Abstract
Background & Aims
The historical prevalence and long-term outcome of undiagnosed celiac disease (CD) are unknown. We investigated the long-term outcome of undiagnosed CD and whether the prevalence of undiagnosed CD has changed during the past 50 years.
Methods
This study included 9,133 healthy young adults at Warren Air Force Base (sera were collected between 1948 and 1954) and 12,768 sex-matched subjects from 2 recent cohorts from Olmsted County, Minnesota, with either similar years of birth (n=5,558) or age at sampling (n=7,210) to that of the Air Force cohort. Sera were tested for tissue transglutaminase and, if abnormal, for endomysial antibodies. Survival was measured during a follow-up period of 45 years in the Air Force cohort. The prevalence of undiagnosed CD between the Air Force cohort and recent cohorts was compared.
Results
Of 9,133 persons from the Air Force cohort, 14 (0.2%) had undiagnosed CD. In this cohort, during 45 years of follow-up, all-cause mortality was greater in persons with undiagnosed CD than among those who were seronegative (hazard ratio=3.9; 95% CI, 2.0–7.5; P<.001). Undiagnosed CD was found in 68 (0.9%) persons with similar age at sampling and 46 (0.8%) persons with similar years of birth. The rate of undiagnosed CD was 4.5-fold and 4-fold greater in the recent cohorts (respectively) than in the Air Force cohort (both P ≤ .0001).
Conclusions
During 45 years of follow-up, undiagnosed CD was associated with a nearly 4-fold increased risk of death. The prevalence of undiagnosed CD appears to have increased dramatically in the United States during the past 50 years.
Keywords: cancer, celiac disease, mortality, prevalence, serology
Celiac disease (CD) is one of the most common chronic inflammatory conditions of the digestive system and is treatable with exclusion of dietary gluten (1). Clinical presentations in CD have been related to the iceberg model of disease; in which classic malabsorption represents the tip of the iceberg and less typical forms are invisible below the waterline (2). Thus, earlier estimates of the prevalence of CD relying on only the presence of the florid malabsorption syndrome with subsequent confirmation by intestinal biopsy are unreliable because many subjects with more subtle manifestations were unrecognized (3,4). Noninvasive screening based on serologic testing within diverse populations has found that CD often remained undiagnosed, and it is currently widely accepted that CD affects about 1% of the American population (5–7). However, the prevalence of undiagnosed CD 50 years ago is unknown because modern serologic testing was not available.
Although currently available data are limited to clinically diagnosed disease, CD seems to be associated with a doubling of mortality and substantial morbidity compared with that in the general population (8–10). Symptomatic CD can be a devastating illness, but many of its effects are preventable or reversible with strict adherence to a gluten-free diet (1,11). In the absence of treatment of symptomatic CD, complications may develop and mortality may increase, but it is unclear whether adverse outcomes occur in all patients with CD or only the small proportion in whom CD becomes clinically obvious. Thus, the natural history of undiagnosed CD remains unclear.
We sought to determine the prevalence of CD in the United States 50 years ago, and the outcome of undiagnosed CD, by testing frozen sera obtained from 1948 to 1954 in a large historical cohort of healthy young persons. The study was designed specifically to test the following hypotheses: 1) undiagnosed CD is associated with excess mortality and 2) the prevalence of CD has dramatically increased in the United States during the past 50 years.
Material and Methods
Subjects
We concurrently tested serum samples for CD antibodies in 3 cohorts (Table 1). The first cohort, the Warren Air Force Base (WAFB) cohort, was a large sample of healthy young men whose serum was collected from 1948 to 1954 and stored frozen as part of a series of studies and surveillance activities related to streptococcal infection (12,13). The cohort was subsequently used successfully to study the prevalence and outcomes of hepatitis C infection. The repository and construction of the study cohort have been described previously (14). To assess a group of persons with years of birth comparable to those in the WAFB cohort, we included a second cohort that was an essentially complete sampling of the older (≥50 years old) population in Olmsted County, Minnesota, during the years 1995 to 2003; the cohort was originally assembled for studies on monoclonal gammopathy of undetermined significance (15). To assess persons whose ages were similar to those of the WAFB cohort at sampling, we included a third cohort that was a convenient sampling of younger men in the general population (18–49 years old) from Olmsted County, Minnesota, during the years 2006 to 2008. Because the purpose for testing present-day cohorts was to compare the prevalence of undiagnosed CD with that in the historical WAFB cohort (>98% males), only male subjects were analyzed.
