Abstract
In humans mutations in DKC1, cause the rare bone marrow failure syndrome dyskeratosis congenita. We have used gene targeting to produce mouse ES cells with Dkc1 mutations that cause DC when in humans. The mutation A353V, the most common human mutation, causes typical DC to very severe DC in humans. Male chimeric mice carrying this mutation do not pass the mutated allele to their offspring. The mutation G402E accounts for a single typical case of DC in a human family. The allele carrying this mutation was transmitted to the offspring with high efficiency. Expression of RNA and protein was reduced compared to wild type animals but no abnormalities of growth and development or in blood values were found in mutant mice. Thus Dkc1 mutations have variable expression in mice, as in humans.
Introduction
Dyskeratosis congenita is a rare skin and bone marrow failure syndrome, associated with a classical triad of mucocutaneous abnormalities, namely abnormal skin pigmentation, leukoplakia and nail dystrophy (Dokal, 2000; Drachtman and Alter, 1995; Vulliamy and Dokal, 2008). Pancytopenia is frequent and bone marrow failure is the most common cause of death. The more common X-linked form is due to mutations in DKC1, encoding dyskerin (Heiss et al., 1998). Dyskerin is one of 4 proteins present in H/ACA box snoRNP particles where it associates with a guide snoRNA which targets the particles to specific uridine residues in nascent rRNA precursors (Meier, 2005). These uridines are then converted to pseudouridine with dyskerin being the active pseudouridine synthase (Ramamurthy et al., 1999; Spedaliere et al., 2000). Pseudouridylation is essential for efficient ribosomal RNA processing which in turn is required for ribosome production (Zebarjadian et al., 1999). The same 4 proteins, including dyskerin are also part of the telomerase RNP particle where they associate with telomerase RNA, which has an H/ACA domain at its 3′ end (Mitchell et al., 1999a; Mitchell et al., 1999b; Pogacic et al., 2000). Telomerase is required for maintenance of the telomeres at the ends of chromosomes whose integrity is essential for chromosome stability (Blackburn, 2005). The precise function of dyskerin in the telomerase complex is unknown as is the effect of the mutations on pseudouridylation and in turn the effect of a decrease in the efficiency of pseudouridylation on ribosome biogenesis. Moreover because of the dual function of dyskerin the relative contributions of rRNA defects and telomerase defects to the pathology of DC are difficult to ascertain (Ruggero et al., 2003; Wong and Collins, 2006).
We are approaching these questions by making targeted Dkc1 mutations in laboratory mice (He et al., 2002) (Mochizuki et al., 2004) (Gu et al., 2008). Mice are particularly favorable for these studies since they show no abnormalities due to lack of telomerase for several generations (Blasco et al., 1997)– so any effects seen in the first generation will likely be due to rRNA defects. Mice with a decrease in expression of Dkc1 due to a downstream targeting event show some characteristics of DC and a specific defect in IRES mediated translation (Ruggero et al., 2003; Yoon et al., 2006), although the significance of these observations has yet to be established. We have previously shown that a null allele of Dkc1 causes embryonic lethality (He et al., 2002). Biochemical analysis of mouse embryonic stem cells with Dkc1 mutations A353V and G402E showed the A353V mutations caused severe telomerase deficiency through a depletion of telomerase RNA whereas the G402E mutation did not (Mochizuki et al., 2004). Both mutations caused detectable decreases in pseudouridylation and rRNA processing. Here we show that both these mutant ES cells contribute to male chimeric mice but that chimeric mice with the A353V mutant allele do not pass on the mutant allele to their offspring. Germ line transmission of the G402E mutant occurred efficiently with male mutant mice showing decreased levels of dyskerin. Long term follow up of the first generation of these mice did not show any abnormalities in growth and development or in hematopoiesis.
Results
Male chimeras do not transmit the A353V allele to their offspring
We have previously described the production and analysis of genetically altered mouse ES cells expressing mutant dyskerins A353V and G402E. Constructs used in the production of these ES cells along with characteristic Southern blot results are shown in Figure 1. These ES cells were injected into blastocysts to produce chimeric mice. In the case of G402E ES cells 2 separate ES cell clones produced male mice with a high degree of chimerism as judged by the high percentage of agouti colored coat. Male chimeras readily produced agouti female offspring which had inherited the G402E mutant dyskerin gene. The mutant dyskerin gene was thereafter transmitted in the usual X-linked manner. The A353V ES cells also produced male chimeras with a high percentage of coat color chimerism but in this case no agouti offspring were produced. Of 20 male chimeric mice produced from 4 separate ES cell clones 14 had progeny but none of the pups were agouti, indicating that there had been no germ line transmission from the A353V ES cells Table 1. Litter size and composition were normal suggesting that mutant embryos were not being produced, rather than that they were dying during embryonic development. The ES cells used in these experiments were the same strain and batch as those used to produce the G402E ES cells which did give good germ line transmission. Karyotyping of 2 of the ES cell lines showed no abnormalities. We conclude the most likely reason for the lack of germ line transmission was failure of sperm production from cells in the male chimera with a Dkc1 gene carrying the A353V mutation.
