Abstract
The mechanisms underlying the pathogenesis of estrogen-induced breast carcinogenesis remain unclear. The present study investigated the roles of estrogen metabolism and oxidative stress in estrogen-mediated mammary carcinogenesis in vivo. Female August Copenhagen Irish (ACI) rats were treated with 17β-estradiol (E2), the antioxidant vitamin C, the estrogen metabolic inhibitor α-naphthoflavone (ANF), or cotreated with E2 + vitamin C or E2 + ANF for up to 8 months. E2 (3 mg) was administered as an subcutaneous implant, ANF was given via diet (0.2%) and vitamin C (1%) was added to drinking water. At necropsy, breast tumor incidence in the E2, E2 + vitamin C and E2 + ANF groups was 82, 29 and 0%, respectively. Vitamin C and ANF attenuated E2-induced alterations in oxidative stress markers in breast tissue, including 8-iso-prostane F2α formation and changes in the activities of antioxidant enzymes superoxide dismutase and glutathione peroxidase. Quantification of 2-hydroxyestradiol (2-OHE2) and 4-hydroxyestradiol (4-OHE2) formation in breast tissue confirmed that ANF inhibited 4-hydroxylation of E2 and decreased formation of the highly carcinogenic 4-OHE2. These results demonstrate that antioxidant vitamin C reduces the incidence of estrogen-induced mammary tumors, increases tumor latency and decreases oxidative stress in vivo. Further, our data indicate that ANF completely abrogates breast cancer development in ACI rats. The present study is the first to demonstrate the inhibition of breast carcinogenesis by antioxidant vitamin C or the estrogen metabolic inhibitor ANF in an animal model of estrogen-induced mammary carcinogenesis. Taken together, these results suggest that E2 metabolism and oxidant stress are critically involved in estrogen-induced breast carcinogenesis.
Introduction
Sex hormones have been implicated in the development of a number of human cancers, and recent data indicate that, in the USA, neoplasia of hormone-responsive tissues accounts for >35% of newly diagnosed cancers in men and >40% of newly diagnosed cancers in women (1). The importance of estrogens in the etiology of breast cancer is widely recognized, and the US government has added steroidal estrogens to the list of known human carcinogens (2–4). In general, elevated lifetime estrogen exposure is considered a major risk factor for breast cancer (5). While a growing body of clinical and epidemiological literature supports a role for estrogen in breast carcinogenesis, the exact mechanisms underlying the initiation and progression of estrogen-related cancers remain elusive (5).
Estrogens exert their carcinogenic effects via estrogen receptor (ER)-dependent mitogenic effects and ER-independent mechanisms (6,7). The ER-independent pathway of estrogen-induced breast carcinogenesis involves metabolic activation of endogenous estrogens by cytochrome P450 enzymes to generate highly reactive genotoxic metabolites (6). Cytochrome P450 1A1 (Cyp1A1) and Cytochrome P450 1B1 (Cyp1B1) are the primary enzymes responsible for the metabolism of 17β-estradiol (E2) into the catechol estrogens 2-hydroxyestradiol (2-OHE2) and 4-hydroxyestradiol (4-OHE2), respectively (8). Tumorigenic estrogen metabolites such as 4-OHE2 undergo oxidative metabolism to generate electrophilic quinones, which readily react with DNA to produce depurinating adducts and mutagenic reactive oxygen species (8–10). DNA adducts produced by the quinone forms of catechol estrogens have been detected in various tissues vulnerable to estrogen-induced carcinogenesis (11,12).
In this study, the antioxidant vitamin C and the E2 metabolic inhibitor α-naphthoflavone (ANF) were used to investigate whether reducing oxidative stress and minimizing the metabolism of E2 to catechol estrogens would reduce estrogen-induced tumor development in vivo. The August Copenhagen Irish (ACI) rat model of estrogen-induced breast cancer was used for the current study, as female ACI rats are uniquely sensitive to estrogen-induced breast carcinogenesis and develop tumors that are estrogen dependent, aneuploid and exhibit genomic instability (13–18). Female ACI rats were treated with vitamin C, ANF, E2 and combinations consisting of either E2 + vitamin C or E2 + ANF for 7, 15, 120 and 240 days. Animals were examined daily for palpable tumors, and at the end of the experiments, animals were euthanized so that various tissues, including breast and liver, could be examined for both histopathological changes as well as alterations in the levels of oxidative stress markers.
Materials and methods
Chemicals
E2, vitamin C, ANF, 2-OHE2, 4-OHE2 and cholesterol were purchased from Sigma–Aldrich (St Louis, MO). A total amount of 250 μCi [6,7-3H(N)]-E2 [specific activity 40–60 Ci (1.48–2.22 TBq)/mmol] was obtained from NEN Radiochemicals (Perkin Elmer, Waltham, MA).