Table 1.
Demographics of Study Cohorts
| Cohort |
|||
|---|---|---|---|
| Present-day |
|||
| Variable | Historical WAFB (n=9,133) | Younga (n=7,210) | Olderb (n=5,558) |
| Mean age (SD) at collection of sample, y | 20.5±2.8 | 37.0±8.8 | 69.9±7.9 |
| Sex, no. (%) | |||
| Male | 6,579 (72.0%) | 7,210 (100%) | 5,558 (100%) |
| Female | 97 (1.1%) | - | - |
| Unknown | 2,457 (26.9%) | - | - |
| Race, no. (%) | |||
| White | 5,774 (63.2%) | 4,793 (66.5%) | 4,804 (86.4%) |
| African American | 668 (7.3%) | 296 (4.1%) | 35 (0.6%) |
| Others | 23 (0.3%) | 489 (6.8%) | 80 (1.4%) |
| Unknown | 2,668 (29.2%) | 1,632 (22.6%) | 639 (11.5%) |
Abbreviation: WAFB, Warren Air Force Base.
Age at sample collection was similar to that of the WAFB cohort.
Years of birth were similar to those of the WAFB cohort.
Study Protocol
Sera were evaluated for tissue transglutaminase antibodies (tTGA) by enzyme-linked immunosorbent assay. Samples with abnormal tTGA results (titers ≥4 U/mL) were tested for endomysial antibodies (EMA) by indirect immunofluorescence. Sera were blindly and simultaneously tested at Mayo Clinic between July 2006 and May 2008.
Feasibility of Testing in 50-Year-Old Sera
When the WAFB cohort serum was used to identify hepatitis C infection (14), the serologic tests used immunoglobulin G isotype. The tests for CD, however, used immunoglobulin A. We expected immunoglobulin A to be very stable when frozen undisturbed. To evaluate its stability after long storage, we assayed 104 random WAFB cohort samples with nephelometry (Minineph; The Binding Site Limited, Birmingham, United Kingdom) and found levels to be in the normal range and comparable (P=.84) to those in a random sample of the present-day cohorts (n=253).
Tissue Transglutaminase Antibodies
Tissue transglutaminase immunoglobulin A antibodies were measured using human recombinant antigen (Bindazyme; The Binding Site Limited, Birmingham, United Kingdom). Result interpretation was as follows: negative, less than 4.0 U/mL; weakly positive, 4 to 10 U/mL; and positive, more than 10 U/mL.
Endomysial Antibody
Endomysial antibody was determined in serum using monkey esophagus (Bindazyme) and visualized by indirect immunofluorescence (16). The result was positive if fluorescence was present at titers of 1:5 or more.
Diagnostic Categories
Diagnostic categories were defined by composite result of the 2 autoantibodies. The retrospective diagnosis of CD was established if both tTGA and subsequent EMA were positive. Celiac disease was ruled out if tTGA was negative. A result was equivocal if tTGA was weakly positive or positive and EMA was negative. This sequential testing paradigm, using subsequent EMA testing for every positive tTGA result, had a sensitivity of 97% and specificity of 100% for the diagnosis of CD by testing 1,000 serum samples from the adult Swedish general population with intestinal biopsies in the Kalixanda study of gastrointestinal symptoms (17).
Outcomes
Survival from the date a serum sample was collected in the historical WAFB cohort was the principal outcome of interest. Vital status records through March 30, 1997, were obtained from the US Department of Veterans Affairs and the US Social Security Administration. Causes of death were obtained from the National Death Index of the National Center for Health Statistics and also from death certificates in the Department of Veterans Affairs claim folder (18). The causes of death were coded by a qualified nosologist using the International Classification of Diseases, ninth revision.
Statistical Analysis
Data were summarized with descriptive statistics. The Kaplan-Meier method was used to estimate survival rates from time of serum collection in the WAFB cohort, and the log-rank test was used to assess the association of survival with serology. A multivariate Cox proportional hazards regression model further estimated this association adjusted for potential confounders such as age, sex, and enlistment status. To include all subjects, age and sex were each categorized, including a category for unknown values. An adjusted hazard ratio and 95% confidence interval (CI) are reported to summarize the risk of death in subjects with undiagnosed CD relative to those with seronegative results.
