Abstract
As part of our effort to understand the mechanism underlying α-tocopheryl succinate [vitamin E succinate (VES)]-mediated antitumor effects, we investigated the signaling pathway by which VES suppresses androgen receptor (AR) expression in prostate cancer cells. VES and, to a greater extent, its truncated derivative TS-1 mediated transcriptional repression of AR in prostate cancer cells but not in normal prostate epithelial cells; a finding that underscores the differential susceptibility of normal versus malignant cells to the antiproliferative effect of these agents. This AR repression was attributable to the ability of VES and TS-1 to facilitate the proteasomal degradation of the transcription factor Sp1. This mechanistic link was corroborated by the finding that proteasome inhibitors or ectopic expression of Sp1 protected cells against drug-induced AR ablation. Furthermore, evidence suggests that the destabilization of Sp1 by VES and TS-1 resulted from the inactivation of Jun N-terminal kinases (JNKs) as a consequence of increased phosphatase activity of protein phosphatase 2A (PP2A). Stable transfection of LNCaP cells with the dominant-negative JNK1 plasmid mimicked drug-induced Sp1 repression, whereas constitutive activation of JNK kinase activity or inhibition of PP2A activity by okadaic acid protected Sp1 from VES- and TS-1-induced degradation. From a mechanistic perspective, the ability of VES and TS-1 to activate PP2A activity underscores their broad spectrum of effects on multiple signaling mechanisms, including those mediated by Akt, mitogen-activated protein kinases, nuclear factor kappaB, Sp1 and AR. This pleiotropic effect in conjunction with low toxicity suggests the translational potential for developing TS-1 into potent PP2A-activating agents for cancer therapy.
Introduction
The translational potential of α-tocopheryl succinate [also known as vitamin E succinate (VES)] in cancer therapy has been the focus of many recent investigations in light of its efficacy in suppressing tumor cell proliferation without incurring toxicity to normal cells (reviewed in refs 1,2). Substantial evidence indicates that VES exhibits a unique ability to target multiple signaling pathways associated with carcinogenesis, tumor progression and metastasis (3–23), including those mediated by nuclear factor kappaB (17,24), protein kinase Cα (25), sphingolipids (13,23), Bcl-2/Bcl-xL (16), androgen receptor (AR) (10), vascular endothelial growth factor (7) and insulin-like growth factor-binding protein-3 (22). Although some of these signaling targets might be cancer type specific, this broad spectrum of action in conjunction of low toxicity underlies the therapeutic value of developing VES into useful agents for cancer treatment or prevention.
Considering the pivotal role of dysregulated AR signaling in prostate carcinogenesis and tumor progression, the effect of VES on suppressing AR expression warrants attention (10). Evidence suggests that targeting AR expression represents a therapeutically relevant strategy to improve the treatment of androgen-independent prostate cancer and ultimately to increase the survival of prostate cancer patients. Thus, in this study, we investigated the mechanism by which VES and its truncated derivative, TS-1 (16), suppress AR expression in prostate cancer cells. We obtained several lines of evidence that VES and, to a greater extent, TS-1 mediated the transcriptional repression of AR by facilitating the proteasomal degradation of the transcription factor Sp1, which, in turn, was attributable to the effect of these agents on inactivating Jun N-terminal kinase (JNK) by increasing protein phosphatase 2A (PP2A) activity. The ability of VES and TS-1 to activate PP2A provides a mechanistic basis to account for their broad spectrum of pharmacological activities against multiple molecular targets relevant to prostate cancer therapy.
