Abstract
While glucocorticoids (GC) exert beneficial effects (anti-inflammatory), they also have adverse effects on the epidermis including decreased epidermal differentiation, decreased keratinocyte proliferation, and decreased cutaneous permeability barrier homeostasis. Thus, the purpose of this study was to develop strategies to prevent these GC toxicities using simultaneous topical treatments in clobetasol-treated mice. While a triple-lipid mixture of stratum-corneum lipids (ceramide, free fatty acid, and cholesterol) was previously shown to reverse the GC-induced abnormality in cutaneous barrier function (Kao, et al. 2003), this lipid mixture did not prevent the GC-induced abnormalities in either keratinocyte proliferation or differentiation. Since activators of PPAR α, β/δ, γ, and LXR, regulate keratinocyte proliferation and differentiation and improve permeability barrier homeostasis, we next assessed the effects of these activators during concurrent GC treatment. Co-application of either ciglitazone (PPAR γ activator), clofibrate (PPARα activator), or 22R (OH) cholesterol (LXR activator) with clobetasol prevented the decrease in involucrin, filaggrin and loricrin expression. In contrast, a PPAR β/δ activator (GW501516) normalized only the expression of involucrin and filaggrin, but not loricrin. Moreover, topical application of PPARα, β/δ, or LXR activators partially prevented the decrease in keratinocyte proliferation in GC-treated murine skin, as measured by PCNA, while no effect was seen after co-treatment with PPAR γ activators. Finally, PPAR γ and PPARβ/δ activators but not PPAR α and LXR activators improved permeability barrier homeostasis in GC treated mice. Together, these studies demonstrate that PPAR and LXR activators can prevent several of the adverse effects of topical GC on the epidermis.
Keywords: keratinocyte proliferation, epidermal differentiation, permeability barrier, involucrin, loricrin, filaggrin, stratum corneum
Introduction
Glucocorticoids (GC) have been the treatment of choice for a large number of skin diseases, mainly due to their high anti-inflammatory potency (1, 2). However, long-term topical GC therapy is well known to induce a wide range of adverse effects, including skin fragility, bruising, and atrophy (3, 4). In the epidermis topical treatment with GC leads to decreased keratinocyte differentiation and decreased epidermal thickness due to an inhibition of keratinocyte proliferation (5-8). Moreover, recent studies have demonstrated that even short term GC treatment impairs permeability barrier recovery after acute barrier disruption, thus impairing permeability barrier homeostasis (9). The delay in epidermal permeability barrier repair after GC treatment is secondary to a decrease in lamellar body (LB) generation attributed to the inhibition of epidermal lipid synthesis (9). Accordingly, topical physiologic lipid replacement reverses the GC-induced abnormalities in permeability barrier homeostasis (9).
The outermost layer of the epidermis, the stratum corneum (SC), is comprised of corneocytes derived from the terminal differentiation of keratinocytes, embedded in a lipid-enriched extra-cellular matrix, that together provide one of the major functions of the skin: the epidermal permeability barrier (10). In addition to changes in ionic gradients that play a major role epidermal homeostasis (11), PPAR α, β/δ γ, and LXR activators are also known to have numerous, diverse and specific effects on epidermal structure and function (12-16). First, both the addition of PPAR/LXR activators to cultured human keratinocytes and the topical application of PPAR/LXR activators to normal mouse skin stimulate expression of keratinocyte differentiation related proteins, such as involucrin, loricrin, profilaggrin, and transglutaminase 1, which together enhance cornified envelope formation (17-21). Second, PPAR/LXR activators increase cholesterol sulfotransferase activity (22), which would increase the synthesis of cholesterol sulfate, (14)and cholesterol sulfate, is itself a potent stimulator of epidermal differentiation and an important inhibitor of corneocyte desquamation (23). Third, topical treatment of murine skin with PPAR/LXR activators improves permeability barrier homeostasis, due to increased: a) epidermal cholesterol, fatty acid, and sphingolipid synthesis, b) lamellar body density and secretion, c) beta-glucocerebrosidase activity, and d) expression of ABCA12, a membrane transporter required for the delivery of glucosylceramides into lamellar bodies (24, 25). These mechanisms all separately enhance permeability barrier homeostasis. Lastly, PPAR and LXR activators regulate keratinocyte proliferation (17, 19, 20, 26). Given these positive attributes the aim of this study was to assess whether activators of PPARs and LXR could prevent the adverse effects of glucocorticoids in normal murine epidermis.
