Abstract
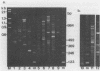
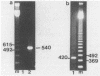
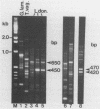
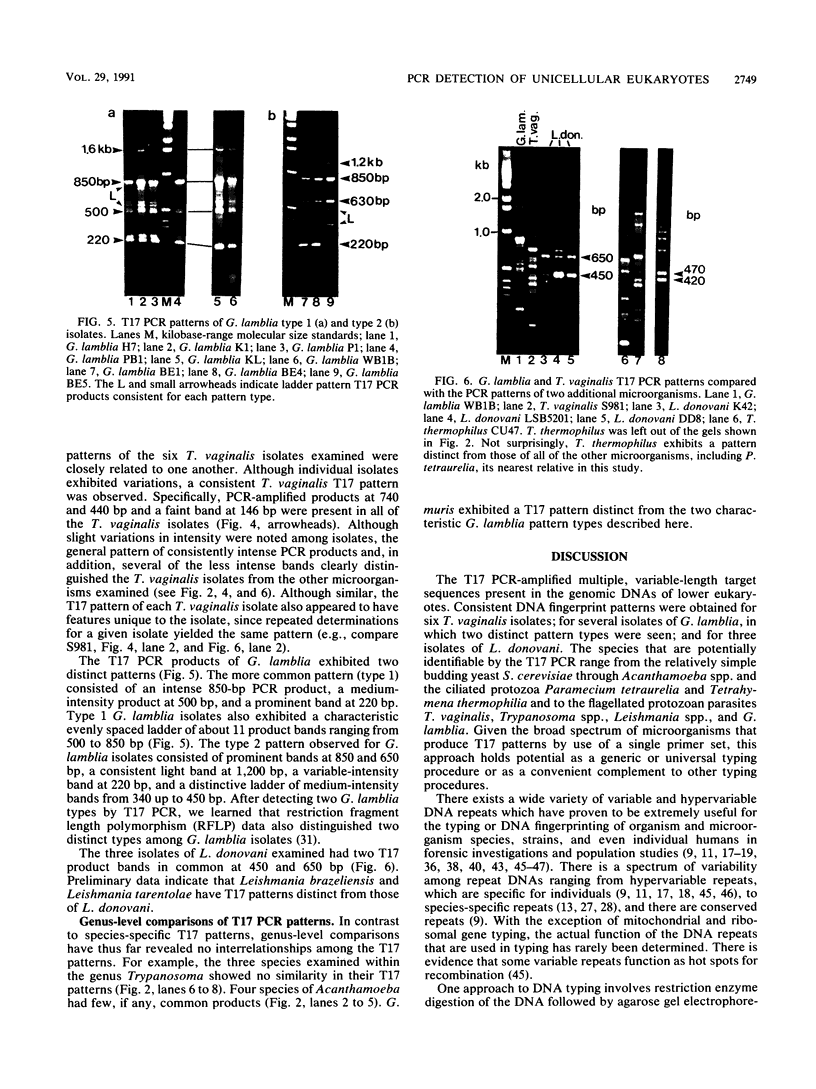
We cloned and sequenced a variable DNA repeat from Trichomonas vaginalis, a flagellated protozoan parasite. Targeting of this repeat in the polymerase chain reaction resulted in complex and intense product patterns for a wide variety of eukaryotic microorganisms, including the pathogenic protozoan parasites T. vaginalis, Giardia lamblia, Leishmania donovani, three species of Trypanosoma, and four species of Acanthamoeba; the nonpathogenic protozoans, Paramecium tetraurelia and Tetrahymena thermophilia; and a yeast, Saccharomyces cerevisiae. Each microorganism exhibited a distinctive pattern of repeats. For example, a characteristic pattern was exhibited by six clinical T. vaginalis isolates. Eight G. lamblia isolates exhibited either one of two characteristic pattern types. There was no reaction with human DNA or DNA from the prokaryotes Ureaplasma urealyticum and Mycoplasma hominis. This approach may facilitate detection of a wide variety of eukaryotic microorganisms by use of a single primer set and holds promise for the development of typing schemes for both T. vaginalis and G. lamblia.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Kasmala L., Metcalfe E., Garza G. E. Phenotypic variation and diversity among Trichomonas vaginalis isolates and correlation of phenotype with trichomonal virulence determinants. Infect Immun. 1986 Aug;53(2):285–293. doi: 10.1128/iai.53.2.285-293.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Suprun-Brown L., Kasmala L. Monoclonal antibody to a major surface glycoprotein immunogen differentiates isolates and subpopulations of Trichomonas vaginalis. Infect Immun. 1986 Apr;52(1):70–75. doi: 10.1128/iai.52.1.70-75.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsopp B., Carrington M., Baylis H., Sohal S., Dolan T., Iams K. Improved characterization of Theileria parva isolates using the polymerase chain reaction and oligonucleotide probes. Mol Biochem Parasitol. 1989 Jun 15;35(2):137–147. doi: 10.1016/0166-6851(89)90116-3. [DOI] [PubMed] [Google Scholar]
- Blanchard A. Ureaplasma urealyticum urease genes; use of a UGA tryptophan codon. Mol Microbiol. 1990 Apr;4(4):669–676. doi: 10.1111/j.1365-2958.1990.tb00636.x. [DOI] [PubMed] [Google Scholar]
- Boerwinkle E., Xiong W. J., Fourest E., Chan L. Rapid typing of tandemly repeated hypervariable loci by the polymerase chain reaction: application to the apolipoprotein B 3' hypervariable region. Proc Natl Acad Sci U S A. 1989 Jan;86(1):212–216. doi: 10.1073/pnas.86.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning D. W., Clemons K. V., Hanson L. H., Stevens D. A. Restriction endonuclease analysis of total cellular DNA of Aspergillus fumigatus isolates of geographically and epidemiologically diverse origin. J Infect Dis. 1990 Nov;162(5):1151–1158. doi: 10.1093/infdis/162.5.1151. [DOI] [PubMed] [Google Scholar]
