Abstract
Effect of caffeic acid on the formation of hydroxyl radicals was examined during xanthone-mediated photosensitization. The reaction was performed on irradiation (λ = 365 nm) of the standard reaction mixture containing 15 µM xanthone, 0.1 M 5,5-dimethyl-1-pyrroline N-oxide (DMPO) and 20 mM phosphate buffer (pH 7.4) using electron paramagnetic resonance (EPR) with spin trapping. Caffeic acid inhibited the formation of hydroxyl radicals. Caffeic acid hardly scavenged both hydroxyl radicals and superoxide radicals under conditions employed in this paper in spite of its ability to act as a hydrogen donor or a reagent for the aromatic hydroxylation, because high concentration of DMPO trapped hydroxyl radicals overwhelmingly. Furthermore, caffeic acid inhibited the formation of hydroxyl radicals in the standard reaction mixture with EDTA under UVA irradiation. Accordingly, the inhibitory effect of caffeic acid on the formation of hydroxyl radicals in the standard reaction mixture under UVA irradiation is not due to its ability to chelate iron. Thus, the inhibitory effect of caffeic acid seems to occur in the standard reaction mixture under UVA irradiation through a novel antioxidation activity, i.e., ability to quench the exited xanthone.
Keywords: radicals, xanthone, quenching, UVA, caffeic acid
Introduction
Of the various oxidative stresses, UV irradiation is one of the primary factors. Chronic exposure to solar UV irradiation of mammalian skin induces a number of biological responses, including erythema, edema, sunburn cell formation, hyperplastic responses, photoaging and skin cancer development [1–3]. Increasing evidences showed that free radicals may be involved in acute sunburn reactions [4–6]. Indeed, hydrogen peroxide, 1O2, O2−• and nitric oxide were observed under UV irradiation [7, 8]. Since UVB (315–280 nm) radiation constitutes only 5% of the solar UV radiation that reaches the surface of the earth, skin damages are not caused entirely by the UVB. Sufficient evidences indicated that UVA (400–315 nm) radiation, which accounts for the major portion of the solar UV radiation also leads to skin damages. The UVA-induced damages would be mediated by endogenous and/or exogenous photosensitizers.
Photosensitizers, xanthone and its derivatives have been extensively examined for the UVA-induced DNA damage [9, 10]. It has been also known that xanthone mediates the photosensitized decomposition of fatty ester hydroperoxides to produce oxyl radicals [11] and hydroxyl radicals [12]. We chose xanthone-mediated photosensitization as a model system to investigate the skin damages by UVA.
Several papers have shown that polyphenols scavenge free radicals through hydrogen-donation and aromatic hydroxylation [13–24]. On the other hand, other studies have indicated that polyphenols inhibit the formation of free radicals and the propagation of free radical reactions through the chelation of transition-metal ions [23–29]. The purpose of the work described here is to examine antioxidation activities of polyphenols through the other mechanism, i.e., quenching an exited photosensitizer.
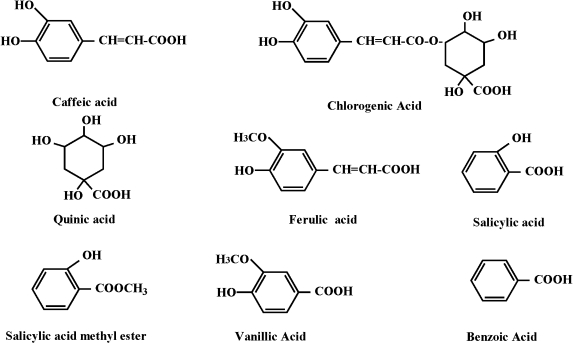
Chlorogenic acid is an ester of caffeic acid with quinic acid (Fig. 1). It is found naturally in various agricultural products such as coffee beans, potatoes and apples. Ferulic acid (Fig. 1) occurs in rice, wheat, olives, citrus fruits and leaves and many other plants. Thus, it is of interest to examine the influence of the polyphenols on the formation of reactive oxygens by UVA in view of their widespread occurrence in food products.
Fig. 1.
Chemical structures of caffeic acid and its related compounds.