Tumor development, estrogen, vitamin C and ANF treatment and histopathologic analysis
Female ACI rats (Harlan Sprague Dawley, Indianapolis, IN) were obtained at 4 weeks of age and housed in the Columbia University animal facility under controlled temperature, humidity and lighting conditions. Animals were fed AIN76A phytoestrogen-free diets (Dyets, Bethlehem, PA) and water was given ad libitum. After a one-week acclimatization period, rats were randomly distributed into six treatment groups. The experimental groups were treated with E2, E2 + vitamin C or E2 + ANF. The respective controls groups were treated with cholesterol, cholesterol + vitamin C or cholesterol + ANF. Vitamin C (1%) was administered in drinking water and ANF was present in the diet (0.2%). Rats in the E2, E2 + vitamin C and E2 + ANF treatment groups were implanted subcutaneously with E2 pellets (3 mg E2 + 17 mg cholesterol). Rats in the cholesterol, cholesterol + vitamin C and cholesterol + ANF control groups were implanted with pellets containing 17 mg cholesterol only. E2 and cholesterol pellets were prepared using a pellet press as described previously (7,19–21). Vitamin C or ANF treatment began 7 days prior to pellet implantation. Each of the six treatment groups (cholesterol, cholesterol + vitamin C, cholesterol + ANF, E2, E2 + vitamin C and E2 + ANF) was divided into four subgroups, containing at least 10 rats each, which underwent their respective treatments for 7, 15, 120 or 240 days. At the end of each of these time periods, animals were anesthetized using isoflurane and euthanized. Mammary (both tumor and normal), uterus, ovary, lung, brain, kidney and liver tissues were removed and snap frozen in liquid nitrogen for future analyses. Frozen tissues were stored at −70°C. A portion of the excised mammary and other tissues was stored in 10% buffered formalin for histopathological and immunohistochemical analyses. Tumor incidence and the number of tumor nodules per rat were counted at the time of dissection. The formalin-fixed tissue was embedded in paraffin, and sections of 4–5 μm thickness were cut. Paraffin-embedded sections of the mammary, liver, brain, uterus, kidney, lung and ovary were stained with hematoxylin and eosin for histopathological evaluation by a pathologist. Histopathological analyses for tumor development and morphological changes were performed on mammary tissue from all rats from the experimental and control groups.
Determination of 8-iso-prostane F2α
Total 8-iso-Prostane F2α (8-isoPGF2α) levels in the mammary, mammary tumor and liver tissue of female ACI rats were quantified using a direct 8-isoPGF2α enzyme immunoassay kit obtained from Assay Designs (Ann Arbor, MI) according to the supplier's instructions as described previously (7,19). Breast and liver tissue homogenates were prepared for use in the 8-isoPGF2α assay according to methods described previously (19). Data are expressed as mean 8-isoPGF2α pg/mg protein ± SE of the mean. Fold changes were calculated by comparing 8-isoPGF2α levels detected in the mammary or liver tissue of E2, E2 + vitamin C or E2 + ANF-exposed animal tissues to levels in the mammary tissue of age-matched controls (i.e. the cholesterol, cholesterol + vitamin C or cholesterol + ANF groups).
Measurement of superoxide dismutase, catalase and glutathione peroxidase enzyme activity
Superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) activities in the mammary, mammary tumor and liver tissues of ACI rats were measured using commercially-available kits from Cayman Chemical Company (Catalog Numbers 706002, 707002 and 703102, Ann Arbor, MI). Breast and liver tissue homogenates were prepared for use in the SOD, GPx and CAT assays according to methods described previously (19). SOD activity is reported as units/microgram protein. GPx activity is reported as nanomole/minute/milligram protein. CAT activity is reported as nanomole/minute/milligram protein. Fold changes in SOD, CAT and GPx activity were calculated by comparing the enzyme activities in the mammary, mammary tumor or liver tissue of E2, E2 + vitamin C and E2 + ANF-treated rats to that of mammary or liver tissue from age-matched controls (i.e. E2 versus cholesterol, vitamin C + E2 versus vitamin C and E2 + ANF versus ANF).
Quantification of 2- and 4-hydroxylation of E2 in ACI rat breast tissue
This method was carried out according to previous descriptions (22,23). Breast tissue from rats in the cholesterol control, E2, vitamin C, vitamin C + E2, ANF and ANF + E2 groups was homogenized in buffer containing 1.14% KCI/10 mM ethylenediaminetetraacetic acid at pH 7.5. Microsomes were isolated according to previously reported methods and stored in buffer containing 0.25 M sucrose/10 mM ethylenediaminetetraacetic acid, pH 7.5 (22–24). Protein concentrations in the microsome samples were determined by using the Pierce bicinchoninic acid Protein kit (Thermo Fisher, Waltham, MA). Radioactivity was assessed using a scintillation counter (Beckman Coulter, Fullterton, CA) and disintegrations per minute were counted and adjusted for microgram protein added to the assay. Data are expressed as a rate (picomolar catechol estrogen/minute/microgram).
Statistical analyses
All data were analyzed using Sigma Plot 8.0 (Systat Software, San Jose, CA). Fisher's exact test was used to compare tumor incidence between two treatment groups or between a treatment group and a specific control group. The average number of tumor nodules per tumor-bearing animal was calculated by dividing the sum of the tumor nodules in all tumor-bearing animals by the total number of tumor-bearing animals. The average number of tumor nodules per rat is expressed as the mean ± SE. After calculating Kaplan–Meier survival curves of tumor occurrence for latency, we used log-rank test to detect differences in the survival curves between two treatment groups. The two sample t-test was used to detect group differences in quantitative variables, including tumor multiplicity (defined as number of tumor nodules per tumor-bearing rat) and specific fold changes in 8-isoPGF2α levels as well as in SOD, GPx and CAT activities.
