Abstract
Systemic Lupus Erythematosus (SLE) is a multisystem autoimmune disease that, despite the advances in immunosuppressive medical therapies, remains potentially fatal in some patients, especially in treatment-refractory patients. Here we reported that impairment of bone marrow mesenchymal stem cells (BMMSCs) and their associated osteoblastic niche deficiency contribute in part to the pathogenesis of SLE-like disease in MRL/lpr mice. Interestingly, allogenic BMMSC transplantation (MSCT) is capable of reconstructing the bone marrow osteoblastic niche and more effectively reverses multi-organ dysfunction as compared to medical immunosuppression with cyclophosphamide (CTX). At the cellular level, MSCT, not CTX treatment, was capable to induce osteoblastic niche reconstruction, possibly contributing to the recovery of regulatory T cells and re-establishment of the immune homeostasis. Based on the promising clinical outcomes in SLE mice, we treated 4 CTX/glucocorticoid treatment-refractory SLE patients using allogenic MSCT and showed a stable 12-18 months disease remission in all treated patients. The patients benefited an amelioration of disease activity, improvement in serologic markers and renal function. These early evidences suggest that allogenic MSCT may be a feasible and safe salvage therapy in refractory SLE patients.
Keywords: Bone marrow mesenchymal stem cells, Transplantation, systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is a common and potentially fatal autoimmune disease in characterized by antibodies associated multi-organ injuries including renal, cardiovascular, neural, musculoskeletal, and cutaneous systems [1]. The pathology of SLE involves the destruction of targeted organ tissues and accumulation of auto-reactive lymphocytes and immune complexes. Although disease severity and organ involvement vary significantly among SLE patients, abnormalities of T and B lymphocytes are universal [1-3]. Moreover, SLE manifests multifaceted immune modulation, including both deficiency and hyperactivity of the immune system. A deeper understanding of the underlying pathology is crucial to develop optimal therapies for the restoration of immune homeostasis without compromising the protective immune response to pathogens [4].
In addition to conventional medical therapies such as cyclophosphamide and mycophenolate mofetil, several new strategies have been developed targeting specific activation pathways relevant to SLE pathogenesis [1, 5]. For instance, B-cell-depleting therapies using the monoclonal antibodies rituximab and epratuzumab have benefitted a specific subpopulation of lupus patients [6]. Recently, hematopoietic stem cell transplantation (HSCT) has been reported to improve disease activity in treatment-refractory SLE patients [7] and reverse organ dysfunction in several animal models [8]. Despite improved supportive care, aggressive immunosuppressive medical therapies, and new therapeutic interventions, a subset of SLE patients continues to suffer significant morbidity and mortality from active disease, with visceral organ involvement. Therefore, it is urgent to develop more effective therapy for SLE disorder, especially for treatment-refractory patients.
Bone marrow mesenchymal stem cells (BMMSCs) are multipotent stem cells capable of differentiating into a variety of cell types including osteoblasts, chondrocytes, adipocytes, and myoblasts [9-11]. The BMMSC/osteoblast lineage plays a critical role in maintaining the HSC niche [12-14] and modulating immune cells including T and B lymphocytes, dendritic cells, and natural killer cells [15-20]. Transplantation of ex vivo-expanded BMMSCs proved effective in treating acute graft-versus-host-disease (GVHD) by inhibiting T lymphocyte function [21-23] and ameliorating HSC engraftment [24,25]. A recent convergence of clinical and basic research has highlighted the potential of using BMMSCs to treat immune diseases [23]. In this study, we found that deficiency of BMMSC/osteoblast function in SLE mouse model leads to impairment of the osteoblastic niche, which may correlate in part, to difficulty of reconstructing immune homeostasis in treatment-refractory SLE patients. Allogenic BMMSC transplantation (MSCT) conferred significant therapeutic effects on SLE mice and treatment-refractory patients by reconstructing the osteoblastic niche and restoring immune homeostasis.
Materials and Methods
Mice
Female C3MRL-Faslpr/J (MRL/lpr) and background matched C3H/HeJ mice were purchased from the Jackson Laboratory. Female immunocompromised mice (Beige XIDIII nude/nude) were purchased from Harlan. All animal experiments were performed under an institutionally approved protocol for the use of animal research (USC #10874 and #10941).
Antibodies
All antibodies used in this study were described in Supplementary MATERIALS AND METHODS.
Bone phenotype analysis
Micro-computed tomography (microCT) and peripheral quantitative CT (pQCT) analyses were performed as previously described [26]. Detailed methods were described in Supplementary MATERIALS AND METHODS. Paraffin sections were used for histological analysis, including H&E staining, TRAP staining and immunohistochemistry as described in Supplementary MATERIALS AND METHODS.
Isolation and culture of mouse BMMSCs
Mouse BMMSCs were isolated and cultured as described previously [26]. The details were described in Supplementary MATERIALS AND METHODS.
Allogenic mouse BMMSC transplantation into MRL/lpr mice
Under general anesthesia, C3H/HeJ-derived BMMSCs (0.1× 106 cells/10g body weight) were infused into MRL/lpr mice via tail vein at different ages of 9 weeks (n=12) and 16 weeks (n=12). In control group, MRL/lpr mice (9-week-old) received PBS (n=12) or cyclophosphamide monohydrate (Sigma) (200μg/g body weight) (n=12) and age-matched C3H/HeJ mice (n=12) were used. All mice were sacrificed at 20 weeks of age for further analysis.
SLE patients
Four patients (three female and one male) at age 16, 17, 20, and 23 were treated with CTX (0.75g/m2 per month) and prednisone (≥20 mg/day) for more than six months. The treatment was ineffective in these patients as shown the SLE disease activity index (SLEDAI) more than 8 [27] and lupus nephritis (24h urine protein≥1g and/or serum creatinine ≥1.5mg/dl) without end-stage renal failure. Four healthy patients’ relatives, at age 19 (male), 42 (male), 43 (male) and 46 (female) were selected as donors. All of the recipients and donors gave informed consent to enroll in the clinical study. This clinical study was approved by the Ethics Committee of the Affiliated Drum Tower Hospital of Nanjing University Medical School and registered at ClinicalTrials.gov (Identifier: NCT00698191).
Culture and expansion of human BMMSCs
Human bone marrow aspirates were collected from iliac of four donors and two SLE patients. The details are described in Supplementary MATERIALS AND METHODS.
Allogenic human BMMSC transplantation in SLE patients
Donor BMMSCs from patients’ family members were intravenously infused in eligible SLE recipients (≥1×106/kg body weight). Prednisone 20-30 mg was administrated to recipient patients prior to the MSCT procedure. Post MSCT maintenance therapy includes a tapering dose of steroid and CTX, with 2 patients completely off CTX at 5-6 months. Specific maintenance therapy for all 4 patients are as followed: 1) patient#1: prednisone 10mg/day and CTX 0.6g/every 2 months for 6 months, then prednisone 5mg/day and CTX 0.6g/every 2 months for 12 months; 2) patients #2: prednisone 10mg/day and CTX 0.6g/every 2 months for 7 months, then prednisone 10mg/day with no CTX for 5 months; 3) patients #3: prednisone 10mg/day and CTX 0.6g/every 2 months for 7 months, then prednisone 5mg/day and CTX 0.6g/every 2 months for 5 months; 4) patients #4: prednisone 10mg/day and CTX 0.6g/every 2 months for 6 months, then prednisone 10mg/day with no CTX for 6 months.
CFU-F assay
CFU-F assay was performed according to previous study (26, and Supplementary MATERIALS AND METHODS).
Cell proliferation assay
The proliferation of BMMSCs was evaluated by BrdU incorporation as previously described (26, and Supplementary MATERIALS AND METHODS).