- Flint J., Boyce A. J., Martinson J. J., Clegg J. B. Population bottlenecks in Polynesia revealed by minisatellites. Hum Genet. 1989 Oct;83(3):257–263. doi: 10.1007/BF00285167. [DOI] [PubMed] [Google Scholar]
- Fouts A. C., Kraus S. J. Trichomonas vaginalis: reevaluation of its clinical presentation and laboratory diagnosis. J Infect Dis. 1980 Feb;141(2):137–143. doi: 10.1093/infdis/141.2.137. [DOI] [PubMed] [Google Scholar]
- Gill P., Jeffreys A. J., Werrett D. J. Forensic application of DNA 'fingerprints'. Nature. 1985 Dec 12;318(6046):577–579. doi: 10.1038/318577a0. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hide G., Cattand P., LeRay D., Barry J. D., Tait A. The identification of Trypanosoma brucei subspecies using repetitive DNA sequences. Mol Biochem Parasitol. 1990 Mar;39(2):213–225. doi: 10.1016/0166-6851(90)90060-y. [DOI] [PubMed] [Google Scholar]
- Hughes M. A., Hommel M., Crampton J. M. The use of biotin-labelled, synthetic DNA oligomers for the detection and identification of Plasmodium falciparum. Parasitology. 1990 Jun;100(Pt 3):383–387. doi: 10.1017/s0031182000078653. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Neumann R., Wilson V. Repeat unit sequence variation in minisatellites: a novel source of DNA polymorphism for studying variation and mutation by single molecule analysis. Cell. 1990 Feb 9;60(3):473–485. doi: 10.1016/0092-8674(90)90598-9. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Kimura H., Shibata M., Kuzushima K., Nishikawa K., Nishiyama Y., Morishima T. Detection and direct typing of herpes simplex virus by polymerase chain reaction. Med Microbiol Immunol. 1990;179(4):177–184. doi: 10.1007/BF00195248. [DOI] [PubMed] [Google Scholar]
- Kiviat N. B., Paavonen J. A., Brockway J., Critchlow C. W., Brunham R. C., Stevens C. E., Stamm W. E., Kuo C. C., DeRouen T., Holmes K. K. Cytologic manifestations of cervical and vaginal infections. I. Epithelial and inflammatory cellular changes. JAMA. 1985 Feb 15;253(7):989–996. [PubMed] [Google Scholar]
- Krieger J. N., Holmes K. K., Spence M. R., Rein M. F., McCormack W. M., Tam M. R. Geographic variation among isolates of Trichomonas vaginalis: demonstration of antigenic heterogeneity by using monoclonal antibodies and the indirect immunofluorescence technique. J Infect Dis. 1985 Nov;152(5):979–984. doi: 10.1093/infdis/152.5.979. [DOI] [PubMed] [Google Scholar]
