Abstract
This study examined the effect of cellcultured Acanthopanax senticosus (A. senticosus) extract on the antioxidative defense system, oxidative stress and cell membrane fluidity in the liver of type 2 diabetes in the C57BL/6J mouse as an animal which is genetically prone to develop insulin resistance and obesity/diabetes. C57BL/6J mice were randomly divided, control diet (N-C), high fat diet (DM-C), control diet plus A. senticosus extract (N-CASM), and high fat diet plus A. senticosus extract (DM-CASM). The mice were orally administered an A. senticosus extract (0.5 g/kg body weight) in the N-CASM and DM-CASM groups once a day for 12 weeks, and distilled water in the N-C and DM-C groups. Cellcultured A. senticosus extract was found to be excellent for strengthening the antioxidative defense system, reducing the generation of reactive oxygen species (ROS) and damaging oxidative substances, and maintaing membrane fluidity (MF) in the liver of type 2 diabetes mouse.
Keywords: Cellcultured Acanthopanax senticosus extract, C57BL/6J mice, antioxidative defense system, oxidative stress, membrane fluidity
Introduction
As a consequence of the increased prevalence of obesity, type 2 diabetes mellitus (T2D) has become a global epidemic, with more than 300 million individuals worldwide projected to be afflicted with T2D by the year 2025 [1]. Previous studies demonstrated that chronic consumption of a high-fat diet induces obesity, insulin resistance, dyslipidemia, and T2D in genetically C57BL/6J mice [2–4]. Reactive oxygen species (ROS) and the oxidative damage that they cause are thought to play a key role in the pathogenesis of diabetes and its complications [5]. It has been proposed that hyperglycemia contributes to the generation in various tissues of free radicals that, in turn, can lead to multiple complications and, at least in part, to the induction of insulin resistance [6, 7]. It has been demonstrated that chronic exposure of cells to high glucose increases intracellular ROS [8–10], and diminished superoxide dismutase (SOD), glutathione peroxidase (GSHpx), catalase (CAT) represent important components of the antioxidant defense enzyme [11] has been found in high-fat diet induced obesity mice.
Acanthopanax sentocisus (A. senticosus) is a common Asian herb known as “Siberian Ginseng” or “Eleutherococcus senticosus” that is used as an adaptogenic medicine [12]. The major active components of A. senticosus are eleutherosides, chiisanosides, isofraxidin, acanthosides, daucosterine, β-sitosterol, sesamine, and savinine [13]. Crude A. senticosus extracts have been used as popular health supplements to treat stress-induced physiological changes [14, 15] as well as antioxidant [16, 17] various allergic conditions [18], inflammation [19], cancer [20] chronic bronchitis, hypertension, ischemic heart disease, and gastric ulcers.
But, crude A. senticosus fertility is weaker than other plants, for theses reasons, now under development through cell cultivation method.
Therefore, in this study, it is intended to prove that seedings of A. senticosus Max. using cell cultivation method has the anti-diabetes effect in the type II diabetes. The present study observed the effect of cellcultured A. senticosus Max. on the antioxidative defense system, oxidative stress and cell membrane fluidity (MF) in the liver of the C57BL6/J mouse which is genetically prone to develop insulin resistance and obesity/diabetes.
Materials and Methods
Materials
All chemicals were obtained from Sigma Chemical Co. (St Louis, MO) unless otherwise indicated. The enzyme assay kits used to determine blood glucose was purchased from Asan Pharm (Asan Pharmaceutical Co., Republic of Korea).
Preparation of Acanthopanax senticosus extract
Cultured A. senticosus cells with a torpedo shape were supplied by Microplants Co., Ltd (Yusung, Korea). A. senticosus cell were allowed to grow in an MS medium (2% sugar) without growth regulators in bio-reactors equipped with an airlift. Culturing for a period of about 10–15 days led the matured somatic embryos to develop plantlets [21]. The A. senticosus cells were dried, ground to a fine powder, and extracted with deionized water (thirty times volume) for 9 h at 80°C. The resulting extracts were filtered, concentrated under vacuum at 60°C to 70 Brix.
Animals and diet
Four-week old male C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME). The animals were maintained on a chow diet (Jeil-jedang, Suwon, Korea) for 1 week, and then randomly divided into 4 groups, control diet (N-C), high fat diet (DM-C), control diet plus A. senticosus extract (N-CASM), and high fat diet plus A. senticosus extract (DM-CASM). The animals were randomly put into groups (n = 10) so that the average weight in each group was comparable. The mice were allowed free access to the diets and water. The mice were orally administered A. senticosus extract (0.5 g/kg body weight) in the N-CASM and DM-CASM groups once a day for 12 weeks, and distilled water in the N-C and DM-C groups. Research Diets (New Brunswick, NJ) manufactured the diets. The animals were housed in a temperature controlled environment with a 12 h light/dark cycle. The food consumption and body weight were measured daily and weekly, respectively.