The Cochran-Mantel-Haenszel test was used to compare the prevalence of CD between the WAFB cohort and both present-day cohorts, stratifying on age at serum draw in comparison to the younger present-day cohort and, separately, year of birth in comparison with the older present-day cohort. A P value less than .05 was considered statistically significant.
Ethical Considerations
This study was approved by the institutional review boards of Mayo Clinic, the University of Minnesota, and the National Academies.
Results
The WAFB Cohort
Demographic Data
The cohort total was 9,133 persons. Of 7,950 whose date of birth was known, 7,511 (94.5%) were younger than 25 years, 426 (5.4%) were 25 to 40 years old, and 13 (0.2%) were older than 40 years at sampling. Of 6,676 persons whose sex was known, 6,579 (98.6%) were men. Among 6,465 persons whose ethnicity was known, 5,774 (89.3%) were white, 668 (10.3%) were African American, and 23 (0.4%) were others.
Serologic Data
Among 9,133 persons tested, the tTGA titer was negative in 9,090 (99.5%), weakly positive in 30 (0.4%), and positive in 13 (0.1%). EMA was positive in 14 (32.6%) of the 43 subjects with positive or weakly positive tTGA results. Undiagnosed CD was found in 14 (0.2%; 95% CI, 0.1%–0.3%), or 1 in 652, persons. The median titer of tTGA in the 14 persons with undiagnosed CD was 17.1 U/mL (range, 5.2–78.8 U/mL). Among subjects with known birth dates, the median age of the group with undiagnosed CD at the time of sampling was 19.7 years (range, 17.4–22.6 years), and the median age of the seronegative group was 20.0 years (range, 14.3–46.4 years). Among persons with undiagnosed CD with known ethnicity (n=9), all were white. No subjects with undiagnosed CD received a clinical diagnosis of CD within the 45-year follow-up period and, therefore, likely remained untreated.
Survival Analysis
Through March 1997, the Kaplan-Meier all-cause mortality rate for the entire cohort was 23.4% (95% CI, 22.5%–24.3%). The mortality rate was higher among subjects with undiagnosed CD (64.3%; 95% CI, 40.6%–88.6%) than among seronegative persons (24.3%; 95% CI, 22.5%–24.3%). Fourteen (of 9,090) seronegative persons in the WAFB cohort lacked valid follow-up data and were thus excluded from the Kaplan-Meier analysis (Figure). During 45 years of follow-up, the hazard ratio for mortality was nearly 4-fold higher for subjects with undiagnosed CD than for seronegative persons (3.9, 95% CI, 2.0–7.5, P<.001), adjusted for age, sex, and enlistment status. Mortality rates were similar among persons with equivocal serologic results and those with seronegative results (data not provided).
Figure.
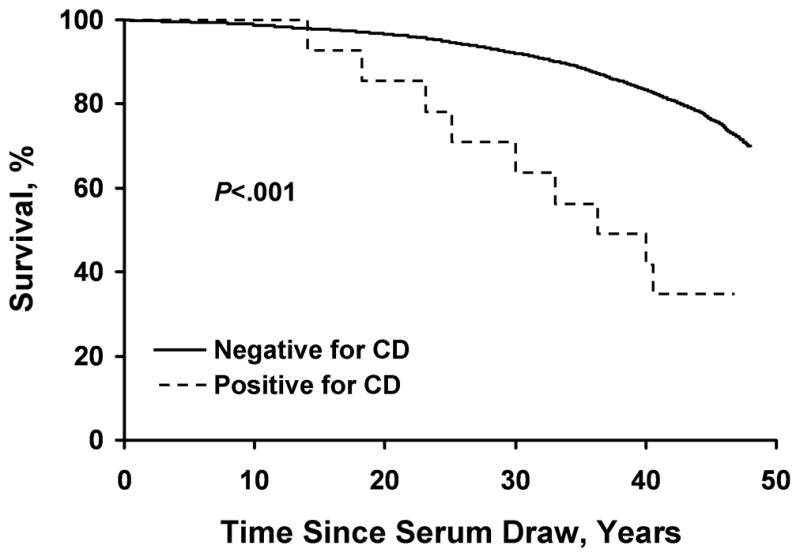
Survival during 45 years of follow-up in 14 subjects with undiagnosed celiac disease (CD) and 9,076 seronegative persons in the Warren Air Force Base cohort.
