Abstract
Tumors metastatic to the bone produce factors that cause massive bone resorption mediated by osteoclasts in the bone microenvironment. Colony stimulating factor (CSF-1) is strictly required for the formation and survival of active osteoclasts, and is frequently produced by tumor cells. Here we hypothesize that the CSF-1 made by tumor cells contributes to bone destruction in osteolytic bone metastases. We show that high level CSF-1 protected osteoclasts from suppressive effects of transforming growth factor β (TGF-β). r3T cells, a mouse mammary tumor cell line that forms osteolytic bone metastases, express abundant CSF-1 in vitro as both a secreted and a membrane-spanning cell-surface glycoprotein, and we show that both the secreted and the cell surface form of CSF-1 made by r3T cells can support osteoclast formation in co-culture experiments in the presence of RankL. Mice with r3T bone metastases have elevated levels of both circulating and bone-associated CSF-1, and the majority of CSF-1 found in bone metastases is associated with the tumor cells. These results support the idea that tumor-cell produced CSF-1 contributes to osteoclast development and survival in bone metastasis.
Keywords: osteoclast, bone metastasis, M-CSF, syngeneic
Introduction
The bone is the most common site of metastasis of breast cancer: nearly 80% of women with metastatic breast cancer have bone metastases at the time of death. While these metastases are difficult to eradicate, the survival of patients with solely bone metastases is significantly higher than if soft tissue metastases are present [1]. Thus, if the bone disease can be stabilized and controlled, prognosis could improve for these patients. However, most breast cancer bone metastases are osteolytic, and can cause extensive, rapid bone loss. This bone loss frequently results in fractures requiring surgery, intractable pain, spinal cord compression resulting from vertebral fractures, and hypercalcemia. Together, these conditions can be debilitating and severely undermine the quality of life for patients with bone metastases. Thus, treatments aimed at reducing the bone loss due to bone metastasis can have extensive benefits for these patients.
Osteoclasts are the normal host cells that are responsible for resorbing bone in normal and abnormal situations. These terminally differentiated, multinucleated cells are highly specialized, forming a tight sealing zone between the cell and the bone surface, secreting protons to dissolve the mineral and proteases to digest the collagen matrix into this space (reviewed in [2]). Osteoclasts differentiate from the hematopoietic lineage, with monocytes being the immediate precursors: these precursors are abundant in bone marrow [3]. Osteoclast differentiation can be completely supported by two cytokines: RankL and CSF-1. In vitro, osteoclast differentiation from bone marrow is dependent on these two proteins; in vivo, mice lacking either RankL or CSF-1 have few osteoclasts, and are severely osteopetrotic, with thick, shortened bones and minimal bone marrow spaces [4]; [5].
Stimulation of osteoclastogenesis and the resultant destruction of bone tissue are required in order for bone metastatic tumors to grow rapidly. This requirement for bone destruction arises from two sources: first, the bony structure physically limits the growth of the tumor, and second, growth factors released from storage in the bone extracellular matrix can accelerate tumor proliferation [6], in what has been called the vicious cycle of bone metastasis [6–8]. Thus, there is a complex interplay of tumor cells and the bone microenvironment that regulates the development of bone metastasis. Several breast cancer cell lines metastatic to bone secrete PTHrP [9,10], which in turn induces RankL production in osteoblasts [11]. Reagents that block RankL-Rank interaction are able to prevent the bone loss associated with bone metastasis [12]. While CSF-1 is also required for osteoclast survival and differentiation [13], its role in bone metastasis is less well understood. CSF-1 has been detected by immunohistochemistry in at least one third of breast carcinomas [14], as well as in ovarian and endometrial tumors [15]. Elevated serum levels of CSF-1 have been detected in breast cancer patients, and higher serum CSF-1 levels were correlated with worse prognosis, and shorter disease free interval [16,17]. These results suggest that CSF-1 expression by tumor cells may facilitate bone metastasis. The metastatic breast cancer cell line MDA-MB231 makes CSF-1 [18], and stimulates osteoclastogenesis in vitro, but it is not clear whether this is a widespread mechanism by which tumor cells support osteoclastogenesis. Here we examine CSF-1 expression in the mouse mammary epithelial cell line r3T that is highly metastatic to the bone, and demonstrate that CSF-1 is likely to be a key tumor-derived factor regulating osteolysis during bone metastasis.
Materials and Methods
Generation of murine osteoclasts in vitro
Mice used for experiments in a 129 (S1, S7) mixed background were bred in-house and handled in accordance with guidelines of The Forsyth Institute Animal Care and Use Committee. Mouse bone marrow osteoclast-like cells were obtained from six to ten- week-old mice as previously described [19]. Briefly, marrow was flushed from femurs and tibia, washed and incubated overnight in bone marrow medium (BMM: α-MEM, 10% heat inactivated FBS (fetal bovine serum, Atlanta Biologicals, Lawrenceville, GA)) supplemented with penicillin and streptomycin (Sigma, St. Louis, MO). Next day, non-adhered cells were layered on histopaque-1083 and cells at the interface were cultured in BMM with mouse CSF-1 (colony stimulating factor-1; Peprotech, Rocky Hill, NJ; 10 ng/ml) and RankL (receptor activator of NF-κB ligand; Peprotech or GST-RANKL purified in the lab; 30–120 ng/ml). After 4 days, osteoclasts were collected by scraping in cell stripper solution (Cellgro, Lawrence, KS) and re-plated at 1×105 cells/ml in 24-well plates and incubated in BMM with or without CSF-1 in the presence or absence of cytokines or other agents as described. For TGF-β treatments, mature osteoclasts were seeded onto coverslips at a cell density of 1×105 cells/ml in 24-well plate and grown in complete medium with CSF-1 and RankL. The next day, the cells were treated with 2 ng/ml TGF-β (transforming growth factor β) plus CSF-1 and RankL. Experiments were performed several times in duplicate.
Cell lines and culture
Mammary epithelial cell line r3T and its precursor cell lines were cultured in α-MEM with 8% FBS, glutamine and pen/strep as described (20). Osteocyte-like cell line MLOY4 [20] was grown in a-MEM with 5% FBS and 5% CS (calf serum) (Gibco). MC3T3 cells were cultured in α-MEM with 10% FBS. Mouse embryonic fibroblast cell lines were grown in D-MEM (Dulbecco’s modified Eagle’s medium) (Gibco) with 10% CS and sub-cultured every three or four days.
Mouse embryonic fibroblast (MEF) cell line preparations and genotyping
Heterozygous Csf1+op mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and bred at The Forsyth Institute. Embryonic fibroblast cell lines were prepared from four embryos as described previously [21]. Briefly, heterozygous Csf1+op mice were mated embryos were removed at 12–16 days. Dissociated embryos were trypsinized and placed into culture. DNA was prepared from ear snips, and PCR amplifications were performed using primers: 5′-TGTGTCCCTTCCTCAGATTACA-3′(forward) and 5′-GGTCTCATCTATTATGTCTTGTACCAGCCAAAA-3′(reverse). Then, PCR products were cut with Bgl I (NEB, Ipswich, MA) and products were separated on 4% Metaphor agarose gel (Cambrex) for high-resolution visualization of fragments.
Cell lysate and conditioned medium (CM) preparations
Cell lysates were prepared from 90% confluent cells by incubation in lysis buffer (20 mM Tris-HCl pH 7.5, 0.6 M KCl, 1% Triton X-100) and protease inhibitors (Sigma, St. Louis, MO) for 30 min on ice, centrifuged and protein concentrations determined by BSA assay. Conditioned medium (CM) was prepared from 90% confluent cells [22]. For westerns, proteins from 100 ul of CM was collected by acetone precipitation prior to loading on the gel.