Sample collection and preparation
All mice were fasted for 12 h prior to sacrificial. Animals were anesthetized using pentothal sodium (40 mg/kg body weight). Their livers were removed immediately, washed in ice-cold saline, and weighted. The removed liver was cut into small pieces and homogenized with a glass Teflon homogenizer in ice-cold 0.1 M potassium phosphate buffer (pH 7.5) equivalent to four times the liver weight. This homogenate was used for the determination of thiobarbituric acid reactive substances (TBARS) contents. The homogenate was centrifuged at 600 g for 10 min, and the resulting supernatant was recentrifuged at 10,000 g for 20 min, in both cases at 4°C. The resulting precipitate, the mitochondrial fraction, was suspended in the above buffer and used as the source of SOD, CAT, superoxide radical, hydrogen peroxide, and carbonyl value and the supernatant was further centrifuged at 105,000 g for 60 min, at 4°C. The supernatant obtained at this point, the cytosolic fraction, was used as the source of the enzymes GSHpx, and hydrogen peroxide and to yield the microsomal fraction used as the source of superoxide radical and carbonyl value. Protein in the liver tissues was determined using the method of Lowry et al. [22].
Activities of defense enzymes
The SOD activity was spectrophotometrically measured using a modified version of the method developed by Marklund and Marklund [23]. Briefly, SOD was detected on the basis of its ability to inhibit superoxide mediated reduction. One unit was determined as the amount of enzyme that inhibited the oxidation of pyrogallol by 50%. The activity was expressed as unit/mg protein/min. The GSHpx activity was measured by the method of Lawrence and Burk [24]. The reaction mixture contained 1 mM glutathione reductase in a 0.1 M Tris-HCl (pH 7.2) buffer. The reaction was initiated by adding 2.5 mM H2O2 and the absorbance was measured at 340 nm for 1 min. A molar extinction coefficient of 6.22 mM−1 cm−1, was used to determine the activity. The activity was expressed as nmol NADPH/mg protein/min. The CAT activity was measured using Aebi’s method [25] a slight modification wherein the hydrogen peroxide decomposition yielding water and oxygen was measured. The mitochondria pellet was dissolved in 1.0 ml of the same homogenization buffer. Ten microliters of the mitochondria solution was added to a cuvette containing 2.89 ml of a 50 mmol/l potassium phosphate buffer (pH 7.4), then the reaction was initiated by adding 0.1 ml of 30 mmol/1 H2O2 to make a final volume of 3.0 ml at 25°C. The decomposition rate of H2O2 was measured at 240 nm for 5 min using a spectrophotometer. A molar extinction coefficient of 0.041 mM−1 cm−1, was used to determine the CAT activity. The activity was defined as the nmol H2O2 decreased/mg protein/min.
Determination of ROS
Superoxide radical content were determined by the Azzi method et al. [26]. The reaction mixture contained 50 mM phosphate buffer (pH 7.5), 90 mM succinate, 150 mM KCl, 30 nM KCN, 0.3 mM cytochrome C and mitochondria or microsome added in that sequence. The mixture was shaken vigorously and the absorbance of the mixture was recorded at 550 nm. After incubated at 37°C. for 2 min the absorption was measured again at 550 nm. The liver cytosolic and mitocondrial hydrogen peroxide levels were measured by Wolff’s method [27]. Fox 1 (Ferrus Oxidation with Xylenol orange) reagent was prepared as the following mixture with 100 µM xylenol orange, 250 µM ammonium ferrus sulfate, 100 mM sorbitol, and 25 mM H2SO4. Fifty microliters of the test sample was added to 950 µL Fox 1 reagent, vortexed, and incubated at room temperature for a minimum of 30 min at which color development is virtually complete. The absorbance was read at 560 nm and the standard was linear in the 0–5 µM H2O2 concentration range. The unit was expressed as micromoles of H2O2 per mg cytosolic or mitochondrial protein.
Measurement of oxidative damage and age
As a marker of lipid peroxidation, the TBARS concentrations were measured in liver homogenates using method of Park et al. [28]. Briefly, 500 µl of a 10%(w/v) tissue homogenate solution was mixed with 2.5 ml of 10% TCA, vortexed, and incubated for 10 min at room temperature. The mixture was centrifuged at 3000 rpm × g for 15 min and the pellet was mixed with 2.5 ml of 0.05 M H2SO4, vortexed and recentrifuged at 3000 rpm × g for 15 min. The pellet was heated at 95°C for 30 min after the addition of 2.5 ml of 0.05 M H2SO4 and 3 ml of TBA. After cooling the reaction, 3 ml of butanol:pyridine (15:1) solution was added and vortexed. The mixture was centrifuged at 3000 rpm × g for 10 min and the resulting coloured layer was measured at 530 nm using malondialdehyde (MDA) made by the hydrolysis of 1,1,3,3-tetramethoxypropane (TMP) as standard. The content of lipofuscin in the liver tissue was measured in compliance with the Fletcher method et al. [29].