Cause of death was available for 6 of the 9 patients with undiagnosed CD who died during follow-up (Table 2). Cause-specific death data were available for 1,912 (88.1%) of the 2,169 seronegative persons who died during follow-up and reflected expected causes of death starting in early adulthood for men: cardiovascular diseases (38.5%), cancer (26.6%), and accidents (14.4%) were the most common. Only 4 persons died because of war-related injuries.
Table 2.
Age at Death, Tissue Transglutaminase Titer, and Cause of Death in 9 Subjects From the Historical WAFB Cohort With Seropositive Results
| Case | Age at Testing/Age at Death, y | Interval From Time of Original Phlebotomy to Death, y | Tissue Transglutaminase Level, U/mL | ICD-9 CM Code | Cause of Death |
|---|---|---|---|---|---|
| 1 | 22/62 | 40 | 49.0 | 492.0 | Emphysema |
| 2 | 17/54 | 37 | 43.8 | 202.8 | Other lymphomas |
| 3 | 22/55 | 33 | 6.0 | 161.9 | Malignant neoplasm of the larynx |
| 4 | 18/48 | 30 | 5.2 | 799.9 | Other unknown and unspecified cause |
| 5 | 20/43 | 23 | 17.5 | 414.0 | Other forms of chronic ischemic heart disease |
| 6 | 19/60 | 41 | 26.6 | 150.9 | Malignant neoplasm of the esophagus |
| 7 | 18/43 | 25 | 6.3 | - | Unknown |
| 8 | 23/37 | 14 | 16.7 | - | Unknown |
| 9 | 20/38 | 18 | 78.8 | - | Unknown |
Abbreviation: ICD-9 CM, International Classification of Diseases Clinical Modification, version 9.
Present-Day Cohort With Similar Years of Birth (Older)
Demographic Data
Among 16,887 older adults (≥50 years of age) from Olmsted County, Minnesota, only the 7,690 (45.5%) men were considered for inclusion. Among these, 5,558 (72.3%) men with years of birth comparable to those in the WAFB cohort were included in this analysis. Among 4,919 (89.0%) persons whose ethnicity was known, 4,804 (97.7%) were white, 35 (0.7%) were African American, and 80 (1.6%) were other.
Serologic Data
Among the 5,558 persons tested, the tTGA titer was negative in 5,501 (99.0%), weakly positive in 19 (0.3%), and positive in 38 (0.7%). EMA was positive in 46 (80.7%) of the 57 subjects with positive or weakly positive tTGA results. Undiagnosed CD was therefore found in 46 (0.8%; 95% CI, 0.6%–1.1%), or 1 in 121, persons. The median tTGA titer in the 46 persons with undiagnosed CD was 20.5 U/mL (range, 5.2–112.3 U/mL). The median age of the undiagnosed CD group at sampling was 65.3 years (range, 58.2–87.7), and the median age of the seronegative group was 68.8 years (range, 56.8–93.4). Among persons with undiagnosed CD whose ethnicity was known (n=41), all were white.
Present-Day Cohort With Similar Age at Sampling (Young)
Demographic Data
Among 17,758 young adults from convenient sampling of community residents 18 to 49 years old, only the 7,210 (40.6%) men were included. Among 5,578 persons whose ethnicity was known, 4,793 (85.9%) were white, 296 (5.3%) were African American, and 489 (8.8%) were other.
Serologic Data
Among the 7,210 persons tested, the tTGA was negative in 7,128 (98.9%), weakly positive in 28 (0.4%), and positive in 54 (0.8%). EMA was positive in 68 (82.9%) of the 82 subjects with positive or weakly positive tTGA results. Undiagnosed CD was found in 68 (0.9%; 95% CI, 0.7%–1.2%), or 1 in 106, persons. The median titer of tTGA in the 68 persons with undiagnosed CD was 21.8 U/mL (range, 4.1–151.7). The median age at sampling was 39.2 years (range, 18.4–49.9) for the undiagnosed CD group, and 21.8 years (range, 18.0–50.0) for the seronegative group. Among the 49 persons with undiagnosed CD whose ethnicity was known, 47 (95.9%) were white.