Materials and Methods
Materials
Adult male and female hairless mice (Skh1), 8 to 10 weeks of age, were purchased from Charles River Laboratories (Wilmington, MA). All animals had free access to food and water ad libitum. Hydroxy acyl ceramides were purchased from Matreya (Pleasant Gap, PA). Palmitic acid, cholesterol, clofibrate, WY-14643 and 22(R) hydroxycholesterol were from Sigma-Aldrich (St. Louis, MO). GW501516 was a gift from Dr Timothy Willson (GlaxoSmithKline, NC, USA). Ciglitazone and TO901317 were from Cayman chemical (Ann Arbor, MI). Affinity purified rabbit primary antibodies specific for mouse filaggrin, loricrin and involucrin were purchased from BabCo (Richmond, CA). Secondary biotinylated goat anti-rabbit IgG, ABC-peroxidase kit and diaminobenzidine were purchased from Vector laboratories (Burlingame, CA). The anti-proliferating cell nuclear antigen (PCNA) was purchased from CalTag Laboratories (Burlingame, CA).
Animal model and tissue preparation
Mice were treated for three consecutive days, twice daily, with a topical application of 35μl of either vehicle (propylene glycol: ethanol, 7:3 v:v) or 0.05% clobetasol alone. A third group of mice were treated with both clobetasol and a PPAR/LXR ligand (PPARα ligand, clofibrate (1 mM) or WY-14643 (10mM); PPARβ/δ ligand, GW501516 (4mM); PPARγ ligand ciglitazone (10mM), or LXR ligand 22(R)hydroxycholesterol (10mM) or TO901317 (10mM)). PPAR and LXR activators were applied 10 min after the clobetasol solution. In separate experiments, a mixture of palmitic acid, cholesterol, and ceramides was applied in a 1 : 1 : 1 mole ratio in a propylene glycol:ethanol (7 : 3 vol/vol) vehicle, as described previously (27, 28).
Barrier recovery measurements
Epidermal barrier disruption was achieved by repeated applications of cellophane tape on the flanks until transepidermal water loss reached 4 to 6 mg/cm2/hr, as determined with an electrolytic water analyzer (Meeco, Warrington, PA). Evaporative water loss was then measured immediately and then at 3, 6 and 24 hours after barrier disruption on three separate marked areas on the mouse flank where barrier disruption was achieved. Experiments were repeated at least twice to confirm results.
Immunohistochemistry
Paraffin-embedded sections were incubated with purified rabbit primary antibodies specific for involucrin, filaggrin and loricrin following blockade of endogenous peroxidase activity and incubation in blocking buffer. After application of secondary biotinylated antibody, staining for these proteins was detected using ABC-peroxidase and revealed by diaminobenzidine as the substrate. Experiments were repeated at least twice to confirm results.
Detection of proliferating cells
Proliferating keratinocytes were quantitated on paraffin-embedded tissue. After incubation in antigen retrieval solution, blockade of endogenous peroxidase activity and incubation in blocking buffer, sections were incubated with anti-PCNA antibody. Staining was revealed by colorimetric reaction using the ABC-peroxidase-diaminobenzidine system. PCNA positive cells were counted in the basal and supra-basal layers on 3 to 5 pictures taken from each section at a 20X magnification. Experiments were repeated at least twice to confirm results.