Measurement of MF
The quantitative measurement of MF employed the fluorescence polarization technique described by Yu et al. [30] with TMA-DPH (1,4-(trimethyl-ammoniumphenyl)-6-phenyl 1-1,3,5-hexatriene) sensing as a fluorescence probe. Membrane preparations (50 µg protein) were suspended in 50 mM Tris-HCl buffer (pH 7.4), mixed with TMA-DPH prepared from a stock solution of 5 mM TMA-DPH solubilized in tetrahydrofurans, and incubated at 37°C for 30 min. Fluorescence polarization was determined using a Perkin Elmer LS-50 fluorescence spectrophotometer equipped with rotating polarizing filters with samples exposed to a wide range of temperatures (15–40°C). Samples were excited at 360 nm, and emission intensity was read at 435 nm. Calculation of the values for polarization (P) and fluorescence anisotropy (r) of the samples were done by the Yu et al. [30].
Statistical analysis
Data from individual experiments are expressed as the mean ± standard deviation. All statistical analysis was performed using SAS software (SAS Institute, Cary, NC). The data were analyzed by two-way analysis of variance (ANOVA) (diet × A. senticosus extract). Pairwise statistical significance was established using a post hoc Student’s t test. When significant differences were indicated found, mean values were compared by Tukey’s test and repeated-measures ANOVA. Statistical significance is defined as p<0.05.
Results
Body weight, food intake, food efficiency ratio, liver weight and blood glucose level
The high fat diet group gained more weight and faster than the control diet group (Table 1). Although, food intake of the groups were not significantly different, the body weights of the mice fed high fat diets were significantly higher than those of the mice fed control diets. A. senticosus extract administration showed no effects on weight in the control diet group; however, in the high fat diet group, the DM-CASM group gained significantly less weight than the DM-C group. The food efficiency ratio (FER) (Table 1) was significantly higher in the high fat diet group than in the control group. A. senticosus extract administration significantly decreased the FER, in both control and high fat diet groups. Weight of hepatic, was significantly increased in the DM-C group than that of N group, but DM-CASM group was significantly decreased compared with HF group. The blood glucose level in the DM-C group was 124% higher than in the N-C group. However, the level of in the DM-CASM group was 18% lower than in the DM-C group (Table 1).
Table 1.
Effects of the Cellcultured Acanthopanax Senticosus extract on body weight gains, food intake, food efficiency ratio and blood glucose in C57BL/6J mice
| Normal |
Diabetic |
ANOVA1 |
|||||
|---|---|---|---|---|---|---|---|
| N-C | N-CASM | DM-C | DM-CASM | D | A | D × A | |
| Initial body weight (g) | 19.52 ± 7.13 | 19.08 ± 6.91 | 19.77 ± 9.27 | 20.23 ± 8.61 | NS | NS | NS |
| Weight gain (g/12 weeks) | 5.82 ± 0.78c | 5.04 ± 0.36c | 16.69 ± 4.27a | 12.49 ± 3.45b | 0.0001 | 0.0185 | NS |
| Food intake (g/12 weeks) | 198.24 ± 0.06 | 223.44 ± 0.27 | 199.08 ± 0.17 | 194.04 ± 0.07 | NS | NS | NS |
| FER2 | 0.029 ± 0.007c | 0.023 ± 0.0024d | 0.086 ± 0.0065a | 0.065 ± 0.0019b | 0.0001 | 0.0001 | 0.0062 |
| Liver weight (g) | 0.85 ± 0.08b | 0.88 ± 0.14b | 1.54 ± 0.47 a | 0.97 ± 0.11b | 0.0001 | 0.0163 | NS |
| Blood glucose (mg/dl) | 95.98 ± 7.36c | 93.49 ± 9.92c | 215.56 ± 16.72a | 176.92 ± 26.39b | 0.0001 | 0.0007 | 1 |
The values represent the mean ± SD of 10 mice per group. Different superscripts in the same row indicate significant differences (p<0.05) among groups by Tukey’s test.
1The degree of significance resulting from the two-way ANOVA is shown with effects of diet (D), administration A. senticosus (A), and the interaction of diet and administration of A. senticosus (D × A) being expressed as the numerical value or as not significant (NS) when p<0.05.
2Food efficiency ratio (FER) was calculated as weight gain weight gain (g/12 weeks)/food intake (g/12 weeks).
Antioxidative defense enzyme activities
Table 2 shows the activity of SOD, an antioxidant enzyme that reduces superoxide radicals to H2O2, which in turn is excreted as H2O based on the activity of GSHpx and CAT, thereby protecting the body from oxygen toxicity. DM-C group had a decreased than the N-C group, whereas, DM-CASM significantly increased than the DM-C group. The GSHpx activity decreased considerably in the DM-C group compared to the N-C group, whereas, the DM-CASM group was 12% higher than the DM-C group, which remained at the normal level. The CAT activity was 28% lower in the DM-C group, compared to the N-C group, whereas, the DM-CASM group remained at the normal level.
Table 2.