Materials and Methods
Chemicals
Xanthone and disodium ethylenediaminetetraacetate (EDTA) were purchased from Wako Pure Chemical Industries (Osaka, Japan). 5,5-Dimethyl-1-pyrroline N-oxide (DMPO), caffeic acid, chlorogenic acid, D-(−)-quinic acid, ferulic acid, salicylic acid methyl ester and vanillic acid were from Tokyo Kasei Kogyo Co. LTD (Tokyo, Japan). Benzoic acid was obtained from Nakarai Chemicals, LTD (Kyoto, Japan). Salicylic acid was from Katayama Chemical (Osaka, Japan). Superoxide dismutase (SOD) and catalase were from Sigma-Aldrich Co. (St. Louise, Mo.).
EPR measurements
The electron paramagnetic resonance (EPR) experiments were carried out on a JES-FR 30 Free Radical Monitor (JEOL Ltd., Tokyo, Japan). Operating conditions of the EPR spectrometer were: power, 4 mW; modulation width, 0.1 mT; time constant, 0.3 s. Magnetic fields were calculated by the splitting of MnO (ΔH3–4 = 8.69 mT).
The standard reaction mixture
The standard reaction mixture contained 15 µM xanthone, 0.1 M DMPO and 20 mM phosphate buffer (pH 7.4) in a quartz test tube (100 mm long × 8 mm i.d.). The DMPO was used as a spin trapping reagent. The 0.1 M DMPO hardly absorbed light at 365 nm. The standard reaction mixtures were exposed to 13 J/cm2 UVA light under aerobic conditions using a 400 W UV lamp and a LXO365 bandpass filter (365 nm) (ASAHI SPECTRA Co., Tokyo, Japan). After 10 min irradiation, the aqueous samples were aspirated into a Teflon tube centered in an EPR microwave cavity. And then, EPR spectra were measured.
The Fenton reaction mixture
The Fenton reaction mixture contained 1 mM H2O2, 1 mM FeSO4 (NH4)2SO4, 0.1 M DMPO and 20 mM phosphate buffer (pH 7.4). The reaction was started by adding FeSO4 (NH4)2SO4. After 2 min reaction, the aqueous samples were aspirated into a Teflon tube centered in an EPR microwave cavity. And then, EPR spectra were measured.
Fluorescence spectroscopy
The fluorescence spectra of the samples were taken using a 650-10S fluorescence spectrophotometer (Hitachi, Ltd., Tokyo, Japan). The sample contained 15 µM xanthone (or 15 µM xanthone with 10 µM caffeic acid) in 50 mM phosphate buffer (pH 7.4). The excitation wavelengths used were 320 nm, 340 nm and 365 nm.
Results
Effect of caffeic acid on the formation of hydroxyl radicals in the standard reaction mixture under UVA irradiation
A previous our paper showed that DMPO traps hydroxyl radicals formed in the standard reaction mixture under UVA (365 nm) irradiation [12]. Several papers indicated that DMPO/OH radical adduct forms in the reaction of singlet oxygen with DMPO [30–34]. To examine whether or not the DMPO/OH radical adduct observed in the standard reaction mixture under UVA (365 nm) irradiation forms through the reaction of singlet oxygen with DMPO, catalase (or SOD) was added. On addition of SOD, the EPR peak height of the DMPO/OH radical adduct was enhanced in the standard reaction mixture under UVA (365 nm) irradiation [12]. While, addition of catalase resulted in the decrease of the EPR peak height of the DMPO/OH radical adduct in the standard reaction mixture under UVA (365 nm) irradiation [12]. Accordingly, the DMPO/OH radical adduct observed in the standard reaction mixture under UVA (365 nm) irradiation seems to be derived from hydrogen peroxide and superoxide anions.
To study effects of caffeic acid on the formation of hydroxyl radicals, EPR measurements were performed for the standard reaction mixture with caffeic acid (or without caffeic acid) under UVA (365 nm) irradiation. On addition of 1 mM caffeic acid, the EPR peak height of the DMPO/OH radical adduct decreased to 6 ± 2% of the standard reaction mixture under UVA irradiation.