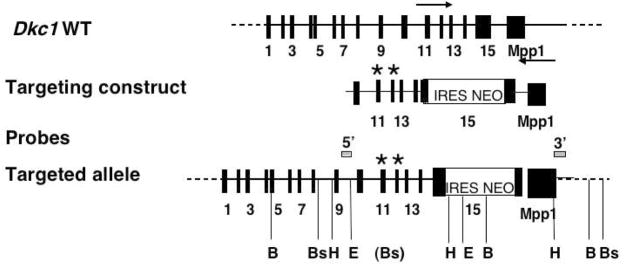
Figure 1.
Targeting constructs used in this study and previously described by Mochizuki et al (Mochizuki et al., 2004). The diagram shows the Dkc1 gene with the exons represented as black rectangles. The arrows indicate the direction of transcription of the Dkc1 and adjacent Mpp1 genes. Targeting constructs consisted of exons 10–15 of the Dkc1 gene with a IRES-NEO element in the 3′ UTR and point mutations in exons 11 (A353V) or 12 (G402E). Probes and restriction enzymes used to analyse the targeted alleles in ES cells and mice are shown. B, BamHI; Bs, BspHI; H, HindIII; E, EcoRI.
Table 1.
Breeding male chimeras from A353V ES cells with C57BL6 females
| ES cell clone | Number of chimeras | Number fertile (had progeny) | Coat color of pups |
|---|---|---|---|
| DC353/60 | 11 | 9 | All black |
| DC353/37 | 2 | 0 | All black |
| DC353/49 | 4 | 3 | All black |
| DC353/115 | 3 | 2 | All black |
The contribution of mutant ES cells to A353V chimeras
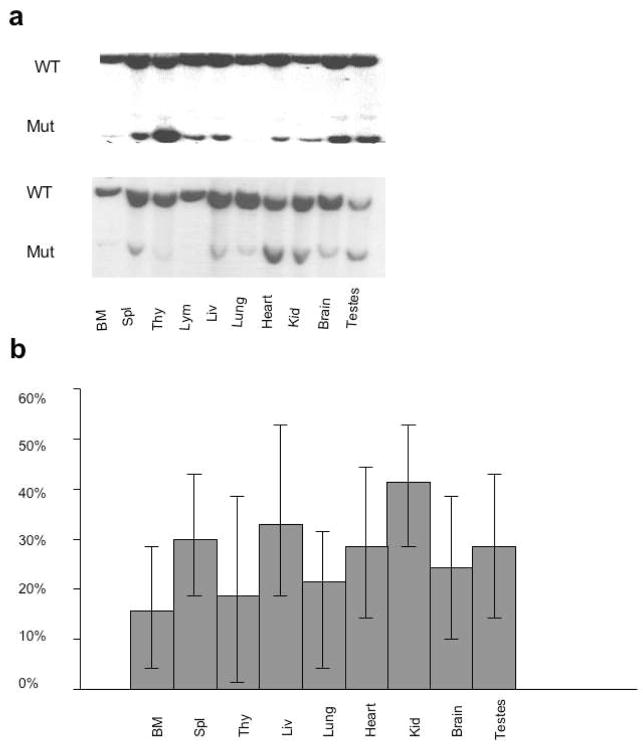
To gain insight into possible reasons for the failure of germ line transmission from male A353V chimeric mice 4 chimeras were sacrificed at 1 year old and DNA extracted from different tissues. We used Southern blotting to estimate the relative contribution of wild type and A353V cells to the different tissues (Figure 2). Although the coat color of these mice suggested a high contribution (80% to 100%) from the ES cells the contribution of mutant cells to the organs and tissues tested was variable but markedly lower. A second set of mice were sacrificed at 1.5 years old and the experiment repeated. Again the contribution of mutant cells to the organs was variable and low and in fact, not significantly different from that measured at 1 year. Altogether 8 chimeras were subjected to this analysis and the results are shown in Fig 2b. There is a generally low contribution of the A353V cells to all tissues and no consistent pattern is seen. Notably the contribution in testes is not particularly low compared with other tissues.