Effects of the Cellcultured Acanthopanax Senticosus extract on SOD, GSHpx, and CAT activity in the liver of type 2 diabetes mouse
| SOD (unit/mg protein/min) | GSHpx (nmol NADPH/mg protein/min) | CAT (nmol/mg protein/min) | ||
|---|---|---|---|---|
| Normal | N-C | 0.48 ± 0.02a | 170.05 ± 4.27a | 69.00 ± 5.30a |
| N-CASM | 0.49 ± 0.03a | 179.97 ± 2.20a | 75.05 ± 3.89a | |
| Diabetic | DM-C | 0.40 ± 0.01c | 150.80 ± 5.65b | 49.90 ± 5.48b |
| DM-CASM | 0.45 ± 0.03b | 168.74 ± 1.04a | 67.90 ± 5.48a |
The values represent the mean ± SD of 10 mice per group. Values with different superscript letters are significantly different at p<0.05 as shown by Tukey’s test.
Changes in ROS contents
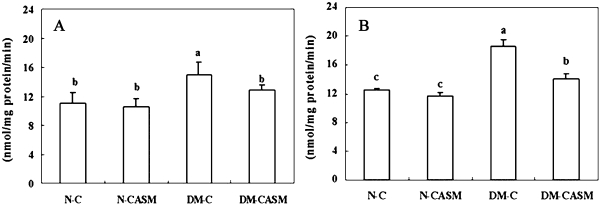
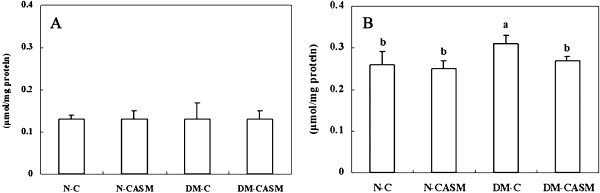
The liver mitochondria and microsome fraction superoxide radical content (Fig. 1) in the DM-C group were 35% and 49% higher than in the N-C group, respectively. However, the activity in the DM-CASM group were 14% and 25% lower than in the DM-C group, respectively. No significant difference was found in the H2O2 content (Fig. 2) in the mitochondria fraction between the experimental groups. But in the case of the cytosol fraction, DM-C group was 19% higher than in the N-C group, whereas, the content in the DM-CASM group was 13% lower than in the DM-C group, which remained at the normal level.
Fig. 1.
Effects of the Cellcultured Acanthopanax Senticosus extract on mitochondria (A) and microsome (B) superoxide radicals in the liver of type 2 diabetes mouse. The values represent the mean ± SD of 10 mice per group. Values with different superscript letters are significantly different at p<0.05 as shown by Tukey’s test.
Fig. 2.
Effects of the Cellcultured Acanthopanax Senticosus extract on mitochondria (A) and cytosol (B) hydrogen peroxixe (H2O2) in the liver of type 2 diabetes mouse. The values represent the mean ± SD of 10 mice per group. Values with different superscript letters are significantly different at p<0.05 as shown by Tukey’s test.
Observation of oxidative damage
The result of TBARS contents as an index oxidative damage was shown in (Table 3). The TBARS concentration in DM-C group was 49% higher than in the N-C group. However, the concentration in the DM-CASM group was 17% lower than in the DM-C group. The lipofuscin contents (Table 3) in the DM-C group was 38% higher than in the N-C group, whereas, in the DM-CASM group was 14% lower in the DM-C group.
Table 3.
Effects of the Cellcultured Acanthopanax Senticosus extract on TBARS and lipofuscin in the liver of type 2 diabetes mouse
| TBARS (MDA nmol/mg protein) | Lipofuscin (µg/mg protein) | ||
|---|---|---|---|
| Normal | N-C | 11.80 ± 0.82c | 11.80 ± 0.82c |
| N-CASM | 10.97 ± 1.61c | 10.97 ± 1.61c | |
| Diabetic | DM-C | 17.62 ± 1.54a | 17.62 ± 1.54a |
| DM-CASM | 14.72 ± 0.79b | 14.72 ± 0.79b |
The values represent the mean ± SD of 10 mice per group. Values with different superscript letters are significantly different at p<0.05 as shown by Tukey’s test.
Observation of MF
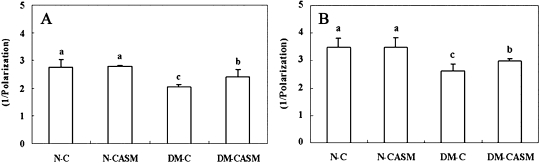
Since the cell membrane requires good fluidity to maintain homeostasis and metabolism in the body, fluidity is an effective index of adult disease. The cell membrane fluidity in the mitochondria and microsome fractions (Fig. 3) of the liver were 26% and 24% lower in the DM-C group, respectively, compared to the N-C group. However, both fractions of the liver were 17% and 14% higher in the DM-CASM group, respectively, compared to the DM-C group.
Fig. 3.