In vitro differentiation assay
In vitro osteogenic and adipogenic induction of mouse BMMSCs were performed as described previously (26, and Supplementary MATERIALS AND METHODS).
In vivo bone formation assay
BMMSCs were subcutaneously transplanted into immunocompromised mice using hydroxyapatite tricalcium phosphate (HA/TCP) as a carrier [28]. Detail methods were described in the Supplementary MATERIALS AND METHODS. At eight weeks post-transplantation, the transplants were harvested for histological analysis (Supplementary MATERIALS AND METHODS).
Reverse transcriptase polymerase chain reaction (RT-PCR) analysis
Total RNA was isolated from cultures. The cDNA was amplified with specific primers (Supplementary MATERIALS AND METHODS). The specific primers were listed on Supplementary Table 1.
Western blot analysis
Western blot analysis was performed as described previously [27]. Detail methods were described in Supplementary MATERIALS AND METHODS.
Measurement of biomarkers in blood serum
urine and spleen. Peripheral blood serum, urine samples, and total protein from spleen were collected from mice. Autoantibodies, albumin, immunoglobulins, RANKL, C-terminal telopeptides of type I collagen, interleukin 6 (IL-6), IL-17 and TGFβ in the serum and spleen were analyzed by ELISA (see Supplementary MATERIALS AND METHODS). The protein concentration in urine was measured using Bio-Rad Protein Assay (Bio-Rad). Peripheral blood serum and urine samples collected from SLE patients were corrected to measure serum C3, blood urea nitrogen (BUN) and urine protein levels in the Clinical Laboratory at the Drum Tower Hospital of Nanjing University Medical School according the laboratory’s instructions.
Histological analysis of kidney, liver and spleen
Kidney, liver and spleen were harvested from mice and fixed. The sections were used for further experiments (Supplementary MATERIALS AND METHODS).
Histometry
Histomorphometric analysis was quantified as described previously [26, 27]. Detailed methods were described in Supplementary MATERIALS AND METHODS.
CD4+ T lymphocyte isolation
CD4+ T lymphocytes were isolated from mouse spleen using a magnetic sorter and mouse CD4+ T lymphocyte isolation kit (Miltenyi Biotec) following manufacture’s instruction. The purity of the CD4+ T cells was >95%.
Flow cytometric analysis
Flow cytometric staining and analysis were performed as previously reported (27, and Supplementary MATERIALS AND METHODS).
Statistical analysis
Student’s t-test was used to analyze significance between two groups. P value of less than 0.05 was considered as a significant difference.
Results
Systemic Lupus Erythematosus (SLE) model MRL/lpr mice showed BMMSC impairment and osteoblastic niche deficiency
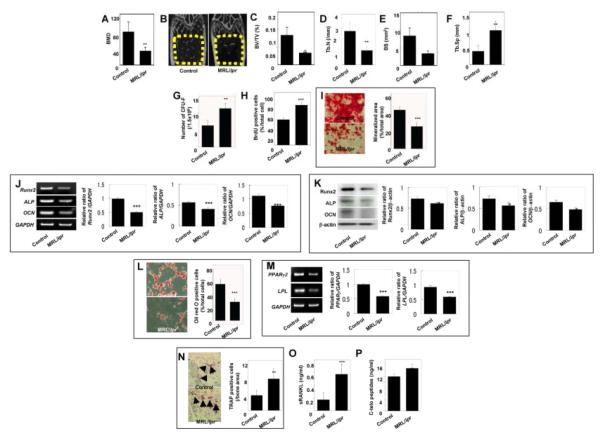
Osteoporosis is commonly reported in SLE patients secondary to long-term use of glucocorticoids and cyclophosphamide [29]. We verified the osteoporotic changes in the skeletal structures of naïve CD95-mutant MRL/lpr mice by micro-radiographic and bone morphometric analyses. The femurs of MRL/lpr mice at age of 20 weeks showed remarkable reduction in bone mineral density (BMD) (Fig. 1A) and significant atrophy of trabecular bone (Fig. 1B) with reduced bone volume (Fig. 1C), trabecular number (Fig. 1D) and bone surface area (Fig. 1E) and increased trabecular separation (Fig. 1F). These findings indicated that the skeletal system of naïve MRL/lpr mice undergoes changes typical of osteoporosis phenotype.
Figure 1.
BMMSC deficiency in MRL/lpr mice (A-F) MicroQCT analysis of the trabecular bone structure of the distal femoral metaphysis at 20-week-old MRL/lpr mice. MRL/lpr mice (MRL/lpr) exhibited significantly decreased bone mineral density (BMD, A). Representative microQCT images of the trabecular bone structure in MRL/lpr mice (n=5) exhibited a significant decrease in bone formation (yellow circle areas, B), bone volume relative to tissue volume (BV/TV, C), trabecular number (Tb.N, D), and bone surface area (BS, E) along with significantly increased trabecular separation (Tb.Sp, F) when compared to the control C3H/HeJ group (Control, n=5; mean±SD; [[P<0.01). (G) The number of CFU-F (mean±SD) in MRL/lpr mice (n=5) increased significantly as compared to the control group (n=5, [[P<0.01). (H) BMMSCs derived from MRL/lpr mice (n=5) showed significantly elevated BrdU-uptake rate. (mean±SD; Control, n=5; [[[P<0.001). (I) Representative images of alizarin red staining of BMMSC cultures under the osteogenic conditions. BMMSCs derived from MRL/lpr mice (n=5) showed significantly decreased calcium accumulation (mean±SD; Control, n=5; [[[P<0.001). (J, K) Semi-quantitative RT-PCR (J) and Western blot (K) analysis showed that MRL/lpr-derived BMMSCs presented significant decrease in the expression of Runx2, ALP, and OCN. Glycerinaldehyd-3-phosphat-dehydrogenase (GAPDH) and β-actin were used as loading controls in RT-PCR and Western blot, respectively. Five repeated tests per group showed similar results ([[[P<0.001; [P<0.05). (L) Representative images of Oil red O staining of BMMSC cultures under the adipogenic conditions. BMMSCs derived from MRL/lpr mice (n=5) showed a significant decreased number of adipocytes (mean±SD; Control, n=5; [[[P<0.001). (M) Semi-quantitative RT-PCR analysis indicated that MRL/lpr-derived BMMSCs had significant decrease in gene expression of PPARγ2 and LPL compared to loading control GAPDH. Five repeated tests per group showed similar results ([[[P<0.001). (N) TRAP staining indicated the increased number of TRAP positive cells (mean±SD) in epiphysis of the distal femurs of MRL/lpr mice (n=5) as compared to the control (Control, n=5; [[P<0.01). (O, P) ELISA revealed that MRL/lpr mice (n=5) have increased levels (mean±SD) of soluble RANKL (sRANKL) (O, [[[P<0.001) and C-terminal telopeptides of type I collagen (C-telopeptides, P, [[P<0.01) in serum as compared to the controls (n=5).