Effect of caffeic acid on the formation of hydroxyl radicals in the standard reaction mixture (or the Fenton reaction mixture) with EDTA
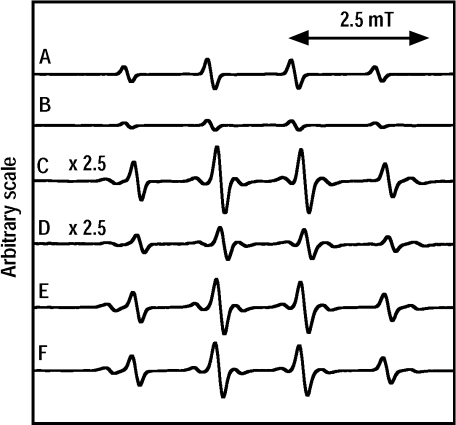
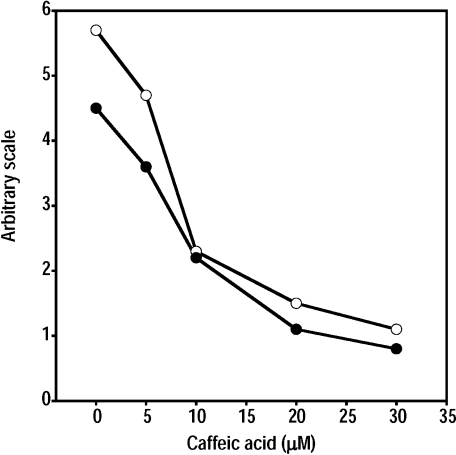
To know whether caffeic acid inhibits the formation of hydroxyl radicals through chelation of iron ions or not, EPR measurements were performed for the standard reaction mixture (or the Fenton reaction mixture) with EDTA (or without EDTA). On addition of caffeic acid (10 µM), the formation of hydroxyl radicals was inhibited in the standard reaction mixture with EDTA (0.1 mM) under UVA irradiation (Fig. 2B). The EPR peak heights of the DMPO/OH radical adduct decreased with increase of caffeic acid concentration in the standard reaction mixture with EDTA (or without EDTA) (Fig. 3). Fifty percent-inhibition concentration (IC50) of caffeic acid was 8.8 µM in the standard reaction mixture without EDTA (Table 1).
Fig. 2.
Effect of caffeic acid on the formation of hydroxyl radicals.
Reaction and EPR conditions were as described in Materials and Methods. (A) standard reaction mixture with 0.1 mM EDTA; (B) standard reaction mixture with 10 µM caffeic acid and 0.1 mM EDTA; (C) Fenton reaction mixture at ×2.5 scale; (D) Fenton reaction mixture with 1 mM caffeic acid at ×2.5 scale; (E) Fenton reaction mixture with 1 mM EDTA; (F) Fenton reaction mixture with 1 mM caffeic acid and 1 mM EDTA.
Fig. 3.
Caffeic acid concentration dependence of the formation of hydroxyl radicals in the standard reaction mixture with EDTA (or without EDTA) under UVA irradiation. Reaction and EPR conditions were as described under Materials and Methods. (closed circle) standard reaction mixture without EDTA; (open circle) standard reaction mixture with EDTA (0.1 mM).
Table 1.
Fifty percent-inhibition concentration (IC50) and absorbance (365 nm) at IC50 for caffeic acid related compounds.
| Compound | IC50 (µM) | Absorbance at IC50 |
|---|---|---|
| Caffeic Acid | 8.8 | 0.006 |
| Chlorogenic Acid | 1000.0 | 2.327 |
| Ferulic Acid | 1100.0 | 0.401 |
| Salicylic Acid | 308.0 | 0.0006 |
| Salicylic Acid Methyl Ester | 1100.0 | 0.002 |
| Vanillic Acid | 320.0 | 0.0054 |
On the other hand, on addition of caffeic acid (1 mM), the formation of hydroxyl radicals was inhibited in the Fenton reaction mixture (43 ± 7% of the Fenton reaction mixture) (Fig. 2D). The inhibitory effect of caffeic acid was reduced in the Fenton reaction mixture with EDTA (95 ± 3% of the Fenton reaction mixture with EDTA) (Fig. 2F).