Measurement of epidermal thickness
Thickness of the epidermal nucleated cell layers was measured on 126X micrographs taken every 2 cm along the epidermis in biopsies from normal control, GC plus vehicle and GC plus ligand-treated skin. The normal epidermal thickness is set at 100% and others are calculated as percentage of normal control. The data presented represents the mean of all measured points +/- SEM. (n=31-45).
Microscopy and imaging
Skin samples were fixed in 4% formalin. Pictures of each section were taken on 5μm thick paraffin sections and examined under a Zeiss Axioplan 2 light microscope (Jena, Germany) at 20X magnification.
Statistics
All data are given as mean ± S.E.M. Statistical analyses were determined using the Student's t test with GraphPad Prism 4 software for Macintosh (version 4.0b, June 2004).
Results and Discussion
SC triple-lipid mixture does not override the negative effects of GC on keratinocyte differentiation or proliferation
Previous studies have shown that a mixture of physiologic lipids, that includes palmitic acid, cholesterol, and ceramides, applied in a 1 : 1 : 1 mole ratio, prevents the adverse effects of GC on permeability barrier homeostasis (9). In contrast, co-application of the same mixture of physiologic lipids did not prevent the GC-induced decrease in expression of the keratinocyte differentiation markers, filaggrin, loricrin and involucrin (Fig.1A). Similarly, when keratinocyte proliferation was measured using PCNA immunohistochemical staining, the physiologic lipid mixture did not prevent the GC-induced decrease (Fig.1B). Thus, while a physiological lipid mixture is capable of improving permeability barrier function in GC treated animals, it does not prevent the full spectrum of epidermal abnormalities induced by GC treatment; i.e. keratinocyte differentiation or proliferation still decrease.
Figure 1. SC triple-lipid mixture does not override the negative effects of GC on keratinocyte differentiation or proliferation.
A mixture of physiologic lipids, that includes palmitic acid, cholesterol, and ceramides, applied in a 1 : 1 : 1 mole ratio, did not prevent the GC-induced decrease in expression of the keratinocyte differentiation markers, filaggrin, loricrin and involucrin (A). This lipid mixture also did not prevent the decrease in keratinocyte proliferation observed after GC treatment measured by PCNA staining (B- PCNA cell count: N= 3; PCNA staining: arrows indicate PCNA positive cells).
Improved permeability barrier homeostasis when PPARβ/δ or PPARγ, but not PPARα or LXR activators, are co-applied with GC
Short-term topical treatment with potent GC compromises permeability barrier homeostasis. We therefore next determined if co-application of PPAR and LXR activators also prevents the development of this abnormality. Interestingly, clofibrate and 22Rhydroxycholesterol, PPARα and LXR activators, respectively, did not improve permeability barrier recovery following acute disruption (data not shown). However, the PPARβ/δ and PPARγ activators, GW501516 and ciglitazone, prevented the negative effects of GC on permeability barrier homeostasis (Fig.2). Why PPARβ/δ and PPARγ activators improve permeability barrier homeostasis, while PPARα and LXR activators were ineffective is unknown.
Figure 2. Improved permeability barrier homeostasis when PPARβ/δ or PPARγ are co-applied with GC.
At 3 and 6 hours after barrier disruption permeability barrier recovery was significantly improved when β/δ ligand (GW501516, 4mM) was co-applied with GC (A; N=8). Similar results were obtained at 3, 6 and 24 hours after PPARγ ligand, ciglitazone co-application (B)(N=5).