Figure 2.
Representation of ES cell derived cells in A353V chimeras. a. Two representative Southern blots are shown. DNA from 1 year old male chimeras was extracted from the tissues indicated, digested with EcoRI and analysed by Southern blotting using a 3′ probe. The upper band is from the wild type allele while the lower band is from the targeted allele containing the A353V mutation. b. The percentage representation of the mutant allele is plotted from analysis of 8 chimeras.
Normal lifespan, fertility and blood values in G402E mice
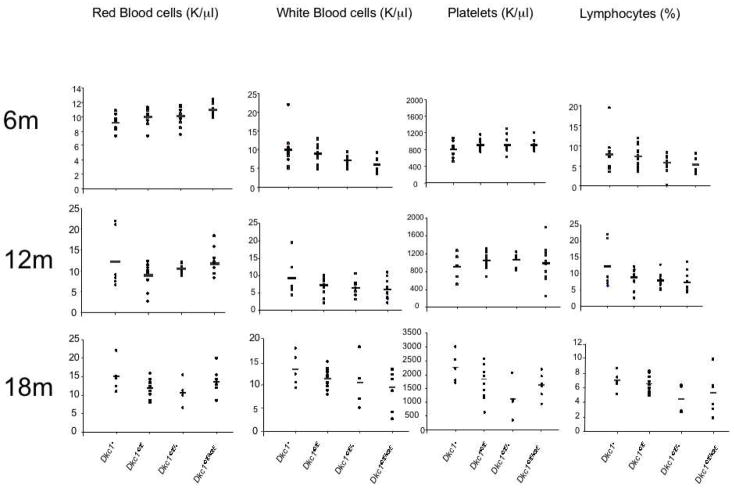
Germ line transmission of the G402E allele was achieved from 2 different mutant ES cells. Offspring from reciprocal crosses show normal X-linked inheritance of the mutant allele. Of 127 offspring produced 62 were male and heterozygous and homozygous female mice and hemizygous and wild type mice were produced in the expected numbers (Table 2). Mutant male mice showed no decrease in fertility. A cohort of male mutant mice, their wild type litter mates, and female homozygous and heterozygous mutant mice were produced. Their survival rates (Figure 3) showed no significant differences. At 6 months, 12 months and 18 months 7 mice from each genotype were sacrificed and blood counts (numbers of rbc, wbc, platelets and lymphocytes) were obtained. No significant differences were observed in the blood counts (Figure 4). Similarly no differences were found in the weight of the animals or in their performance in a wound healing assay (data not shown). At 18 months all animals were sacrificed and carefully examined, major organs being submitted for histological examination (Figure 5). There was no significant difference in tumor incidence (mice developing a tumor were 1/18 male Dkc1402E, 1/11 female Dkc1402E/+, 0/12 male Dkc1+ and 0/10 female Dkc1402E/402E). No significant differences in histological appearance were noted.
Table 2.
Analysis of 128 offspring of crosses between male DKC402E and female DKC1402E/+ mice.
| Genotype | Obtained (%) | Expected % |
|---|---|---|
| Male Dkc1402E | 33 (26) | 25 |
| Male Dkc1+ | 34 (27) | 25 |
| Female Dkc1402E/402E | 30 (23) | 25 |
| Female Dkc1402E/+ | 31 (24) | 25 |
Figure 3.
Lifespan of Dkc1402E mice. The graph shows the lifespan of male hemizygotes, and female heterozygotes and homozygotes compared with wild type C57BL6 mice.
Figure 4.
Peripheral blood counts in mice carrying the Dkc1G402E allele and wild type mice at 6, 12 and 18 months.
Figure 5.
Bone marrow from mutant and wild type mice showing normal cellularity in the mutant mouse.
