Abstract
To evaluate the latent risk of acidosis in commercially available total parenteral nutrition (TPN) products, three types of commercially available TPN products were compared in postoperative patients. Sixty-four hospitalized patients with gastro-intestinal disease who undertook curative gastro intestinal resection were studied prospectively and administered with TPN solutions. Three types of commercially available TPN products were assigned randomly to eligible patients. Serial studies of blood acid-base status, serum electrolytes, and urinary acid-base status were conducted in the three groups administered with different TPN solutions. Patients received appropriate electrolytic solutions on the operation day and TPN solution from 2 to 7 days after operation. There were no differences among any of the serum electrolytes in the three groups. In one group, urinary pH decreased slightly and urinary net acid excretion (NAE) increased significantly after administration. This TPN product contains about 40 mEq/L of non-metabolizable acid to avoid the Maillard reaction that produces a complex of glucose and amino acids. Urinary NAE did not change in the other two groups. These TPN products do not use non-metabolizable acid to adjust pH. The present results suggest that the non-metabolizable acid may be a risk factor of metabolic acidosis.
Keywords: acidosis, total parenteral nutrition, Maillard reaction, acid-base imbalance, Humans
Introduction
It is well known that malnutrition generates a predisposition for postoperative complications, increased incidence of infection [1], and prolonged hospital stays [2]. Metabolic disturbances occur in malnourished patients undergoing major surgery and patients with total parenteral nutrition (TPN) [3, 4]. Acid-base imbalance, i.e., metabolic acidosis resulting from infusates of intravenous nutrition, is observed frequently during TPN therapy [5]. Further acidosis occurs because of metabolic abnormalities, such as thiamine deficiency, and an excess of lactic acid induced by the bolus injection of D-fructose, as well as an excess infusion of anionic components such as chloride salts provided by the administration of synthetic L-amino acids [6–8]. Moreover, acidosis could be related to either the excess amount of titratable acidity in the infusates or hydrogen ions released by the metabolism of nitrogen sources [9]. The problem may be overcome by the use of thiamine hydrochloride, management of the infusion rate, or adding free salts or acetate of cationic amino acid to the preparation [1]. However, even with such interventions hyperchloremic metabolic acidosis has been reported during TPN therapy in Japan [10]. Previously, it was reported that the acid load with hydrochloric acid in TPN solutions may cause severe hyperchloremic metabolic acidosis in rabbits [11], although acetic acid may not produce acidemia for patients, because hydrogen ions from the acetic acid are neutralized by bicarbonate ions generated from the metabolism of acetate ions. However, this hypothesis has not yet been proven by clinical research. In addition, the regulation of the kind or the amount of acid for adjusting the pH is not prescribed by the guidelines of the American Society for Parenteral and Enteral Nutrition (ASPEN) or the Japanese Society for Parenteral and Enteral Nutrition (JSPEN).
This study investigated the effects of three types of commercial TPN products on plasma acid-base balance.
Materials and Methods
Study population
The study was approved by the institutional review board of Nagoya University Hospital, and the patients gave written informed consent before enrollment. It was assumed that the annual incidence of acidosis was lower than 5%. In this situation, the sample size was calculated by the coordinator of the Critical Appraisal Skills Program JAPAN (CASPjp: http://caspjp.umin.ac.jp). Patients with gastrointestinal carcinoma who had undergone curative resection, such as gastrectomy, proctocolectomy, or cholecystectomy, and were admitted to the first surgical unit of Nagoya University Hospital were enrolled. Because patients with renal dysfunction may have an increased risk of acidosis, those with renal insufficiency (50 mL/min: calculated creatinine clearance using the Cockcroft-Gault formula) were excluded. Patients with metabolic acidosis or pulmonary complications were also excluded.
Nutritional management
All patients were managed according to a standard treatment protocol. Central venous catheters were placed and all patients received a crystalloid infusion (4.5% dextrose and 0.2% sodium chloride fluid: Solita T3® Ajinomoto Pharmaceuticals) before their operations. After the operation, eligible patients were administered TPN solutions. The compositions of the three TPN solutions used in this study are shown in Table 1, and these were based on the guidelines of ASPEN and JSPEN.