Immunoblotting and ELISA
Western blotting was as described previously [23]. CSF -1 was detected with biotinylated goat anti-CSF antibody (#AF416, R&D Systems, Minneapolis, MN); anti-beta-actin antibody (Sigma) was used for controls. CSF-1 levels in lysates and serum samples obtained at euthanasia were measured using enzyme-linked immunosorbent assay (ELISA). This was performed according to the instructions for the mouse CSF-1 ELISA Duoset Kit (R &D systems), substituting antibody AF416 for the monoclonal antibody supplied as capture antibody with the kit. We were unable to detect secreted CSF-1 using the Duoset kit as supplied, and hypothesize that the monoclonal antibody does not detect glycosylated CSF-1. ELISA conditions and antibody concentrations were optimized in our laboratory. Briefly, a 96 well plate was coated with antibody AF416 (2 μg/ml) overnight at room temperature. After blocking and sample incubation according to the manufacturer’s instructions, the washed plate was then incubated with detection antibody (# BAF416, R&D Systems, 0.2 μg/ml) for 1 h and then with Streptavidin-HRP for 20 min. Optical density of each well was determined immediately using a microplate reader set to 450 nm. All standards and samples were assayed in duplicate.
RNA extraction and PCR analysis
Cells were washed three times with PBS and total RNA was harvested in TRIzol reagent (Invitrogen, Carlsbad, CA) and purified according to manufacturer’s instructions. RNA samples were monitored on agarose gel and yield was calculated. One microgram of RNA was converted to cDNA with MMLV reverse transcriptase (Gibco) in 20 μl reaction. The resulting cDNA samples were used in PCR analysis of soluble CSF-1 (Pa:5′-CCACTTGTAGAACAGGAGGCCC-3′; Pb: 5′ GCTTGAGGGCAAGAGAAGTACC-3′) and cell-surface associated CSF-1 (Pa:5′-CCACTTGTAGAACAGGAGGCCC-3′; Pc: 5′ CCAAGAACTGCAACAACAGCTTTGC-3′ [24], and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), (5′-GGGTGTGAACCACGAGAAAT-3′, 5′-CCTTCCACAATGCCAAAGTT-3′) expression. cDNAs made from cell lines and tumor-bearing mouse bones were used in real-time PCR analysis of total CSF-1 RNA with primers: 5′-CTCATGAGCAGGAGTATTGCCA-3′, 5′-ATTTGACTGTCGATCAACTGCTG-3′. Conditions for qPCR were as described previously [25] using Sybr green: the CSF-1 assay was performed using a PCR-amplified fragment of the mouse CSF-1 cDNA as standard, and had an efficiency of 101%. Numbers of molecules of CSF-1 was divided by molecules of GAPDH to calculate relative CSF-1 expression. Controls without reverse transcriptase were performed for all samples. Tumor cell numbers in bone samples were determined using a qPCR assay for the r3T cells described previously [9].
Flow cytometric analysis
The r3T cells were re-suspended in PBS buffer containing 5% FBS and incubated with biotinylated mouse-CSF-1 antibody (BAF416, R&D Systems) for 45 min at 4 °C. Cells were washed with PBS buffer containing 5% FBS, collected and stained with PE-conjugated avidin for 45 min at 4 °C. Labeled cells were analyzed using Coulter® Epics® Altra™ flow cytometer (Beckman Coulter, Fullerton, CA). Signal of 1×106 cells was measured.
Mitomycin C (MMC) treatment and glutaraldehyde fixation
For co-culture experiments, confluent cell lines were incubated with 20 μg/ml mitomycin (MMC) (Sigma) at 37°C for 4 hr. Treated cells were washed, trypsinized and plated at 1×104 cells/ml in 24-well plates and cultured overnight in complete medium. Alternatively, cells were fixed with glutaraldehyde as described earlier [26]. Briefly, cells were grown in 24-well plates to 70% confluence, fixed with 2.5 % glutaraldehyde for 1 min and immediately quenched with 1.5% glycine in PBS for 20 min. Fixed cells were washed and incubated overnight in complete medium before co-culturing with mature osteoclasts. Each treatment was repeated in three separate experiments in duplicate.
TRAP and DAPI staining
Osteoclast cells were fixed in 3% p (Sigma) for 20 min at room temperature, permeabilized in 70% ethanol for 1 min, washed with PBS, and stained for TRAP (tartrate-resistant acid phosphatase) using fast red solution (0.2 M sodium acetate, pH 5.0, Naphtol AS-MX phosphate (Sigma) (0.5 mg/ml), fast red TR salt (Sigma) (1.1 mg/ml) at 37°C for 10 min. TRAP positive cells were photographed and were evaluated blind by quantification of the number cells with two or more nuclei from five different areas. Cells grown on coverslips were mounted in antifade-mounting medium containing 25 mg/ml DABCO (1, 4 diazabicyclo octane) (Sigma) and 0.5 μg/ml DAPI (4, 6-diamaino-2-2phenylindole) (Sigma). Experiments were performed multiple times in duplicate.
Immunohistochemistry
Bone metastases from mice injected with r3T cells were obtained as described previously [9]. Briefly, r3T cells (1 × 106/mouse) were injected into the left ventricle of the heart, and animals were sacrificed 18–24 days later. Negative control bones from CSF1op/op mice were from 12-day pups. Bones were fixed in 4% paraformaldehyde and decalcified in formic acid/sodium citrate buffer. Sections were rehydrated and blocked in 5% serum, incubated in primary anti-mouse CSF-1 antibody (AF416,1:50; R&D Systems) overnight at 4°C or anti-cathepsin K [27] antiserum (1:4000) for 2 hr at room temperature. After secondary biotinylated anti-goat rabbit antibody for CSF-1 and anti-rabbit for cathepsin K (1:300; Vector), the sections were reacted with biotin-avidin HRP complex (ABC reagent, Vector), and visualized with 3,3-diaminobenzidine (DAB) tetrahydrochloride. Nuclei were counterstaining with hematoxylin or 1% fast green. Sections incubated with only secondary antibody were used as controls and showed no staining.
Statistical analysis
Experiments were performed in duplicate in multiple times as indicated. The results are confirmed in several experiments, and are presented as either representative results or mean ± SEM. Graph Pad Prism software was used to calculate standard deviations and p values using unpaired t-tests.
Results
CSF-1 overcomes TGF-β inhibition of osteoclast survival
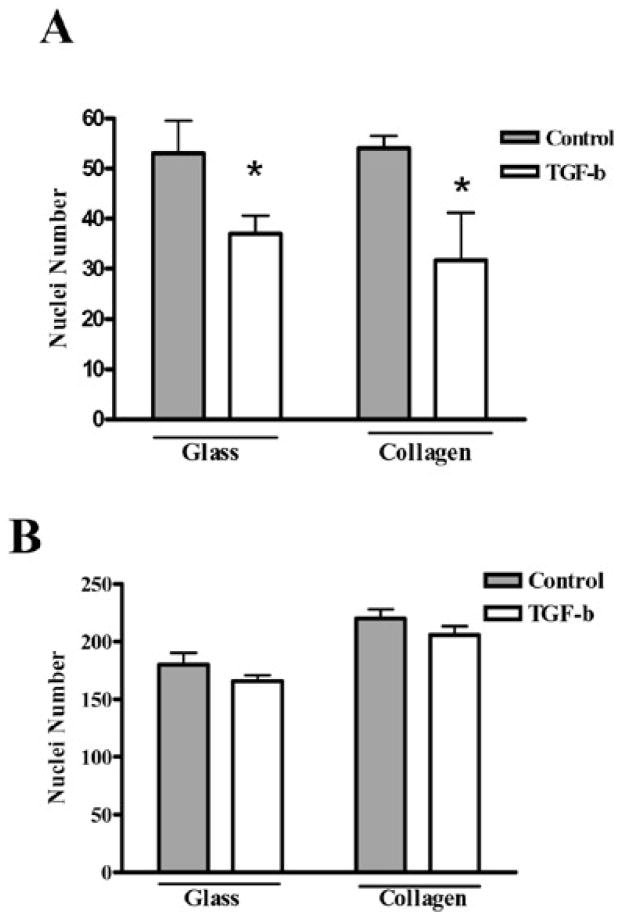
Earlier studies have shown that conditioned medium from cancer cells producing higher level of CSF-1 supports higher levels of bone resorption, suggesting that CSF-1 originating from cancer cells may contribute to osteoclast activity at the metastatic site by enhancing their survival [28]. To assess the direct effect of CSF-1 on osteoclast survival, mature osteoclasts cultured on glass and collagen-coated coverslips were treated with 2 ng/ml TGF-β in the presence of RankL (120 ng/ml) and CSF-1 (10 or 100 ng/ml). The cells that survived TGF-β treatment were determined by staining cell nuclei with DAPI. The DAPI stained nuclei were counted from six different random fields for each experimental condition. The number of DAPI stained nuclei decreased significantly after TGF-β treatment in the presence of 10 ng/ml CSF-1 (* p< 0.05) (Fig. 1A). However, when the CSF-1 level was increased to 100 ng/ml in the same experimental conditions, the effect of TGF-β on cell survival was blocked, and there was no significant difference in nuclei numbers between treated and untreated cells (Fig. 1B). These results suggest that high level expression of CSF-1 in tumors may enhance osteoclast survival in the presence of TGF-β, in addition to supporting osteoclast differentiation. There are multiple cell types in the vicinity of tumors that secrete CSF-1, including osteoblasts and stromal cells. However, several metastatic cell lines have also been shown to secrete CSF-1. Thus, we then asked whether CSF-1 made by tumor cells themselves are a major source of this cytokine in bone metastases.