Molecular characteristics of G402E mice
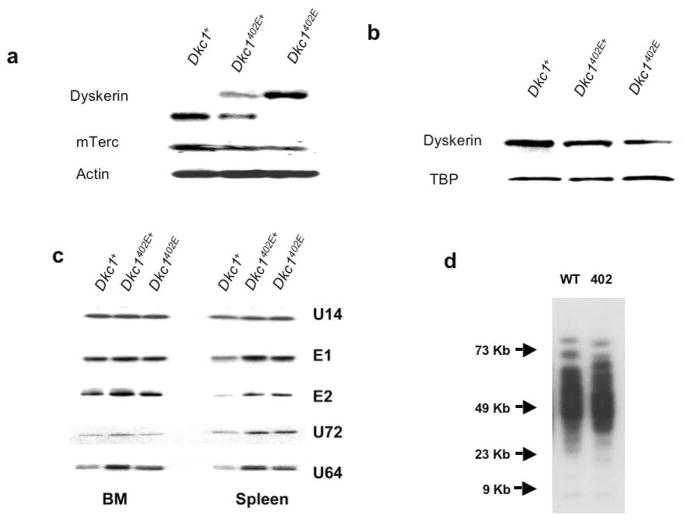
G402E mice appeared to be perfectly healthy and normal in terms of blood counts, lifespan, cancer incidence, fertility and by histological examination. In a previous study (Mochizuki et al., 2004) we had compared G402E ES cells, A353V ES cells and wild type ES cells. We found A353V ES cells had greatly reduced levels of Terc RNA, decreased telomerase activity and an increased rate of telomere loss compared with wild type ES cells while G402E mice had normal levels of Terc, normal telomerase activity and no detectable telomere loss in culture. However both A353V and G402E ES cells had slightly decreased levels of dyskerin mRNA and protein compared to wild type ES cells. Moreover A353V ES cells had decreased levels of some, but not all H/ACA snoRNAs. In this respect G402E ES cells had marginally decreased levels of some H/ACA snoRNAs, but these did not coincide with those decreased in the A353V ES cells. It was important therefore to examine the molecular consequences of the G402E mutation in mice – to see whether the changes seen in ES cells were present in mouse tissues, despite the apparent lack of any phenotype in these mice. We thus extracted RNA and protein from tissues of adult G402E male mice as well as heterozygous female mice and control C57BL6 mice. Figure 6 shows that in both spleen and bone marrow levels of dyskerin mRNA were similar in all genotypes. Moreover in female heterozygotes the amount of mutant mRNA is similar to that of wild type RNA indicating no significant growth advantage for wild type over mutant cells following random X-inactivation. However levels of dyskerin protein were clearly decreased in male mutant cells and were intermediate in female heterozygous cells. Similarly levels of mTerc were decreased in mutant cells and intermediate in heterozygotes. Levels of H/ACA snoRNAs were found to vary between tissues in adult mice but overall the picture is similar to that seen in ES cells with some H/ACA RNAs actually increased in the mutant mice.
Figure 6.
Molecular analysis of mice carrying the Dkc1G402E allele. a. Northern blot of RNA from the spleen of 6 month old wild type and mutant mice. The mutant mRNA is larger than the wild type mRNA because it contains the IRES neo element. Note the decrease in the level of telomerase RNA mTerc in the mutant cells. b. Western blotting of nuclear protein from the liver of wild type and mutant mice. The steady state level of dyskerin is decreased in the mutant liver. c Northern blotting of RNA from the bone marrow and spleen of wild type and mutant mice. The blot was hybridized with oligonucleotide probes representing a subset of H/ACA snoRNAs. d. Telomere lengths in DNA extracted from the spleens of 6 month old mice with the indicated genotypes.
Discussion
We have presented our findings on the production of mice with point mutations in the Dkc1 gene that are copies of mutations that cause dyskeratosis congenita in humans. In the case of mutation A353V we find that male chimeras do not pass the mutated allele to their male or female pups. By contrast germ line transmission of the G402E allele is efficient but mice with this mutation are essentially indistinguishable from normal mice.