Since T-lymphocyte over-activation has been associated with BMMSC impairment and osteoporosis [26, 30, 31], we next examined whether T-lymphocytes are over-activated in MRL/lpr mice and their effects on BMMSCs. We showed that BMMSCs derived from MRL/lpr mice (MRL/lpr-BMMSCs) displayed an increase in the number of colony-forming unit fibroblasts (CFU-F), representing the number of clonogenic mesenchymal progenitors, as compared to control mice (Fig. 1G), and an elevated proliferation rate by bromodeoxyuridine (BrdU) incorporation assay (Fig. 1H). MRL/lpr-BMMSCs also showed impairment of osteogenic differentiation, shown here as a decreased mineralization in osteo-inductive cultures (Fig. 1I), and decreased levels of osteogenic gene expression, including runt-related transcription factor 2 (Runx2), alkaline phosphatase (ALP), and osteocalcin (OCN) assessed by both semi-quantitative RT-PCR (Fig. 1J) and Western blot analyses (Fig. 1K). The in vitro findings were further confirmed with in vivo studies in immunocompromised mice (Fig. 3D), showing reduced bone nodule formation when subcutanously transplanted using HA/TCP as a carrier. Additionally, MRL/lpr-BMMSCs demonstrated impairment of adipogenic differentiation as shown by decreased numbers of lipid-specific Oil red O-positive cells (Fig. 1L) and reduced expression of adipocyte-specific genes, peroxisome proliferator-activated receptor γ2 (PPARγ2) and lipoprotein lipase (LPL) by semi-quantitative RT-PCR (Fig. 1M). These findings suggest that BMMSCs derived from MRL/lpr mice were functionally impaired compared to control mice.
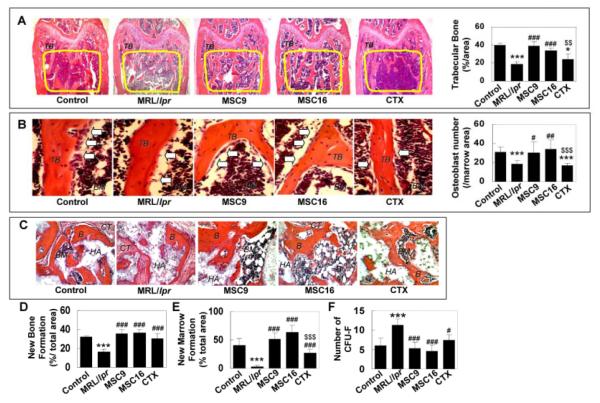
Figure 3.
Allogenic MSCT reconstructed trabecular bone and osteoblastic niche in MRL/lpr mice (A) MRL/lpr mice (n=6) showed decreased trabecular bone (TB) formation (yellow circle area, mean±SD) when compared to control mice (n=6). MSCT (MSC9, n=6; MSC16, n=6) exhibited a significant increase in the trabecular bone volume. However, CTX treatment failed to recover trabecular bone. [[[[P<0.001 vs. Control; [P<0.05 vs. Control; ###P<0.001 vs. MRL/lpr; $$P<0.01 vs. MSCT (MSC9 and MSC16)]. (B) The number of osteoblasts (open arrows) per bone marrow area (mean±SD) in the distal femoral metaphysis was significantly decreased in MRL/lpr mice (n=6) compared to controls (n=6). MSCT (MSC9, n=6; MSC16, n=6) were able to significantly recover osteoblast numbers in MRL/lpr mice, but CTX treatment (n=6) was not capable of recovering the number. [[[P<0.01 vs. Control; [[[P<0.001 vs. Control; #P<0.05 vs. MRL/lpr; ##P<0.01 vs. MRL/lpr; $$$P<0.001 vs. MSCT (MSC9 and MSC16)]. BM: bone marrow. (C-E) In vivo osteogenic assay showed that newly bone (B) and hematopoietic marrow (BM) formation (mean±SD) were significantly decreased in MRL/lpr-BMMSC transplants (n=6) compared to the control group (n=6). MSCT (MSC9, n=6, and MSC16, n=6), as well as CTX treatment (n=6), can significantly improve BMMSC-mediated newly bone and hematopoietic marrow formation in vivo. CT: connective tissue, HA: HA/TCP. H&E staining. Original magnification; X200. [[[[P<0.001 vs. Control; ###P<0.0051vs. MRL/lpr; $$$P<0.001 vs. MSCT (MSC9 and MSC16)]. (F) The number of CFU-F (mean±SD) in MRL/lpr mice (n=6) increased significantly as compared to control group (n=6). All treatments (MSC9, n=6; MSC16, n=6; CTX, n=6) significantly reduced the number of CFU-F to the control level. ([[[P<0.001 vs. Control, #P<0.05 vs. MRL/lpr, ###P<0.001 vs. MRL/lpr).
In contrast to BMMSC/osteoblast lineage, osteoclasts play a significant role in the maintenance of bone homeostasis by their bone resorption function. We examined osteoclast activity in MRL/lpr mice and found an increased number of tartrate-resistant acid phosphatase (TRAP) positive osteoclasts in the distal femur epiphysis of MRL/lpr mice (Fig. 1N), elevated serum levels of runt-related NF-κB ligand (RANKL), a critical factor for osteoclastogenesis, (Fig. 1O) and bone resorption marker C-terminal telopeptides of type I collagen (Fig. 1P) as compared to control mice. These findings revealed that over-activated osteoclasts in MRL/lpr mice potentially contribute to bone loss in SLE-like disease.
Allogenic BMMSC Transplantation (MSCT) improves multiple organ function in MRL/lpr mice
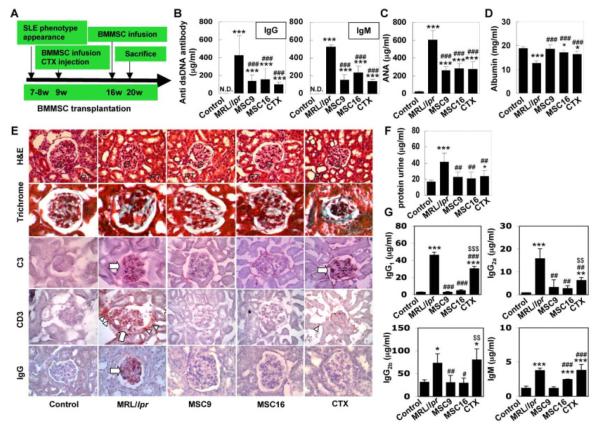
SLE-like multi-systemic autoimmune disorders usually appear at age 7-8 weeks in MRL/lpr mice. To explore the effects of early versus late treatment interventions, we infused allogenic BMMSCs into MRL/lpr mice either at an early stage of the SLE disorder (9 weeks of age, MSCT9), or at the matured stage (16 weeks of age, MSCT16) (Fig. 2A). Cyclophosphamide (CTX) treatment at 9 weeks of age was used as a conventional treatment control (Fig. 2A). It has been reported that autoantibodies play a crucial role in multiple organ impairment in SLE patients [32]. Consistent with human findings, MRL/lpr mice showed a remarkable increase in circulating autoantibodies, specifically anti-double strand DNA (dsDNA) IgG and IgM antibodies (Fig. 2B), anti-nuclear antibody (ANA) (Fig. 2C), and immunoglobulins including IgG1, IgG2a, IgG2b and IgM (Supplementary Fig. 1A) in the peripheral blood. Initiating MSCT at both early and matured stages, 9- and 16-week-olds, benefited a significantly reduction in serum levels of anti dsDNA antibody IgG and IgM, ANA, immunoglobulins IgG1, IgG2a, IgG2b and IgM (Fig. 2B, 2C, Supplementary Fig. 1A). In addition, decreased serum albumin levels in MRL/lpr mice were observed after MSCT (Fig. 2D). When compared to MSCT, conventional CTX treatment only partially reduced levels of serum autoantibodies, immunoglobulin IgG2a and recovered albumin level in MRL/lpr mice (Fig. 2B-2D, Supplementary Fig. 1A). In addition, unlike MSCT, CTX treatment failed to reduce circulating immunoglobulins IgG1, IgG2b and IgM in MRL/lpr mice (Supplementary Fig. 1A).
Figure 2.