Fig. 1.
High CSF-1 concentration overcomes TGF-β effect on osteoclast survival. Mature osteoclasts were cultured on glass or collagen-coated coverslips overnight, and then treated with or without 2 ng/ml TGF- β for 48 hr in the presence of 10 ng/ml CSF-1 (A) or 100 ng/ml CSF-1 (B). DAPI stained nuclei were counted from six different random fields at 40x magnification. Mean results of three experiments are shown. (* p< 0.05, TGF- β vs. control)
r3T cells make CSF-1
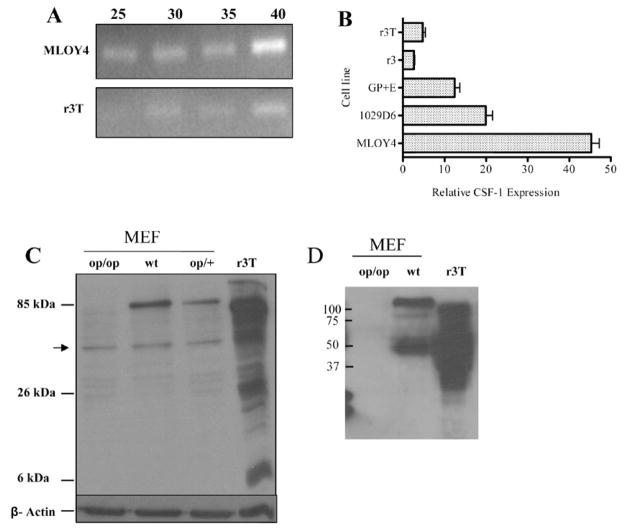
The r3T cells have been described in detail earlier: these cells form osteolytic bone metastases with high efficiency after injection into the arterial circulation of syngeneic mice [29]. Semiquantitative RT-PCR analysis (Fig. 2A) showed that r3T cells produce detectable CSF-1 mRNA; the osteocyte-like cell line MLOY4 [20], which expresses high-level CSF-1, was used a positive control. The r3T cells were derived by in vitro transformation of a cell line from a carcinogen induced mammary tumor [29] – quantitative PCR showed that these cells as well as its parental, less metastatic cell lines 1029 D6, GP+E and r3 all express CSF-1, although all these cell lines express less CSF-1 than MLOY4 (Fig. 2B). Western blot analysis confirmed expression of CSF-1 protein in r3T cell lysates. CSF-1 is a multiply glycosylated protein migrating with a molecular mass ranging from 25 to 100 kDa under reducing conditions [30] and various glycosylated and sulfated forms of the protein have been observed [31]. The predominant form of CSF-1 detected in r3T lysates in our immunoblots was about 80 kDa (Figure 2C, lane 4): a slightly larger, ~85 kDa protein band was detected in wild type and heterozygous MEF cell lines (Figure 2C, lanes 2 and 3), but not in CSF-1-deficient (op/op) MEF cells (Figure 2C, lane 1). The reason for the difference in migration of CSF-1 in these different cell lines is unclear, but may reflect different patterns of glycosylation or sulfation. Since reducing agent was added to all samples, the different forms probably do not represent dimers [31]. Several lower molecular weight bands were seen in both CSF-1-deficient and r3T cells (arrow, Figure 2C); the identity of these bands is unknown. Similar levels of β-actin were found in all cells confirming similar protein loading. The secreted form of CSF-1 was also identified by western blotting of conditioned medium from different cell lines (Figure 2D). The predominant band seen in MEF and r3T cells migrated at about 50kD, while additional smaller forms were seen in the r3T cells. There was no CSF-1 protein detectable in the conditioned medium from the CSF-1op/op MEF cells. The 80–85 kD band was not observed in the conditioned medium samples, suggesting that these bands represent membrane associated precursors.
Fig. 2.
Analysis of CSF-1 expression in r3T cells. A: semi-quantitative PCR analysis for CSF-1 in MLOY4 (upper panel) and r3T cells (lower panel). The number of PCR cycles is indicated above each lane. B: qPCR analysis of CSF-1 expression in r3T cells compared to the parental cell lines (1029 D6, GP+E, r3) and MLOY4. CSF-1 expression was quantified by real-time PCR, and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression. C: western analysis of CSF-1 expression in cell lysates from CSF-1 deficient (op/op) MEF (lane 1), wild type MEF (lane 2), heterozygous MEF (lane 3) and r3T cell lines (lane 4). The approximate molecular weight of major bands are indicated: the arrow indicates a band seen in all lanes including the CSF1- deficient cells. The membrane was subsequently stripped and re-probed with β-actin antibody as loading control. D: western analysis of CSF-1 expression in conditioned medium from CSF-1 deficient (op/op) MEFs (lane 1), WT MEFs (lane 2) and r3T cells (lane 3). Positions of molecular markers in kD are indicated.
r3T cells express both the soluble and cell-surface forms of CSF-1
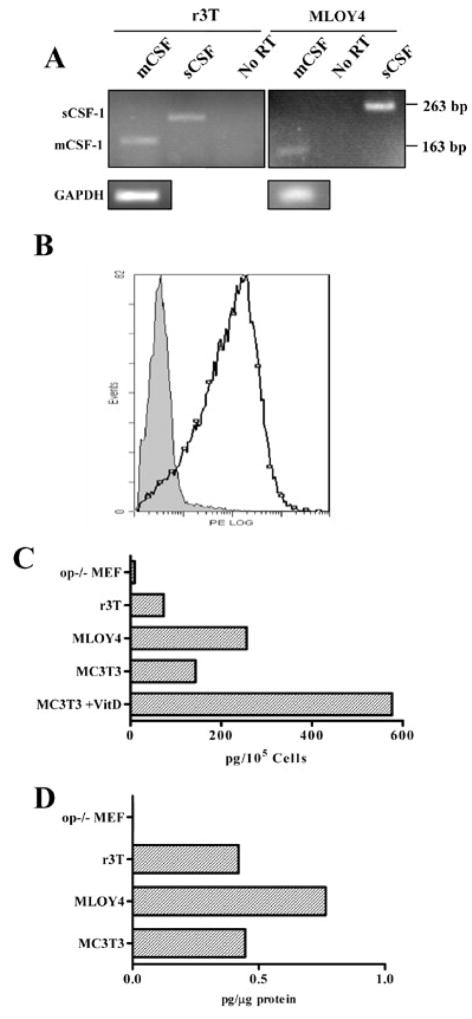
CSF-1 can be expressed as both secreted and cell-surface associated forms: the secreted forms include both proteoglycans and glycosylated molecules [32,33]. These molecules are derived from 5 different CSF-1 mRNAs: differential splicing whereby alternative splice acceptor sites in Exon 6 are used is responsible for the production of cell-surface associated vs secreted proteins [34]. A 4kb mRNA codes for a protein that is expressed on the cell surface and is rapidly cleaved to generate soluble CSF (sCSF-1), while a 1.4 kb mRNA is translated to a transmembrane form (csCSF-1) that is cleaved only slowly [24]. An additional alternative splice site at the 3′ end generates mRNAs with different 3′ untranslated sequences [35]. To examine whether r3T cells express mRNA encoding the cell-surface associated (csCSF-1) and soluble (sCSF-1) forms of CSF-1, total RNA was prepared from r3T and MLOY4 cells, and the alternatively spliced forms of CSF-1 exon 6 in r3T cells were identified by RT-PCR. Primers Pa and Pb [24] generate a 263 bp PCR product, which contains the full exon 6, representing sCSF-1 mRNA, while primers Pa and Pc amplify a 163 bp product [24] from the alternatively spliced mRNA (csCSF-1). Both these products were amplified from r3T cells (Fig. 3A, lanes 1 and 2). Similar results were seen for MLOY4 cells (Fig. 3A, lanes 4 and 6). GAPDH-specific primers yielded a 100 bp PCR product (Fig. 3A). Confirmation that the r3T cells produce the cell surface form of CSF-1 was obtained by FACS analysis, where the majority of unfixed r3T cells stained strongly for cell-surface CSF-1 (Fig. 3B). CSF-1 produced by r3T cells was quantified by ELISA for mouse CSF-1. sCSF-1 was detected by ELISA of conditioned medium, while csCSF-1 was estimated by assaying cell lysates, which should contain both cell-surface associated CSF-1 and any precursor forms in transit to the cell membrane. The levels of both CSF-1 isoforms in r3T cells (73 pg/105 cells in CM, Fig. 3C; 0.42 pg/μg protein in cell lysate, Fig. 3D) were lower than that of MLOY4 cells (256 pg/105 cells in CM, Fig. 3C; 0.76 pg/μg protein in cell lysate, Fig. 3D). Interestingly, the expression of both forms of CSF-1 in r3T cells was similar to that in osteoblastic MC3T3 cells. Expression of soluble CSF-1 in MC3T3 cells was increased following incubation with 100 nM Vitamin D (MC3T3 stimulated). As expected [36], CSF-1 levels were below the limit of detection in CSF-1 deficient (op/op) MEF cells (Fig. 3C, D).