The mutation A353V in humans is the most common mutation causing X-linked dyskeratosis congenita (Knight et al., 1999) (Vulliamy et al., 2006). It is found in 40% of cases and is often found as a de novo mutation. The mutation is associated with a wide range of severity of DC. Although there has been no published information on the effects of X-linked DC on male fertility it has been reported that testicular atrophy is common in X-linked DC patients (MIM305000). It is conceivable therefore that spermatogenesis in the male chimeric mice produces only sperm with a normal Dkc1 gene. It appears curious that the male chimeras produced from injecting blastocysts with the A353V ES cells had very high rates of chimerism (80%–100%) as judged by the coat color (ES cells carrying a gene for agouti and the recipient blastocysts being black) but examination of internal organs by DNA extraction and Southern blotting shows a low contribution of the mutated Dkc1 allele in most tissues. A possible explanation is that the mutant ES cells may not have been pure and during expansion were outgrown by wild type ES cells with respect to the Dkc1 gene. These would then form an agouti coat but would have a wild type Dkc1 gene. Further proliferative advantage of these cells in spermatogenesis may then occur. We did not observe any obvious growth defects in the A353V ES cells but did not test them in competition with wild type cells. A353V ES cells had a significant decrease in telomerase RNA abundance and telomerase activity and were detectably deficient in the levels of some snoRNAs. They also had a small but reproducible decrease in the rate of rRNA production (Mochizuki et al., 2004). It is possible that these biochemical defects, which suggested the A353V mutation would stand a good chance of producing a mouse DC model, led to a failure of the mutant cells to contribute to the sperm in the chimeric mice. If that is the case it is not clear how such a problem can be overcome, since most ES cells used in gene targeting are male. Possible solutions are to use female ES cells or to attempt to “rescue” the defect in the mutant ES cells with an ectopically expressed Dkc1 gene that can be segregated from the A353V gene by breeding. In a third approach we are trying to produce targeted mice with an inducible Dkc1 mutation.
One of the key questions we hoped to answer in this work was whether the defects caused by pathogenic Dkc1 mutations were due to defects in telomerase or those in other aspects of dyskerin function, chiefly ribosome biogenesis. Mice with no telomerase activity due to homozygous deletion of either Terc or Tert have no defects in the first generation but, as telomeres get shorter in subsequent generations they show defects with increasing severity in the third and later generations (Blasco et al., 1997) (Liu et al., 2002). We reasoned that any defects we saw with Dkc1 mutations in the first generation were unlikely to be due to telomerase defects. This reasoning would lead us to conclude that the failure of the A353V cells to give germ line transmission is not due to telomerase deficiency. Because these experiments have involved the competition between cells of different genotype in the chimera however the situation is more complex. The failure to obtain germ line transmission may in fact be due to a proliferative advantage of wild type cells over mutant cells, particularly in the lineage leading to sperm production. Telomerase deficiency in a competitive situation such as this has not been directly tested before so we cannot formally exclude telomerase deficiency, via a mechanism other than telomere length maintenance, as a factor that may be contributing. It is unlikely that the failure to obtain germ line transmission was due to the ES cells used since the cells used were the same batch as those used in the G402E experiment which was performed in parallel and which resulted in perfect transmission of the targeted gene.
With the G402E mutation we found germ line transmission of the targeted allele and inheritance thereafter with normal Mendelian frequencies. In the first generation of mice however there were no significant abnormalities caused by this mutation. There are several possible explanations for this finding. First it is possible that the mutant dyskerin effects telomerase function but not ribosome biogenesis or some other function of dyskerin and thus we do not see any effect in the first generation of mice since even with no telomerase several generations of shortening are required before phenotypes due to short telomeres are seen as discussed above (Blasco et al., 1997) (Liu et al., 2002). This was in fact our expectation when undertaking these experiments. However we do not see very significant reduction in Terc levels in G402E ES cells or in mouse tissues. We see a slight reduction in the amount of dyskerin and in the amount of Terc RNA in mouse tissues but this does not approach the reduction in TERC RNA seen in human cells with pathogenic dyskerin mutations (Mitchell et al., 1999b; Wong et al., 2004) or that seen in our A353V ES cells (Mochizuki et al., 2004). Whether this reduction in telomerase levels would be sufficient to cause a phenotype in mice with short telomeres can be determined by breeding with late generation telomerase deficient mice. Similarly we do not see a dramatic reduction in the abundance of particular H/ACA snoRNAs in either ES cells or mouse tissues. The second possibility is that the G402E amino acid change does not have the same effect on the mouse protein as it does on the human protein. We note that the 402G residue is very well conserved but that the amino acid sequence flanking this residue is less well conserved. It is feasible that the mouse structure can accommodate the change to a glutamic acid residue at position 402 better than the human protein. This might be the case if the residue interacts with residues that are different in the mouse and human proteins. However though the crystal structure of the archael orthologue of dyskerin has been determined (Hamma et al., 2005; Li and Ye, 2006; Rashid et al., 2006) it does not include the part of the molecule containing residue 402. A third and more troubling possibility is that perturbations in dyskerin function do not affect mouse development and physiology in the same way as they do human development because of complex physiological differences between the two species.
The results found with the G402E mutation contrast with those we previously found with the deletion mutant Dkc1Δ15, which is a copy of a human mutation (Gu et al., 2008). In this case there was no clear phenotype in mutant male mice but female heterozygotes show a clear growth advantage of the wild type over the mutant cells following random X-inactivation. No such growth advantage is seen with the G402E mutation (Figure 6a).