Table 1.
The composition of the three TPN solutions
| AMINO | PN | UNI | |
|---|---|---|---|
| N+ (mEq/L) | 38.9 | 45.5 | 40 |
| K+ (mEq/L) | 30 | 27.3 | 27 |
| Mg2+ (mEq/L) | 5.6 | 5.5 | 6 |
| Ca2+ (mEq/L) | 5.6 | 7.3 | 6 |
| Cl− (mEq/L) | 38.9 | 45.5 | 59 |
| SO42− (mEq/L) | 5.6 | 5.5 | |
| Acetate− (mEq/L) | 60 | 36.4 | 10 |
| Gluconate− (mEq/L) | 5.6 | 7.3 | 6 |
| Lactate− (mEq/L) | — | — | 35 |
| Malate2− (mEq/L) | — | — | 17 |
| P (mmol/L) | 6.7 | 7.3 | 8.1 |
| Zn (µmol/L) | 10 | 18.2 | 20 |
| Glucose (g/L) | 111.3 | 163.6 | 175 |
| Fructose (g/L) | 55.3 | — | — |
| Xylitol (g/L) | 28 | — | — |
| Total free amino acid (g/L) | 33.3 | 27.3 | 30 |
| pH | 5.56 | 5.1 | 4.36 |
| Titratable acidity (mEq/L) | 24.3 | 31.9 | 44.1 |
| Sodium sulfite (g/L) | 0.4 | 0.04 | 0.48 |
| HCL(mEq/L) | — | — | 35.1–39.1 |
AMINO: Branched chain amino acid (BCAA) rich solution and glucose, fructose and xylitol solution in a separate cavity bag; Aminotoripa®; PN: Milk composition amino acid solution and glucose solution contained in a separate cavity bag; PNtwin®; UNI: BCAA rich solution and glucose solution in same cavity bag; Unicaliq®
TPN product randomization
After written consent was obtained from the patients before operation, the attending physician called the CASPjp call center and the TPN products were assigned randomly using a computer-generated random number. Sixty-four patients who had undergone curative resection were randomized into the three TPN product groups. The TPN solutions used were three general commercial products: Aminotoripa® (AMINO: Branched chain amino acid (BCAA) rich solution with glucose, fructose, and xylitol solution in a separate cavity bag; Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan), PNtwin® (PN: Milk composition amino acid solution and glucose solution contained in a separate cavity bag; Ajinomoto Pharma Co., Ltd., Tokyo, Japan), and Unicaliq® (UNI: BCAA rich solution and glucose solution in same cavity bag; Terumo Corp., Tokyo, Japan). Patients received appropriate electrolytic solutions on operation day and TPN solution from 2 to 7 days after operation. All patients received multivitamins during this study. Blood and urine samples were collected before operation and 1, 3, 5, and 7 days after operation. Serum electrolytes, creatinine, blood urea nitrogen (BUN), lactic acid, pyruvic acid, and blood acid-base status were calculated by using standard automated laboratory techniques. Twenty-four hour urine collections for the measurements of urine electrolytes, pH, creatinine, and urinary NAE were also performed. Urinary NAE was determined by a titrimetric method [12] and was the sum of titratable acid and ammonium concentrations minus the bicarbonate concentration. The titratable endpoint pH was 7.40 at 0 Pa as a PCO2.
Outcome measures
This randomized clinical study was conducted to discover the acidosis risk of TPN therapy, and the primary outcome measure was acidosis as an adverse event. However, considering the patients’ safety, it was difficult to measure the harm of TPN therapy using a randomized clinical study method. Therefore, this study initially evaluated the acidosis risk in animal subjects, and urinary acid excretion was confirmed as a risk factor of acidosis. NAE was correlative with arterial blood pH (p<0.0001). Thus, urinary total acid excretion used as a surrogate outcome of the acidosis risk during TPN therapy.
Statistical analysis
Efficacy was analyzed according to the intention to treat approach. All statistical analyses were performed using StatView for Windows software (Version 5.0, SAS Institute, Cary, NC). All data were presented as means ± s.e. Student’s t test was used for the comparison of data; a p value of less than 0.05 was considered statistically significant.