Effect of caffeic acid on the formation of hydroxyl radicals in the standard reaction mixture with SOD under UVA irradiation
To know whether or not caffeic acid scavenges the O2−•, EPR measurements were performed for the standard reaction mixture with SOD under UVA irradiation. On addition of caffeic acid (1 mM) to the standard reaction mixture with SOD under UVA irradiation, the EPR peak height of the DMPO/OH radical adduct decreased to 13 ± 7% of the standard reaction mixture with SOD under UVA irradiation.
Absorbances of caffeic acid at 365 nm
To assess the effects of the UVA absorption of caffeic acid on the inhibition, absorbances at 365 nm were measured for caffeic acid at IC50 (Table 1). Caffeic acid hardly absorbed the light at 365 nm.
Effect of caffeic acid related compounds on the formation of hydroxyl radicals in the standard reaction mixture under UVA irradiation
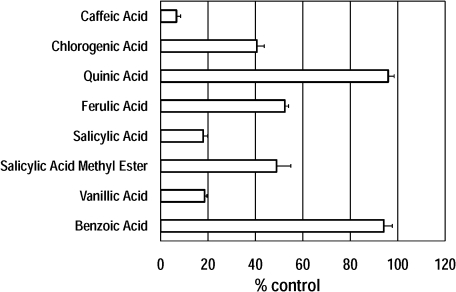
Effect of caffeic acid related compounds on the formation of hydroxyl radicals was examined in the standard reaction mixture under UVA irradiation. On addition of chlorogenic acid [or ferulic acid, or salicylic acid, or salicylic acid methyl ester, or vanillic acid], the EPR peak height of the DMPO/OH radical adduct decreased to 41 ± 3% [or 52 ± 2%, or 19 ± 1%, or 49 ± 6%, or 18 ± 2%] of the standard reaction mixture under UVA irradiation (Fig. 4). On the other hand, quinic acid (or benzoic acid) showed no effect (Fig. 4).
Fig. 4.
Effect of caffeic acid and its related compounds on the formation of hydroxyl radicals in the standard reaction mixture under UVA irradiation. Caffeic acid, (1 mM) and its related compounds (1 mM) were added to the standard reaction mixture. The control value 100% represents the level of DMPO/OH radical adduct formed in the standard reaction mixture under UVA irradiation. The respective values are means ± SD of three determinations. Reaction and EPR conditions were as described under Materials and Methods.
Fifty percent-inhibition concentrations (IC50) of the caffeic acid related compounds in the standard reaction mixture under UVA irradiation
Fifty percent-inhibition concentrations (IC50) were obtained by measuring concentration dependence of the EPR peak heights of the DMPO/OH radical adduct for caffeic acid related compounds in the standard reaction mixture under UVA irradiation (Table 1). Fifty percent-inhibition concentrations of chlorogenic acid, ferulic acid, salicylic acid, salicylic acid methyl ester, vanillic acid are as follows: chlorogenic acid, 1000 µM; ferulic acid, 1100 µM; salicylic acid, 308 µM; salicylic acid methyl ester, 1100 µM; vanillic acid, 320 µM.
Absorbances of caffeic acid related compounds at 365 nm
To assess the effects of the UVA absorption of caffeic acid related compounds on the inhibition, absorbances at 365 nm were measured for caffeic acid related compounds at IC50 (Table 1). Ferulic acid, salicylic acid, salicylic acid methyl ester, vanillic acid hardly absorbed the light at 365 nm. The absorbance of chlorogenic acid was 2.327 at IC50.
Fluorecence spectrum of xanthone (or xanthone with caffeic acid)
To know whether or not caffeic acid quenches the exited xanthone, fluorescence spectra were measured for 15 µM xanthone (or 15 µM xanthone with 10 µM caffeic acid). Xanthone produced emission maxima at λ = 393 nm upon excitation with either 320 nm or 340 nm or 365 nm. The fluorescence spectrum of xanthone was not affected by the addition of caffeic acid, indicating that caffeic acid does not quench the first excited singlet state of xanthone.
