Metabolic syndrome and asymptomatic peripheral artery disease in subjects over 60 years of age. Diabetes Care. 2006 Jan;29(1):148–150. doi: 10.2337/diacare.29.1.148. [DOI] [PubMed] [Google Scholar]
- 37.Koenig W, Sund M, Frohlich M, et al. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999 Jan 19;99(2):237–242. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- 38.Best LG, Zhang Y, Lee ET, et al. C-reactive protein as a predictor of cardiovascular risk in a population with a high prevalence of diabetes: the Strong Heart Study. Circulation. 2005 Aug 30;112(9):1289–1295. doi: 10.1161/CIRCULATIONAHA.104.489260. [DOI] [PubMed] [Google Scholar]
- 39.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006 Jul;116(7):1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vincent MA, Montagnani M, Quon MJ. Molecular and physiologic actions of insulin related to production of nitric oxide in vascular endothelium. Curr Diab Rep. 2003 Aug;3(4):279–288. doi: 10.1007/s11892-003-0018-9. [DOI] [PubMed] [Google Scholar]
- 41.Faxon DP, Fuster V, Libby P, et al. Atherosclerotic Vascular Disease Conference: Writing Group III: pathophysiology. Circulation. 2004 Jun 1;109(21):2617–2625. doi: 10.1161/01.CIR.0000128520.37674.EF. [DOI] [PubMed] [Google Scholar]
- 42.Fowkes FG, Housley E, Riemersma RA, et al. Smoking, lipids, glucose intolerance, and blood pressure as risk factors for peripheral atherosclerosis compared with ischemic heart disease in the Edinburgh Artery Study. Am J Epidemiol. 1992 Feb 15;135(4):331–340. doi: 10.1093/oxfordjournals.aje.a116294. [DOI] [PubMed] [Google Scholar]
- 43.Vu JD, Vu JB, Pio JR, et al. Impact of C-reactive protein on the likelihood of peripheral arterial disease in United States adults with the metabolic syndrome, diabetes mellitus, and preexisting cardiovascular disease. Am J Cardiol. 2005 Sep 1;96(5):655–658. doi: 10.1016/j.amjcard.2005.04.038. [DOI] [PubMed] [Google Scholar]