PPARα, PPARγ and LXR activators normalize differentiation in GC- treated murine skin
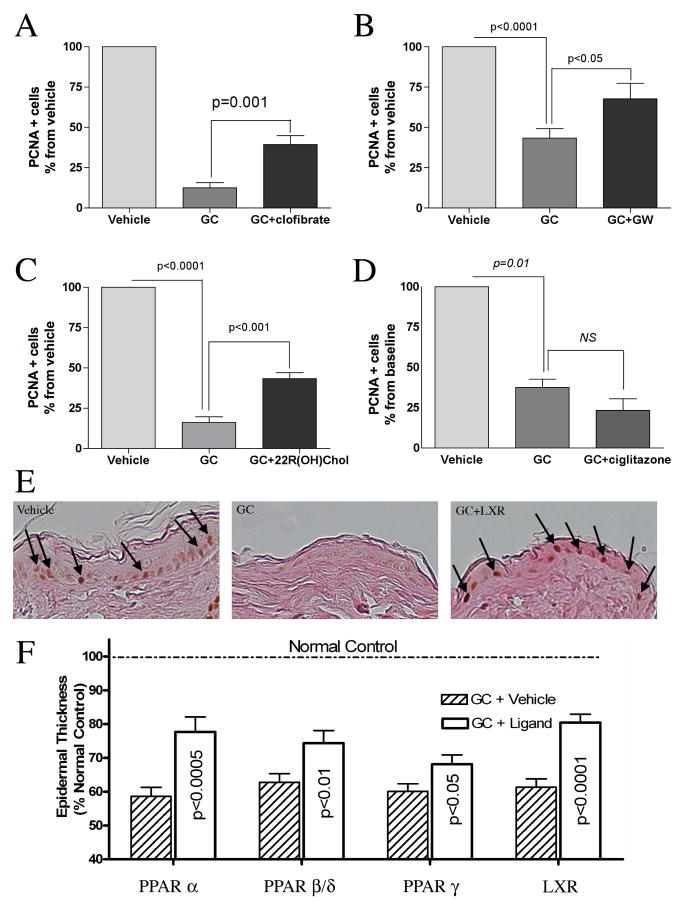
Since nuclear hormone receptor activators are known to stimulate keratinocyte differentiation in normal skin, we next asked whether they could prevent the adverse effects of GC on the expression of differentiation markers in the epidermis. Topical co-applications of two classes of PPAR activators, PPARα (clofibrate, Fig.3A) and PPARγ (ciglitazone, Fig.3B) or of the LXR activator (22R hydroxycholesterol, Fig.4) with clobetasol blocked the GC-induced decrease in the expression of the keratinocyte differentiation markers, involucrin, filaggrin and loricrin. In contrast, an activator of PPARβ/δ (GW501516) only prevented the decrease in filaggrin and involucrin expression, but did not prevent the decrease in the expression of loricrin when co-applied with clobetasol (Fig.5). These results demonstrate that PPAR and LXR activators can prevent, at least in part, the GC-induced decrease in keratinocyte differentiation.
Figure 3. PPARα and PPARγ activators normalizes differentiation in GC- treated murine skin.
As shown in immunohistochemical staining for the keratinocyte differentiation markers, co-application of clofibrate (PPARα ligand)(1mM) with clobetasol normalizes the expression of filaggrin (FIL), involucrin (INV) and loricrin (LOR)(A). Similarly, co-application of ciglitazone (PPARγ activator) (10mM) with clobetasol normalizes these differentiation markers (B). N= 3.
Figure 4. LXR activator normalizes differentiation in GC- treated murine skin.
Immunohistochemical staining for the keratinocyte differentiation markers shows that co-application of the LXR activator 22R hydroxycholesterol (10mM) with clobetasol normalizes the expression of filaggrin (FIL), involucrin (INV) and loricrin (LOR). N= 3.
Figure 5. PPARβ/δ activator GW501516 inhibited the decrease in filaggrin and involucrin, but not loricrin expression, on GC- treated murine epidermis.
Application of the PPARβ/δ activator GW501516 (4mM) on GC-treated murine epidermis normalized the levels of two keratinocyte differentiation markers, filaggrin (FIL) and involucrin (INV). However, this PPARβ/δ ligand did not prevent the decrease in loricrin (LOR) after GC treatment. N= 3.