Materials and methods
Reagents, antibodies and plasmids
VES and the proteasome inhibitors MG132 and epoxomicin were purchased from EMD Chemicals (San Diego, CA) and Sigma-Aldrich (St Louis, MO), respectively. TS-1 {succinic acid mono-[2-(4,8-dimethylnonyl)-2,5,7,8-tetramethylchroman-6-yl] ester} is a truncated derivative of VES with an improved antiproliferative potency (16). Stock solutions of these agents were made in dimethyl sulfoxide (DMSO) and added to medium with a final DMSO concentration of 0.1%. Antibodies against various proteins were obtained from the following sources. Mouse monoclonal antibodies: AR and prostate-specific antigen, Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit antibodies: Sp1, Santa Cruz Biotechnology; poly (adenosine diphosphate-ribose) polymerase, p-Ser473-Akt, p-Thr308-Akt, Akt, p-extracellular signal-regulated kinase (ERK), ERK, p-JNK, JNK, p-p38 and p38, Cell Signaling Technology (Beverly, MA). The AR promoter-luciferase reporter vector (hAR-Luc) was constructed as described previously (26). The dominant-negative JNK1 plasmid pCDNA3-Flag-JNK1a1 was obtained from Addgene (Cambridge, MA). Hemagglutinin (HA)-ubiquitin plasmid and the constitutively active JNK plasmid Flag-MKK7-JNK1 encoding MKK7-JNK1 fusion protein with constitutive JNK activity (27) were kind gifts from Dr Hung-Wen Chen (Institute of Biological Sciences, Academia Sinica, Taipei, Taiwan) and Dr Roger Davis (University of Massachusetts Medical School, Worcester, Massachusetts), respectively. The pCMVSp1 plasmid was purchased from OriGene Technologies (Rockville, MD).
Cell culture
LNCaP androgen-dependent (p53+/+) and PC-3 androgen-non-responsive (p53−/−) prostate cancer cells were purchased from the American Type Culture Collection (Manassas, VA) and cultured in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum (FBS). Normal prostate epithelial cells (PrECs) were obtained from Lonza (Allendale, NJ) and maintained in prostate epithelial growth media supplemented with a growth factor kit suggested by the vendor. All cell types were cultured at 37°C in a humidified incubator containing 5% CO2. Cells in log phase growth were harvested by trypsinization for use in the 3-(4,5-dimethylthiazole-2-yl)-2,5-biphenyl tetrazolium bromide viability assay. LNCaP cells were plated in poly-D-lysine-coated culture flasks in order to assist cell adherence to the surface. Prior to drug treatment, cells were plated in a density of 12 000 cells/cm2 surface area in the respective culture medium for 24–48 h, followed by individual test agents in 2.5% FBS-supplemented RPMI medium.
Immunoblotting
Cells cultured in T25 flasks were collected by scraping, and cell pellets were washed once with phosphate-buffered saline. Cells were lysed in a lysis buffer consisting of 1% sodium dodecyl sulfate (SDS), 10 mM ethylenediaminetetraacetic acid (EDTA) and 50 mM Tris–HCl, pH 8.1, in the presence of a commercial protease inhibitor cocktail from Sigma-Aldrich (2 mM 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (AEBSF), 1 mM EDTA, 130 μM bestatin, 14 μM E-64, 1 μM leupeptin and 0.3 μM aprotinin). Following a 10 s sonication using 20% output in a Virsonic 300 sonicator (Virtis, Gardiner, NY) to disrupt cellular organelles and genomic DNA, cell lysates were centrifuged at 15 200g for 15 min. One microliter of the suspension was used for protein determination using a colorimetric bicinchoninic acid assay (Pierce, Rockford, IL), and to the remaining solution was added an equivalent volume of 2× SDS–polyacrylamide gel electrophoresis sample loading buffer (62.5 mM Tris–HCl, pH 6.8, 4% SDS, 5% β-mercaptoethanol, 20% glycerol and 0.1% bromophenol blue) and boiled for 5 min. Equal amounts of proteins were resolved in 8% SDS–polyacrylamide gels and transferred to nitrocellulose membranes using a semidry transfer cell. The transblotted membrane was washed twice with Tris-buffered saline containing 0.1% Tween 20 (TBST). After blocking with TBST containing 5% non-fat milk for 40 min, the membrane was incubated with the appropriate primary antibody in TBST–1% non-fat milk at 4°C overnight. All primary antibodies were diluted 1:1000 in 1% non-fat milk-containing TBST. After treatment with the primary antibody, the membrane was washed three times with TBST for a total of 15 min, followed by incubation with goat anti-rabbit or anti-mouse immunoglobulin G–horseradish peroxidase conjugates (diluted 1:2000) for 1 h at room temperature and four washes with TBST for a total of 1 h. The immunoblots were visualized by enhanced chemiluminescence.