Fig. 3.
r3T cells express both cell-surface associated and secreted CSF-1. A: different primer sets were used to identify csCSF (163 bp) or sCSF (263 bp). Lanes 1–3, r3T cDNA, lanes 4–6, MLOY4 cDNA. No-reverse transcriptase controls (no RT) confirm that the bands seen result from transcripts, not genomic DNA. PCR for GAPDH (lower panels) was used to demonstrate similar cDNA levels. B: FACS analysis of CSF-1 surface expression on r3T cells. Biotin-labeled anti CSF-1 antibody was used at 1 μg/reaction for flow cytometry, and detected with PE-avidin. Solid peak: No primary antibody. Open peak: CSF-1 antibody. C, D: quantification of CSF-1 levels in different cell lines by ELISA. C: sCSF-1 levels were determined in conditioned medium of indicated cell lines by ELISA. Results were represented as pg sCSF/1×105 cells. D: CSF-1 level in cell lysates was normalized to total protein.
r3T cells in co-culture support osteoclast survival
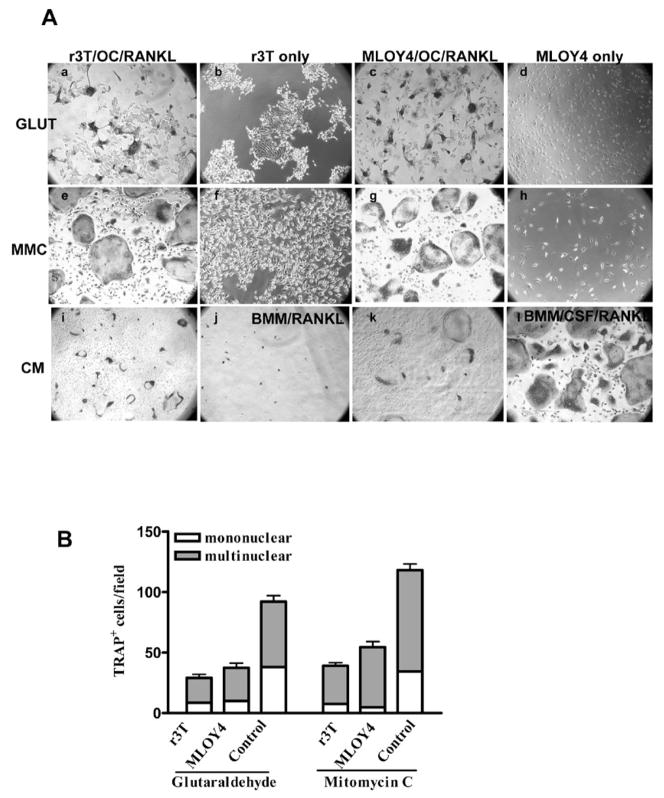
CSF-1 is an obligate growth factor for osteoclasts: adherent osteoclast-like cells differentiated from mouse bone marrow precursors are unable to survive and differentiate into mature osteoclasts in the absence of CSF-1 even in the presence of RankL (Fig. 4A; panel j). However, these osteoclast-like cells when co-cultured with r3T cells were able to differentiate into mature osteoclasts in the presence of RankL but without exogenous CSF-1 as shown by the formation of TRAP-positive multinucleated cells (Fig. 4A; Panels a and e). To test the involvement of both CSF-1 isoforms in osteoclast formation, r3T cells were pretreated with mitomycin C (MMC) or glutaraldehyde prior to incubation with osteoclast precursor cells. Mitomycin C is a cross-linking agent that mitotically inactivates cells, and is used to prepare feeder cells: cells treated with MMC will express both cell surface and secreted CSF-1. Glutaraldehyde, on the other hand, kills the cells and prevents the continued synthesis and processing of sCSF-1, but preserves the structure and bioactivity of csCSF-1 already present on the cell membrane [37]: CSF-1 was undetectable by ELISA in conditioned medium harvested from glutaraldehyde-fixed cells (data not shown). When incubated in co-culture with osteoclast-like cells and RankL, both glutaraldehyde-fixed (Fig. 4A; panel a) and MMC-treated (Fig. 4A; panel e) r3T cells were able to support osteoclast survival. The ability of r3T and MLOY4 cells to support osteoclast survival and differentiation into mature TRAP-positive cells was similar (compare Fig. 4A panels a, c, e, g; Fig 4B). The TRAP-positive cells produced in these co-cultures were mostly multinuclear (Fig. 4B) and were able to form pits on dentine slices (data not shown). On the other hand, when these osteoclast-like cells were co-cultured with Csf1op/op MEF cells which do not synthesize CSF-1, osteoclasts did not survive (data not shown). The effect of sCSF-1 alone on osteoclast survival was tested using conditioned medium harvested from r3T or MLOY4 cells as an exogenous CSF-1 source. As expected, these conditioned media were able to support osteoclast survival in the presence of RankL, but the effect was less than that seen in the co-culture experiments (Fig. 4A; panels i, k). Osteoclast precursors cultured with soluble purified CSF-1 and RankL were used as a positive control in all experiments to monitor osteoclast precursor preparation and experimental conditions (Fig. 4A; panel l).
Fig. 4.
Effect of cell-surface associated and soluble CSF-1 forms on osteoclast formation. A: r3T or MLOY4 cells were treated with glutaraldehyde (GLUT, panels a–d) or mitomycin C (MMC, panels e–h). Treated cells were co-cultured with osteoclast precursors (OC) and RankL (panels a,c,e,g) or cultured alone (r3T only, panels b,f; MLOY4 only, panels c,g). Osteoclast precursors were incubated with RankL and conditioned medium (CM) from r3T (panel i), or MLOY4 cells (panel k) as indicated. Negative controls (BMM medium with RANKL, but no added CSF-1, panel j) and positive controls (BMM medium with 10 ng/ml CSF-1, panel l) are also shown. Different preparations of r3T and MLOY4 cells were plated and incubated overnight. Next day, osteoclast-like cells were added in the presence of complete medium plus RankL. Cultures were stained for TRAP after 3 days. B: TRAP positive mono- and multi-nucleated osteoclast cell numbers were determined from five random fields for each co-culture condition. Control – osteoclast precursors cultured in complete medium plus RankL and purified CSF-1(10 ng/ml) Representative results of multiple experiments are shown.