Comparative Prevalence of CD Over Time
The prevalence of undiagnosed CD was 4 times higher in the older present-day cohort and 4.5 times higher in the younger present-day cohort than in the WAFB cohort (P≤.0001 for both by Cochran-Mantel-Haenszel test) (Table 3). There was a higher percentage, among those who had EMA testing, of weakly positive tTGA results (as compared to positive) in the WAFB cohort (30/43, 69.8%) than in the older present-day cohort (19/57, 33.3%) and the young present-day cohort (28/54, 34.2%) (P<.001 for both).
Table 3.
Summary of Diagnostic Categories According to Cohort
| Cohort |
|||
|---|---|---|---|
| Present-day |
|||
| Diagnostic Category | Historical WAFB (n=9,133) | Younga (n=7,210) | Olderb (n=5,558) |
| Celiac disease, no. (%) | 14 (0.2%) | 68 (0.9%)c | 46 (0.8%)c |
| Equivocal serology, no. (%) | 29 (0.3%) | 14 (0.2%) | 11 (0.2%) |
| No celiac disease, no. (%) | 9,090 (99.5%) | 7,128 (98.9%) | 5,501 (99.0%) |
Abbreviation: WAFB, Warren Air Force Base.
Age at sample collection was similar to that of the WAFB cohort.
Years of birth were similar to those of the WAFB cohort.
P≤.0001 (Cochran-Mantel-Haenszel test) compared with the historical cohort, stratifying on age at sample drawa or year of birthb.
Discussion
This study yielded 2 major findings. First, undiagnosed CD was associated with a nearly 4-fold increased risk of death compared with subjects without serologic evidence of CD. Second, the prevalence of CD appears to have increased dramatically in the United States during the past 50 years.
These results are important because, by testing a unique collection of sera obtained from 1948 to 1954, we were able to study the long-term natural history of CD. Our results confirm recent data (19) showing that undiagnosed CD is associated with a significant excess mortality over time. Thus, early detection and treatment of presymptomatic CD appear logical if we assume that strict adherence to a gluten-free diet has the same positive effect in undiagnosed CD as previously shown in symptomatic CD (11). Exploring the potential benefits of early detection and intervention with a gluten-free diet is also important because undiagnosed CD results in excess mortality during middle age. However, both the low number of cases with undiagnosed CD and the missing data render specific comparison unreliable on cause of death between subjects with seronegative results and subjects with undiagnosed CD. Whether treatment actually improves survival in patients with clinically silent CD remains unknown. The benefits, if any and cost-effectiveness of treatment should be prospectively assessed, including any influences on cumulative morbidity, quality of life, and mortality in silent CD (20). For ethical reasons, it is unlikely that a subject found to have CD, albeit silent, can be randomized to no treatment.
This study suggests that the prevalence of CD has dramatically increased more than 4-fold in the United States during the past 50 years, consistent with the finding in a recent study from Europe (21). Reasons for the increased prevalence of CD over time are unknown. However, because human genetic changes in response to environmental challenges are extremely slow, the most likely explanation may be environmental, such as a change in quantity, quality, or processing of cereal. Several major changes in wheat genetics, bread processing, and enzymatic modification of wheat prolamins as a result of industry food processing have occurred in the past 40 years (22,23). Changing patterns of early childhood infection may also affect the prevalence of autoimmune diseases (“hygiene hypothesis”) (24,25), but the host immune system-microbial interactions are complex and some infections (ie, rotavirus) may increase the risk of CD autoimmunity in genetically predisposed children (26). The hygiene hypothesis is likely only a partial explanation for the increasing prevalence because CD is a global health problem that affects both developed and developing countries (5).
For the cohort of subjects with a similar year of birth (older present-day cohort), the higher prevalence of CD compared with that in the historical WAFB cohort cannot be solely explained by a higher detection rate as a result of using modern serologic testing (27), because the same modern serologic tests were used for diagnosis in both. This finding further supports our theory that an unidentified environmental factor or factors are responsible for the changing prevalence of CD in the United States over time. The potential role of highly processed nutrients as modifiers of gene expression (nutrigenomics) that may alter the risk for development of CD in genetically susceptible individuals is intriguing but difficult to prove experimentally (28). Because our data are cross-sectional, the exact point in time when the change in prevalence occurred and the actual trend over time are unknown.