Results
ANF and vitamin C significantly decrease the development of E2-induced mammary tumors
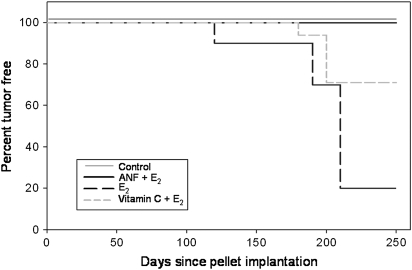
Mammary tissue from all animals in the control groups (cholesterol, cholesterol + vitamin C and cholesterol + ANF) displayed normal lobular architecture, consisting of ducts surrounded by small lobules (Figure 1). No morphological differences were detected between mammary tissues from any of the control groups (Figure 1). Analysis of mammary tissue from all rats in the E2-treated experimental groups (i.e. E2, vitamin C + E2 and ANF + E2-treated animals) revealed hyperplastic lobular units that expanded into the stromal fat (Figure 1). Lobular hyperplasia and compression of stromal fat progressively increased with duration of E2 exposure (data not shown). Mammary tissue from rats treated with E2, E2 + vitamin C or E2 + ANF were not morphologically different from one another, indicating that neither vitamin C nor ANF interfered with the proliferative response of the mammary gland to E2 (Figure 1). No mammary tumors were observed in control rats (Table I). The incidence of mammary tumors in rats treated with E2 was equal to 82% following 240 days (Table I). Strikingly, cotreatment of rats with ANF + E2 completely abrogated the development of mammary tumors (Table I). Mammary tumor incidence in rats in the E2 + vitamin C group was equal to 29% after 240 days treatment (Table I). The first palpable breast tumor appeared after 128 days in the E2-treatment group, whereas in the E2 + vitamin C group, the first palpable tumor did not appear until day 183 (Table I, Figure 2). Indeed, according to Kaplan–Meier survival curve analysis, average tumor latency was significantly longer for animals in the vitamin C + E2 group relative to those in the E2 group (Figure 2). Tumor multiplicity was not significantly reduced by vitamin C; however, the data suggest a trend toward decreased tumor multiplicity in the vitamin C + E2 group relative to the E2 group (Table I). Histopathological examination revealed no differences in tumor type or morphology between tumors collected from animals in the E2 and E2 + vitamin C groups. Mammary tumors from both groups were classified as adenocarcinomas (data not shown). Breast tumors from E2-treated rats showed evidence of invasion, whereas tumors from animals in the E2 + vitamin C group were mostly encapsulated (data not shown).
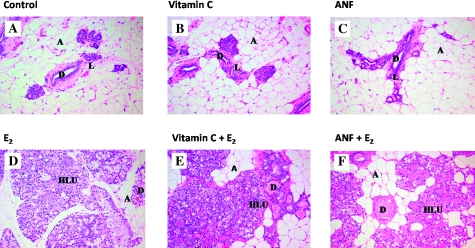
Fig. 1.
Mammary tissue from the E2, E2 + vitamin C, E2 + ANF and their respective control groups (i.e. cholesterol, cholesterol + vitamin C and cholesterol + ANF) after 240 days. Female ACI rats were treated with E2, E2 + vitamin C or E2 + ANF for 240 days. Animals in the E2, E2 + vitamin C and E2 + ANF groups were implanted with E2 pellets (subcutaneous, 3 mg E2 + 17 mg cholesterol) for 240 days. Control rats were implanted with pellets containing 17 mg cholesterol only. Vitamin C-treated rats received vitamin C (1%) in drinking water and ANF-treated rats were given ANF via diet (0.2% in food). (A–C) The mammary glands of ACI rats exposed to cholesterol, cholesterol + vitamin C or cholesterol + ANF show normal lobular architecture (L) with branched ducts (D) and normal distribution of fat/adipose tissue (A) (all panels ×100). (D–F) Mammary glands from rats in the E2, vitamin C + E2 and ANF + E2 groups show increased proliferation with dilated ducts containing inspissated secretions (D) and increased proliferation and expansion of terminal lobular units (HLU) accompanied by compression of and expansion into the surrounding fat/adipose tissue (A) (all panels ×100).
Table I.
Tumor incidence and multiplicity by treatment group
| Treatment | Tumor incidence | Tumor multiplicity | Day of first tumor appearance |
| Cholesterol | 0 (0/10) | NA | NA |
| 17β-Estradiol (E2) | 81.8% (9/11) | 3.1 ± 0.7 | 128 |
| Vitamin C | 0 (0/17) | NA | NA |
| Vitamin C + E2 | 29.4% (5/17)* | 1.6 ± 0.4 | 183 |
| α-naphthoflavone (ANF) | 0 (0/18) | NA | NA |
| ANF + E2 | 0 (0/17)* | NA | NA |
NA, not applicable.
Column one lists the different treatments each group of animals received. The percentage of animals that developed tumors during the 240 day treatment period (tumor incidence) is listed in column two. The symbol indicates ‘*’ indicates a P value <0.05 comparing tumor incidence between the E2 treatment group and the E2 + vitamin C and E2 + ANF treatment groups. P-values were calculated using Fisher's exact test. The number of tumors per tumor-bearing rat (tumor multiplicity) is listed in column three. Column four lists the day of first tumor appearance for animals in each group.
Fig. 2.
Vitamin C and ANF prevent the development of E2-induced breast tumors. Female ACI rats were treated with E2, E2 + vitamin C or E2 + ANF as described in the Materials and Methods section. Kaplan–Meier survival curves for tumor occurrence were plotted for each treatment group, and the log-rank test was used to detect differences in the tumor latency curves between groups. Animals in the control groups (cholesterol, cholesterol + vitamin C and cholesterol + ANF) did not develop any tumors and are represented by the same line on the graph (Control). Tumor latency was significantly longer in the vitamin C + E2 group compared with the E2 group (P = 0.01).