CSF-1 levels are elevated in the plasma from tumor-bearing mice and in bone metastases
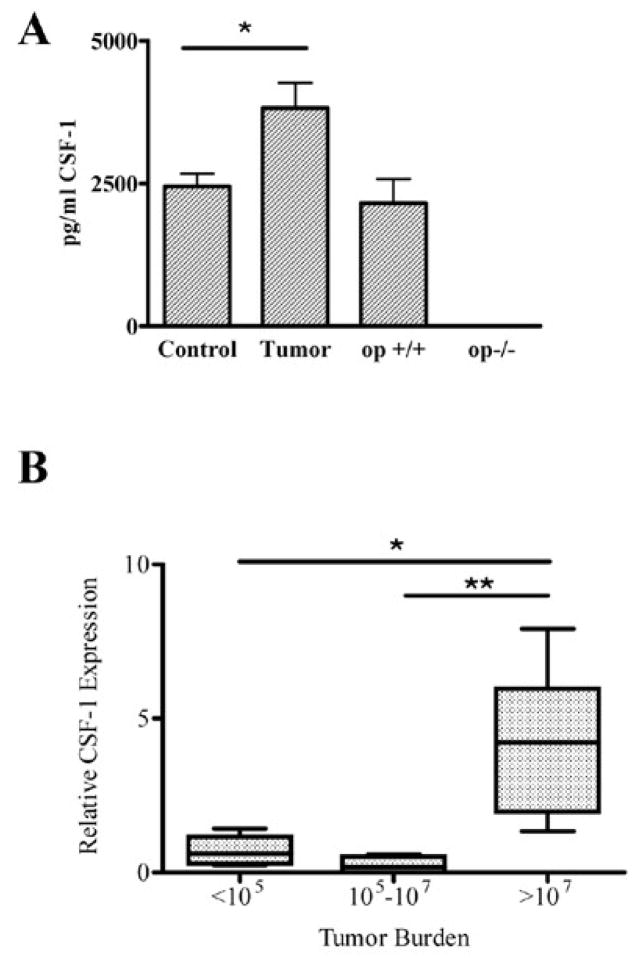
When injected into the arterial circulation, r3T cells form bone metastases, primarily in the proximal tibia and distal femur [9,29], although some metastases are found in other organs [38]. The contribution of r3T-derived tumors to plasma CSF-1 levels was estimated by measuring CSF-1 levels in plasma from control and tumor-bearing mice at the time of sacrifice; we found that plasma levels of CSF-1 were increased by 40% (p<0.01) in tumor-bearing mice compared to control mice. (Fig. 5A). As expected, CSF-1op/op mice had no detectable CSF-1 level in the serum, while their WT littermates had levels similar to control mice. To estimate the CSF-1 production associated with metastatic bone lesions, CSF-1 mRNA was measured by qPCR in bones from tumor bearing and control mice. In this experiment, the tumor burden in each bone sample was estimated using a qPCR assay for tumor cells as described previously [9]. Using RNA isolated from the same bone samples, CSF-1 expression was determined by qPCR analysis using primers for total CSF-1, and was normalized to GAPDH expression. Normalized CSF-1 expression was significantly increased in bones with the highest tumor burden (p< 0.02; Fig. 5B). Together, these results demonstrate that CSF-1 levels are elevated in bone metastases.
Fig. 5.
CSF-1 levels in tumor-bearing mice. A: CSF-1 levels were determined by ELISA in serum samples from control mice (n=9) and mice with bone metastases (n=10). Serum from two week old wild type (op+/+) (n=3) and CSF-1-deficient mice (op/op, n=2) confirmed the specificity of the ELISA (* p< 0.02). B: relative CSF-1 expression was determined by qPCR in cDNA prepared from bones from tumor-bearing mice samples, and normalized to GAPDH expression. Tumor burden in each bone was determined by qPCR and represents the number of r3T cells/bone [9]. n=4 (<105); 6 (105–107); 8 (>107)) (* p< 0.002; ** p< 0.02).
CSF-1 expression in vivo in bone metastasis
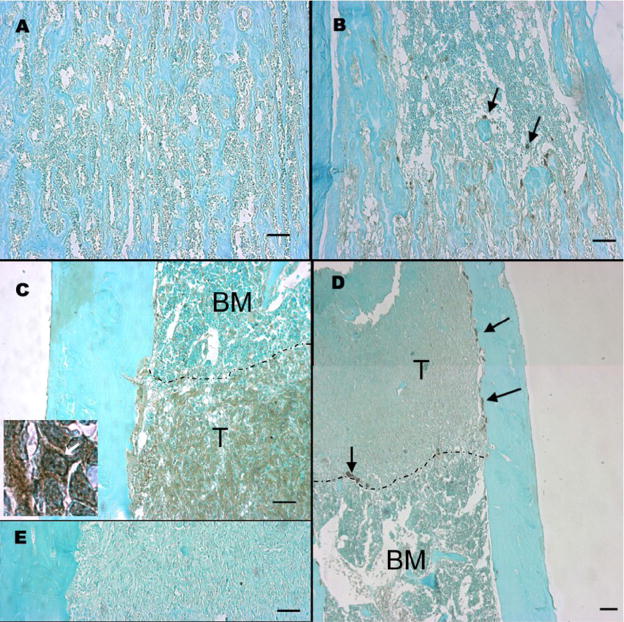
To estimate the relative contribution of host and tumor cells to the CSF-1 in the tumor, we examined CSF-1 expression at the protein level by immunohistochemistry in tibiae from tumor-bearing mice. In normal bones from young mice, CSF-1 was detected in scattered cells associated with trabeculae, possibly osteoblasts (Figure 6B, arrows). In tumor bearing bones, CSF-1 expression was widespread throughout the tumor (Fig. 6C), and expression in the tumor was much higher than in nearby normal bone marrow. While it is difficult to distinguish host cells within the tumor, it is clear that tumor expression of CSF-1 is strong, and is likely the predominant source of CSF-1 in these tumors by virtue of the preponderance of tumor cells present. The high power image (inset, Figure 6C) shows strong staining at the edge of some cells (arrow), consistent with membrane expression. Antibody specificity was confirmed by comparing the reactivity of the CSF-1 antibody on sections from op/op and WT tibiae from 3-week old mice (Fig. 6A, B) and by use of no primary antibody controls (Fig. 6E). Interestingly, multinucleated cathepsin K positive osteoclasts were located only in close proximity to tumor cells (Fig. 6D, arrows), suggesting that cell-cell contact with tumor cells may be important for osteoclast differentiation and survival in response to tumor development in the bone.
Fig. 6.
CSF-1 expression in normal and tumor-bearing bones determined by immunohistochemistry. A: Femur from CSF-1op/op mouse, 2–3 weeks of age, CSF-1 antibody. B: femur from a WT mouse, 2–3 weeks of age, CSF-1 antibody. C: tibia from a WT mouse (4–5 months of age) injected with r3T cells, CSF-1 antibody. Inset: higher magnification (100x) of the same sample: arrow indicates potential membrane staining. T- tumor cells, BM- bone marrow. Dashed line indicates tumor border. D: Same sample as C, cathepsin K antibody. The image is a merge of two fields. E: same sample as C without primary antibody. Black bars represent 100 μm. Antibody staining is visualized as brown staining; sections were counterstained with methyl green.
Discussion
CSF-1 has been identified in a variety of different tumor types, and has been suggested to enhance tumor development and metastasis [39], possibly through an effect on tumor-associated macrophages [40]. We hypothesize that CSF-1 expression in metastatic tumor cells is additionally important because of its ability to stimulate osteoclast function, promoting the osteolysis that accompanies breast cancer metastases. This role of tumor-expressed CSF-1 may be independent of its function in enhancing metastasis.
Several groups have shown previously that CSF-1 is expressed in human osteolytic cell lines such as MDA-MB-231 [18,41,42]. Here, we extend these previous observations in showing that metastatic tumor cells express both the secreted and cell-surface forms of CSF-1, and that the cell surface form alone can support osteoclast differentiation and survival. Further, we show that elevated CSF-1 levels can protect osteoclasts from deleterious effects of TGF-β, and that CSF-1 levels are elevated in bone metastases: immunohistochemical analyses suggested that tumor cells are the predominant source of CSF-1 in these bone metastases. Together these results are consistent with the hypothesis that high level CSF-1 expression due to the presence of tumor cells at the site of bone metastasis facilitates osteoclast differentiation and survival. These results are also consistent with studies in breast cancer patients, where plasma CSF-1 levels were found to be higher in patients with more advanced breast cancer [16,17]. While these studies did not identify patients with bone metastasis it is estimated that 80% of patients with metastatic breast cancer have bone metastases [1], consistent with the idea that elevated CSF-1 expression in breast cancer supports development of bone metastasis.