Discussion
The conversion of singlet oxygen (1O2) to hydroxyl radical (HO•) has been extensively examined during photosensitization of merocyanone 540 (or rose bengal, or anthrapyrazole, or uroporphyrin, or methylene blue) by a spin trapping technique with DMPO [31–34]. Addition of catalase resulted in the decrease of the EPR peak height of the DMPO/OH radical adduct in the standard reaction mixture under UVA (365 nm) irradiation [12]. The result indicated that H2O2 is an intermediate in the formation of DMPO/OH radical adduct in the standard reaction mixture under UVA irradiation [12]. On addition of SOD, the EPR peak height of the DMPO/OH radical adduct was enhanced in the standard reaction mixture under UVA (365 nm) irradiation [13], suggesting that the O2−• is involved in the the formation of DMPO/OH radical adduct. Therefore, the conversion of singlet oxygen (1O2) to hydroxyl radical (HO•) is not main reaction path in the standard reaction mixture under UVA irradiation.
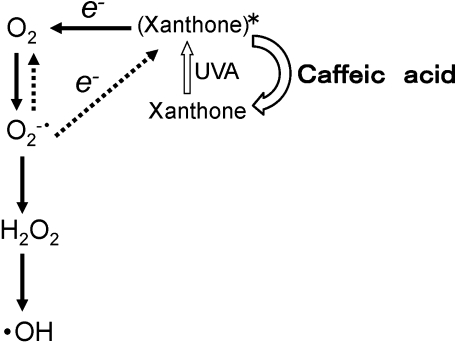
A possible reaction path for the formation of hydroxyl radicals is as follows (Scheme 1) [12]. The excited sensitizer (xanthone)*, which forms under UVA irradiation releases an electron to molecular oxygen (Eq. 1). Then, superoxide radical forms. The superoxide radical formed dismutes to form H2O2 and molecular oxygen spontaneously (Eq. 2).
Scheme 1.
A possible mechanism for the inhibitory effect of caffeic acid on the formation of hydroxyl radicals in the standard reaction mixture under UVA irradiation.
(Xanthone)* + O2 ↔ (Xanthone)+ + O2−• (1)
O2−• + O2−• + 2H+ → H2O2 + O2 (2)
The Fenton reaction appears to participate in the standard reaction mixture under UVA irradiation (Eq. 3). The Fe2+ in the reaction is possibly supplied through the reduction of Fe3+ by superoxide anions [12].
H2O2 + Fe2+ → HO• + OH− + Fe3+ (3)
To evaluate at which steps caffeic acid inhibits the formation of hydroxyl radicals in the standard reaction mixture under UVA irradiation, EPR measurements were performed for the standard reaction mixture with EDTA (or SOD) under UVA irradiation (Fig. 2). Caffeic acid could inhibit the HO• formation (Eq. 3) through chelation of iron ions [26, 29]. Indeed, caffeic acid inhibited the formation of hydroxyl radicals in the Fenton reaction mixture, but not in the presence of EDTA (Fig. 2), suggesting that caffeic acid inhibits the hydroxyl radical formation in the Fenton reaction mixture through chelation of iron ions. On the other hand, on addition of caffeic acid to the standard reaction mixture under UVA irradiation, the EPR peak height of DMPO/OH radical adduct decreased even in the presence of EDTA (Fig. 2). These results indicate that the caffeic acid does not inhibit the HO• formation through the chelation of iron ions in the standard reaction mixture under UVA irradiation.
To know whether or not caffeic acid scavenges hydroxyl radicals through the hydrogen-donating [14–24] or the aromatic hydroxylation [13] in the standard reaction mixture under UVA irradiation, caffeic acid was added into the hydroxyl radical generating system, the Fenton reaction mixture with EDTA (Fig. 2F). On addition of 1 mM caffeic acid to the Fenton reaction mixture with EDTA, no EPR peak height change of the DMPO/OH radical adduct was observed. The result indicates that caffeic acid does not scavenge hydroxyl radicals under this reaction conditions. This is due to the high concentration of DMPO (0.1 M) which traps hydroxyl radicals overwhelmingly. Therefore, caffeic acid does not seem to scavenge hydroxyl radicals in the standard reaction mixture under UVA irradiation.