A mouse model of dyskeratosis congenita, derived by targeted alteration of the mouse Dkc1 locus, has been described by others (Ruggero et al., 2003; Yoon et al., 2006). In this case a selection cassette inserted downstream of the wild type Dkc1 gene decreases the steady state level of mRNA in mutant male mice four fold. Levels of dyskerin protein were not examined. These mice were reported to have severe anemia, dyskeratosis of the skin and a dramatic increase in tumor formation in the first generation. These effects are thought to be mediated via reduced IRES mediated transcription of certain cell division control genes by an unknown mechanism. It is interesting that the G402E male mice described here, which have a decreased level of mutated dyskerin show none of these features. At present we have no explanation for this conundrum but note that the mice described by Ruggero et al (Ruggero et al., 2003) resulted from an aberrant rearrangement event and the effect of the rearrangement on the expression of neighboring loci, particularly Mpp1 has not been reported. A mouse with a pathogenic dyskerin mutation that causes impaired growth detected in heterozygous females by mosaic analysis has recently been described (Gu et al., 2008). Interestingly the growth disadvantage was apparent in the first generation but was completely dependent on telomerase, as shown by genetic experiments.
In summary two point mutations in the mouse Dkc1 gene, both of which are copies of mutations causing DC in humans do not produce phenocopies of the disease in mice. In diseases like DC caused by modulation, rather than ablation of function it may be particularly challenging to alter the function of a protein in such a way as to copy the precise effect on development and physiology in an experimental animal.
Methods
The production of chimeric mice strains and generation of dyskerin mutant mice
Production of ES cells containing Dkc1 mutations A353V and G402E has been previously described (Mochizuki et al., 2004). ES cells were injected into blastocysts for the generation of chimeric mice. Male chimeric mice were bred with C57BL/6J or SV129 female mice for the production of heterozygous female mice. Homozygous (female) or hemizygous (male) mice were produced in subsequent generations. Mutant loci were detected using the following PCR primers
DC11F 5′ TGA TGA CTA CAG CAG TGA CTA CAG CAG TGA TTT C 3′
DC402mut 5′ GTG TTG TCT GTG GGT TTC T CA 3′
DC353mut 5′ CAA TTG CAC TCA TGA CTA CAG T 3′
DC12R5′ TAA TCC TGT TTC CAT GTG GC -3′.
The mice were bred and maintained in the animal facility, medical school of Washington University in St. Louis. All animal received care in compliance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85–23, revised 1985).
The long term follow-up of Dkc1 mutant mice
All mice came were of mixed C57BL/6J and SV129 background. We separated the mice into four groups according to their genotype. All the mice were fed a standard diet and weighed at 1 month, 3 months, 6 months, 12 months and 18 months. The coat color changes, defective wound healing (Malinda et al., 1999), and reduced life span as well as tumor incidence were monitored over a period of 18 months. At the end of 18 months, all animals were autopsied and all tissues were examined regardless of their pathological status. The sternum, spleen, liver, kidney and intestine were fixed in buffered 10% formalin, embedded in paraffin, and stained with hematoxylin and eosin (H&E).
Peripheral blood analysis
200ul peripheral blood was collected by bleeding the mutant mice and their wild type (WT) littermate controls at1 month, 3 months, 6 months, 12 months and 18 months old. The red blood cells (RBC), white blood cells (WBC), hemoglobin (HB) and platelets counts were determined using a Hemavet (CDC Technologies, Inc.). The percentage of B and T lymphocytes was determined with Flowcytometric analysis after incubation for 30 minutes at room temperature with the following monoclonal antibodies conjugated with phycoerythrin (PE) or fluorescein isothiocyanate (FITC); CD11b, Gr-1, B220, CD4, CD8.
Analysis of Telomere Lengths
Telomere lengths were measured by in-gel hybridization following the procedure of Zhu et al. (Zhu et al., 1998). Cell suspensions were prepared from liver, spleen and bone marrow tissue, suspended in cold PBS, and washed once with cold PBS. 5 × 106 cells were embedded in one agarose plug following instructions from the manufacturer (CHEF agarose plug kit; Bio-Rad). DNA embedded in the agarose plug was digested by incubating the plugs with 100 units/ml each of HinfI and RsaI for 18–28 hours at 37° C. Digested DNA was separated on a 1% agarose gel for 14 hours at 6 V/cm at a constant pulse of 5 s using the CHEF DR-III pulse-field gel electrophoresis apparatus. The gel was analyzed by in-gel hybridization with [-32P] 5′ end-labeled oligonucleotide (CCCTAA)4 probe.