Results
Of the 76 patients in the surgical ward who received curative operations during the study period, 12 were excluded because of renal insufficiency. Sixty-four eligible patients were assigned randomly to the three groups. The demographics for each group were similar (Table 2). Consent was sought from the 64 eligible patients; all patients agreed to participate in this study. Twenty one, 22, and 21 of the 64 patients were allocated to the AMINO, PN, and UNI groups, respectively. The three groups of patients showed no important differences in baseline characteristics. No patient was withdrawn from the study after randomization. All patient data were included in this study.
Table 2.
The characteristics of patients at entry to the study
| Characteristic | AMINO (n = 21) | PN (n = 22) | UNI (n = 21) |
|---|---|---|---|
| Age (years) | 60.5 ± 2.6 | 55.7 ± 2.9 | 62.1 ± 2.6 |
| Height (cm) | 162.9 ± 2.0 | 161.5 ± 1.7 | 157.7 ± 1.8 |
| Weight (kg) | 69.7 ± 2.0 | 56.9 ± 2.1 | 53.2 ± 2.5 |
| Serum creatinine (mg/dL) | 0.83 ± 0.04 | 0.80 ± 0.04 | 0.76 ± 0.05 |
| Creatinine clearance (mL/min) | 75.1 ± 3.6 | 83.9 ± 5.9 | 73.8 ± 5.1 |
| Gender (male/female) | 7/14 | 7/15 | 9/12 |
| Type of resection gastrectomy | 4 | 9 | 6 |
| proctocolectomy | 8 | 4 | 7 |
| hepatectomy | 2 | 4 | 1 |
| pancreatectomy | 2 | 3 | |
| cholecystectomy | 2 | 2 | 1 |
| others | 3 | 3 | 3 |
Data are shown as mean ± s.e. Creatinine clearance calculated by using the Cockcroft–Gault formula.
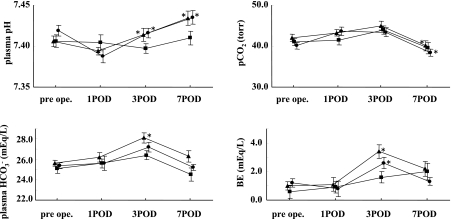
The time courses of plasma pH, HCO3−, pCO2, and blood base excess (BE) levels in the patients after administration of the TPN solutions are shown in Figure 1. The pH of arterial blood increased significantly in the AMINO and PN group after administration. The pCO2 of arterial blood decreased significantly in the AMINO and PN group at 6 days after administration compared with postoperative day (POD) 1. The HCO3− concentration of arterial blood increased significantly in the PN group 1 day after administration. BE increased significantly in the AMINO and PN groups 1 day after administration. There were no significant differences in anion gap among the three groups during the study period. No patients suffered from metabolic alkalosis or acidosis. All patients did not change their body weight during this study.
Fig. 1.
Time courses of plasma pH, plasma HCO3−, pCO2, and BE levels after administration of AMINO (circle), PN (triangle), and UNI (square) TPN solutions. Values are mean ± s.e. *p<0.05 vs POD 1. BE; blood base excess, POD: postoperative day.
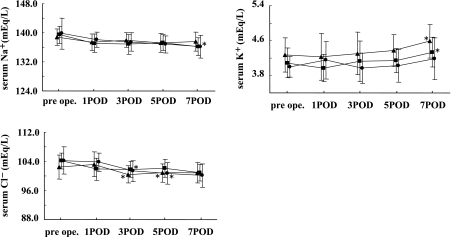
The time courses of serum electrolyte levels after administration of the TPN solutions are shown in Fig. 2. The concentrations of serum electrolytes such as Na+, K+, and Cl−, changed slightly but were within normal limits. There were no significant changes in the concentration of serum creatinine, BUN, urine electrolytes, and urine creatinine during this study (data not shown).
Fig. 2.
Time courses of serum electrolyte levels after administration of AMINO (circle), PN (triangle), and UNI (square) TPN solutions. Values are mean ± s.e. *p<0.05 vs POD 1.