- Krieger J. N., Holmes K. K., Spence M. R., Rein M. F., McCormack W. M., Tam M. R. Geographic variation among isolates of Trichomonas vaginalis: demonstration of antigenic heterogeneity by using monoclonal antibodies and the indirect immunofluorescence technique. J Infect Dis. 1985 Nov;152(5):979–984. doi: 10.1093/infdis/152.5.979. [DOI] [PubMed] [Google Scholar]
- Krieger J. N., Tam M. R., Stevens C. E., Nielsen I. O., Hale J., Kiviat N. B., Holmes K. K. Diagnosis of trichomoniasis. Comparison of conventional wet-mount examination with cytologic studies, cultures, and monoclonal antibody staining of direct specimens. JAMA. 1988 Feb 26;259(8):1223–1227. doi: 10.1001/jama.259.8.1223. [DOI] [PubMed] [Google Scholar]
- Krieger J. N. Urologic aspects of trichomoniasis. Invest Urol. 1981 May;18(8):411–417. [PubMed] [Google Scholar]
- Krieger J. N., Wolner-Hanssen P., Stevens C., Holmes K. K. Characteristics of Trichomonas vaginalis isolates from women with and without colpitis macularis. J Infect Dis. 1990 Feb;161(2):307–311. doi: 10.1093/infdis/161.2.307. [DOI] [PubMed] [Google Scholar]
- Lane D. J., Pace B., Olsen G. J., Stahl D. A., Sogin M. L., Pace N. R. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6955–6959. doi: 10.1073/pnas.82.20.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medlin L., Elwood H. J., Stickel S., Sogin M. L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene. 1988 Nov 30;71(2):491–499. doi: 10.1016/0378-1119(88)90066-2. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Bracha R., Rozenblatt S., Garfinkel L. I. Repetitive DNA elements characteristic of pathogenic Entamoeba histolytica strains can also be detected after polymerase chain reaction in a cloned nonpathogenic strain. Infect Immun. 1990 Jun;58(6):1660–1663. doi: 10.1128/iai.58.6.1660-1663.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash T. E., McCutchan T., Keister D., Dame J. B., Conrad J. D., Gillin F. D. Restriction-endonuclease analysis of DNA from 15 Giardia isolates obtained from humans and animals. J Infect Dis. 1985 Jul;152(1):64–73. doi: 10.1093/infdis/152.1.64. [DOI] [PubMed] [Google Scholar]
- Riley D. E., Goldman M. A., Gartler S. M. Nucleotide sequence of the 3' nuclease-sensitive region of the human phosphoglycerate kinase 1 (PGK1) gene. Genomics. 1991 Sep;11(1):212–214. doi: 10.1016/0888-7543(91)90121-t. [DOI] [PubMed] [Google Scholar]
- Riley D. E. Very rapid nucleotide sequence analysis of improved, double-stranded minipreps. Gene. 1989 Jan 30;75(1):193–196. doi: 10.1016/0378-1119(89)90396-x. [DOI] [PubMed] [Google Scholar]
- Ross B. C., Raios K., Jackson K., Sievers A., Dwyer B. Differentiation of Mycobacterium tuberculosis strains by use of a nonradioactive Southern blot hybridization method. J Infect Dis. 1991 Apr;163(4):904–907. doi: 10.1093/infdis/163.4.904. [DOI] [PubMed] [Google Scholar]