Allogenic MSCT reduced levels of autoantibodies and improved renal function in MRL/lpr mice (A) The scheme of allogenic MSCT and CTX treatment procedures. (B) ELISA quantified that levels of anti dsDNA IgG and IgM antibodies (mean±SD) were significantly increased in the peripheral blood of MRL/lpr mice (n=6) when compared to the undetectable level (N.D.) in controls (n=6). MSCT at 9 weeks (MSC9, n=6) and at 16 weeks (MSC16, n=6) and CTX treatment (CTX, n=6) were able to reduce levels of anti dsDNA IgG and IgM, but failed to reduce the levels of anti dsDNA IgG and IgM at the undetectable level as shown in controls. ([[[P<0.001 vs. Control; ###P<0.001 vs. MRL/lpr). (C) MSCT (MSC9, n=6, and MSC16, n=6) and CTX treatment (n=6) were able to significantly reduce anti nuclear antibody (ANA) (mean±SD) in MRL/lpr mice (n=6), which was significantly increased compared to the control (n=6). But the levels at the post treatments were higher than the control. ([[[P<0.001 vs. Control; ###P<0.001 vs. MRL/lpr). (D) MSC9 (n=6) appeared to increase albumin level (mean±SD) compared to the level in MRL/lpr mice (n=6), which were significantly decreased compared to the control (n=6). MSC16 (n=6) and CTX treatments (n=6) were also able to significantly elevate the levels, which were still significantly lower than the control. ([[[P<0.001 vs. Control; [P<0.05 vs. Control; ###P<0.001 vs. MRL/lpr). (E) MSCT, as well as CTX treatment, reduced basal membrane disorder and mesangium cell over-growth in glomerular (G) (upper panels, H&E staining; upper second panels, trichrome staining). RT: renal tubule. Immunohistochemistry showed MSCT was able to diminish C3 deposition in glomerular (open arrow) of MRL/lpr group, however, CTX treatment failed to reduce C3 in glomerular (open arrow) (middle panels). All treatments were capable of infiltration of CD3-positve cells and reducing IgG deposition in glomerular of MRL/lpr group (lower panels). (F) All treatments (MSC9, n=6; MSC16, n=6; CTX, n=6) significantly reduced urine protein levels (mean±SD) in MRL/lpr mice, which significantly increased when compared to control mice (n=6). ([[[P<0.001 vs. Control; [P<0.05 vs. Control; ##P<0.01 vs. MRL/lpr). (G) Markedly increased urine immunoglobulins (IgG1, IgG2a, IgG2b and IgM) (mean±SD) in MRL/lpr mice (n=6) were significantly reduced after allogenic MSCT (MSC9, n=6; MSC16, n=6). CTX treatment (n=6) was not effectively in reducing the immunoglobulins levels. [[[[P<0.005 vs. Control; [P<0.05 vs. Control; ###P<0.005 vs. MRL/lpr; #P<0.05 vs. MRL/lpr; $$$P<0.005 vs. MSCT (MSC9 and MSC16); $P<0.05 vs. MSCT (MSC9 and MSC16)].
As expected, MRL/lpr mice showed renal disorders such as nephritis with glomerular basal membrane disorder, mesangial cell over-growth, deposition of complement component 3 (C3) and IgG, and infiltration of CD3-positive cells (Fig. 2E). In addition, we found presence of increments of urine protein (Fig. 2F) and immunoglobulins including IgG1, IgG2a, IgG2b and IgM in MRL/lpr mice (Fig. 2G). In general, MSCT at both early and matured stages was able to improve renal disorders (Fig. 2E-2G), specifically restoring kidney glomerular structure, and reducing C3 and glomerular IgG deposition (Fig. 2E). Moreover, enzyme-linked immunosorbent assay (ELISA) data showed that MSCT was able to elevate serum C3 level and reduce urine C3 level (Supplementary Fig. 1B). Although CTX treatment could reduce glomerular IgG deposition, it did not restore the glomerular structure and C3 accumulation as compared to MSCT (Fig. 2E, Supplementary Fig. 1B). In response to either MSCT or CTX treatment, MRL/lpr mice showed reduced urine protein levels at 4 weeks post treatment (Fig. 2F). However, MSCT, but not CTX treatment, was capable to completely restore renal function, shown as normalization of serum and urine creatinine levels in MRL/lpr mice, in comparison to disease-free control mice (Supplementary Fig. 1C). These experimental evidences indicated that MSCT is a superior therapeutic approach for treating nephritis in MRL/lpr mice and capable to restore renal function.
Further histological analysis with H&E, Oil red O, and Periodic Acid-Schiff staining revealed presence of inflammatory cells infiltrates in the portal triads, fatty degeneration, and glycogen degradation in hepatocytes (Supplementary Fig. 2), suggesting liver impairment in MRL/lpr mice. Interestingly, MSCT at two stages, as well as CTX treatment, were capable to reverse all SLE-associated histological changes in the liver (Supplementary Fig. 2). These findings indicate that MSCT-associated suppression of autoantibodies may partly contribute to the amelioration of multi-organ dysfunction in MRL/lpr mice.
Allogenic MSCT ameliorates osteoporosis-like phenotype in MRL/lpr mice, and improves the osteoblastic niche
To examine whether MSCT is capable of recovering skeletal disorder in MRL/lpr mice, we analyzed the bone phenotype and BMMSC function in MSCT-treated MRL/lpr mice compared to CTX-treated and non-treated mice. Bone histomorphometric analysis with H&E and TRAP staining revealed that MSCT at both early and matured stages was capable of promoting trabecular bone formation and inhibiting osteoclastogenesis (Fig. 3A and Supplementary Fig. 3A, 3B). A balance between bone formation and resorption is necessary for maintaining bone integrity. It has been recognized that osteoblasts, differentiated from their progenitor BMMSCs, contribute to niche organization for HSC in the bone marrow compartment [12-14]. Consistent with this observation, we showed that MSCT significantly improved osteoblastic niche reconstruction in MRL/lpr mice (Figure 3B) evidenced by an increase in new bone and marrow formation. However, CTX treatment was not able to improve bone volume and reconstruct osteoblastic niche (Fig. 3A, 3B), but slightly suppress osteoclastogenesis (Supplementary Fig. 3A, 3B). At the molecular level, the expression of osteoprotegerin (OPG) and RANKL were down-regulated and up-regulated in splenocytes of MRL/lpr mice, respectively (Supplementary Fig. 4). MSCT was capable to restore OPG and suppress RANKL expression in MRL/lpr mice. However, CTX treatment only suppressed RANKL (Supplementary Fig. 4).
BMMSCs isolated from MSCT-treated MRL/lpr mice showed restoration of osteogenic differentiation demonstrated as an increase in calcium deposition (Supplementary Fig. 5A), elevated levels of osteogenic genes and proteins, including ALP and OCN (Supplementary Fig. 5B, 5C), under osteogenic inductive conditions. CTX treatment mildly promoted osteogenic differentiation, but was not able to restore changes in the osteogenic genes in MRL/lpr mice (Supplementary Fig. 5). BMMSCs derived from MSCT-treated MRL/lpr mice showed significantly increased new bone formation (Fig. 3C, 3D) and osteoblastic niche regeneration (Fig. 3C, 3E) when transplanted into immunocompromised mice. In addition, MSCT appeared to suppress the high colony formation of BMMSCs derived from MRL/lpr shown as a significant reduction in number of CFU-F in treated mice (Fig. 3F). CTX treatment showed similar treatment effects to MSCT, however at a lower extent (Fig. 3C, 3E and Supplementary Fig. 5). These findings suggest that allogenic MSCT provided an optimal therapy for improving bone volume and BMMSC function in MRL/lpr mice as compared to conventional CTX.