RNA isolation and reverse transcription–polymerase chain reaction
LNCaP cells were subject to total RNA isolation by using a Trizol reagent (Invitrogen Corporation, Carlsbad, CA). RNA concentrations were determined by measuring absorption at 260 nm in a spectrophotometer. Aliquots of 2 μg of total RNA from each sample were reverse transcribed to complementary DNAs using the iScript complementary DNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions. Polymerase chain reaction (PCR) primers used were as follows: AR, 5′-ACACATTGAAGGCTATGAATGTC-3′ and 5′-TCACTGGGTGTGGAAATAGATG-GG3′; Sp1, 5′-GGCGAGAGGCCATTTATGTGT-3′ and 5′-TGCATGACGTTGATGCCACT-3′; vascular endothelial growth factor (28), 5′-CCATGAACTTTCTGCTGTCTT-3′ and 5′-ATCGCATCAGGGGCACACAG-3′; Mdm2 (29), 5′-TCAGGATTCAGTTTCAGATCAG-3′ and 5′-CATTTCCAATAGTCAGCTAAGG-3′; DNA methyltransferase 1 exon 1–6 (30), 5′-ATCGCCTCTCTCCGTTTGGTA-3′ and 5′-TGGACTCATCCGATTTGGCT-3′. PCR products were resolved electrophoretically in 1.2% agarose gels and visualized by ethidium bromide staining.
Transfection and luciferase assay
Cells were transfected with 5 μg of the AR-linked luciferase reporter (hAR-Luc) plasmid in an Amaxa Nucleofector using a cell line-specific nucleofector kit according to the manufacturer's protocol (Amaxa, Gaithersburg, MD) and then seeded in T25 flasks at 3 × 105 cells per flask for 48 h. The transfection efficiency was determined by transfecting cells with 3 μg of pmaxGFP plasmid followed by fluorescence microscopy to detect green fluorescent protein expression. For each transfection, herpes simplex virus thymidine kinase promoter-driven Renilla reniformis luciferase was used as an internal control for normalization. For the luciferase reporter gene assay, after transfection, cells were cultured in 24-well plates in 10% FBS-supplemented RPMI 1640 for 48 h, subjected to different treatments in 2.5% FBS-supplemented medium for the indicated times, collected and lysed with passive lysis buffer (Promega Corporation, Madison, WI). Aliquots of lysates (50 μl) were mixed with 75 μl of luciferase substrate (Promega) in 96-well plates, and luciferase activities were monitored in a MicroLumaPlus LB96V luminometer (Berthold Technologies, Oak Ridge, TN) with the WinGlow software package. All transfection experiments were carried out in six replicates.
Immunoprecipitation
LNCaP cells were cotransfected with 5 μg each of Flag-Sp1 and HA-ubiquitin plasmids in an Amaxa Nucleofector using a LNCaP-specific nucleofector kit. These transiently transfected cells were seeded in 6-well plates at 2 × 105 per well. After 48 h incubation, cells were exposed to VES or TS-1 at the indicated concentration for 48 h and lysed by a radioimmunoprecipitation assay lysis buffer (Santa Cruz Biotechnology) in the presence of a freshly prepared cocktail of phosphatase and protease inhibitors (2 mM AEBSF, 1 mM EDTA, 130 μM bestatin, 14 μM E-64, 1 μM leupeptin and 0.3 μM aprotinin, 2 mM imidazole, 1 mM sodium fluoride, 1.15 mM sodium molybdate, 1 mM sodium orthovanadate and 4 mM sodium tartrate dihydrate). After centrifugation at 13 000g for 15 min, the supernatants were collected, preincubated with protein A/G agarose (Santa Cruz Biotechnology) for 15 min and centrifuged at 1000g for 5 min. Supernatant (20 μl) was stored at 4°C to be used as input, whereas the remaining supernatant was exposed to 4 μg of anti-Sp1 antibodies at 4°C for 12 h, followed by protein A/G agarose beads at 4°C for another 2 h. After brief centrifugation, immunoprecipitates were collected, washed with the aforementioned lysis buffer four times, suspended in 2× SDS sample buffer and subjected to western blot analysis with antibodies against HA and Flag.