PPARα, PPARβ/δ, and LXR activators increase proliferation in GC- treated murine epidermis
Epidermal thinning is commonly observed in glucocorticoid treated skin as a result of decreased keratinocyte proliferation. Thus, we next assessed the ability of PPAR and LXR activators to prevent the decrease in keratinocyte proliferation induced by topical clobetasol treatment. Topical co-applications of the PPARα activator, clofibrate, the PPAR β/δ activator, GW501516, or the LXR activator, 22R hydroxycholesterol, partially prevented the decrease in keratinocyte proliferation induced by GC-treatment (Fig.6 - A,B,C,E). In contrast, the PPARγ activator, ciglitazone, did not affect the GC-induced decrease in keratinocyte proliferation (Fig.6D). Thus, activation of PPARα, PPARβ/δ, and LXR can reduce the inhibition of keratinocyte proliferation induced by GC treatment. Finally, we measured the thickness of the epidermal nucleated cell layers after PPAR or LXR application onto GC treated epidermis. The results paralleled those observed with the PCNA staining with an increase in epidermal thickness after PPARα, β/δ and LXR treatment. However, while PPARγ did not affect keratinocyte proliferation, it did induce a significant increase in epidermal thickness (Fig.6F).
Figure 6. PPARα, PPARβ/δ, and LXR activators increase proliferation in GC-treated murine epidermis.
Topical application of either PPARα activator (clofibrate, 1mM)(A)(N=3), β/δ ligand (GW501516, 4mM)(B)(N=3) or LXR activator (22R hydroxycholesterol, 10mM)(C, E)(N= 3) increased keratinocyte proliferation in GC-treated murine epidermis, as measured by PCNA. In contrast, no effect on proliferation was seen after ciglitazone (PPARγ ligand) co-treatment (D)(N= 3). All ligands significantly reversed the decrease in the thickness of the epidermal nucleated layers observed after GC treatment (F). The data presented represents the mean of all measured points +/- SEM. (N=31-45).
In conclusion, a physiological triple lipid mixture can correct the GC induced defects in epidermal permeability barrier homeostasis but it does not reverse the GC-induced abnormalities in differentiation and proliferation (Table 1). In contrast, we show here that topical treatment with PPARs or LXR activators prevents the GC induced abnormalities in differentiation and proliferation, while only PPARβ/δ and PPARγ activators improve the decrease in permeability barrier function induced by GC treatment. Further experiments are needed to assess the molecular mechanisms by which PPAR and LXR activators protect from the adverse effects of glucocorticoids and the mechanisms underlying the different effects observed with the SC triple lipid mixture, PPAR activators, and LXR ligands on barrier homeostasis, epidermal differentiation and keratinocyte proliferation, in both normal and glucocorticoid treated epidermis.
Table 1. Effects of NHR ligands after glucocorticoid treatment on murine epidermal homeostasis.
| GC | GC+Lipids | GC+PPARα | GC+PPARδ | GC+PPARγ | GC+LXR | |
|---|---|---|---|---|---|---|
| Barrier recovery | Decreaseda | Improveda | No change | Improved | Improved | No change |
| Differentiation: - INV | Decreased | No effect | Increased | Increased | Increased | Increased |
| - FIL | Decreased | No effect | Increased | Increased | Increased | Increased |
| - LOR | Decreased | No effect | Increased | No change | Increased | Increased |
| Mitogenesis (PCNA) | Decreased | No effect | Increased | Increased | No effect | Increased |
data from Kao et al., 2003.
These studies suggest that it will be possible to develop strategies to ameliorate the epidermal abnormalities that occur with topical GC treatment. If similar favorable results can be demonstrated in humans one maybe able to take advantage of the anti-inflammatory beneficial effects of topical GC treatment without the toxic side effects on the epidermis. Importantly, topical PPAR and LXR activators demonstrate potent anti-inflammatory activity in a variety of inflammatory models (18, 29, 30), so that the addition of such compounds, many of which are naturally occurring lipids, to topical GC preparations would likely enhance the anti-inflammatory effects of GC. Finally, preliminary studies in animal models and humans have suggested that PPAR activators may have beneficial effects on skin diseases, such as psoriasis and atopic dermatitis, suggesting that the addition of such compounds to GC could result in added clinical benefit (26, 31-36).