Allogenic MSCT restores the immune system via CD4+CD25+Foxp3+ cell, Th17 cells, and plasma cells
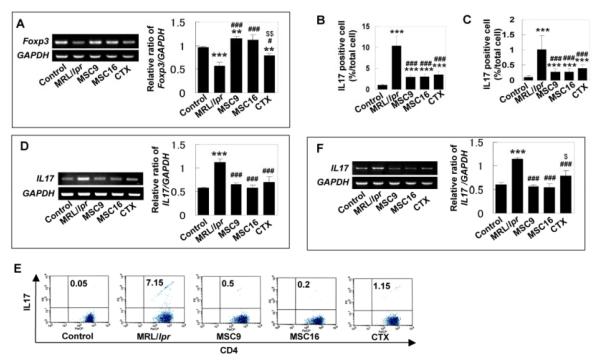
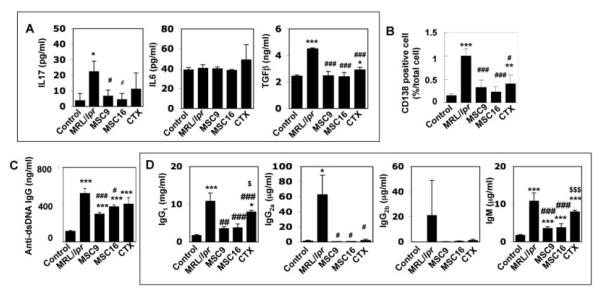
It has been suggested that CD4+CD25+Foxp3+ cells prevent pathogenic autoimmunity by suppressing proliferation and production of pro-inflammatory cytokines in effector immune cells, such as helper T-lymphocytes [33]. In contrast, Th17 cells, an inflammatory IL17 producing cell, have been linked to the pathogenesis of autoimmune diseases [34] and bone destruction [35]. To explore whether MSCT affects the immune balance between CD4+CD25+Foxp3+ cells and Th17 cells in SLE-like disorders, we studied Foxp3+ cells and Th17 cells in spleen and bone marrow of MRL/lpr mice. MSCT at both early and matured stages was able to restore Foxp3+ cells in MRL/lpr mice (Fig. 4A). However, CTX treatment only slightly increased the level of Foxp3 (Fig. 4A). Immunohistochemical analysis showed that IL17-positive cells were significantly increased in bone marrow and spleen of MRL/lpr mice when compared to control mice, and were specifically suppressed by MSCT (Fig. 4B, 4C). Flow cytometry also demonstrated that the increased in CD4+IL17+ T-lymphocytes in bone marrow and spleen of MRLlpr mice was significantly reduced by MSCT at both early and matured stages, as compared to CTX treatment (Fig. 4E). Semi-quantitative RT-PCR analysis further confirmed the decreased IL17 gene expression in both bone marrow and spleen of MSCT mice (Fig. 4D, 4F). Likewise, CTX treatment also reduced IL17 levels in MRL/lpr mice (Fig. 4B-4F). Moreover, ELISA analysis showed that IL17 levels were remarkably increased in spleen of MRL/lpr mice and MSCT, but not CTX treatment, significantly suppressed the elevated IL17 levels (Fig. 5A).
Figure 4.
The numbers of Foxp3+ cells and Th17 cells contributed to pathological process in MRL/lpr mice (A) Semi-quantitative RT-PCR confirmed decreased Foxp3 gene expression in bone marrow of MRL/lpr mice and increased Foxp3 expression in the treatment groups. The results were representative of five independent experiments ([[[P<0.001 vs. control; [[P<0.01 vs. control; ###P<0.001 vs. MRL/lpr; #P<0.05 vs. MRL/lpr; $$P<0.01 vs. MSCT). (B) Immunohistochemical staining with anti-IL17 antibody indicated that number of IL17 positive cells (mean±SD, arrows) was significantly increased in bone marrow (BM) of MRL/lpr mice (n=6). MSCT (MSC9, n=6; MSC16, n=6), as well as CTX treatment (n=6), significantly reduced IL17-positive cells in MRL/lpr bone marrow, but still showed higher level than that in control group ([[[P<0.001 vs. Control, ###P<0.001 vs. MRL/lpr). (C) Immunohistochemical staining using anti-IL17 antibody showed that number of IL17 positive cells (mean±SD, arrows) was significantly increased in spleen of MRL/lpr (n=6) compare to control group (n=6) and treatment group (MSC9; n=6, MSC16; n=6, CTX; n=6) ([[[P<0.001 vs. Control; ###P<0.001 vs. MRL/lpr). (D) Semi-quantitative RT-PCR revealed high expression of IL17 in bone marrow of MRL/lpr and this increased level of IL17 was decreased in MSCT and CTX treatment groups. The results were representative of five independent experiments ([[[P<0.001 vs. control; ###P<0.001 vs. MRL/lpr). (E) Flow cytometry revealed that MRL/lpr mice had significantly increased level of CD4+IL17+ T lymphocytes in spleen compared to control group. The CD4+IL17+ cells were markedly decreased in MSCT and CTX groups. (F) Semi-quantitative RT-PCR confirmed increased IL17 expression in spleen of MRL/lpr and reduced IL17 expression in the treatment groups. The results were representative of five independent experiments ([[[P<0.001 vs. control; ###P<0.001 vs. MRL/lpr; $P<0.05 vs. MSCT).
Figure 5.
Allogenic MSCT reduced number of CD138 positive plasma cells and the capability of autoantibodies and immunoglobulins (A) ELISA confirmed the decreased levels of IL17 following MSCT (right panel: MSC9, n=5; MSC16, n=5) compared to MRL/lpr mice (n=5). However, CTX treatment (CTX, n=5) failed to show the efficiency. On the other hand, IL6 levels showed no changes, but the levels of total TGFβ were changed similar to that of IL17. [[[[P<0.005 vs. Control (n=5), [P<0.05 vs. Control, ###P<0.005 vs. MRL/lpr, #P<0.05 vs. MRL/lpr]. (B) Immunohistochemical staining revealed that MRL/lpr mice (n=6) had increased number of CD138 positive plasma cells (mean±SD, arrows) in bone marrow as compared to control mice (n=6). MSCT (MSC9, n=6; MSC16, n=6) and CTX treatment (n=6) resulted in a significantly decreased number of CD138 positive plasma cells in the bone marrow. ([[P<0.01 vs. Control; [[[P<0.001 vs. Control; #P<0.05 vs. MRL/lpr; ###P<0.001 vs. MRL/lpr). (C) ELISA quantified that levels of anti dsDNA IgG antibodies (mean±SD) were significantly increased in spleen of MRL/lpr mice (n=5) when compared to that of controls (n=5). MSCT at 9 weeks (MSC9, n=5) and at 16 weeks (MSC16, n=5) and CTX treatment (CTX, n=5) treatment were able to reduce levels of anti dsDNA IgG, but not significant against CTX group. [[[P<0.001 vs. Control, ###P<0.005 vs. MRL/lpr, #P<0.05 vs. MRL/lpr. (D) ELISA showed MSCT (MSC9, n=5; MSC16, n=5) reduced immunoglobulins (IgG1, IgG2a, IgG2b and IgM) levels (mean±SD) in MRL/lpr mice (n=5). CTX treatment (CTX, n=5) also showed efficient effect on IgG1, IgG2a, and IgG2b, but not for IgM. [[[[P<0.005 vs. Control, [[P<0.01 vs. Control, [P<0.05 vs. Control, ###P<0.005 vs. MRL/lpr, ##P<0.01 vs. MRL/lpr, #P<0.05 vs. MRL/lpr, $$$P<0.005 vs. MSCT (MSC9 and MSC16), $P<0.05 vs. MSCT (MSC9 and MSC16)].