Quantification of 2- and 4-hydroxylation of E2 in ACI rat breast
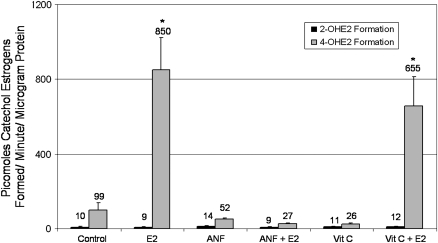
The 2- and 4-hydroxylase activity assay was used in order to examine estrogen metabolism in the breast tissue of rats treated with cholesterol, E2, vitamin C and ANF groups. In rat breast, 4-hydroxylation of E2 represents the major pathway of estrogen metabolism, whereas 2-hydroxylation makes up a relatively minor fraction of reactions. This assay was performed in order to confirm that ANF treatment inhibited the formation of 4-OHE2, as has been reported previously (25–28). Microsomes from rats in the cholesterol, cholesterol + vitamin C and cholesterol + ANF groups catalyzed the formation of very little 2- and 4-OHE2 (Figure 3). No significant differences were observed between the formation of 2- or 4-OHE2 in the cholesterol, cholesterol + vitamin C or cholesterol + ANF groups, vitamin C or ANF groups. Similarly, microsomes from rats in the E2, vitamin C + E2 and ANF + E2 groups catalyzed very little 2-hydroxylation of E2, and 2-OHE2 generation in these groups was not significantly different from formation observed in control, vitamin C or ANF-treated animals. However, 4-OHE2 formation by microsomes from rats in the E2 and vitamin C + E2 groups was greatly increased compared with rats from the cholesterol and cholesterol + vitamin C groups, respectively (Figure 3). Notably, microsomes from rats in the ANF + E2 group catalyzed very little 4-hydroxylation of E2 (Figure 3). 4-OHE2 formation in the ANF + E2 group was comparable with 4-OHE2 formation observed in cholesterol- and cholesterol + ANF- treated control animals and was significantly lower than 4-OHE2 generation observed in microsomes from E2 and vitamin C + E2-treated rats (P < 0.01). These results confirm that ANF treatment prevents 4-hydroxlation of E2 in ACI rat breast tissue.
Fig. 3.
Quantification of 2- and 4-hydroxylation of E2 in rat breasts. The 2- and 4-hydroxylase activity assay was used in order to examine estrogen metabolism in the breast tissue of rats from the cholesterol, cholesterol + vitamin C, cholesterol + ANF, E2, vitamin C + E2 and ANF + E2 groups. Radioactive 3H-E2 was used to trace the formation of catechol estrogens by rat breast microsomes. The 2- and 4-OHE2 generated during the reaction were separated from one another by using a thin layer chromatography method, and the amounts of each radioactive catechol estrogen were quantified by using a scintillation counter and corrected for the amount of microsomal protein added to the reactions. Data are expressed as picomoles catechol estrogen formed/minute/μg protein ± standard error. The symbol ‘*’ indicates a P value <0.05 compared with control values. The absolute values are listed above each bar. No differences in 2-hydroxyestradiol (2-OHE2) formation were detected between any of the treatment groups. 4-OHE2 generation was significantly higher than control levels in the E2 and vitamin C + E2 groups. The presence of ANF inhibits E2-induced increases in 4-hydroxylation in rat breast.
ANF and vitamin C treatment reduce E2-associated 8-isoPGF2α formation
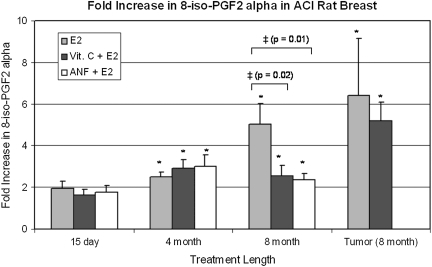
8-isoPGF2α, a known marker of lipid peroxidation and oxidative stress, was used to assess oxidative stress in mammary, mammary tumor and liver tissues from ACI rats (29). No significant differences in liver 8-isoPGF2α levels were detected between control animals and those in the E2, E2 + vitamin C or E2 + ANF groups after 240 days treatment (data not shown). As reported recently, mammary tissue 8-isoPGF2α levels were increased in a time-dependent manner following exposure to E2 (19). Vitamin C and ANF suppressed E2-induced elevations in mammary 8-isoPGF2α formation. At the 240 day time point, mammary tissue from rats in the vitamin C + E2 or ANF + E2 groups displayed significantly smaller fold increases in 8-isoPGF2α levels relative to rats in the 240 day E2 treatment group (Figure 4). As reported recently, mammary tissue 8-isoPGF2α levels in rats treated with E2 for 240 days were ∼5-fold higher than in age-matched cholesterol-treated controls (19). In contrast, mammary 8-isoPGF2α levels were elevated 2.5- and 2.4-fold after 240 days treatment with vitamin C + E2 and ANF + E2, respectively (Figure 4).
Fig. 4.
Vitamin C and ANF attenuate E2-associated increases in 8-isoPGF2α. Female ACI rats were treated with cholesterol, cholesterol + vitamin C, cholesterol + ANF, E2, E2 + vitamin C or E2 + ANF as described in the Materials and Methods section. 8-isoPGF2α levels were measured in mammary tissue from animals in each of these groups. 8-isoPGF2α levels were assessed in mammary tumor tissue as well. Fold changes were calculated for E2-treated animals relative to age-matched cholesterol-treated controls. Fold changes were calculated for E2 + vitamin C-treated animals relative to age-matched vitamin C-treated controls. Fold changes were calculated for E2 + ANF-treated animals relative to age-matched ANF-treated controls. Fold change data for tumor tissue were determined by comparing 8-isoPGF2α levels in tumor tissue with levels detected in non-tumor mammary tissue from age-matched control rats. These data are reported as an average of values obtained for at least seven different animals ± standard error. The symbol ‘*’ indicates a P value <0.05 relative to each group's respective controls. The symbol ‘‡’ indicates a P value <0.05 comparing E2 and E2 + vitamin C groups or E2 and E2 + ANF groups.