To examine whether or not caffeic acid scavenges the O2−•, EPR measurements were performed for the standard reaction mixture with SOD under UVA irradiation. On addition of caffeic acid to the standard reaction mixture with SOD under UVA irradiation, the EPR peak height of the DMPO/OH radical adduct decreased to 13 ± 7% of the standard reaction mixture with SOD under UVA irradiation. On the other hand, caffeic acid showed a similar inhibitory effect in the standard reaction mixture without SOD (6 ± 2%) under UVA irradiation. In the presence of SOD, the reaction (Eq. 2) is fast enough to ignore the effect of caffeic acid. If caffeic acid scavenges the O2−• (Eq. 2) in the absence of SOD, the scavenging effect could be reduced in the presence of SOD. This is not the case for this reaction. Therefore, caffeic acid does not appear to scavenge the O2−• in the standard reaction mixture under UVA irradiation.
To assess whether the inhibitory effect is due to the UVA absorption of caffeic acid, and caffeic acid related compounds or not, UVA (365 nm) absorbances of these compounds were measured at IC50. Caffeic acid, ferulic acid, salicylic acid, salicylic acid methyl ester and vanillic acid hardly absorbed light at 365 nm, suggesting that the inhibitory effect is not due to the absorption of these compounds at 365 nm (Table 1). Therefore, caffeic acid hardly inhibits the step from xanthone to (xanthone)*.
Caffeic acid has no influence on all the steps in the Scheme 1 except for the step from (xanthone)* to xanthone. Thus, caffeic acid seems to inhibit the formation of hydroxyl radicals in the standard reaction mixture under UVA irradiation through a novel antioxidant action, i.e., ability to quench the excited xanthone. Fluorescence spectrum of xanthone (or xanthone with caffeic acid) indicated that caffeic acid does not quench the first excited singlet state of xanthone. Caffeic acid may quench the excited triplet state of xanthone which can interact with the triplet state of oxygen to form superoxide. Since the excited triplet state of xanthone is generally stable compared with singlet one, It can be formed from the excited singlet state of xanthone.
Of the compounds examined, caffeic acid, chlorogenic acid, ferulic acid, salicylic acid, salicylic acid methyl ester and vanillic acid showed the inhibitory effect. Since these compounds have phenol moieties, the phenol moieties may be essential for the inhibitory effects. Of the compounds examined, caffeic acid is the most potent inhibitor. That may be due to the intermolecular interaction between caffeic acid and xanthone.
References
- 1.Mukhtar H., Elmets C.A. Photocarcinogenesis: Mechanisms, models and human health implications. Photochem. Photobiol. 1996;63:355–447. doi: 10.1111/j.1751-1097.1996.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 2.Goihman-Yahr M. Skin aging and photoaging: An outlook. Clin. Dermatol. 1996;14:153–160. doi: 10.1016/0738-081x(95)00150-e. [DOI] [PubMed] [Google Scholar]
- 3.Young A.R. Cumulative effects of ultraviolet radiation on the skin: Cancer and photoaging. Semin. Dermatol. 1990;9:25–31. [PubMed] [Google Scholar]
- 4.Taira J., Mimura K., Yoneya T., Hagi A., Murakami A., Makino K. Hydroxyl radical formation by UV-irradiated epidermal cells. J. Biochem. 1992;111:693–695. doi: 10.1093/oxfordjournals.jbchem.a123820. [DOI] [PubMed] [Google Scholar]
- 5.Jurkiewicz B.A., Buettner G.R. Ultraviolet light-induced free radical formation in skin: An electron paramagnetic resonance study. Photochem. Photobiol. 1994;59:1–4. doi: 10.1111/j.1751-1097.1994.tb04993.x. [DOI] [PubMed] [Google Scholar]