Since MSCT is capable of suppressing the levels of autoantibodies in MRL/lpr mice, we examined whether MSCT regulates CD138-positive plasma cells, an immunoglobulin producing cell. In MRL/lpr mice, CD138-positive cells were significantly increased compared to control mice by immunohistochemistry (Fig. 5B). MSCT reduced the number of CD138-positive cells in MRL/lpr mice (Fig. 5B). ELISA also showed that allogenic MSCT was able to reduce the levels of anti-dsDNA IgG, and immunoglobulins, IgG1, IgG2a, IgG2b and IgM, in spleen of MRL/lpr mice (Fig. 5C, 5D). Although CTX appeared capable of inhibiting the number of CD138-positive cells and the production of both autoantibodies and immunoglobulins, this conventional treatment was not as effective as MSCT (Figs. 5B, 5C). These results suggest that allogenic MSCT is a more improved therapy with a better treatment effect than conventional CTX in SLE-like MRL/lpr mice, possibly through the modulation of multiple immune cells.
BMMSCs derived from SLE patients showed osteogenic impairment
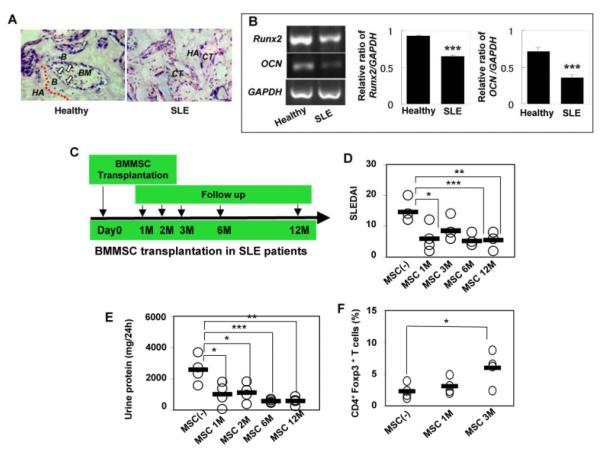
Previous study suggested that BMMSCs from SLE patients might show differentiation impairment similar to those observed in SLE mice [36]. To verify the findings in humans, we isolated BMMSCs from two SLE patients and characterized their osteogenic differentiation properties in vivo using subcutaneous transplantation in immunocompromised mice. BMMSCs derived from SLE patients showed significantly decreased bone forming capacity and impaired reconstruction of bone marrow in vivo as compared to BMMSCs from matched normal healthy subjects (Fig. 6A). Furthermore, semi-quantitative RT-PCR analysis revealed decreased expression of osteogenic genes Runx2 and OCN in BMMSCs from SLE patients (Fig. 6B). Given the evidence that some advanced stage SLE patients may have experienced a suppression of CD34+ bone marrow cells [37], it is postulated that the decrease in CD34+ subset may correlate with the osteoblastic niche deficiency in the bone marrow of SLE patients.
Figure 6.
Allogenic MSCT was an effective treatment for treatment-refractory SLE patients (A) In vivo osteogenic assay revealed that newly bone formation and bone marrow reconstruction were diminished in SLE patients’ BMMSC transplants (SLE, n=2) as compared to normal BMMSC transplants (Control, n=2). Arrows indicate osteoblasts lining on the bone surface. B; bone, BM; bone marrow, CT; connective tissue, HA; HA/TCP. H&E staining. Original magnification; X200. (B) Semi-quantitative RT-PCR analysis revealed that SLE patients’ BMMSCs showed a decrease in the expression of osteogenic genes Runx2 and OCN as compared to BMMSCs from healthy donor controls. GAPDH was used as a loading control ([[[P<0.001 vs. control). (C) The scheme of MSCT and CTX treatment in treatment-refractory SLE patients. (D) MSCT led significant decrement of the score of SLEDAI in the recipients at one ([P<0.05), six ([[[P<0.005), and twelve ([[P<0.01) months post-transplantation compared to the original indexes prior to MSCT. (E) MSCT showed capable of maintaining reduced urine protein levels in SLE patients at one ([P<0.05), two ([P<0.05), six ([[[P<0.005) and twelve ([[[P<0.005) months after MSCT compared to the original levels. (F) CD4+Foxp3+ cells in the peripheral blood were significantly elevated in the patients three months post-transplantation (n=4) ([P<0.05) compared to the initial levels (n=4), but not in one-month post-MSCT.
Allogenic MSCT is a safe and feasible salvage therapy in patients with refractory SLE
Since our animal study showed that MSCT, but not CTX treatment, offered improved clinical outcomes and reversed multi-organ dysfunction in SLE, we hypothesized that MSCT may be capable of curing CTX-refractory SLE patients. To test this hypothesis, we conducted a pilot clinical study to assess the efficacy and safety of MSCT in a small cohort group of SLE patients. Three female and one male patients in the age range of 16 to 23 years old, with treatment-refractory SLE for duration of 12-51 months were enrolled for allogenic MSCT (Table 1). All subjects met the revised criteria for SLE established by the American College of Rheumatology (ACR, 1997) and had been previously treated with CTX and high dose of prednisone (more than 20 mg/day) (Table 1). Patient eligibility criteria also included lupus glomerulonephritis (class III, IV, V) with severe elevation of increment of 24-hour urine protein levels and/or serum creatinine ≥1.5mg/dl. Bone marrow was collected from patients’ healthy family member and ex vivo expanded in culture under GLP/GMP protocols. MSCT were infused at ≥1×106cells/kg body weight. Primary outcomes were overall survival and disease remission defined as requiring no further high dose of immunosuppressive medications except the low maintenance doses of corticosteroids and CTX. Post MSCT maintenance therapy includes a tapering dose of steroid and CTX, with 2 patients completely off CTX at 6 months, and 2 patients on low dose of CTX at 0.6 mg/every 2 months. Secondary outcomes included systemic lupus erythematosus disease activity index (SLEDAI) [27], complement C3, and renal function monitored by 24-hour urine protein and serum creatinine levels. Our short-term clinical outcome in 12-18 months follow up post-MSCT showed no allogenic MSCT-related complications including cardiovascular, pulmonary insufficiencies, infection, malignancy, and metabolic disturbances. Assessment of SLEDAI indicated the improvement of disease activity in all allogenic MSCT-treated patients at each follow-up period (Fig. 6D). All recipients were followed up for 12-18 months and showed recovery of kidney function with low baseline 24-hour urine protein levels (Fig.6E). Serum C3 level improved at one-month post MSCT in all patients, from 0.4775±0.1134g/L to 0.7750±0.0826g/L. These early clinical data demonstrate safety and efficacy of MSCT in SLE patients and improvement of disease activities at post allogenic MSCT. Further long-term follow ups and additional patient enrollment are in progress.
Table 1.
Patient Information
| Patient No. |
Sex | Age(Years) | History of SLE (Months) |
Previous Drug Treatment | Follow up Post-MSCT (Months) |
|
|---|---|---|---|---|---|---|
| Predonisone | CTX | |||||
| 1 | F | 23 | 12 | 20mg/d | 14.4g/total | 18 |
| 2 | F | 20 | 29 | 25mg/d | 19.2g/total | 12 |
| 3 | M | 17 | 24 | 30mg/d | 19.2g/total | 12 |
| 4 | F | 16 | 51 | 20mg/d | 19.2g/total | 12 |
Interestingly, we also found increased levels of CD4+Foxp3+ cells followed allogenic MSCT in 3 SLE patients with statistical significance at three-month post MSCT (Fig. 6F). The MSCT-associated increased level of CD4+Foxp3+ cells in these treated patients correlates with similar findings of recovery of Foxp3+ cells in MRL/lpr mice followed MSCT (Fig. 4A). Further studies are needed to uncover the underlying mechanisms of MSCT induced immune regulation in ameliorating SLE disease activities in refractory patients.