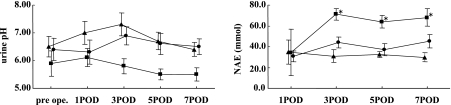
The time courses of urine pH and NAE levels after administration are shown in Figure 3. Urinary pH did not change in the AMINO and PN groups, but decreased in the UNI group after administration, whereas urinary NAE increased significantly in the UNI group after administration.
Fig. 3.
Time courses of urine pH and NAE levels after administration of AMINO (circle), PN (triangle), and UNI (square) TPN solutions. Values are mean ± s.e. *p<0.05 vs POD 1. NAE; net acid excretion.
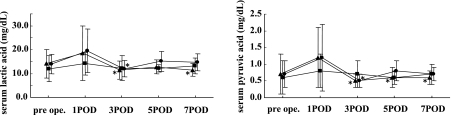
The time courses of lactic acid and pyruvic acid levels are shown in Fig. 4. The serum concentration of lactic acid decreased significantly in the AMINO group at POD 3 and in the PN group at POD 3 and 7 compared to POD 1. The serum concentration of pyruvic acid decreased significantly in the AMINO group at POD 3 and in the PN group on POD 3, 5, and 7 compared to POD 1. There were no significant changes in the serum concentration of lactic acid or pyruvic acid in the UNI groups.
Fig. 4.
Time courses of serum lactic and pyruvic acid levels after administration of AMINO (circle), PN (triangle), and UNI (square) TPN solutions. Values are mean ± s.e. *p<0.05 vs POD 1.
Discussion
Acidosis during TPN therapy could be related to the presence of hydrochloric salts in synthetic L-amino-acid preparations [5–8]. ASPEN and JSPEN have developed clinical guidelines for TPN therapy. However, these guidelines have not commented on the risk of the acid load induced from pH adjustment agents in TPN solutions.
Recently, hydrochloric acid has been added to bagged commercial TPN solutions to avoid Maillard reaction, which is induced by D-glucose and amino acids, and the UNI solution uses hydrochloric salts of synthetic L-amino-acid to avoid this reaction. Organic acids such as acetic acid are added to the TPN solutions to stabilize the pH of the products after heat sterilization [13]. Therefore, two types of acids are added to commercial TPN solutions to maintain the quality of the formula. As a result, the titratable acidity of the nutrient solution consists of hydrochloric acid and organic acids. In general, titratable acidity is higher in solutions containing hydrochloric salts, and the adjusted TPN solutions have higher titratable acidity compared to the standard solutions. Terashima et al. suggested that metabolic acidosis is caused by the high titratable acidity induced from not only non-metabolizable acids but also from metabolizable acids in commercial TPN solutions [9]. This study focused on acids in TPN solutions as a cause of metabolic acidosis and investigated the effect of non-metabolizable and metabolizable acids on the plasma acid-base balance. The present findings suggested that the amount of titratable acidity is not related to the incidence of acid load. Only non-metabolizable acids such as hydrochloric acid influence the patient’s acid load.
Metabolic acidosis is a pH imbalance in which the body has accumulated too much acid and does not have enough bicarbonate to effectively neutralize the effects of the acid. Previously, we reported that the acid load with hydrochloric acid in TPN solutions may cause severe hyperchloremic metabolic acidosis in rabbits [11]. In the present study, urinary pH decreased slightly and NAE increased significantly after administration in UNI group, but other two groups did not change urinary pH and NAE. Urine becomes more acidic when the body is in acidosis. Since urine is a waste, the acid in the urine are lost for the body and this contributes to return the body pH to a normal value. However, there is no regulation that pharmaceutical companies should indicate the amount of added acid in the attached documents for their products. This situation is dangerous for physicians because they cannot determine the risk of acidosis when they order TPN prescriptions. Therefore, information regarding the potential inorganic acid content in commercial TPN solutions should be included in the attached documents for TPN products.
The present study focused on acids in TPN solutions as a cause of metabolic acidosis and investigated the effect of metabolizable and non-metabolizable acids on the plasma acid-base balance. The present results suggest that the amount of titratable acidity is not related to the incidence of acid load. Only non-metabolizable acids such as hydrochloric acid influence serum pH in patients. Non-metabolizable acid may be a risk factor of metabolic acidosis. Therefore, pharmaceutical companies should inform medical professionals of the amount of non-metabolizable acid in TPN solutions to help to avoid metabolic acidosis.