Particular strengths of our study included use of a unique collection of 50-year-old sera, availability of 45-year follow-up data, and overall large sample size. Several potential limitations of this study should also be considered, including the lack of diagnostic confirmation of CD by intestinal biopsy. However, the very high specificity of the sequential testing paradigm strongly supports the serologic-based diagnosis of CD and makes false-positive results due to disorders associated with single-antibody positivity (especially tTGA) very unlikely (19,29). Survival estimates should be interpreted cautiously because they are based on a limited number of cases of undiagnosed CD and because the deaths are known through only March 1997. Moreover, potential confounders were not systematically evaluated (especially smoking habits, in that some deaths in persons with undiagnosed CD could be smoking-related); thus, the effect of those factors in risk of death in persons with undiagnosed CD is unknown. However, most studies have reported an inverse association between cigarette smoking and CD (30–33).
The major limitation of this study is that the historical WAFB cohort and the present-day cohorts are heterogeneous, although every effort was made to make the cohorts comparable in terms of sex and age. Clinically diagnosed CD is more common in women (female:male ratio, 2–3:1) (1), but screening-detected CD was equally prevalent in men and women (7). Although our analysis is limited to male subjects, it is therefore unlikely that our results are biased by sex selection. In addition, the WAFB cohort is composed of male subjects from various parts of the United States, and the present-day cohorts are from the Upper Midwest. The incidence of clinically detected CD has increased recently in Olmsted County, Minnesota (27), but serologic evidence of CD in the present-day cohorts (~1%) was similar to that in other settings in the United States and Europe (5,7). Thus, the difference in the prevalence of undiagnosed CD between the WAFB cohort and present-day cohorts is not explained by a higher risk for CD in the Olmsted County cohorts. African American participation was also higher in the WAFB cohort than in both present-day cohorts, but none of the WAFB subjects with undiagnosed CD were known to be African American, and only 1 subject with undiagnosed CD was known to be African American in both present-day cohorts combined. Therefore, we believe that this imbalance is not confounding the prevalence rate comparisons. In fact, excluding known African Americans in a post-hoc analysis, we found that the rate of undiagnosed CD among WAFB subjects was still substantially less than in the present-day cohorts (data not provided). Finally, we cannot assess the impact that a potential shift in age at onset of CD during the past 50 years may have had on the observed differences in prevalence because all subjects were adults at the time of sampling in the WAFB cohort and the present-day cohorts. However, this possibility seems unlikely as the rate of diagnosis of CD during childhood in Olmsted County has been consistently low for the past 50 years (27).
Thus, using a unique collection of sera with the longest follow-up to date, we showed that undiagnosed CD was associated with a nearly 4-fold increased risk of death compared with that in subjects without serologic evidence of CD. This result is clinically relevant. Moreover, our finding that the prevalence of CD seems to have increased dramatically during the past 50 years suggests that CD is emerging as a substantial public health concern in the United States.
Acknowledgments
Funding/Support: This work was supported in part by the National Institutes of Health (NIH) under Ruth L. Kirschstein National Research Service Award/Training Grant in Gastrointestinal Allergy and Immunology Research T32 AI07047 (A.R.-T.), NIH grants DK57892, DK070031, AR30582 (J.A.M.), DK61617 (W.R.K.), and CA62242 (R.A.K.), and the CTSA grant 1UL1RR024150-01 from the National Center for Research Resources.
Abbreviations
- CD
celiac disease
- CI
confidence interval
- EMA
endomysial antibody
- tTGA
tissue transglutaminase antibody
- WAFB
Warren Air Force Base
Footnotes
Portions of this manuscript have been published in abstract form: Gastroenterology. 2008 Apr;134 Suppl 1:A-80-1.