- 6.Shindo Y., Witt E., Han D., Packer L. Dose-response effects of acute ultraviolet irradiation on antioxidants and molecular markers of oxidation in murine epidermis and dermis. J. Invest. Dermatol. 1994;102:470–475. doi: 10.1111/1523-1747.ep12373027. [DOI] [PubMed] [Google Scholar]
- 7.Katiyar S.K., Afag F., Perez A., Mukhtar H. Green tea polyphenol (−)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis. 2001;22:287–294. doi: 10.1093/carcin/22.2.287. [DOI] [PubMed] [Google Scholar]
- 8.Pathak M.A., Joshi P.C. Production of active oxygen species (1O2 and O2−•) by psoralens and ultraviolet radiation (320–400 nm) Biochim. Biophys. Acta. 1984;798:115–126. doi: 10.1016/0304-4165(84)90018-7. [DOI] [PubMed] [Google Scholar]
- 9.Hirakawa K., Yoshida M., Oikawa S., Kawanishi S. Base oxidation at 5' site of GG sequence in double-stranded DNA induced by UVA in the presence of xanthone analogues: Relationship between the DNA-damaging abilities of photosensitizers and their HOMO energies. Photochem. Photobiol. 2003;77:349–355. doi: 10.1562/0031-8655(2003)077<0349:boasog>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Hirakawa K., Yoshida M., Nagatsu A., Mizukami H., Rana V., Rawat M.S.M., Oikawa S., Kawanishi S. Chemopreventive action of xanthone derivatives on photosensitized DNA damage. Photochem. Photobiol. 2005;81:314–319. doi: 10.1562/2004-07-29-RA-252. [DOI] [PubMed] [Google Scholar]
- 11.Adam W., Andler S., Saha-Moller C.R. DNA cleavage induced by oxyl radicals generated in the photosensitized decomposition of fatty ester hydroperoxides derived from oleic and linoleic acid. Arch. Biochem. Biophys. 1998;349:261–266. doi: 10.1006/abbi.1997.0353. [DOI] [PubMed] [Google Scholar]
- 12.Mori H., Iwahashi H. Superoxide dismutase enhances the formation of hydroxyl radicals in the reaction mixture containing xanthone under an UVA irradiation. Biosci. Biotechnol. Biochem. 2007;71:3014–3018. doi: 10.1271/bbb.70412. [DOI] [PubMed] [Google Scholar]
- 13.Grootveld M., Halliwell B. Aromatic hydroxylation as a potential measure of hydroxyl-radical formation in vivo. Biochem. J. 1986;237:499–504. doi: 10.1042/bj2370499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Husain S.R., Cillard J., Lillard P. Hydroxyl radical scavengeing activity of flavonoids. Phytochemistry. 1987;26:2489–2491. [Google Scholar]
- 15.Sichel G., Corsaro C., Scalia M., Bilio A.J.D., Bonomo R.P. In vitro scavenger activity of some flavonoids and melanins against O2−•. Free Radic. Biol. Med. 1991;11:1–8. doi: 10.1016/0891-5849(91)90181-2. [DOI] [PubMed] [Google Scholar]
- 16.Cotelle N., Bernier J.L., Henichart J.P., Catteau J.P., Gaydou E., Wallet J.C. Scavenger and antioxidant properties of ten synthetic flavones. Free Radic. Biol. Med. 1992;13:211–219. doi: 10.1016/0891-5849(92)90017-b. [DOI] [PubMed] [Google Scholar]
- 17.Scott B.C., Butler J., Halliwell B., Aruom O.I. Evaluation of the antioxidant actions of ferulic acid and catechins. Free Radic. Res. Commun. 1993;19:241–253. doi: 10.3109/10715769309056512. [DOI] [PubMed] [Google Scholar]
- 18.Jovanovic S.V., Steenken S., Tosic M., Marjanovic B., Simic M.G. Flavonoids as antioxidants. J. Am. Chem. Soc. 1994;116:4846–4851. [Google Scholar]
- 19.Hanasaki Y., Ogawa S., Fukui S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic. Biol. Med. 1994;16:845–850. doi: 10.1016/0891-5849(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 20.Sanz M.J., Ferrandiz M.L., Cejudo M., Terencio M.C., Gil B., Bustos G., Ubeda A., Gunasegaran R., Alcaraz M.J. Influence of a series of natural flavonoids on free radical generating systems and oxidative stress. Xenobiotica. 1994;24:689–699. doi: 10.3109/00498259409043270. [DOI] [PubMed] [Google Scholar]