Acknowledgments
These studies were supported by the National Institutes of Health (grants AR 19098, AR-39448(PP), and AR-049932) and by the Medical Research Service, Department of Veterans Affairs Medical Center. We gratefully acknowledge the excellent editorial assistance of Joan Wakefield.
Abbreviations
- GC
glucocorticoids
- PPAR
peroxisome proliferators-activated receptor
- LXR
liver X receptor
- N
number of animals in each experimental group
References
- 1.Schafer-Korting M, Kleuser B, Ahmed M, Holtje HD, Korting HC. Glucocorticoids for human skin: new aspects of the mechanism of action. Skin pharmacology and physiology. 2005;18:103–114. doi: 10.1159/000084907. [DOI] [PubMed] [Google Scholar]
- 2.Jackson S, Gilchrist H, Nesbitt LT., Jr Update on the dermatologic use of systemic glucocorticosteroids. Dermatologic therapy. 2007;20:187–205. doi: 10.1111/j.1529-8019.2007.00133.x. [DOI] [PubMed] [Google Scholar]
- 3.Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006;54:1–15. doi: 10.1016/j.jaad.2005.01.010. quiz 16-18. [DOI] [PubMed] [Google Scholar]
- 4.Schoepe S, Schacke H, May E, Asadullah K. Glucocorticoid therapy-induced skin atrophy. Experimental dermatology. 2006;15:406–420. doi: 10.1111/j.0906-6705.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 5.du Vivier A, Phillips H, Hehir M. Applications of glucocorticosteroids. The effects of twice-daily vs once-every-other-day applications on mouse epidermal cell DNA synthesis. Archives of dermatology. 1982;118:305–308. doi: 10.1001/archderm.118.5.305. [DOI] [PubMed] [Google Scholar]
- 6.Laurence EB, Christophers E. Selective action of hydrocortisone on postmitotic epidermal cells in vivo. The Journal of investigative dermatology. 1976;66:222–229. doi: 10.1111/1523-1747.ep12482145. [DOI] [PubMed] [Google Scholar]
- 7.Sheu HM, Lee JY, Chai CY, Kuo KW. Depletion of stratum corneum intercellular lipid lamellae and barrier function abnormalities after long-term topical corticosteroids. The British journal of dermatology. 1997;136:884–890. [PubMed] [Google Scholar]
- 8.Sheu HM, Tai CL, Kuo KW, Yu HS, Chai CY. Modulation of epidermal terminal differentiation in patients after long-term topical corticosteroids. The Journal of dermatology. 1991;18:454–464. doi: 10.1111/j.1346-8138.1991.tb03115.x. [DOI] [PubMed] [Google Scholar]
- 9.Kao JS, Fluhr JW, Man MQ, et al. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. The Journal of investigative dermatology. 2003;120:456–464. doi: 10.1046/j.1523-1747.2003.12053.x. [DOI] [PubMed] [Google Scholar]
- 10.Elias PM, Feingold KR. Permeability barrier homeostasis. In: Elias PM, Feingold KR, editors. Skin Barrier. New York: Taylor & Francis; 2006. pp. 337–362. [Google Scholar]
- 11.Micallef L, Belaubre F, Pinon A, et al. Effects of extracellular calcium on the growth-differentiation switch in immortalized keratinocyte HaCaT cells compared with normal human keratinocytes. Experimental dermatology. 2008 doi: 10.1111/j.1600-0625.2008.00775.x. [DOI] [PubMed] [Google Scholar]
- 12.Kuenzli S, Saurat JH. Peroxisome proliferator-activated receptors in cutaneous biology. The British journal of dermatology. 2003;149:229–236. doi: 10.1046/j.1365-2133.2003.05532.x. [DOI] [PubMed] [Google Scholar]