The cell surface form of CSF-1 is found in numerous cell types, including osteoblasts, where expression of this form, in the absence of soluble CSF-1 can support osteoclastogenesis in vitro [26,37]: these authors suggested that the amount of cell surface-associated CSF-1 needed to effect osteoclastogenesis might be less than that of the secreted form, due to its increased local concentration. In vivo, csCSF-1 alone can correct the osteoclast deficiency in CSF-1 deficient op/op mice when overexpressed in osteoblasts [43], but this form of CSF-1 is less effective than WT when expressed under the control of the same CSF-1 promoter [34]. Our observations that either mitomycin c or glutaraldehyde treated r3T cells producing soluble and/or cell-surface associated CSF-1 supported formation of osteoclasts in in vitro co-culture systems in the presence of RANKL are consistent with these findings. While mitomycin c treated r3T cells provided both CSF-1 forms to the murine osteoclast-like cells in co-culture, glutaraldehyde treated r3T cells provided only the cell-surface associated form of CSF-1, and osteoclastogenesis was efficient under these conditions. Overall our in vitro co-culture results indicated that both CSF-1 forms expressed by the r3T metastatic breast tumor cells are active and support osteoclastogenesis. The role of cell-cell contact in the development of bone metastases is still unclear, but in our model, osteoclasts are only found in close proximity to the tumor cells (Fig. 6), suggesting that cell surface proteins or poorly diffusible factors are required for tumor-associated osteoclast differentiation and/or survival. Tumor cell-associated CSF-1 may be one such factor. Of course, RANKL, which can also be expressed as a secreted or membrane-associated protein[44], is additionally required for osteoclast differentiation and survival in all these experiments [12]: we have shown previously that this cytokine is not expressed by r3T cells [9], so host cells are the sole source of this critical cytokine in this model.
Using a sarcoma model in syngeneic mice, Clohisy and co-workers demonstrated that tumor cells alone can provide sufficient CSF-1 for the development of osteolytic lesions in mice deficient for CSF-1 expression [45]. This work clearly demonstrates the importance of tumor-derived CSF-1 in supporting osteoclastogenesis in development of bone metastases. These experiments were performed using tumor cell injection directly into the bone, where a large bolus of tumor cells are delivered directly into the bone microenvironment. Whether this is also the case in a breast cancer model where tumor cells are introduced into the arterial circulation is still unclear. Our results suggest that tumor-associated CSF-1 in a breast cancer model may also be sufficient for osteoclast development in the absence of host CSF-1: further experiments are required to test this idea.
Osteopontin (OPN)-deficient mice are resistant to bone resorption in vivo due to defects in osteoclast migration and resorption [46,47]. We have shown that this osteoclast defect in the absence of osteopontin is overridden by tumor cells, in that bone resorption associated with osteolytic bone metastasis is unaffected by osteopontin deficiency [9]. OPN affects osteoclast function at least partially through its interaction with the αvβ3 integrin [19]. In vitro, the osteoclast differentiation defect observed in β3-integrin deficient mice can be overcome in the presence of high concentrations of CSF-1, suggesting crosstalk between signaling pathways stimulated by integrins and growth factors in osteoclasts [48]. Here we suggest that the same mechanism may occur during bone metastasis. High level expression of CSF-1 in bone metastases may mimic the effects seen in vitro, stimulating normal osteoclast function in the absence of β3-integrin-signaling initiated by osteopontin. While tumor-cell produced CSF-1 does not override the osteoclast defect in β3-integrin-defiecient mice [49], the defect in the OPN −/− mice is less severe, and may be affected by CSF-1 overexpression. We show that breast cancer cells metastatic to bone produce both soluble and cell-surface associated CSF-1, and that both forms are able to support osteoclast survival in vitro. CSF-1 expression is increased in bones and serum of mice with osteolytic lesions, and is predominantly localized to tumor cells in these lesions. Finally, increased levels of CSF-1 can protect osteoclasts from suppressive effects of TGF-β, further supporting the idea that overexpression of CSF-1 by bone metastatic tumor cells supports osteoclasts superactivation.
We have observed that osteoclasts associated with bone metastases are larger than those found in some other form of rapid bone resorption, such as during tooth eruption (data not shown). Typically mouse osteoclasts in vivo have two-ten nuclei [50], while those associated with bone resorption can have dozens of nuclei. For instance, in bone metastases associated with sarcoma cells, tumor associated osteoclasts were significantly larger than in control bones [45]. We suggest that this larger size reflects “superactivation” of osteoclasts in vivo, and indeed it has been shown that larger osteoclasts (>10 nuclei) are more active resorbers than are small osteoclasts (<5 nuclei) [51], and that they express higher levels of c-fms and the αvβ3 integrin than do small osteoclasts [50]. These observations are consistent with our hypothesis that tumor-associated osteoclasts are less sensitive to the absence of OPN, since their increased αvβ3 levels may allow adequate signaling in the presence of lower-affinity ligands. We further suggest that abnormally high levels of CSF-1 provided by tumor cells can contribute to osteoclast superactivation. Indeed, CSF-1 increases the size and resorptive activity of mature rabbit osteoclasts [52]. It is possible that cell-surface associated CSF-1 may be more effective at increasing osteoclast size than is secreted CSF-1, due to its higher concentration in the area of osteoclast activity. In CSF-1 deficient op/op mice with few osteoclasts, osteolytic tumor cells that express CSF-1 induce the formation of osteoclasts at the site of the tumor, but not systemically [45], further supporting the idea that CSF-1 expression by tumors functions primarily locally. It is not known if the sarcoma cells in these experiments expressed cell-surface CSF-1.
Our immunohistochemistry results clearly show that CSF-1 is widely distributed in tumor cells within the bone (Figure 6). While it is difficult to identify host cells such as osteoblasts in these images, it is clear that the expression in tumor cells is not significantly lower than in host cells, since the staining is quite uniform throughout all the area of the tumor. In normal, non tumor-bearing bones, scattered individual cells associated with the trabecular bone, possibly osteoblasts, express the highest amount of CSF-1. Thus, in bone metastases arising from r3T cells, the tumor cells seem to be the main source of CSF-1, because there are so many more of these cells than the host cells. Both membrane and cytoplasmic staining is seen in these sections when viewed at high magnification (data not shown), suggesting that both the forms of CSF-1 made by the r3T cells in vitro are also made in vivo in the bone microenvironment.
TGF-β is abundant in bone matrix, which is a major storage site, and has complex effects on osteolysis (reviewed in [53]; [54] ). Osteoclastic bone resorption releases TGF-β that can modulate bone formation and resorption. TGF-β released from bone matrix during osteolysis stimulates tumor cells to produce more PTHrP, which in turn stimulates RankL production by tumor cells, activating osteoclasts. This vicious cycle of tumor cell bone microenvironment interaction results in bone destruction and tumor growth [55]. TGF-β has also been shown to have dose dependent inhibitory effects on osteoclast differentiation and causes apoptosis of osteoclasts in mixed bone marrow cultures [56]. While TGF-β stimulates osteoclast development in the presence of CSF-1 and RankL, this effect is most pronounced in early times of culture, with minimal to no effect at later times [57]. Thus, our observation that TGF-β suppresses the number of cells in mature osteoclast cultures is consistent with its role in osteoclast apoptosis, and may reflect an inhibitory effect of this cytokine on late stages of osteoclast life span. The effect of high levels of CSF-1 in blocking this effect of TGF-β thus suggests that increased levels of this growth factor due to tumor cell expression can enhance osteoclast survival at multiple levels, counteracting suppressive effects of TGF-β released during osteolysis.
Conclusions
Here we demonstrate for the first time that the r3T cells produce CSF-1, and that CSF-1 levels are elevated in bone metastases, most likely in the tumor cells themselves. Cell surface expression of CSF-1 on r3T cells can function to support osteoclastogenesis. Since elevated CSF-1 levels can both support osteoclastogenesis and confer resistance to TGF-β these results are consistent with a central role for tumor-expressed CSF-1 in the development of osteolytic bone metastases. Future experiments are planned to test this hypothesis.