ANF and vitamin C prevent E2-induced alterations in the activities of antioxidant enzymes
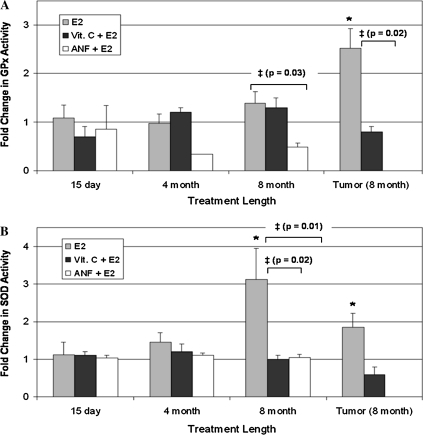
The activities of the antioxidant enzymes GPx, CAT and SOD were quantified in mammary, mammary tumor and liver tissues from rats in all of the experimental and control groups. No significant alterations in GPx or SOD activity were detected in mammary tissue from rats treated with vitamin C + E2 or ANF + E2 for 7, 15, 120 or 240 days relative to their respective age-matched controls (Figure 5). Furthermore, mammary tumors from animals in the E2 + vitamin C group did not display changes in SOD or GPx activity relative to control breast tissue (Figure 5). The lack of change in SOD or GPx activity in animals from the vitamin C + E2 or ANF + E2 groups is in contrast with increases in SOD and GPx activity observed in mammary and mammary tumor tissue from animals treated with E2 alone (Figure 5). No alterations in CAT activity were detected in mammary tissue from animals in the E2 + ANF or E2 + vitamin C groups (data not shown). No changes in SOD, GPx or CAT enzyme activity were observed between the liver tissues of control and E2, vitamin C + E2 or ANF + E2-treated rats (data not shown).
Fig. 5.
Vitamin C and ANF decrease E2-induced changes in antioxidant enzyme activities. Female ACI rats were treated with cholesterol, E2, cholesterol + vitamin C, E2 + vitamin C, ANF or E2 + ANF as described in the Materials and Methods section. The activity levels of SOD and GPx were measured in mammary tissue and mammary tumor tissue from animals in each of the treatment groups. Fold changes in enzyme activity were calculated for animals in the E2, E2 + vitamin C and E2 + ANF groups relative to enzyme activity levels in age-matched control animals. Fold change data for tumor tissue were determined by comparing enzyme activity levels in tumor tissue to levels observed in non-tumor mammary tissue from age-matched controls. These data are reported as an average of values obtained for at least seven different animals ± standard error. (A) Fold changes in GPx activity in mammary tissue from rats treated with E2, E2 + vitamin C and E2 + ANF. (B) Fold changes in SOD activity in mammary tissue from rats treated with E2, E2 + vitamin C and E2 + ANF. The symbol ‘*’ indicates a P value <0.05 relative to each group's respective controls. The symbol ‘‡’ indicates a P value <0.05 comparing the E2 + vitamin C and E2 + ANF groups to the E2 group.
Discussion
Both epidemiological and experimental evidence implicate estrogens in the pathogenesis of breast cancer. Despite extensive research, the mechanisms by which estrogens exert their carcinogenic effects remain elusive (5,30). Current data suggest that tumor induction by E2 depends on E2 metabolism and subsequent oxidative stress (7,27,31–33). The present study was designed to evaluate the importance of oxidative stress and estrogen metabolism in breast carcinogenesis using an in vivo model. Female ACI rats were treated with E2 with or without simultaneous treatment with vitamin C or ANF. Vitamin C, an antioxidant, was used to reduce the burden of oxidative stress during E2 treatment. ANF, a Cyp inhibitor, has previously been shown to inhibit estrogen metabolism and decrease the formation of catechol estrogens (25,34). In this study, ANF was used to minimize E2 metabolism and reduce the production of catechol estrogens. Catechol estrogens, such as 4-OHE2, can form depurinating DNA adducts and/or produce oxidative stress via metabolic redox cycling, a pathway that is proposed to play an important role in hormonal carcinogenesis (7,30).
Demonstrating the critical importance of estrogen metabolism and oxidative stress in breast carcinogenesis, ANF treatment completely abrogated the development of mammary tumors, and vitamin C treatment significantly reduced tumor incidence and significantly increased tumor latency in female ACI rats (Figure 2, Table I). Moreover, the results of our histopathological analyses showed that vitamin C and ANF had no effect on proliferation of mammary tissue in ACI rats, such that mammary tissues from animals in the E2, vitamin C + E2 and ANF + E2 groups were not markedly different from one another (Figure 1). The proliferative response of the breast to E2 remains intact in animals from each of the treatment groups, and the decrease in E2 metabolism and oxidative stress in the E2 + ANF and E2 + vitamin C groups is probably responsible for the dramatic reduction in breast cancer observed in these groups. ANF has previously been shown to inhibit Cyp activity and E2 metabolism (25,26,35).
In contrast to the distinct effects of E2 treatment on mammary tissue, no significant morphological changes were observed in kidneys, uteri, lungs or brains from rats in the E2, vitamin C + E2 or ANF + E2 groups relative to tissues collected from age-matched controls (data not shown). The ANF dose (0.2% wt/wt in diet) chosen for use in this study effectively prevented the development of estrogen-dependent renal tumors in Syrian hamsters without dramatically elevating liver tumor incidence; however, higher ANF doses (0.4% wt/wt in diet) augmented development of liver tumors in hamsters (27,28).