- 13.Schmuth M, Jiang YJ, Dubrac S, Elias PM, Feingold KR. Thematic review series: skin lipids. Peroxisome proliferator-activated receptors and liver X receptors in epidermal biology. Journal of lipid research. 2008;49:499–509. doi: 10.1194/jlr.R800001-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Sertznig P, Seifert M, Tilgen W, Reichrath J. Peroxisome proliferator-activated receptors (PPARs) and the human skin: importance of PPARs in skin physiology and dermatologic diseases. American journal of clinical dermatology. 2008;9:15–31. doi: 10.2165/00128071-200809010-00002. [DOI] [PubMed] [Google Scholar]
- 15.Michalik L, Wahli W. Peroxisome proliferator-activated receptors (PPARs) in skin health, repair and disease. Biochimica et biophysica acta. 2007;1771:991–998. doi: 10.1016/j.bbalip.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Russell LE, Harrison WJ, Bahta AW, Zouboulis CC, Burrin JM, Philpott MP. Characterization of liver X receptor expression and function in human skin and the pilosebaceous unit. Experimental dermatology. 2007;16:844–852. doi: 10.1111/j.1600-0625.2007.00612.x. [DOI] [PubMed] [Google Scholar]
- 17.Hanley K, Jiang Y, He SS, et al. Keratinocyte differentiation is stimulated by activators of the nuclear hormone receptor PPARalpha. The Journal of investigative dermatology. 1998;110:368–375. doi: 10.1046/j.1523-1747.1998.00139.x. [DOI] [PubMed] [Google Scholar]
- 18.Schmuth M, Haqq CM, Cairns WJ, et al. Peroxisome proliferator-activated receptor (PPAR)-beta/delta stimulates differentiation and lipid accumulation in keratinocytes. The Journal of investigative dermatology. 2004;122:971–983. doi: 10.1111/j.0022-202X.2004.22412.x. [DOI] [PubMed] [Google Scholar]
- 19.Hanley K, Ng DC, He SS, et al. Oxysterols induce differentiation in human keratinocytes and increase Ap-1-dependent involucrin transcription. The Journal of investigative dermatology. 2000;114:545–553. doi: 10.1046/j.1523-1747.2000.00895.x. [DOI] [PubMed] [Google Scholar]
- 20.Komuves LG, Schmuth M, Fowler AJ, et al. Oxysterol stimulation of epidermal differentiation is mediated by liver X receptor-beta in murine epidermis. The Journal of investigative dermatology. 2002;118:25–34. doi: 10.1046/j.0022-202x.2001.01628.x. [DOI] [PubMed] [Google Scholar]
- 21.Mao-Qiang M, Fowler AJ, Schmuth M, et al. Peroxisome-proliferator-activated receptor (PPAR)-gamma activation stimulates keratinocyte differentiation. The Journal of investigative dermatology. 2004;123:305–312. doi: 10.1111/j.0022-202X.2004.23235.x. [DOI] [PubMed] [Google Scholar]
- 22.Jiang YJ, Kim P, Elias PM, Feingold KR. LXR and PPAR activators stimulate cholesterol sulfotransferase type 2 isoform 1b in human keratinocytes. Journal of lipid research. 2005;46:2657–2666. doi: 10.1194/jlr.M500235-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Strott CA, Higashi Y. Cholesterol sulfate in human physiology: what's it all about? Journal of lipid research. 2003;44:1268–1278. doi: 10.1194/jlr.R300005-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Jiang YJ, Lu B, Kim P, et al. PPAR and LXR activators regulate ABCA12 expression in human keratinocytes. The Journal of investigative dermatology. 2008;128:104–109. doi: 10.1038/sj.jid.5700944. [DOI] [PubMed] [Google Scholar]
- 25.Man MQ, Choi EH, Schmuth M, et al. Basis for improved permeability barrier homeostasis induced by PPAR and LXR activators: liposensors stimulate lipid synthesis, lamellar body secretion, and post-secretory lipid processing. The Journal of investigative dermatology. 2006;126:386–392. doi: 10.1038/sj.jid.5700046. [DOI] [PubMed] [Google Scholar]