Discussion
Clinically, BMMSCs have been successfully utilized to treat a variety of human diseases such as bone fracture [38], severe aplastic anemia [39], and acute graft-versus-host-disease (GVHD) [22], suggesting that BMMSCs are a promising population of postnatal stem cells for clinical therapies. In this study, we used MRL/lpr mice as a SLE mouse model to investigate the role of the BMMSC/osteoblast lineage in SLE disorder and compared MSCT mediated therapy to conventional CTX treatment in SLE. MRL/lpr mice were generated by the insertion of the early transposable element ETn in the Fas gene, which causes a striking reduction in Fas mRNA expression and is associated clinically with marked acceleration of the lupus-like disease [40]. Levels of circulating immune complexes rise enormously from about three months of age in MRL-lpr/lpr but not in MRL mice. In addition, a markedly increase in serum interleukin 12 (IL-12) and nitric oxide have been attributed to the development of autoimmunity [41]. Although SLE mice have been well-characterized [42], we identified a significant osteoporosis phenotype marked by elevated osteoclast activity, as seen in estrogen deficient osteoporosis [30], and impairment of BMMSCs caused by T cell over-activation [43]. The deficiency of BMMSCs in SLE conditions supports the rationale of using allogenic, not autologous, BMMSCs for SLE treatment in this study. Recognizing that the bone marrow microenvironment in SLE can potentially injure osteoblasts and their progenitor BMMSCs [36, 44], we hypothesized that osteoblastic niche dysfunction might contribute to the pathology of SLE and therefore restoration of the SLE-impaired niche will provide a better treatment outcome. Since the BMMSC/osteoblast lineage plays an important role in regulating the immune function and maintaining the HSC niche [15-20], re-establishment of the osteoblastic niche can improve the overall immune function as seen in allogenic MSCT-treated mice showing improvement of Foxp3+ cells, Th17 cells, and plasma cells. Overall, MSCT resulted in better therapeutic outcomes than CTX treatment, specifically improving immune system, recovering renal function, and restoring SLE-degenerative changes in multi-organs, including the skeletal, renal and hepatic systems, in MRL/lp. The new cell-based therapeutic approach appears more effective than conventional therapy without the drug-related cytotoxicities. Supported by the clinical benefits of MSCT in SLE mice [45], we tested the feasibility of MSCT in a small defined group of CTX-refractory SLE patients. A possible explanation that MSCT showed superior therapeutic effect to CTX may associate with the fact that MSCT, but not CTX, is capable of recovering bone formation and marrow genesis and therefore, contributing to the reorganization of the osteoblastic niche. CD4+Foxp3+ cells are naturally occurring suppressor T-cells that regulate a wide variety of immune functions and provide protection from autoimmune diseases, GVHD, transplant rejection, and overwhelming tissue destruction during infections. Thus, CD4+ Foxp3+ cells are critical in the induction and maintenance of immune tolerance [46]. Foxp3 is developed as a specific marker of natural regulatory T cells (nTregs) and induced regulatory T cells (iTregs) [47-49]. In animal studies, the induction of Foxp3 positive T cells markedly reduces autoimmune disease severity in models of diabetes, multiple sclerosis, asthma, inflammatory bowel disease, thyroiditis and renal disease [50]. In human disease, alterations in numbers and functions of Foxp3-expressed cells are found in a number of diseases such as SLE [51]. Our data showed that MSCT restored Foxp3+ cell levels in both MRL/lpr mice and SLE patients. The combination treatment using MSCT with CTX in the SLE mice failed to yield an optimal therapeutic effect as compared to MSCT in terms of bone mineral density improvement (data not shown).
Lupus nephritis is one of the major complications of SLE disorder and its treatment remains a clinical challenge. Classical immunosuppressive agents were able to slow the progression to end-stage renal failure but do not target specific immune dysfunctions [31]. The patients with treatment-refractory SLE enrolled in this study failed to maintain adequate renal functions secondary to lupus nephritis. MSCT treatment significantly improved renal function in these patients, and maintained an improved overall survival and disease remission during the current follow ups.
HSC transplantation has shown promising results in treating SLE patients. Because allogenic BMMSC from a patient’s family member without HLA match is an easily accessible stem cell resource, MSCT may offer another effective cell therapy with fewer side effects. In our 12-18 month follow-up period, we did not find any clinical signs of adverse effects. The therapeutic outcomes of allogenic MSCT were significant in patients with treatment-refractory SLE shown as reduced SLEDAI score, urine protein level, and improved quality of life. In addition, MSCT may be valuable at different stages of SLE, as we administered MSCT to MRL/lpr mice at both early and matured stages of SLE and their therapeutic effects were almost the same.
In this study, we demonstrated that SLE is associated with osteoblastic niche deficiency and proposed a new ready-to-use cell-based therapeutic approach for SLE patients that may directly target the immune system. Allogenic MSCT ameliorates SLE through multiple mechanisms, including re-establishing the osteoblastic niche, recovering Foxp3+ cells, and down-regulating Th17 levels. Using a small pilot clinical study, we have demonstrated early promising findings supporting the efficacy and safety of using allogenic MSCT in patients with treatment-refractory SLE during our short-term follow ups. Further long-term follow ups are in progress and our study is currently in the active recruiting phase to further confirm the efficacy and safety of MSCT as a new treatment modality in SLE.
Supplementary Material
Acknowledgments
This research was supported by the grant from California Institute for Regenerative Medicine (RN1-00572 for S.S. and A.L.) and partially supported by the grants from NIDCR/NIH R01DE017449 and R21 DE017632 (to S.S. for animal study), the National Natural Science Foundation of China (No. 30772014 for L.S.), the Chinese Education Ministry (20050315001 for L.S), and the Jiangsu Province 135 Talent Foundation (RC2007002 for L.S.). We thank Dr. Larry Fisher at NIDCR/NIH for providing ALP and OCN antibodies.