Abbreviations
- TPN
total parenteral nutrition
- NAE
net acid excretion
- CASPjp
Critical Appraisal Skills Programme JAPAN
- BCAA
Branched chain amino acid
- BUN
blood urea nitrogen
- TA
titratable acid
- BE
base excess
- Aminotoripa®
AMINO
- PNtwin®
PN
- Unicaliq®
UNI
- POD
postoperative day
References
- 1.Fong Y.M., Marano M.A., Barber A., He W., Moldawer L.L., Bushman E.D., Coyle S.M., Shires G.T., Lowry S.F. Total parenteral nutrition and bowel rest modify the metabolic response to endotoxin in humans. Ann. Surg. 1989;210:449–457. doi: 10.1097/00000658-198910000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore F.A., Moore E.E., Haenel J.B. Clinical benefits of early post-injury enteral feeding. Clin. Intensive Care. 1995;6:21–27. [PubMed] [Google Scholar]
- 3.Kappers-Klunne M.C., Degener J.E., Stijnen T., Abels J. Complications from long-term indwelling central venous catheters in haematologic patients with special reference to infection. Cancer. 1989;64:1747–1752. doi: 10.1002/1097-0142(19891015)64:8<1747::aid-cncr2820640832>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 4.Buzby G. The veterans affairs total parenteral nutrition cooperative study group. Perioperative TPN in surgical patients. N. Engl. J. Med. 1991;325:525–532. doi: 10.1056/NEJM199108223250801. [DOI] [PubMed] [Google Scholar]
- 5.Kushner R.F. Total parenteral nutrition-associated metabolic acidosis. J. Parenter. Enteral Nutr. 1986;10:306–310. doi: 10.1177/0148607186010003306. [DOI] [PubMed] [Google Scholar]
- 6.Chan J.C., Asch M.J., Lin S., Hays D.M. Hyperalimentation with amino acid and casein hydrolysate solutions. Mechanism of acidosis. JAMA. 1972;220:1700–1705. [PubMed] [Google Scholar]
- 7.Heird W.C., Dell R.B., Driscoll J.M. Jr., Grebin B., Winters R.W. Metabolic acidosis resulting from intravenous alimentation mixtures containing synthetic amino acids. N. Engl. J. Med. 1972;287:943–948. doi: 10.1056/NEJM197211092871901. [DOI] [PubMed] [Google Scholar]
- 8.Caldwell M.D., O’Neill J.A. Jr., Meng H.C., Stahlman M.H. Evaluation of a new amino acid source for use in parenteral nutrition. Ann. Surg. 1977;185:153–161. doi: 10.1097/00000658-197702000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terashima H., Miura O., Hatakeyama S., Hirayama K., Ohkubo S. Hyperchloremic metabolic acidosis associated with TPN solutions. Jpn. JPEN. 1998;20:359–368. [Google Scholar]
- 10.Japanese Government, author. Ministry of Health and Welfare Total parenteral nutrition-associated acidosis. Adverse Drug React. Info. 1994;128 [Google Scholar]
- 11.Sugiura S., Inagaki K., Noda Y., Nagai T., Nabeshima T. Acid load during total parenteral nutrition: Comparison of hydrochloric acid and acetic acid on plasma acid-base balance. Nutrition. 2000;16:260–263. doi: 10.1016/s0899-9007(99)00304-4. [DOI] [PubMed] [Google Scholar]
- 12.Chan J.C. The rapid determination of urinary titratable acid and ammonium and evaluation of freezing as a method of preservation. Clin. Biochem. 1972;5:94–98. doi: 10.1016/s0009-9120(72)80014-6. [DOI] [PubMed] [Google Scholar]
- 13.Lebowitz M.H., Masuda J.Y., Beckerman J.H. The pH and acidity of intravenous infusion solutions. JAMA. 1971;215:1937–1940. [PubMed] [Google Scholar]