- 21.Rice-Evans C.A., Miller N.J., Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 22.Masaki H., Okamoto N., Sakaki S., Sakurai H. Protective effects of hydroxybenzoic acids and their esters on cell damage induced by hydroxyl radicals and hydrogen peroxides. Biol. Phar. Bull. 1997;20:304–308. doi: 10.1248/bpb.20.304. [DOI] [PubMed] [Google Scholar]
- 23.Paganga G., Al-Hashim H., Khodr H., Scott B.C., Aruoma O.I., Hider R.C., Halliwell B., Rice-Evans C.A. Mechanisms of antioxidant activites of quercetin and catechin. Redox Report. 1996;2:359–364. doi: 10.1080/13510002.1996.11747075. [DOI] [PubMed] [Google Scholar]
- 24.Brown J.E., Khodr H., Hider R.C., Rice-Evans C.A. Structure dependence of flavonoid interactions with Cu2+ ions: implications for their antioxidant properties. Biochem. J. 1998;330:1173–1178. doi: 10.1042/bj3301173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Afanas’ev I.B., Dorozhko A.I., Brodskii A.V., Kostyuk V.A., Potapovitch A.I. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem. Pharmacol. 1989;38:1763–1769. doi: 10.1016/0006-2952(89)90410-3. [DOI] [PubMed] [Google Scholar]
- 26.Iwahashi H., Ishii T., Sugata R., Kido R. The effect of caffeic acid and its related catechols on hydroxyl radical formation by 3-hydroxyanthanthranilic acid, ferric chloride, and hydrogen peroxide. Arch. Biochem. Biophys. 1990;276:242–247. doi: 10.1016/0003-9861(90)90033-u. [DOI] [PubMed] [Google Scholar]
- 27.Morel I., Lescoat G., Cogrel P., Sergent O., Pasdeloup N., Brissot P., Cillard P., Cillard J. Antioxidant and iron-chelating activities of the flavonoids catechin, quercetin and diosmetin on iron-loaded rat hepatocyte cultures. Biochem. Pharmacol. 1993;45:13–19. doi: 10.1016/0006-2952(93)90371-3. [DOI] [PubMed] [Google Scholar]
- 28.van Acker S.A.B.E., van Den Berg D.-J., Tromp M.N.J.L., van Bennekom W.P., van Der Vijgh W.J.F., Bast A. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med. 1996;20:331–342. doi: 10.1016/0891-5849(95)02047-0. [DOI] [PubMed] [Google Scholar]
- 29.Iwahashi H. Some polyphenols inhibit the formation of pentyl radical and octanoic acid radical in the reaction mixture of linoleic acid hydroperoxide with ferrous ions. Biochem. J. 2000;346:265–273. [PMC free article] [PubMed] [Google Scholar]
- 30.Harbour J.R., Issler S.L., Hair M.L. Singlet oxygen and spin trapping with nitrones. J. Am. Chem. Soc. 1980;102:7778–7779. [Google Scholar]
- 31.Feix J.B., Kalyanaraman B. Production of singlet oxygen-derived hydroxyl radical adducts during merocyanine-540-mediated photosensitization: analysis by ESR-spin trapping and HPLC with electrochemical detection. Arch. Biochem. Biophys. 1991;291:43–51. doi: 10.1016/0003-9861(91)90103-p. [DOI] [PubMed] [Google Scholar]
- 32.Bilski P., Reszka K., Bilska M., Chignell C.F. Oxidation of the spin trap 5,6-dimethyl-1-pyrroline N-oxide by singlet oxygen in aqueous solution. J. Am. Chem. Soc. 1996;118:1330–1338. [Google Scholar]
- 33.Takeshita K., Olea-Azar C.A., Mizuno M., Ozawa T. Singlet oxygen-dependent hydroxyl radical formation during uroporphyrin-mediated photosensitization in the presence of NADPH. Antioxid. Redox Signal. 2000;2:355–362. doi: 10.1089/ars.2000.2.2-355. [DOI] [PubMed] [Google Scholar]
- 34.Nishizawa C., Takeshita K., Ueda J., Mizuno M., Suzuki K., Ozawa T. Hydroxyl radical generation caused by the reaction of singlet oxygen with a spin trap, DMPO, increases significantly in the presence of biological reductants. Free Radic. Res. 2004;38:385–392. doi: 10.1080/1071576042000191772. [DOI] [PubMed] [Google Scholar]