Effects of the Cellcultured Acanthopanax Senticosus extract on mitochondria (A) and microsome (B) membrane fluidity in the liver of type 2 diabetes mouse. The values represent the mean ± SD of 10 mice per group. Values with different superscript letters are significantly different at p<0.05 as shown by Tukey’s test.
Discussion
The current study examined the effects of cellcultured A. senticosus extract on the antioxidative defense system, oxidative stress and cell membrane fluidity in the liver of the C57BL6/J mouse as an animal whose auto obesity/diabetes was caused by a high fat diet. These results support previous studies in which a high fat diet increased body weight [31, 32]. There were no significant difference found among the experimental groups, the high fat diet led to significantly higher weight gain compared with the control diet, which resulted in higher FER. Thus mice fed a high fat diet showed a more rapid growth than the mice fed control diet. However, the higher FER exhibited by the A. senticosus extract administration, respectively, in control diet and high fat diet group whereas there was no significant effect on body weight in the control diet group. These results suggest that cellcultured A. senticosus extract can suppress the increase of weight gain induced by a high fat diet. Also, it was observed that A. senticosus extract decreased blood glucose in DM-CASM group. As reported previoudly by Park et al. [33], A. senticosus stem bark ethanol extract-treated mice showed marked decreases in plasma glucose level relative to the obese control mice. Oxidative stress means the production stress means the production of highly reactive oxygen radicals that are toxic to the cells, particularly to the cell membrane in which these radicals interact with the lipid bilayer and produce lipid peroxides [34]. Endogenous antioxidant enzymes (e.g., SOD, GSHpx and CAT) are responsible for the detoxication of deleterious oxygen radicals [35, 36]. From the current results of observing the activities of the antioxidative defense system in the liver, the SOD activity in the DM-C group was significantly decreased than that of N-C group, but the DM-CASM group was significantly increased than that of DM-C group. The reason for the decrease in the DM-C group, was suggested that the induction of oxidative stress by high fat diet. Therefore, it is possible that the hydrogen peroxide generated from superoxide radical directly reduces the levels of SOD [37]. GSHpx and CAT activities decreased considerably in the DM-C group compared to the N-C group, respectively. However, the DM-CASM groups remained the same as that in the N-C group. An increase in GSHpx and CAT activities indicates that A. senticosus extract helps in the restoration of vital molecules such as NAD, cytochrome, and glutathione. The inactivity of the antioxidant enzymes GSHpx and CAT in the diabetes groups was attributed to peroxidative damage to the tissues caused by being fed a high fat diet, while the administration of A. senticosus extract contributed to maintaining the optimum condition of the cell membrane organelles, essential for enzyme activity, by protecting them from peroxidation. This result confirms that A. senticosus acts as an excellent antioxidant even for the liver, which plays an important role in glucose metabolism, and it is a major site of insulin clearance [38]. The phenolic compounds in A. senticosus, such as isofraxidin and eleutherosides B and E from the stem bark [39]; eleutheroside E2 and isomaltol 3-O-α-D-glucopyranoside from the roots [40]; chiisanoside, chiisano-genin, and hyperin from the leaves [41]; as well as protocatechuic acid, syringin, chorogenic acid, caffeic acid, liriodendrin, and isofraxidin in ethanol extract of whole A. senticosus [42], have a protective effect against oxidative damage. In addition, Lee et al. reported that there were no changes in GOT and GPT levels after 6 months treatment with A. senticosus [12]. From this data suggested that A. senticosus supplementation has no side effects.
ROS was generated as free radicals in the body, such as superoxide anion, hydroxyl radical, and hydrogen peroxide, which are known to induce many adult diseases and promote the aging process. For example, Laura’s report [43] stated that ROS induce Alzheimer’s disease. Recent evidence showed that increased flux of free fatty acid (FFA), glucose, or hexosamine could raise mitochondrial ROS production, leading to increased intracellular oxidative stress [44, 45]. Among the free radicals which are known to induce aging or adult diseases, the superoxide radical content in the mitochondria and microsome fractions of the liver was found to be considerably higher in the DM-C group compared to the N-C group, which coincides with a previous report of increased ROS in diabetes [46]. However, in the both fractions, the activity in the DM-CASM group was significantly decreased compared to the DM-C group, respectively. In the case of cytosol fraction, showed that the hydrogen peroxide concentration was increased in DM-C group compared to the N-C group, but the DM-CASM group was significantly decreased compared to the DM-C group.