- 26.Demerjian M, Man MQ, Choi EH, et al. Topical treatment with thiazolidinediones, activators of peroxisome proliferator-activated receptor-gamma, normalizes epidermal homeostasis in a murine hyperproliferative disease model. Experimental dermatology. 2006;15:154–160. doi: 10.1111/j.1600-0625.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- 27.Man MM, Feingold KR, Thornfeldt CR, Elias PM. Optimization of physiological lipid mixtures for barrier repair. The Journal of investigative dermatology. 1996;106:1096–1101. doi: 10.1111/1523-1747.ep12340135. [DOI] [PubMed] [Google Scholar]
- 28.Mao-Qiang M, Brown BE, Wu-Pong S, Feingold KR, Elias PM. Exogenous nonphysiologic vs physiologic lipids. Divergent mechanisms for correction of permeability barrier dysfunction. Arch Dermatol. 1995;131:809–816. doi: 10.1001/archderm.131.7.809. [DOI] [PubMed] [Google Scholar]
- 29.Sheu MY, Fowler AJ, Kao J, et al. Topical peroxisome proliferator activated receptor-alpha activators reduce inflammation in irritant and allergic contact dermatitis models. The Journal of investigative dermatology. 2002;118:94–101. doi: 10.1046/j.0022-202x.2001.01626.x. [DOI] [PubMed] [Google Scholar]
- 30.Fowler AJ, Sheu MY, Schmuth M, et al. Liver X receptor activators display anti-inflammatory activity in irritant and allergic contact dermatitis models: liver-X-receptor-specific inhibition of inflammation and primary cytokine production. The Journal of investigative dermatology. 2003;120:246–255. doi: 10.1046/j.1523-1747.2003.12033.x. [DOI] [PubMed] [Google Scholar]
- 31.Ellis CN, Varani J, Fisher GJ, et al. Troglitazone improves psoriasis and normalizes models of proliferative skin disease: ligands for peroxisome proliferator-activated receptor-gamma inhibit keratinocyte proliferation. Arch Dermatol. 2000;136:609–616. doi: 10.1001/archderm.136.5.609. [DOI] [PubMed] [Google Scholar]
- 32.Bongartz T, Coras B, Vogt T, Scholmerich J, Muller-Ladner U. Treatment of active psoriatic arthritis with the PPARgamma ligand pioglitazone: an open-label pilot study. Rheumatology (Oxford, England) 2005;44:126–129. doi: 10.1093/rheumatology/keh423. [DOI] [PubMed] [Google Scholar]
- 33.Kippenberger S, Loitsch SM, Grundmann-Kollmann M, et al. Activators of peroxisome proliferator-activated receptors protect human skin from ultraviolet-B-light-induced inflammation. The Journal of investigative dermatology. 2001;117:1430–1436. doi: 10.1046/j.0022-202x.2001.01537.x. [DOI] [PubMed] [Google Scholar]
- 34.Staumont-Salle D, Abboud G, Brenuchon C, et al. Peroxisome proliferator-activated receptor alpha regulates skin inflammation and humoral response in atopic dermatitis. The Journal of allergy and clinical immunology. 2008;121:962–968. doi: 10.1016/j.jaci.2007.12.1165. e966. [DOI] [PubMed] [Google Scholar]
- 35.Varani J, Bhagavathula N, Ellis CN, Pershadsingh HA. Thiazolidinediones: potential as therapeutics for psoriasis and perhaps other hyperproliferative skin disease. Expert opinion on investigational drugs. 2006;15:1453–1468. doi: 10.1517/13543784.15.11.1453. [DOI] [PubMed] [Google Scholar]
- 36.Venkatraman MS, Chittiboyina A, Meingassner J, et al. Alpha-Lipoic acid-based PPARgamma agonists for treating inflammatory skin diseases. Archives of dermatological research. 2004;296:97–104. doi: 10.1007/s00403-004-0480-5. [DOI] [PubMed] [Google Scholar]