RNA isolation and Northern-blotting
Total RNA was isolated from mouse tissues cells using TRIzol (Invitrogen, Carlsbad CA). For analysis of snoRNAs, 20μg of total RNA was electrophoresed through 6% poly-acrylamide-7M urea gels and transferred onto Zeta-probe membranes (Bio-Rad, Richmond, CA) using Trans-blot SD semi-dry blotting apparatus (Bio-Rad). The sequence of the oligonucleotides used to probe these blots was as before (Mochizuki et al., 2004).
Western-blot
Nuclear extract were prepared by using NE-PER nuclear and Cytoplasmic Extraction Reagents (PIERCE). Protein concentration were measured by using the Bio-Rad protein assay (Bio-Rad). Mouse monoclonal antibody to TATA-box binding protein (TBP) was used as nuclear loading control (Abcam, ab818). To detect dyskerin filters were probed with primary rabbit antiserum against raised against a mixture of 2 peptides (AA 74–88 CGSNPLKREIGDYIR and AA 382–396 GLLDKHGKPTDNTPA) and supplied by Eurogentec Belgium, followed by a horseradish peroxidase-conjugated secondary antibody, and then developed using ECL plus Western Blotting Detection System (Amersham).
Statistical Analysis
The data is expressed as mean ± standard error of the mean (SEM). Comparisons between two groups were performed using an unpaired t- test, and comparisons between multiple groups were analyzed using ANOVA. P < 0.05 was considered statistically significant.
Acknowledgments
We thank Sandra Navarette for skilled technical assistance. We would like to thank the NIH and NCI for financial support through grants to PJM and MB.
References
- Blackburn EH. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005;579:859–862. doi: 10.1016/j.febslet.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- Dokal I. Dyskeratosis congenita in all its forms. Br J Haematol. 2000;110:768–779. doi: 10.1046/j.1365-2141.2000.02109.x. [DOI] [PubMed] [Google Scholar]
- Drachtman RA, Alter BP. Dyskeratosis congenita. Dermatol Clin. 1995;13:33–39. [PubMed] [Google Scholar]
- Gu BW, Bessler M, Mason PJ. A pathogenic dyskerin mutation impairs proliferation and activates a DNA damage response independent of telomere length in mice. Proc Natl Acad Sci U S A. 2008;105:10173–10178. doi: 10.1073/pnas.0803559105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamma T, Reichow SL, Varani G, Ferre-D’Amare AR. The Cbf5-Nop10 complex is a molecular bracket that organizes box H/ACA RNPs. Nat Struct Mol Biol. 2005;12:1101–1107. doi: 10.1038/nsmb1036. [DOI] [PubMed] [Google Scholar]
- He J, Navarrete S, Jasinski M, Vulliamy T, Dokal I, Bessler M, Mason PJ. Targeted disruption of Dkc1, the gene mutated in X-linked dyskeratosis congenita, causes embryonic lethality in mice. Oncogene. 2002;21:7740–7744. doi: 10.1038/sj.onc.1205969. [DOI] [PubMed] [Google Scholar]
- Heiss NS, Knight SW, Vulliamy TJ, Klauck SM, Wiemann S, Mason PJ, Poustka A, Dokal I. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet. 1998;19:32–38. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- Knight SW, Heiss NS, Vulliamy TJ, Greschner S, Stavrides G, Pai GS, Lestringant G, Varma N, Mason PJ, Dokal I, Poustka A. X-linked dyskeratosis congenita is predominantly caused by missense mutations in the DKC1 gene. Am J Hum Genet. 1999;65:50–58. doi: 10.1086/302446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Ye K. Crystal structure of an H/ACA box ribonucleoprotein particle. Nature. 2006;443:302–307. doi: 10.1038/nature05151. [DOI] [PubMed] [Google Scholar]
- Liu Y, Kha H, Ungrin M, Robinson MO, Harrington L. Preferential maintenance of critically short telomeres in mammalian cells heterozygous for mTert. Proc Natl Acad Sci U S A. 2002;99:3597–3602. doi: 10.1073/pnas.062549199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinda KM, Sidhu GS, Mani H, Banaudha K, Maheshwari RK, Goldstein AL, Kleinman HK. Thymosin beta4 accelerates wound healing. J Invest Dermatol. 1999;113:364–368. doi: 10.1046/j.1523-1747.1999.00708.x. [DOI] [PubMed] [Google Scholar]