Because type 2 diabetes is a chronic disease, prevention of oxidative stress needs to be achieved not only periodically but as long as the hyperglycemia persists. Lipid peroxidation is recognized as the most important substance which damaged by various toxicity compounds and drugs or pathophysiological phenomenon by disease. This is caused by the augmentation of oxidative stress and the decrease of an antioxidative defense system in vivo. The contents of TBARS in DM-C group was significantly higher than in the N-C group. However, the concentration of the DM-CASM group was significantly lower than that of the DM-C group. The observed effect of A. senticosus extract on lipofuscin that is consumptiveness aging pigment produced by free radicals that estimate the degree of aging and leads to diseases in adult people by toxicity function in the cell and promotes aging. The lipofuscin contents in the DM-C group was higher than in the N-C group whereas, in the DM-CASM group was significantly lower than in the DM-C group. In the current study, when the fluidity in the cell membrane of the liver was observed, mitochondria and microsome fractions both showed a decrease in the DM-C group compared to the N-C groups. It would appear that the cell membranes connected by double bonds of polyunsaturated fatty acid in the diabetes-induced groups lost fluidity due to the generation of ROS, such as superoxide radical and hydrogen peroxide, whereas the activity of antioxidant enzyme increased with the administration of A. senticosus extract, resulting in significantly increased cell membrane fluidity in the DM-CASM group compared to the DM-C group.
In conclusion, A. senticosus extract strengthened the antioxidative defense system with an increased activity of antioxidant enzymes, such as SOD, GSHpx and CAT, in the liver of the type 2 obesity/diabetes exeperimental mice. Accordingly, A. senticosus extract was found to reduce the accumulation of ROS, such as superoxide radical and H2O2, which decrease the generation of oxidative damage substances, such as TBARS and lipofuscin, increase the membrane fluidity lowered by oxidative damage.
Acknowledgments
This work was supported by the Ministry of Health & Welfare Grant (03-PJ1-PG10-22000-0015).
Abbreviations
- CAT
catalase
- FER
food efficiency ratio
- GSHpx
glutathione-peroxidase
- MDA
malondialdehyde
- MF
membrane fluidity
- NIDDM
non-insulin dependent diabetes mellitus
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TBARS
thiobarbituric acid reactive substances
References
- 1.Wei-jian J. Sirtuins: novel targets for metabolic disease in drug development. Biochem. Biophy. Res. Commun. 2008;373:341–344. doi: 10.1016/j.bbrc.2008.06.048. [DOI] [PubMed] [Google Scholar]
- 2.Wouters K., van Gorp P.J., Bieghs V., Gijbels M.J., Duimel H., Lutjohann D., Kerksiek A., van Kruchten R., Maeda N., Staels B., van Bilsen M., Shiri-Sverdlov R., Hofker M.H. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology. 2008;48:474–486. doi: 10.1002/hep.22363. [DOI] [PubMed] [Google Scholar]
- 3.Stewart L.K., Soileau J.L., Ribnicky D., Wang Z.Q., Raskin I., Poulev A., Majewski M., Cefalu W.T., Gettys T.W. Quercetin transiently increases energy expenditure but persistently decreases circulating markers of inflammation in C57BL/6J mice fed a high-fat diet. Metabolism. 2008;57:S39–S46. doi: 10.1016/j.metabol.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin C.C., Lin C.H., Lin W.L. Effects of Momordica charantia on insulin resistance and visceral obesity in mice on high-fat diet. Diabetes Res. Clin. Pract. 2008;81:134–143. doi: 10.1016/j.diabres.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Fiala E., Westendorf J., West I.C. Radicals and oxidative stress in diabetes. Toxicology. 2000;146:83–92. doi: 10.1016/s0300-483x(00)00140-2. [DOI] [PubMed] [Google Scholar]
- 6.Edelstein D., Dimmeler S., Ju Q., Sui C., Brownlee M. Biochemistry and molecular cell biology of diabetic complications. J. Clin. Invest. 2001;108:1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marfella R., Quagliaro L., Nappo F., Ceriello A., Giugliano D. Acute hyperglycemia induces an oxidative stress in healthy subjects. J. Clin. Invest. 2001;108:635–636. doi: 10.1172/JCI13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ha H., Lee H.B. Reactive oxygen species as glucose signaling molecules in mesangial cells cultured and under high glucose. Kidney Int. 2000;77:S19–S25. doi: 10.1046/j.1523-1755.2000.07704.x. [DOI] [PubMed] [Google Scholar]