Footnotes
References
- 1.Rahman A, Isenberg DA. Systemic Lupus Erythematosus. N Engl J Med. 2008;358:929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 2.Kyttaris VC, Juang YT, Tsokos GC. Immune cells and cytokines in systemic lupus erythematosus: an update. Curr Opin Rheumatol. 2005;17:518–522. doi: 10.1097/01.bor.0000170479.01451.ab. [DOI] [PubMed] [Google Scholar]
- 3.Crispin JC, Tsokos GC. Novel molecular targets in teh treatmetn of systemic lupus erythematosus. Autoimmun Rev. 2008;7:256–261. doi: 10.1016/j.autrev.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramanujam M, Davidson A. Targeting of the immune system in systemic lupus erythematosus. Expert Rev Mol Med. 2008;21:10, e2. doi: 10.1017/S1462399408000562. [DOI] [PubMed] [Google Scholar]
- 5.Pego-Reigosa JM, Isenberg DA. System in systemic lupus erythematosus: pharmacological developments and recommendations for a therapeutic strategy. Expert Opin Investig Drugs. 2008;17:31–41. doi: 10.1517/13543784.17.1.31. [DOI] [PubMed] [Google Scholar]
- 6.Tieng AT, Peeva E. B-cell-directed therapies in systemic lupus erythematosus. Semin Arthritis Rheum. 2008;38:218–227. doi: 10.1016/j.semarthrit.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Burt RK, Traynor A, Statkute L, et al. Nonmyeloablative hematopoietic stem cell transplantation for systemic lupus erythematosus. JAMA. 2006;295:527–535. doi: 10.1001/jama.295.5.527. [DOI] [PubMed] [Google Scholar]
- 8.Smith-Berdan S, Gille D, Weissman IL, et al. Reversal of autoimmune disease in lupus-prone New Zealand black/New Zealand white mice by nonmyeloablative transplantation of purified allogeneic hematopoietic stem cells. Blood. 2007;110:1370–1378. doi: 10.1182/blood-2007-03-081497. [DOI] [PubMed] [Google Scholar]
- 9.Friedenstein AJ, Chailakhyan RK, Latsinik NV, et al. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues.Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1998;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 11.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 13.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 14.Arai F, Hirao A, Ohmura M, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 16.Uccelli A, Pistoia V, Moretta L. Mesenchymal stem cells: A new strategy for immunosuppression? Trends Immunol. 2007;28:219–226. doi: 10.1016/j.it.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 18.Rasmusson I, Le Blanc K, Sundberg B, et al. Mesenchymal stem cells stimulate antibody secretion in human B cells. Scand. J Immunol. 2007;65:336–343. doi: 10.1111/j.1365-3083.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- 19.Ramasamy R, Fazekasova H, Lam EW, et al. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83:71–76. doi: 10.1097/01.tp.0000244572.24780.54. [DOI] [PubMed] [Google Scholar]
- 20.Spaggiari GM, Capobianco A, Becchetti S, et al. Mesenchymalstemcell-natural killer cell interactions: Evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 21.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 22.Le Blanc K, Frassoni F, Ball L, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Armstrong MA, Li G. Mesenchymal stem cells in immunoregulation. Immunol Cell Biol. 2006;84:413–421. doi: 10.1111/j.1440-1711.2006.01458.x. [DOI] [PubMed] [Google Scholar]
- 24.Koç ON, Gerson SL, Cooper BW, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 25.Noort WA, Kruisselbrink AB, in’t Anker PS, et al. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34 cells in NOD/SCID mice. Exp Hematol. 2002;30:870–878. doi: 10.1016/s0301-472x(02)00820-2. [DOI] [PubMed] [Google Scholar]
- 26.Miura M, Chen XD, Allen MR, et al. A crucial role of Caspase-3 in osteogenic differentiation of bone marrow stromal stem cells. J Clin Invest. 2004;114:1704–1713. doi: 10.1172/JCI20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 28.Shi S, Gronthos S, Chen S, et al. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol. 2002;20:587–591. doi: 10.1038/nbt0602-587. [DOI] [PubMed] [Google Scholar]
- 29.Lane NE. Therapy Insight: osteoporosis and osteonecrosis in systemic lupus erythematosus. Nat Clin Pract Rheumatol. 2006;2:562–569. doi: 10.1038/ncprheum0298. [DOI] [PubMed] [Google Scholar]
- 30.Raisz LG. Pathogenesis of osteoporosis:concepts,conflict, and prospects. J Clin Invest. 2005;115:3318–3325. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamaza T, Miura Y, Bi Y, et al. Pharmacologic stem cell based intervention as a new approach to osteoporosis treatment in rodents. PLoS ONE. 2008;3:e2615. doi: 10.1371/journal.pone.0002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hostmann A, Jacobi AM, Mei H, Hiepe F, Dörner T. Peripheral B cell abnormalities and disease activity in systemic lupus erythematosus. Lupus. 2008;17:1064–1069. doi: 10.1177/0961203308095138. [DOI] [PubMed] [Google Scholar]
- 33.La Cava A. T-regulatory cells in systemic lupus erythematosus. Lupus. 2008;17:421–425. doi: 10.1177/0961203308090028. [DOI] [PubMed] [Google Scholar]
- 34.Garrett-Sinha LA, John S, Gaffen SL. IL-17 and the Th17 lineage in systemic lupus erythematosus. Curr Opin Rheumatol. 2008;20:519–525. doi: 10.1097/BOR.0b013e328304b6b5. [DOI] [PubMed] [Google Scholar]
- 35.Sato K, Suematsu A, Okamoto K, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun LY, Zhang HY, Feng XB, et al. Abnormality of bone marrow-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Lupus. 2007;16:121–128. doi: 10.1177/0961203306075793. [DOI] [PubMed] [Google Scholar]
- 37.Sun LY, Zhou KX, Feng XB, et al. Abnormal surface markers expression on bone marrow CD34+ cells and correlation with disease activity in patients with systemic lupus erythematosus. Clin Rheumatol. 2007;26:2073–2079. doi: 10.1007/s10067-007-0621-2. [DOI] [PubMed] [Google Scholar]
- 38.El-Badri NS, Hakki A, Ferrari A, et al. Autoimmune disease: is it a disorder of the microenvironment? Immunol Res. 2008;41:79–86. doi: 10.1007/s12026-007-0053-8. [DOI] [PubMed] [Google Scholar]
- 39.Cordeiro AC, Isenberg DA. Novel therapies in lupus - focus on nephritis. Acta Reumatol Port. 2008s [PubMed] [Google Scholar]
- 40.Drappa J, Brot N, Elkon KB. The Fas protein is expressed at high levels on CD4+CD8+ thymocytes and activated mature lymphocytes in normal mice but not in the lupus-prone strain, MRL lpr/lpr. Proc Natl Acad Sci U S A. 1993;90:10340–10344. doi: 10.1073/pnas.90.21.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang FP, et al. The role of interleukin 12 and nitric oxide in the development of spontaneous autoimmune disease in MRL/MP-lpr/lpr mice. J Exp Med. 1996;183:1447–1459. doi: 10.1084/jem.183.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quarto R, Mastrogiacomo M, Cancedda R, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 43.Neubert K, Meister S, Moser K, et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med. 2008;14:748–755. doi: 10.1038/nm1763. [DOI] [PubMed] [Google Scholar]
- 44.Fouillard L, Bensidhoum M, Bories D, et al. Engraftment of allogeneic mesenchymal stem cells in the bone marrow of a patient with severe idiopathic aplastic anemia improves stroma. Leukemia. 2003;17:474–476. doi: 10.1038/sj.leu.2402786. [DOI] [PubMed] [Google Scholar]
- 45.Zhou K, Zhang H, Jin O, et al. Transplantation of human bone marrow mesenchymal stem cell ameliorates the autoimmune pathogenesis in MRL/lpr mice. Cell Mol Immunol. 2008;5:417–424. doi: 10.1038/cmi.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banham AH, Powrie FM, Suri-Payer E. FOXP3(+) regulatory T cells: Current controversies and future perspectives. Eur J Immunol. 2006;36:2832–2836. doi: 10.1002/eji.200636459. [DOI] [PubMed] [Google Scholar]
- 47.Hori S, Nomura T, Sakaguchi S. “Control of regulatory T cell development by the transcription factor Foxp3”. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 48.Fontenot JD, Gavin MA, Rudensky AY. “Foxp3 programs the development and function of CD4+CD25+ regulatory T cells”. Nature Immunology. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 49.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. “Regulatory T cell lineage specification by the forkhead transcription factor Foxp3”. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 50.Suri-Payer E, Fritzsching B. “Regulatory T cells in experimental autoimmune disease”. Springer Semin Immunopathol. 2006;28:3–16. doi: 10.1007/s00281-006-0021-8. [DOI] [PubMed] [Google Scholar]
- 51.Alvarado-Sánchez B, Hernández-Castro B, Portales-Pérez D, Baranda L, Layseca-Espinosa E, Abud-Mendoza C, Cubillas-Tejeda A, González-Amaro R. “Regulatory T cells in patients with systemic lupus erythematosus”. J Autoimmun. 2006;27:110–118. doi: 10.1016/j.jaut.2006.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.