Acknowledgments
The authors appreciate expert technical help provided by Craig Zetterberg, the kind gift of MC3T3 cells from Dr. Toshi Kawai, and the gift of MLOY4 cells from Lynda Bonewald. Special thanks to Meena Chellaiah for advice on osteoclast culture and the kind gift of GST-RANKL plasmid. Supported by NIH #DK067685.
Abbreviations
- CSF-1
colony stimulating factor -1
- csCSF-1
cell-surface-associated colony stimulating factor -1
- sCSF
soluble colony stimulating factor -1
- qPCR
quantitative PCR
- BMM
bone marrow medium
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- MMC
mitomycin C
- CM
conditioned medium
- MEF
mouse embryo fibroblast
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin TJ, Moseley JM. Mechanisms in the skeletal complications of breast cancer. Endocr Relat Cancer. 2000;7:271–284. doi: 10.1677/erc.0.0070271. [DOI] [PubMed] [Google Scholar]
- 2.Teitelbaum SL. Osteoclasts: what do they do and how do they do it? Am J Pathol. 2007;170:427–435. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4:638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 4.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD, Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- 6.Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, Massague J, Mundy GR, Guise TA. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guise TA, Yin JJ, Taylor SD, Kumagai Y, Dallas M, Boyce BF, Yoneda T, Mundy GR. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest. 1996;98:1544–1549. doi: 10.1172/JCI118947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chirgwin JM, Guise TA. Molecular mechanisms of tumor-bone interactions in osteolytic metastases. Crit Rev Eukaryot Gene Expr. 2000;10:159–178. [PubMed] [Google Scholar]
- 9.Natasha T, Kuhn M, Kelly O, Rittling SR. Override of the osteoclast defect in osteopontin-deficient mice by metastatic tumor growth in the bone. Am J Pathol. 2006;168:551–561. doi: 10.2353/ajpath.2006.050480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guise TA. Parathyroid hormone-related protein and bone metastases. Cancer. 1997;80:1572–1580. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1572::aid-cncr7>3.3.co;2-d. [DOI] [PubMed] [Google Scholar]
- 11.Yoneda T, Hiraga T. Crosstalk between cancer cells and bone microenvironment in bone metastasis. Biochem Biophys Res Commun. 2005;328:679–687. doi: 10.1016/j.bbrc.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 12.Canon JR, Roudier M, Bryant R, Morony S, Stolina M, Kostenuik PJ, Dougall WC. Inhibition of RANKL blocks skeletal tumor progression and improves survival in a mouse model of breast cancer bone metastasis. Clin Exp Metastasis. 2008;25:119–129. doi: 10.1007/s10585-007-9127-1. [DOI] [PubMed] [Google Scholar]
- 13.Biskobing DM, Fan X, Rubin J. Characterization of MCSF-induced proliferation and subsequent osteoclast formation in murine marrow culture. J Bone Miner Res. 1995;10:1025–1032. doi: 10.1002/jbmr.5650100706. [DOI] [PubMed] [Google Scholar]
- 14.Kacinski BM, Scata KA, Carter D, Yee LD, Sapi E, King BL, Chambers SK, Jones MA, Pirro MH, Stanley ER. FMS (CSF-1 receptor) and CSF-1 transcripts and protein are expressed by human breast carcinomas in vivo and in vitro. Oncogene. 1991;6:941–952. [PubMed] [Google Scholar]
- 15.Kacinski BM. CSF-1 and its receptor in breast carcinomas and neoplasms of the female reproductive tract. Mol Reprod Dev. 1997;46:71–74. doi: 10.1002/(SICI)1098-2795(199701)46:1<71::AID-MRD11>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Scholl SM, Lidereau R, de la RA, Le Nir CC, Mosseri V, Nogues C, Pouillart P, Stanley FR. Circulating levels of the macrophage colony stimulating factor CSF-1 in primary and metastatic breast cancer patients. A pilot study Breast Cancer Res Treat. 1996;39:275–283. doi: 10.1007/BF01806155. [DOI] [PubMed] [Google Scholar]
- 17.Lawicki S, Szmitkowski M, Wojtukiewicz M. The pretreatment plasma level and diagnostic utility of M-CSF in benign breast tumor and breast cancer patients. Clin Chim Acta. 2006;371:112–116. doi: 10.1016/j.cca.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 18.Mancino AT, Klimberg VS, Yamamoto M, Manolagas SC, Abe E. Breast cancer increases osteoclastogenesis by secreting M-CSF and upregulating RANKL in stromal cells. J Surg Res. 2001;100:18–24. doi: 10.1006/jsre.2001.6204. [DOI] [PubMed] [Google Scholar]
- 19.Chellaiah MA, Hruska KA. The integrin alpha(v)beta(3) and CD44 regulate the actions of osteopontin on osteoclast motility. Calcif Tissue Int. 2003;72:197–205. doi: 10.1007/s00223-002-1025-6. [DOI] [PubMed] [Google Scholar]
- 20.Zhao S, Zhang YK, Harris S, Ahuja SS, Bonewald LF. MLO-Y4 osteocyte-like cells support osteoclast formation and activation. J Bone Miner Res. 2002;17:2068–2079. doi: 10.1359/jbmr.2002.17.11.2068. [DOI] [PubMed] [Google Scholar]
- 21.Rittling SR, Denhardt DT. p53 mutations in spontaneously immortalized 3T12 but not 3T3 mouse embryo cells. Oncogene. 1992;7:935–942. [PubMed] [Google Scholar]
- 22.Christensen B, Kazanecki CC, Petersen TE, Rittling SR, Denhardt DT, Sorensen ES. Cell type-specific post-translational modifications of mouse osteopontin are associated with different adhesive properties. J Biol Chem. 2007;282:19463–19472. doi: 10.1074/jbc.M703055200. [DOI] [PubMed] [Google Scholar]
- 23.Rittling SR, Feng F. Detection of mouse osteopontin by western blotting. Biochem Biophys Res Comm. 1998;250:287–292. doi: 10.1006/bbrc.1998.9306. [DOI] [PubMed] [Google Scholar]
- 24.Rubin J, Fan X, Thornton D, Bryant R, Biskobing D. Regulation of Murine Osteoblast Macrophage Colony-Stimulating Factor Production by 1,25(OH)2D3. Calcified Tissue International. 1996;59:291–296. doi: 10.1007/s002239900125. [DOI] [PubMed] [Google Scholar]
- 25.Yates B, Zetterberg C, Rajeev V, Reiss M, Rittling SR. Promoter-independent regulation of vimentin expression in mammary epithelial cells by val(12)ras and TGFbeta. Exp Cell Res. 2007;313:3718–3728. doi: 10.1016/j.yexcr.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao GQ, Sun B, Hammond EE, Spencer EN, Horowitz MC, Insogna KL, Weir EC. The cell-surface form of colony-stimulating factor-1 is regulated by osteotropic agents and supports formation of multinucleated osteoclast-like cells. J Biol Chem. 1998;273:4119–4128. doi: 10.1074/jbc.273.7.4119. [DOI] [PubMed] [Google Scholar]
- 27.Anway MD, Wright WW, Zirkin BR, Korah N, Mort JS, Hermo L. Expression and localization of cathepsin k in adult rat sertoli cells. Biol Reprod. 2004;70:562–569. doi: 10.1095/biolreprod.103.018291. [DOI] [PubMed] [Google Scholar]
- 28.Gallet M, Mentaverri R, Sevenet N, Brazier M, Kamel S. Ability of breast cancer cell lines to stimulate bone resorbing activity of mature osteoclasts correlates with an anti-apoptotic effect mediated by macrophage colony stimulating factor. Apoptosis. 2006;11:1909–1921. doi: 10.1007/s10495-006-9507-z. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Rittling SR. Novel murine mammary epithelial cell lines that form osteolytic bone metastases: effect of strain background on tumor homing. Clin Exp Metastasis. 2003;20:111–120. doi: 10.1023/a:1022675031185. [DOI] [PubMed] [Google Scholar]