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007 Oct 25;357(17):1731–43. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 2.Rostom A, Murray JA, Kagnoff MF. American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastroenterology. 2006 Dec;131(6):1981–2002. doi: 10.1053/j.gastro.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Talley NJ, Valdovinos M, Petterson TM, Carpenter HA, Melton LJ., 3rd Epidemiology of celiac sprue: a community-based study. Am J Gastroenterol. 1994 Jun;89(6):843–6. [PubMed] [Google Scholar]
- 4.Rossi TM, Albini CH, Kumar V. Incidence of celiac disease identified by the presence of serum endomysial antibodies in children with chronic diarrhea, short stature, or insulin-dependent diabetes mellitus. J Pediatr. 1993 Aug;123(2):262–4. doi: 10.1016/s0022-3476(05)81699-3. [DOI] [PubMed] [Google Scholar]
- 5.Catassi C. The world map of celiac disease. Acta Gastroenterol Latinoam. 2005;35(1):37–55. [PubMed] [Google Scholar]
- 6.Not T, Horvath K, Hill ID, Partanen J, Hammed A, Magazzu G, et al. Celiac disease risk in the USA: high prevalence of antiendomysium antibodies in healthy blood donors. Scand J Gastroenterol. 1998 May;33(5):494–8. doi: 10.1080/00365529850172052. [DOI] [PubMed] [Google Scholar]
- 7.Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003 Feb 10;163(3):286–92. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen OH, Jacobsen O, Pedersen ER, Rasmussen SN, Petri M, Laulund S, et al. Non-tropical sprue: malignant diseases and mortality rate. Scand J Gastroenterol. 1985 Jan;20(1):13–8. doi: 10.3109/00365528509089626. [DOI] [PubMed] [Google Scholar]
- 9.Logan RF, Rifkind EA, Turner ID, Ferguson A. Mortality in celiac disease. Gastroenterology. 1989 Aug;97(2):265–71. doi: 10.1016/0016-5085(89)90060-7. [DOI] [PubMed] [Google Scholar]
- 10.Corrao G, Corazza GR, Bagnardi V, Brusco G, Ciacci C, Cottone M, et al. Club del Tenue Study Group. Mortality in patients with coeliac disease and their relatives: a cohort study. Lancet. 2001 Aug 4;358(9279):356–61. doi: 10.1016/s0140-6736(01)05554-4. [DOI] [PubMed] [Google Scholar]
- 11.Collin P, Reunala T, Pukkala E, Laippala P, Keyrilainen O, Pasternack A. Coeliac disease: associated disorders and survival. Gut. 1994 Sep;35(9):1215–8. doi: 10.1136/gut.35.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denny FW, Wannamaker LW, Brink WR, Rammelkamp CH, Jr, Custer EA. Prevention of rheumatic fever; treatment of the preceding streptococcic infection. J Am Med Assoc. 1950 May 13;143(2):151–3. doi: 10.1001/jama.1950.02910370001001. [DOI] [PubMed] [Google Scholar]
- 13.Thomas RJ, Conwill DE, Morton DE, Brooks TJ, Holmes CK, Mahaffey WB. Penicillin prophylaxis for streptococcal infections in United States Navy and Marine Corps recruit camps, 1951–1985. Rev Infect Dis. 1988 Jan–Feb;10(1):125–30. doi: 10.1093/clinids/10.1.125. [DOI] [PubMed] [Google Scholar]
- 14.Seeff LB, Miller RN, Rabkin CS, Buskell-Bales Z, Straley-Eason KD, Smoak BL, et al. 45-Year follow-up of hepatitis C virus infection in healthy young adults. Ann Intern Med. 2000 Jan 18;132(2):105–11. doi: 10.7326/0003-4819-132-2-200001180-00003. [DOI] [PubMed] [Google Scholar]
- 15.Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002 Feb 21;346(8):564–9. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 16.Chorzelski TP, Beutner EH, Sulej J, Tchorzewska H, Jablonska S, Kumar V, et al. IgA anti-endomysium antibody: a new immunological marker of dermatitis herpetiformis and coeliac disease. Br J Dermatol. 1984 Oct;111(4):395–402. doi: 10.1111/j.1365-2133.1984.tb06601.x. [DOI] [PubMed] [Google Scholar]
- 17.Aro P, Ronkainen J, Storskrubb T, Bolling-Sternevald E, Carlsson R, Johansson SE, et al. Valid symptom reporting at upper endoscopy in a random sample of the Swedish adult general population: the Kalixanda study. Scand J Gastroenterol. 2004 Dec;39(12):1280–8. doi: 10.1080/00365520410008141. [DOI] [PubMed] [Google Scholar]