- 9.Park S.H., Choi H.J., Lee J.H., Woo C.H., Kim J.H., Han H.J. High glucose inhibits renal proximal tubule cell proliferation and involves PCK, oxidative stress, and TGF-beta 1. Kidney Int. 2001;59:1695–1705. doi: 10.1046/j.1523-1755.2001.0590051695.x. [DOI] [PubMed] [Google Scholar]
- 10.Peiro C., Lafuente N., Matesanz N., Llergo J.L., Vallejo S., Rodriguez-Manas L., Sanchez-Ferrer C.F. High glucose induces cell death of cultured human aortic smooth muscle cells through the formation of hydrogen peroxide. Br. J. Pharmacol. 2001;133:967–974. doi: 10.1038/sj.bjp.0704184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee Y.M., Choi J.S., Kim M.H., Jung M.H., Lee Y.S., Song J. Effects of dietary genistein on hepatic lipid metabolism and mitochondrial function in mice fed high-fat diets. Nutrition. 2006;22:956–964. doi: 10.1016/j.nut.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Lee Y.J., Chung H.Y., Kwak H.K., Yoon S. The effects of A. senticosus supplementation on serum lipid profiles, biomarkers of oxidative stress, and lymphocyte DNA damage in postmenopausal women. Biochem. Biophy. Res. Commun. 2008;375:44–48. doi: 10.1016/j.bbrc.2008.07.097. [DOI] [PubMed] [Google Scholar]
- 13.Davydov M., Krikorian A.D. Eleutherococcus senticosus (Rupr. & Maxium.) Maxium. (Araliaceae) as an adaptogen: a cross look. J. Ethnopharmacol. 2000;72:345–393. doi: 10.1016/s0378-8741(00)00181-1. [DOI] [PubMed] [Google Scholar]
- 14.Fujikawa T., Yamaquchi A., Morita L., Takeda H., Nishibe S. Protective effects of Acanthopanax senticosus Harms from Hokkaido and its components on gastric ulcer in restrained cold water stressed rats. Biol. Pharm. Bull. 1996;19:1227–1230. doi: 10.1248/bpb.19.1227. [DOI] [PubMed] [Google Scholar]
- 15.Gaffney B.T., Hugel H.M., Rich P.A. Panax ginseng and Eleutherococcus senticosus may exaggerate an already existing biphasic response to stress via inhibition of enzymes which limit the binding of stress hormones to their receptors. Med. Hypotheses. 2001;56:567–572. doi: 10.1054/mehy.2000.1163. [DOI] [PubMed] [Google Scholar]
- 16.Lee S.H., Son D.W., Ryu J.Y., Lee Y.S., Jung S.H., Kang J.G., Lee S.Y., Ki H.S., Shin K.H. Anti-oxidant activities of Acanthopanax senticosus stems and their ligan components. Arch. Pharm. Res. 2004;27:106–110. doi: 10.1007/BF02980055. [DOI] [PubMed] [Google Scholar]
- 17.Lee Y.S., Jung S.H., Lim S.S., Ji J., Lee S., Shin K.H. Effects of the water extract from the stem bark of on hyperlipidemia in rats. Korean J. Pharm. 2001;32:103–107. [Google Scholar]
- 18.Yi J.M., Hong S.H., Kim H.K., Song H.J., Kim H.M. Effect of Acanthopanax senticosus stem on mast cell-dependemt anaphylaxis. J. Ethonopharmcol. 2002;79:347–352. doi: 10.1016/s0378-8741(01)00403-2. [DOI] [PubMed] [Google Scholar]
- 19.Lin Q.Y., Jin L.J., Cao Z.H., Xu Y.P. Inhibition of inducible nitric oxide synthase by Acanthopanax senticosus extract in RAW264.7 macrophage. J. Ethnopharmacol. 2008;23:231–236. doi: 10.1016/j.jep.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Yamazaki T., Shimosaka S., Sasaki H., Matsumura T., Tukiyama T., Tokiwa T. (+)-Syringaresinol-di-O-beta-D-glucoside, a phsnolic compound from Acanthopanax senticosus Harms, suppresses proinflammatory mediators in SE982 human synovial sarcoma cells by inhibiting activating protein-1 and/or nuclear factor-kappaB activities. Toxicol. In Vitro. 2007;21:1530–1537. doi: 10.1016/j.tiv.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Choi Y.E., Kim J.W., Yoon E.S. High frequency of plant production via somatic embryogenesis from callus or cell suspension cultures in Eleutherococcus senticosus. Ann. Bot (Lond). 1999;83:309–314. [Google Scholar]
- 22.Lowry O.H., Roseborough N.J., Farr A.L., Randall R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 23.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 24.Lawrence R.A., Burk R.F. Glutathione peroxidase activity in selenium deficient rat liver. Biochem. Biophy. Res. Commun. 1976;71:952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- 25.Aebi H., Wyss S.R., Scherz B., Skvaril F. Heterogeneity of erythrocyte catalase. Isolation and characterixation of normal and variant erythrocyte catalase and their subunits. Eur. J. Biochem. 1974;48:137–145. doi: 10.1111/j.1432-1033.1974.tb03751.x. [DOI] [PubMed] [Google Scholar]