ANF and vitamin C reduced E2-associated elevations in oxidative stress in ACI rats. Mammary tissue from rats cotreated with either vitamin C + E2 or ANF + E2 for 240 days displayed significantly smaller fold increases in 8-isoPGF2α levels, an established marker of oxidative stress in vitro and in vivo, relative to rats treated with E2 only (Figure 4) (36). Similarly, ANF and vitamin C attenuated E2-induced increases in the activities of the antioxidant enzymes SOD and GPx (Figure 5). The implication of these findings is that animals exposed to E2 undergo increases in oxidative stress and compensate for this stress by enhancing the activities of detoxifying enzymes, such as SOD and GPx. The observation that animals treated simultaneously with E2 and either vitamin C or ANF do not display compensatory increases in the activities of antioxidant enzymes and have significantly smaller increases in 8-isoPGF2α than do animals treated with E2 only suggests that the vitamin C and ANF suppress E2-induced oxidative stress and at least partially block the receptor-independent/genotoxic metabolite pathway of tumor induction by E2. Detection of increased SOD activity in breast tissue from rats treated with E2 for 240 days without corresponding increases in GPx or CAT activity suggests that H2O2 may accumulate in the breast tissue of E2-treated rats. Increases in SOD activity were not detected in breast tissue from animals treated with E2 + vitamin C or E2 + ANF, indicating that accumulation of H2O2 may not occur in these animals. Furthermore, no changes in 8-isoPGF2α levels or antioxidant enzyme activities were found in liver tissue from rats treated with E2 or E2 + vitamin C for any of the time points (data not shown), suggesting that oxidative changes may only occur in estrogen-target tissues.
The present study is the first report demonstrating the inhibition of estrogen-induced breast carcinogenesis by antioxidant vitamin C or the estrogen metabolic inhibitor ANF in an animal model of estrogen-induced breast cancer. The results of the present investigation are in agreement with previous reports that ANF and vitamin C reduce estrogen-induced renal carcinogenesis in hamsters (27,28,37–39). Presumably, the protective effects of vitamin C and ANF stem from their abilities to prevent oxidation of estrogens and reduce the generation of carcinogenic metabolites. The carcinogenicity of 4-OHE2, the principal genotoxic metabolite of E2, has been demonstrated in the hamster renal tumor model as well as in the CD-1 mouse uterine tumor model (32,33). In human breast tumor biopsies, 4-hydroxlation of E2 was elevated relative to measurements in normal human breast tissue (40,41). Surprisingly, a recent study showed that 4-OHE2 exposure was not sufficient to produce mammary tumors in female ACI rats (42). While this result seems paradoxical, it is possible that exposure to exogenous 4-OHE2 does not produce target organ concentrations great enough to induce cancer development, as 4-OHE2 is more water soluble than E2 and is cleared more quickly. The authors suggest that it may be critical for 4-OHE2 to be formed in or near the target tissue in order to reach sufficient levels and effectively initiate tumorigenesis (42).
Further illustrating the importance of oxidative stress in initiating breast carcinogenesis, a number of epidemiologic investigations have revealed that vitamin C in the form of fruits and vegetables may lower breast cancer risk. A meta-analysis of 12 case–control studies related to diet and breast cancer demonstrated that vitamin C intake via fruit and vegetable consumption reduces breast cancer risk (relative risk for highest versus lowest quintile of consumer, 0.69; P < 0.0001) (43). Unfortunately, in prospective studies, the purported inverse association between breast cancer risks and vitamin C intake did not materialize (44–48). However, upon examination of specific risk categories, two well-designed prospective trials detected significant decreases in breast cancer among women in the quintile of highest vitamin C-rich food consumption compared with women in the lowest quintile. Similarly, the Nurses’ Health Study has shown that premenopausal women with a family history of breast cancer who consume an average of 205 mg/day of vitamin C from food have a 63% lower risk of breast cancer than those who consumed an average of 70 mg/day (P = 0.002) (49). The Swedish Mammography Cohort, a population-based mammography screening program, reported a 39% lower risk of breast cancer among overweight (BMI >25 kg/m2) women who consumed an average of 110 mg/day of vitamin C, relative to overweight women who consumed just 31 mg/day (P = 0.004) (50).
Taken together, the results of the present study demonstrate that while inhibition of oxidative stress significantly reduces breast tumorigenesis, inhibition of estrogen metabolism, a prerequisite for the generation of reactive oxygen species and cellular damage by genotoxic metabolites, completely abrogates breast tumor development in this animal model. Thus, estrogen metabolism-mediated oxidative stress plays an important role in the process of E2-induced breast carcinogenesis. Use of vitamin C and ANF to minimize the contribution of the ER-independent pathway of breast cancer induction significantly reduced mammary tumor development in the ACI rat model of estrogen-dependent breast carcinogenesis. These data strongly suggest that E2 metabolism and oxidative stress are critical factors in the development of mammary tumors following E2 exposure, as even partially decreasing the action of this receptor-independent pathway significantly impairs tumor development.
Funding
National Institutes of Health (ES009089, CA 109551 to H.K.B.).