- 18.Patterson BH, Bilgrad R. Use of the National Death Index in cancer studies. J Natl Cancer Inst. 1986 Oct;77(4):877–81. [PubMed] [Google Scholar]
- 19.Metzger MH, Heier M, Maki M, Bravi E, Schneider A, Lowel H, et al. Mortality excess in individuals with elevated IgA anti-transglutaminase antibodies: the KORA/MONICA Augsburg cohort study 1989–1998. Eur J Epidemiol. 2006;21(5):359–65. doi: 10.1007/s10654-006-9002-4. Epub 2006 Apr 29. [DOI] [PubMed] [Google Scholar]
- 20.Collin P. Should adults be screened for celiac disease? What are the benefits and harms of screening? Gastroenterology. 2005 Apr;128(4 Suppl 1):S104–8. doi: 10.1053/j.gastro.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 21.Lohi S, Mustalahti K, Kaukinen K, Laurila K, Collin P, Rissanen H, et al. Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther. 2007 Nov 1;26(9):1217–25. doi: 10.1111/j.1365-2036.2007.03502.x. [DOI] [PubMed] [Google Scholar]
- 22.Cabrera-Chavez F, Rouzaud-Sandez O, Sotelo-Cruz N, Calderon de la Barca AM. Transglutaminase treatment of wheat and maize prolamins of bread increases the serum IgA reactivity of celiac disease patients. J Agric Food Chem. 2008 Feb 27;56(4):1387–91. doi: 10.1021/jf0724163. Epub 2008 Jan 15. [DOI] [PubMed] [Google Scholar]
- 23.Cronin CC, Shanahan F. Why is celiac disease so common in Ireland? Perspect Biol Med. 2001 Summer;44(3):342–52. doi: 10.1353/pbm.2001.0045. [DOI] [PubMed] [Google Scholar]
- 24.Rautava S, Ruuskanen O, Ouwehand A, Salminen S, Isolauri E. The hygiene hypothesis of atopic disease: an extended version. J Pediatr Gastroenterol Nutr. 2004 Apr;38(4):378–88. doi: 10.1097/00005176-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Kondrashova A, Mustalahti K, Kaukinen K, Viskari H, Volodicheva V, Haapala AM, et al. Epivir Study Group. Lower economic status and inferior hygienic environment may protect against celiac disease. Ann Med. 2008;40(3):223–31. doi: 10.1080/07853890701678689. [DOI] [PubMed] [Google Scholar]
- 26.Stene LC, Honeyman MC, Hoffenberg EJ, Haas JE, Sokol RJ, Emery L, et al. Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am J Gastroenterol. 2006 Oct;101(10):2333–40. doi: 10.1111/j.1572-0241.2006.00741.x. [DOI] [PubMed] [Google Scholar]
- 27.Murray JA, Van Dyke C, Plevak MF, Dierkhising RA, Zinsmeister AR, Melton LJ., 3rd Trends in the identification and clinical features of celiac disease in a North American community, 1950–2001. Clin Gastroenterol Hepatol. 2003 Jan;1(1):19–27. doi: 10.1053/jcgh.2003.50004. [DOI] [PubMed] [Google Scholar]
- 28.Ross SA. Nutritional genomic approaches to cancer prevention research. Exp Oncol. 2007 Dec;29(4):250–6. [PubMed] [Google Scholar]
- 29.Peracchi M, Trovato C, Longhi M, Gasparin M, Conte D, Tarantino C, et al. Tissue transglutaminase antibodies in patients with end-stage heart failure. Am J Gastroenterol. 2002 Nov;97(11):2850–4. doi: 10.1111/j.1572-0241.2002.07033.x. [DOI] [PubMed] [Google Scholar]
- 30.Snook JA, Dwyer L, Lee-Elliot C, Khan S, Wheeler DW, Nicholas DS. Adult coeliac disease and cigarette smoking. Gut. 1996 Jul;39(1):60–2. doi: 10.1136/gut.39.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vazquez H, Smecuol E, Flores D, Mazure R, Pedreira S, Niveloni S, et al. Relation between cigarette smoking and celiac disease: evidence from a case-control study. Am J Gastroenterol. 2001 Mar;96(3):798–802. doi: 10.1111/j.1572-0241.2001.03625.x. [DOI] [PubMed] [Google Scholar]
- 32.Austin AS, Logan RF, Thomason K, Holmes GK. Cigarette smoking and adult coeliac disease. Scand J Gastroenterol. 2002 Aug;37(8):978–82. doi: 10.1080/003655202760230973. [DOI] [PubMed] [Google Scholar]
- 33.Patel AH, Loftus EV, Jr, Murray JA, Harmsen WS, Zinsmeister AR, Sandborn WJ. Cigarette smoking and celiac sprue: a case-control study. Am J Gastroenterol. 2001 Aug;96(8):2388–91. doi: 10.1111/j.1572-0241.2001.04040.x. [DOI] [PubMed] [Google Scholar]