- 26.Azzi A., Montecucco C., Richter C. The use of acetylated ferric cytochrome c for the detection of superoxide radicals produced in biological membranes. Biochem. Biophy. Res. Commun. 1975;65:597–603. doi: 10.1016/s0006-291x(75)80188-4. [DOI] [PubMed] [Google Scholar]
- 27.Wolff S.P. Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol. 1994;223:182–189. [Google Scholar]
- 28.Park S.Y., Bok S.H., Jeon S.M., Park Y.B., Lee S.J., Jeong T.S., Choi M.S. Effect of rutin and tannic acid supplements on cholesterol metabolism in rats. Nutr. Res. 2002;22:283–295. [Google Scholar]
- 29.Fletcher B.L., Dillard C.J., Tappel A.L. Measurement of fluorescent lipid peroxidation products in biological systems and tissues. Anal. Biochm. 1973;52:1–9. doi: 10.1016/0003-2697(73)90327-8. [DOI] [PubMed] [Google Scholar]
- 30.Choi J.H., Yu B.P. Unsuitability of TBA test as a lipid peroxidation maker due to prostaglandin synthesis in the aging kidney. Age. 1990;13:61–64. [Google Scholar]
- 31.Cha Y.S., Soh J.R., Kim J.W. Acanthopanax senticosus extract prepared from cultured cells improves lipid parameters in rats fed with a high fat diet. J. Korean Soc. Nytraceut. Food. 2003;8:40–45. [Google Scholar]
- 32.Lin S., Thomas T.C., Storlien L.H., Huang X.F. Development of high fat diet-induced obesity and leptin resistance in C57BL/6J mice. Int. J. Obes. Relat. Metab. Disord. 2000;24:639–646. doi: 10.1038/sj.ijo.0801209. [DOI] [PubMed] [Google Scholar]
- 33.Park S.H., Lee S.G., Kang S.K., Chung S.H. Acanthopanax senticosus reverses fatty liver disease and hyperglycemia in ob/ob mice. Arch. Pharm. Res. 2006;29:768–776. doi: 10.1007/BF02974078. [DOI] [PubMed] [Google Scholar]
- 34.Sakaguchi S., Furusawa S. Oxidative stress and septic shock: metabolic aspects of oxygen-derived free radicals generated in the liver during endotoxemia. FEMS. Immunol. Med. Microbiol. 2006;47:166–177. doi: 10.1111/j.1574-695X.2006.00072.x. [DOI] [PubMed] [Google Scholar]
- 35.Del Maestro R.F. An approach to free radicals in medicine and biology. Acta Physiol. Scand. Suppl. 1980;492:153–168. [PubMed] [Google Scholar]
- 36.Stern L.Z., Ringel S.P., Ziter F.A., Menander-Huber K.B., Ionasescu V., Pellegrino R.J., Snyder R.D. Drug trial of superoxide dismutase in Duchenne’s muscular dystrophy. Arch. Neurol. 1982;39:34234–34236. doi: 10.1001/archneur.1982.00510180020004. [DOI] [PubMed] [Google Scholar]
- 37.Hodgson E.K., Fridovich I. The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: chemiluminescence and peroxidation. Biochemistery. 1975;14:5299–5303. doi: 10.1021/bi00695a011. [DOI] [PubMed] [Google Scholar]
- 38.Duckworth W.C., Hamel F.G., Peavy D.E. Hepatic metabolism of insulin. Am. J. Med. 1988;85:71–76. doi: 10.1016/0002-9343(88)90399-3. [DOI] [PubMed] [Google Scholar]
- 39.Nishible S., Kinoshita H., Takeda H., Okano G. Phenolic compounds from stem bark of Acanthopanax senticosus and their pharmacological effect in chronic swimming stress rats. Chem. Pharm. Bull. (Tokyo) 1990;38:1763–1765. doi: 10.1248/cpb.38.1763. [DOI] [PubMed] [Google Scholar]
- 40.Li Q., Jia Y., Xu L., Wang X., Shen Z., Liu Y., Bi K. A new lignan glycoside from Eleutherococcus senticosus. Planta. Med. 2001;67:776–778. doi: 10.1055/s-2001-18352. [DOI] [PubMed] [Google Scholar]
- 41.Lee S., Shin D.S., Oh K.B., Shin K.H. Antibacterial compounds from the leaves of Acanthopanax senticosus. Arch. Pharm. Res. 2003;26:106–110. doi: 10.1007/BF03179929. [DOI] [PubMed] [Google Scholar]
- 42.Li Q., Jia Y., Xu L., Wang X., Shen Z., Liu Y., Bi K. Simultaneous determination of protocatechiu acid, syringin, chlorogenic acid, caffeic acid, liriodendrin and isofraxidin in Acanthopanax senticosus Harms by HPLC-DAD. Biol. Pharm. Bull. 2006;29:532–534. doi: 10.1248/bpb.29.532. [DOI] [PubMed] [Google Scholar]
- 43.Laura J.H., Michael A.T., Juan C.T. Increased susceptibility of Alzhemier’s disease temporal cortex to oxygen free radical-mediated process. Free Radic. Biol. Med. 1997;23:183–190. doi: 10.1016/s0891-5849(96)00573-4. [DOI] [PubMed] [Google Scholar]
- 44.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 45.Nishikawa T., Edelstein D., Du K.L., Yamagishi S., Matsumura T., Kaneda Y., Yorek M.A., Beebe D., Oates P.J., Hammes H.P., Giardino I., Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 46.Kwag O.G., Kim S.O., Choi J.H., Rhee I.K., Choi M.S., Rhee S.J. Vitamin E improves microsomal phospholipase A2 activity and the arachidonic acid cascade in kidney of diabetic rats. J. Nutr. 2001;131:1297–1301. doi: 10.1093/jn/131.4.1297. [DOI] [PubMed] [Google Scholar]