- Spence M. R., Hollander D. H., Smith J., McCaig L., Sewell D., Brockman M. The clinical and laboratory diagnosis of Trichomonas vaginalis infection. Sex Transm Dis. 1980 Oct-Dec;7(4):168–171. doi: 10.1097/00007435-198010000-00004. [DOI] [PubMed] [Google Scholar]
- Stevens D. A., Odds F. C., Scherer S. Application of DNA typing methods to Candida albicans epidemiology and correlations with phenotype. Rev Infect Dis. 1990 Mar-Apr;12(2):258–266. doi: 10.1093/clinids/12.2.258. [DOI] [PubMed] [Google Scholar]
- Su-Lin K. E., Honigberg B. M. Antigenic analysis of Trichomonas vaginalis strains by quantitative fluorescent antibody methods. Z Parasitenkd. 1983;69(2):161–181. doi: 10.1007/BF00926952. [DOI] [PubMed] [Google Scholar]
- Torian B. E., Connelly R. J., Stephens R. S., Stibbs H. H. Specific and common antigens of Trichomonas vaginalis detected by monoclonal antibodies. Infect Immun. 1984 Jan;43(1):270–275. doi: 10.1128/iai.43.1.270-275.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upcroft P., Mitchell R., Boreham P. F. DNA fingerprinting of the intestinal parasite Giardia duodenalis with the M13 phage genome. Int J Parasitol. 1990 May;20(3):319–323. doi: 10.1016/0020-7519(90)90146-e. [DOI] [PubMed] [Google Scholar]
- Vincent R. D., Goewert R., Goldman W. E., Kobayashi G. S., Lambowitz A. M., Medoff G. Classification of Histoplasma capsulatum isolates by restriction fragment polymorphisms. J Bacteriol. 1986 Mar;165(3):813–818. doi: 10.1128/jb.165.3.813-818.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvesvara G. S. Classification of Acanthamoeba. Rev Infect Dis. 1991 Mar-Apr;13 (Suppl 5):S369–S372. doi: 10.1093/clind/13.supplement_5.s369. [DOI] [PubMed] [Google Scholar]
- Wahls W. P., Wallace L. J., Moore P. D. Hypervariable minisatellite DNA is a hotspot for homologous recombination in human cells. Cell. 1990 Jan 12;60(1):95–103. doi: 10.1016/0092-8674(90)90719-u. [DOI] [PubMed] [Google Scholar]
- Wong Z., Wilson V., Jeffreys A. J., Thein S. L. Cloning a selected fragment from a human DNA 'fingerprint': isolation of an extremely polymorphic minisatellite. Nucleic Acids Res. 1986 Jun 11;14(11):4605–4616. doi: 10.1093/nar/14.11.4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöstemeyer J. Strain-dependent variation in ribosomal DNA arrangement in Absidia glauca. Eur J Biochem. 1985 Jan 15;146(2):443–448. doi: 10.1111/j.1432-1033.1985.tb08671.x. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- de Bièvre C., Dauguet C., Nguyen V. H., Ibrahim-Granet O. Polymorphism in mitochondrial DNA of several Trichophyton rubrum isolates from clinical specimens. Ann Inst Pasteur Microbiol. 1987 Nov-Dec;138(6):719–727. doi: 10.1016/0769-2609(87)90149-9. [DOI] [PubMed] [Google Scholar]