- 30.Hohensinner PJ, Kaun C, Rychli K, Niessner A, Pfaffenberger S, Rega G, de MR, Maurer G, Ullrich R, Huber K, Wojta J. Macrophage colony stimulating factor expression in human cardiac cells is upregulated by tumor necrosis factor-alpha via an NF-kappaB dependent mechanism. J Thromb Haemost. 2007;5:2520–2528. doi: 10.1111/j.1538-7836.2007.02784.x. [DOI] [PubMed] [Google Scholar]
- 31.Price LK, Choi HU, Rosenberg L, Stanley ER. The predominant form of secreted colony stimulating factor-1 is a proteoglycan. J Biol Chem. 1992;267:2190–2199. [PubMed] [Google Scholar]
- 32.Pixley FJ, Stanley ER. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 2004;14:628–638. doi: 10.1016/j.tcb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Douglass TG, Driggers L, Zhang JG, Hoa N, Delgado C, Williams CC, Dan Q, Sanchez R, Jeffes EWB, Wepsic HT, Myers MP, Koths K, Jadus MR. Macrophage colony stimulating factor: Not just for macrophages anymore! A gateway into complex biologies. International Immunopharmacology. 2008;8:1354–1376. doi: 10.1016/j.intimp.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 34.Dai XM, Zong XH, Sylvestre V, Stanley ER. Incomplete restoration of colony-stimulating factor 1 (CSF-1) function in CSF-1-deficient Csf1op/Csf1op mice by transgenic expression of cell surface CSF-1. Blood. 2004;103:1114–1123. doi: 10.1182/blood-2003-08-2739. [DOI] [PubMed] [Google Scholar]
- 35.Ladner MB, Martin GA, Noble JA, Wittman VP, Warren MK, McGrogan M, Stanley ER. cDNA cloning and expression of murine macrophage colony-stimulating factor from L929 cells. Proc Natl Acad Sci USA. 1988;85:6706–6710. doi: 10.1073/pnas.85.18.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan GR, Dai XM, Dominguez MG, Tong W, Chuan F, Chisholm O, Russell RG, Pollard JW, Stanley ER. Rescue of the colony-stimulating factor 1 (CSF-1)-nullizygous mouse (Csf1(op)/Csf1(op)) phenotype with a CSF-1 transgene and identification of sites of local CSF-1 synthesis. Blood. 2001;98:74–84. doi: 10.1182/blood.v98.1.74. [DOI] [PubMed] [Google Scholar]
- 37.Yao GQ, Sun BH, Weir EC, Insogna KL. A role for cell-surface CSF-1 in osteoblast-mediated osteoclastogenesis. Calcif Tissue Int. 2002;70:339–346. doi: 10.1007/s00223-001-1079-x. [DOI] [PubMed] [Google Scholar]
- 38.Kuhn M, Shah S, Natasha T, Rittling SR. A mouse model of breast cancer metastasis to the choroid of the eye. Clin Exp Metastasis. 2005;22:685–690. doi: 10.1007/s10585-006-9012-3. [DOI] [PubMed] [Google Scholar]
- 39.Fingleton B. Molecular targets in metastasis: lessons from genomic approaches. Cancer Genomics Proteomics. 2007;4:211–221. [PubMed] [Google Scholar]
- 40.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 41.Gallet M, Sevenet N, Dupont C, Brazier M, Kamel S. Breast cancer cell line MDA-MB 231 exerts a potent and direct anti-apoptotic effect on mature osteoclasts. Biochem Biophys Res Commun. 2004;319:690–696. doi: 10.1016/j.bbrc.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 42.Schwaninger R, Rentsch CA, Wetterwald A, van der HG, van Bezooijen RL, van der PG, Lowik CW, Ackermann K, Pyerin W, Hamdy FC, Thalmann GN, Cecchini MG. Lack of noggin expression by cancer cells is a determinant of the osteoblast response in bone metastases. Am J Pathol. 2007;170:160–175. doi: 10.2353/ajpath.2007.051276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao GQ, Wu JJ, Sun BH, Troiano N, Mitnick MA, Insogna K. The cell surface form of colony-stimulating factor-1 is biologically active in bone in vivo. Endocrinology. 2003;144:3677–3682. doi: 10.1210/en.2002-221071. [DOI] [PubMed] [Google Scholar]
- 44.Ohshiba T, Miyaura C, Inada M, Ito A. Role of RANKL-induced osteoclast formation and MMP-dependent matrix degradation in bone destruction by breast cancer metastasis. Br J Cancer. 2003;88:1318–1326. doi: 10.1038/sj.bjc.6600858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clohisy DR, Ogilvie CM, Ramnaraine ML. Tumor osteolysis in osteopetrotic mice. J Orthop Res. 1995;13:892–897. doi: 10.1002/jor.1100130613. [DOI] [PubMed] [Google Scholar]
- 46.Ishijima M, Tsuji K, Rittling SR, Yamashita T, Kurosawa H, Denhardt DT, Nifuji A, Noda M. Resistance to unloading-induced three-dimensional bone loss in osteopontin-deficient mice. J Bone Miner Res. 2002;17:661–667. doi: 10.1359/jbmr.2002.17.4.661. [DOI] [PubMed] [Google Scholar]
- 47.Yoshitake H, Rittling SR, Denhardt DT, oda MN. Osteopontin-deficient mice are resistant to ovariectomy-induced bone resorption [published erratum appears in Proc Natl Acad Sci U S A 1999 Sep 14;96(19):10944] Proc Natl Acad Sci USA. 1999;96:8156–8160. doi: 10.1073/pnas.96.14.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faccio R, Takeshita S, Zallone A, Ross FP, Teitelbaum SL. c-Fms and the alphavbeta3 integrin collaborate during osteoclast differentiation. J Clin Invest. 2003;111:749–758. doi: 10.1172/JCI16924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bakewell SJ, Nestor P, Prasad S, Tomasson MH, Dowland N, Mehrotra M, Scarborough R, Kanter J, Abe K, Phillips D, Weilbaecher KN. Platelet and osteoclast beta3 integrins are critical for bone metastasis. Proc Natl Acad Sci USA. 2003;100:14205–14210. doi: 10.1073/pnas.2234372100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trebec DP, Chandra D, Gramoun A, Li K, Heersche JN, Manolson MF. Increased expression of activating factors in large osteoclasts could explain their excessive activity in osteolytic diseases. J Cell Biochem. 2007;101:205–220. doi: 10.1002/jcb.21171. [DOI] [PubMed] [Google Scholar]
- 51.Lees RL, Sabharwal VK, Heersche JN. Resorptive state and cell size influence intracellular pH regulation in rabbit osteoclasts cultured on collagen-hydroxyapatite films. Bone. 2001;28:187–194. doi: 10.1016/s8756-3282(00)00433-6. [DOI] [PubMed] [Google Scholar]
- 52.Lees RL, Heersche JN. Macrophage colony stimulating factor increases bone resorption in dispersed osteoclast cultures by increasing osteoclast size. J Bone Miner Res. 1999;14:937–945. doi: 10.1359/jbmr.1999.14.6.937. [DOI] [PubMed] [Google Scholar]
- 53.Janssens K, ten DP, Janssens S, Van HW. Transforming growth factor-beta1 to the bone. Endocr Rev. 2005;26:743–774. doi: 10.1210/er.2004-0001. [DOI] [PubMed] [Google Scholar]
- 54.Quinn JMW, Gillespie MT. Modulation of osteoclast formation. Biochem Biophys Res Comm. 2005;328:739–745. doi: 10.1016/j.bbrc.2004.11.076. [DOI] [PubMed] [Google Scholar]
- 55.Kozlow W, Guise TA. Breast cancer metastasis to bone: mechanisms of osteolysis and implications for therapy. J Mammary Gland Biol Neoplasia. 2005;10:169–180. doi: 10.1007/s10911-005-5399-8. [DOI] [PubMed] [Google Scholar]
- 56.Hughes DE, Dai A, Tiffee JC, Li HH, Mundy GR, Boyce BF. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-[beta] Nat Med. 1996;2:1132–1136. doi: 10.1038/nm1096-1132. [DOI] [PubMed] [Google Scholar]
- 57.Lari R, Fleetwood AJ, Kitchener PD, Cook AD, Pavasovic D, Hertzog PJ, Hamilton JA. Macrophage lineage phenotypes and osteoclastogenesis--complexity in the control by GM-CSF and TGF-beta. Bone. 2007;40:323–336. doi: 10.1016/j.bone.2006.09.003. [DOI] [PubMed] [Google Scholar]