- Meier UT. The many facets of H/ACA ribonucleoproteins. Chromosoma. 2005;114:1–14. doi: 10.1007/s00412-005-0333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JR, Cheng J, Collins K. A box H/ACA small nucleolar RNA-like domain at the human telomerase RNA 3′ end. Mol Cell Biol. 1999a;19:567–576. doi: 10.1128/mcb.19.1.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999b;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- Mochizuki Y, He J, Kulkarni S, Bessler M, Mason PJ. Mouse dyskerin mutations affect accumulation of telomerase RNA and small nucleolar RNA, telomerase activity, and ribosomal RNA processing. Proc Natl Acad Sci U S A. 2004;101:10756–10761. doi: 10.1073/pnas.0402560101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogacic V, Dragon F, Filipowicz W. Human H/ACA small nucleolar RNPs and telomerase share evolutionarily conserved proteins NHP2 and NOP10. Mol Cell Biol. 2000;20:9028–9040. doi: 10.1128/mcb.20.23.9028-9040.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthy V, Swann SL, Paulson JL, Spedaliere CJ, Mueller EG. Critical aspartic acid residues in pseudouridine synthases. J Biol Chem. 1999;274:22225–22230. doi: 10.1074/jbc.274.32.22225. [DOI] [PubMed] [Google Scholar]
- Rashid R, Liang B, Baker DL, Youssef OA, He Y, Phipps K, Terns RM, Terns MP, Li H. Crystal structure of a Cbf5-Nop10-Gar1 complex and implications in RNA-guided pseudouridylation and dyskeratosis congenita. Mol Cell. 2006;21:249–260. doi: 10.1016/j.molcel.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Ruggero D, Grisendi S, Piazza F, Rego E, Mari F, Rao PH, Cordon-Cardo C, Pandolfi PP. Dyskeratosis congenita and cancer in mice deficient in ribosomal RNA modification. Science. 2003;299:259–262. doi: 10.1126/science.1079447. [DOI] [PubMed] [Google Scholar]
- Spedaliere CJ, Hamilton CS, Mueller EG. Functional importance of motif I of pseudouridine synthases: mutagenesis of aligned lysine and proline residues. Biochemistry. 2000;39:9459–9465. doi: 10.1021/bi001079n. [DOI] [PubMed] [Google Scholar]
- Vulliamy TJ, Dokal I. Dyskeratosis congenita: the diverse clinical presentation of mutations in the telomerase complex. Biochimie. 2008;90:122–130. doi: 10.1016/j.biochi.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Vulliamy TJ, Marrone A, Knight SW, Walne A, Mason PJ, Dokal I. Mutations in dyskeratosis congenita: their impact on telomere length and the diversity of clinical presentation. Blood. 2006;107:2680–2685. doi: 10.1182/blood-2005-07-2622. [DOI] [PubMed] [Google Scholar]
- Wong JM, Collins K. Telomerase RNA level limits telomere maintenance in X-linked dyskeratosis congenita. Genes Dev. 2006;20:2848–2858. doi: 10.1101/gad.1476206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JM, Kyasa MJ, Hutchins L, Collins K. Telomerase RNA deficiency in peripheral blood mononuclear cells in X-linked dyskeratosis congenita. Hum Genet. 2004;115:448–455. doi: 10.1007/s00439-004-1178-7. [DOI] [PubMed] [Google Scholar]
- Yoon A, Peng G, Brandenburger Y, Zollo O, Xu W, Rego E, Ruggero D. Impaired control of IRES-mediated translation in X-linked dyskeratosis congenita. Science. 2006;312:902–906. doi: 10.1126/science.1123835. [DOI] [PubMed] [Google Scholar]
- Zebarjadian Y, King T, Fournier MJ, Clarke L, Carbon J. Point mutations in yeast CBF5 can abolish in vivo pseudouridylation of rRNA. Mol Cell Biol. 1999;19:7461–7472. doi: 10.1128/mcb.19.11.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Hathcock KS, Hande P, Lansdorp PM, Seldin MF, Hodes RJ. Telomere length regulation in mice is linked to a novel chromosome locus. Proc Natl Acad Sci U S A. 1998;95:8648–8653. doi: 10.1073/pnas.95.15.8648. [DOI] [PMC free article] [PubMed] [Google Scholar]