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- ACI
August Copenhagen Irish
- ANF
α-naphthoflavone
- CAT
catalase
- E2
17β-estradiol
- ER
estrogen receptor
- GPx
glutathione peroxidase
- 8-isoPGF2α
8-iso-prostane F2α
- 2-OHE2
2-hydroxyestradiol
- 4-OHE2
4-hydroxyestradiol
- SOD
superoxide dismutase
References
- 1.Henderson BE, et al. Hormonal carcinogenesis. Carcinogenesis. 2000;21:427–433. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]
- 2.International Association for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon: IARC; 1987. [Google Scholar]
- 3.International Association for Research on Cancer. Monographs on the Evaluation of Carcinogenic Risks to Humans: Hormonal Contraception and Postmenopausal Hormone Therapy. Lyon: IARC; 1999. [Google Scholar]
- 4.National Toxicology Program. Report on Carcinogens. National Toxicology Program, Research Triangle Park, NC: 2002. [Google Scholar]
- 5.Key T, et al. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J. Natl Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 6.Yager JD, et al. Estrogen carcinogenesis in breast cancer. N. Engl. J. Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 7.Bhat HK, et al. Critical role of oxidative stress in estrogen-induced carcinogenesis. Proc. Natl Acad. Sci. USA. 2003;100:3913–3918. doi: 10.1073/pnas.0437929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavalieri EL, et al. A unifying mechanism in the initiation of cancer and other diseases by catechol quinones. Ann. N. Y. Acad. Sci. 2004;1028:247–257. doi: 10.1196/annals.1322.029. [DOI] [PubMed] [Google Scholar]
- 9.Roy D, et al. Estrogen, DNA damage and mutations. Mutat. Res. 1999;424:107–115. doi: 10.1016/s0027-5107(99)00012-3. [DOI] [PubMed] [Google Scholar]
- 10.Yager JD. Endogenous estrogens as carcinogens through metabolic activation. J. Natl Cancer Inst. Monogr. 2000;27:67–73. doi: 10.1093/oxfordjournals.jncimonographs.a024245. [DOI] [PubMed] [Google Scholar]
- 11.Singh KP, et al. Somatic mutations in stilbene estrogen-induced Syrian hamster kidney tumors identified by DNA fingerprinting. J. Carcinog. 2004;3:4. doi: 10.1186/1477-3163-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavalieri E, et al. Estrogens as endogenous genotoxic agents—DNA adducts and mutations. J. Natl Cancer Inst. Monogr. 2000;27:75–93. doi: 10.1093/oxfordjournals.jncimonographs.a024247. [DOI] [PubMed] [Google Scholar]
- 13.Harvell DM, et al. Rat strain-specific actions of 17beta-estradiol in the mammary gland: correlation between estrogen-induced lobuloalveolar hyperplasia and susceptibility to estrogen-induced mammary cancers. Proc. Natl Acad. Sci. USA. 2000;97:2779–2784. doi: 10.1073/pnas.050569097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shull JD, et al. Ovary-intact, but not ovariectomized female ACI rats treated with 17beta-estradiol rapidly develop mammary carcinoma. Carcinogenesis. 1997;18:1595–1601. doi: 10.1093/carcin/18.8.1595. [DOI] [PubMed] [Google Scholar]
- 15.Arnerlov C, et al. Intratumoral variations in DNA ploidy and s-phase fraction in human breast cancer. Anal. Cell. Pathol. 2001;23:21–28. doi: 10.1155/2001/430674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li JJ, et al. Ploidy differences between hormone- and chemical carcinogen-induced rat mammary neoplasms: comparison to invasive human ductal breast cancer. Mol. Carcinog. 2002;33:56–65. doi: 10.1002/mc.10022. [DOI] [PubMed] [Google Scholar]
- 17.Li JJ, et al. Estrogen mediates Aurora-A overexpression, centrosome amplification, chromosomal instability, and breast cancer in female ACI rats. Proc. Natl Acad. Sci. USA. 2004;101:18123–18128. doi: 10.1073/pnas.0408273101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weroha SJ, et al. Overexpression of cyclins D1 and D3 during estrogen-induced breast oncogenesis in female ACI rats. Carcinogenesis. 2006;27:491–498. doi: 10.1093/carcin/bgi278. [DOI] [PubMed] [Google Scholar]
- 19.Mense S, et al. Estrogen-induced breast cancer: alterations in breast morphology and oxidative stress as a function of estrogen exposure. Toxicol. Appl. Pharmacol. 2008;232:78–85. doi: 10.1016/j.taap.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han X, et al. DNA single-strand breaks in kidneys of Syrian hamsters treated with steroidal estrogens: hormone-induced free radical damage preceding renal malignancy. Carcinogenesis. 1994;15:997–1000. doi: 10.1093/carcin/15.5.997. [DOI] [PubMed] [Google Scholar]
- 21.Wang MY, et al. Induction by estrogens of lipid peroxidation and lipid peroxide-derived malonaldehyde-DNA adducts in male Syrian hamsters: role of lipid peroxidation in estrogen-induced kidney carcinogenesis. Carcinogenesis. 1995;16:1941–1945. doi: 10.1093/carcin/16.8.1941. [DOI] [PubMed] [Google Scholar]
- 22.Roy D, et al. Comparison of assays for catechol estrogen synthase activity: product isolation vs radioenzymatic catechol-O-methyltransferase-coupled procedures. J. Steroid Biochem. 1989;33:243–249. doi: 10.1016/0022-4731(89)90300-2. [DOI] [PubMed] [Google Scholar]
- 23.Zhu BT, et al. Conversion of estrone to 2- and 4-hydroxyestrone by hamster kidney and liver microsomes: implications for the mechanism of estrogen-induced carcinogenesis. Endocrinology. 1994;135:1772–1779. doi: 10.1210/endo.135.5.7956900. [DOI] [PubMed] [Google Scholar]
- 24.Dignam JD, et al. NADPH-cytochrome P-450 reductase from rat liver: purification by affinity chromatography and characterization. Biochemistry. 1977;16:1116–1123. doi: 10.1021/bi00625a014. [DOI] [PubMed] [Google Scholar]
- 25.Shimada T, et al. Selectivity of polycyclic inhibitors for human cytochrome P450s 1A1, 1A2, and 1B1. Chem. Res. Toxicol. 1998;11:1048–1056. doi: 10.1021/tx980090+. [DOI] [PubMed] [Google Scholar]
- 26.Guengerich FP, et al. Cytochrome P450 1B1: a target for inhibition in anticarcinogenesis strategies. Mutat. Res. 2003;523–524:173–182. doi: 10.1016/s0027-5107(02)00333-0. [DOI] [PubMed] [Google Scholar]
- 27.Liehr JG, et al. DNA adduct formation in liver and kidney of male Syrian hamsters treated with estrogen and/or alpha-naphthoflavone. Carcinogenesis. 1991;12:385–389. doi: 10.1093/carcin/12.3.385. [DOI] [PubMed] [Google Scholar]
- 28.Li JJ, et al. High incidence of hepatocellular carcinomas after synthetic estrogen administration in Syrian golden hamsters fed alpha-naphthoflavone: a new tumor model. J. Natl Cancer Inst. 1984;73:543–547. doi: 10.1093/jnci/73.2.543. [DOI] [PubMed] [Google Scholar]
- 29.Milne GL, et al. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat. Protoc. 2007;2:221–226. doi: 10.1038/nprot.2006.375. [DOI] [PubMed] [Google Scholar]
- 30.Cavalieri E, et al. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim. Biophys. Acta. 2006;1766:63–78. doi: 10.1016/j.bbcan.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Castagnetta LA, et al. Tissue content of hydroxyestrogens in relation to survival of breast cancer patients. Clin. Cancer Res. 2002;8:3146–3155. [PubMed] [Google Scholar]
- 32.Li JJ, et al. Estrogen carcinogenesis in Syrian hamster tissues: role of metabolism. Fed. Proc. 1987;46:1858–1863. [PubMed] [Google Scholar]
- 33.Newbold RR, et al. Induction of uterine adenocarcinoma in CD-1 mice by catechol estrogens. Cancer Res. 2000;60:235–237. [PubMed] [Google Scholar]
- 34.Guengerich FP. Cytochromes P450, drugs, and diseases. Mol. Interv. 2003;3:194–204. doi: 10.1124/mi.3.4.194. [DOI] [PubMed] [Google Scholar]
- 35.Li SA, et al. Estrogen 2- and 4-hydroxylase activity, catechol estrogen formation, and implications for estrogen carcinogenesis in the hamster kidney. Cancer Res. 1985;45:181–185. [PubMed] [Google Scholar]
- 36.Milne GL, et al. Human biochemistry of the isoprostane pathway. J. Biol. Chem. 2008;283:15533–15537. doi: 10.1074/jbc.R700047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liehr JG. Dual role of oestrogens as hormones and pro-carcinogens: tumour initiation by metabolic activation of oestrogens. Eur. J. Cancer Prev. 1997;6:3–10. doi: 10.1097/00008469-199702000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Liehr JG. 4-hydroxylation of oestrogens as a marker for mammary tumours. Biochem. Soc. Trans. 1999;27:318–323. doi: 10.1042/bst0270318. [DOI] [PubMed] [Google Scholar]
- 39.Li SA, et al. Changes in estrogen receptor levels during DES-induced hepatocarcinogenesis in the Syrian hamster fed alpha-naphthoflavone. J Steroid Biochem. 1981;15:387–392. doi: 10.1016/0022-4731(81)90302-2. [DOI] [PubMed] [Google Scholar]
- 40.Liehr JG, et al. 4-Hydroxylation of estrogens as marker of human mammary tumors. Proc. Natl Acad. Sci. USA. 1996;93:3294–3296. doi: 10.1073/pnas.93.8.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rogan EG, et al. Relative imbalances in estrogen metabolism and conjugation in breast tissue of women with carcinoma: potential biomarkers of susceptibility to cancer. Carcinogenesis. 2003;24:697–702. doi: 10.1093/carcin/bgg004. [DOI] [PubMed] [Google Scholar]
- 42.Turan VK, et al. The effects of steroidal estrogens in ACI rat mammary carcinogenesis: 17beta-estradiol, 2-hydroxyestradiol, 4-hydroxyestradiol, 16alpha-hydroxyestradiol, and 4-hydroxyestrone. J. Endocrinol. 2004;183:91–99. doi: 10.1677/joe.1.05802. [DOI] [PubMed] [Google Scholar]
- 43.Howe GR, et al. Dietary factors and risk of breast cancer: combined analysis of 12 case-control studies. J. Natl Cancer Inst. 1990;82:561–569. doi: 10.1093/jnci/82.7.561. [DOI] [PubMed] [Google Scholar]
- 44.Garland M, et al. Antioxidant micronutrients and breast cancer. J. Am. Coll. Nutr. 1993;12:400–411. doi: 10.1080/07315724.1993.10718329. [DOI] [PubMed] [Google Scholar]
- 45.Hunter DJ, et al. A prospective study of the intake of vitamins C, E, and A and the risk of breast cancer. N. Engl. J. Med. 1993;329:234–240. doi: 10.1056/NEJM199307223290403. [DOI] [PubMed] [Google Scholar]
- 46.Kushi LH, et al. Intake of vitamins A, C, and E and postmenopausal breast cancer. The Iowa Women's Health Study. Am. J. Epidemiol. 1996;144:165–174. doi: 10.1093/oxfordjournals.aje.a008904. [DOI] [PubMed] [Google Scholar]
- 47.Rohan TE, et al. Dietary fiber, vitamins A, C, and E, and risk of breast cancer: a cohort study. Cancer Causes Control. 1993;4:29–37. doi: 10.1007/BF00051711. [DOI] [PubMed] [Google Scholar]
- 48.Verhoeven DT, et al. Vitamins C and E, retinol, beta-carotene and dietary fibre in relation to breast cancer risk: a prospective cohort study. Br. J. Cancer. 1997;75:149–155. doi: 10.1038/bjc.1997.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang S, et al. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J. Natl Cancer Inst. 1999;91:547–556. doi: 10.1093/jnci/91.6.547. [DOI] [PubMed] [Google Scholar]
- 50.Michels KB, et al. Dietary antioxidant vitamins, retinol, and breast cancer incidence in a cohort of Swedish women. Int. J. Cancer. 2001;91:563–567. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1079>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]