Chromatin immunoprecipitation
After drug treatment, LNCaP cells (2 × 107) in 50 ml of phosphate-buffered saline were cross-linked with 1.35 ml of 37% formaldehyde (final concentration 1%) for 15 min at room temperature. Glycine solution (1 M) was added to a final concentration of 125 mM to stop the cross-linking reaction. Cells were harvested and washed twice with 5 ml of phosphate-buffered saline, and the cell pellets were lysed in a chromatin immunoprecipitation (ChIP) lysis buffer containing (50 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid–KOH at pH 7.5, 140 mM NaC1, 1% Triton X-100, 0.1% sodium deoxycholate, 2 mM AEBSF, 1 mM EDTA, 130 μM bestatin, 14 μM E-64, 1 μM leupeptin and 0.3 μM aprotinin). The suspension was sonicated at 20% output in a Virsonic 300 sonicator with six sets of 10 s pulses (resulting in an average fragment size of 0.8–0.2 kb) and centrifuged for 10 min at 15 000g at 4°C. One microliter aliquots of the transparent supernatants were taken for determining protein concentrations by bicinchoninic acid assays. Immunoprecipitation was carried out as described above. Aliquots of 1 mg proteins were used for immunoprecipitation using 4 μg of anti-Sp1 antibody followed by protein A/G agarose beads. The immunoprecipitates were successively washed twice with 1 ml of ChIP lysis buffer, twice with 1 ml of a high salt ChIP lysis buffer (50 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid–KOH at pH 7.5, 500 mM NaC1, 1% Triton X-100, 0.1% sodium deoxycholate, 2 mM AEBSF, 1 mM EDTA 130 μM bestatin, 14 μM E-64, 1 μM leupeptin and 0.3 μM aprotinin), twice with 1 ml of ChIP wash buffer (10 mM Tris pH 8.0; 250 mM LiCl; 0.5% NP-40; 0.5% sodium deoxycholate; 1 mM EDTA) and twice with 1 ml of TE buffer (10 mM Tris, pH 7.5, 1 mM EDTA). The immunocomplex was eluted by addition of 75 μl of elution buffer (50 mM Tris, pH 8.0, 1% SDS, 10 mM EDTA) and was incubated at 65°C for 10 min. The resulting supernatant was collected after brief centrifugation, and the pellets were eluted again with another 75 μl of elution buffer. The combined supernatant was incubated at 65°C overnight in the presence of NaCl at a final concentration of 200 mM. Ten microgram aliquots (1%) of the original total proteins were added to 150 μl of elution buffer and were incubated at 65°C overnight in the presence of NaCl at a final concentration of 200 mM as the input control. Finally, samples were processed for DNA purification using a PCR purification kit (Qiagen, Valencia, CA), and the recovered DNA was eluted with 50 μl of 10 mM Tris–HCl, pH 8.5. One microliter aliquots were used for PCR with primers spanning two adjacent Sp1-binding sites on the AR promoter, located at 429–442 of 5′-UTR of the AR gene (31): 5′-AGCTGCTAAAGACTCGGAGG-3′ and 5′-GGAGTTACCTCTCTGCAAAC-3′. E2TAK taq polymerase (Takara Bio, Inc., Shiga, Japan) and the corresponding buffer system were used for amplification of PCR products.
PP2A activity assay
PP2A activity in drug-treated cells was determined by using a PP2A Immunoprecipitation Phosphatase Assay Kit (Millipore, Billerica, MA) according to the manufacturer's instructions. LNCaP cells were exposed to DMSO, VES or TS-1 at the indicated concentrations in 2.5% FBS-supplemented medium for 12 h and subjected to cell lysis in a phosphate-free lysis buffer containing 20 mM imidazole-HCl, pH 7.0, 2 mM EDTA, 2 mM ethyleneglycol-bis(aminoethylether)-tetraacetic acid, 2 mM AEBSF, 1 mM EDTA, 130 μM bestatin, 14 μM E-64, 1 μM leupeptin and 0.3 μM aprotinin. The suspension was sonicated (Virtis) at 20% output for 10 s, followed by centrifugation at 2000g for 5 min. One microliter aliquots of the supernatants were taken for protein determination by bicinchoninic acid assays, and the remaining supernatants were used for phosphatase activity assays. Aliquots of cell lysates containing 400 μg of proteins were combined with 4 μg of anti-PP2Ac antibody (Millipore, Billerica, MA), to which was added PP2A assay buffer (20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid pH 7.0, 100 mM NaCl) to a final volume of 500 μl followed by 40 μl of Protein A–agarose. Mixtures were incubated at 4°C for 2 h and briefly centrifuged. The immunocomplexes were washed and used for the phosphatase activity assay. The amounts of PP2A in the immunocomplexes were determined semiquantitatively by western blotting.
Results
Differential effect of VES and TS-1 on suppressing AR expression in LNCaP cells versus normal PrECs
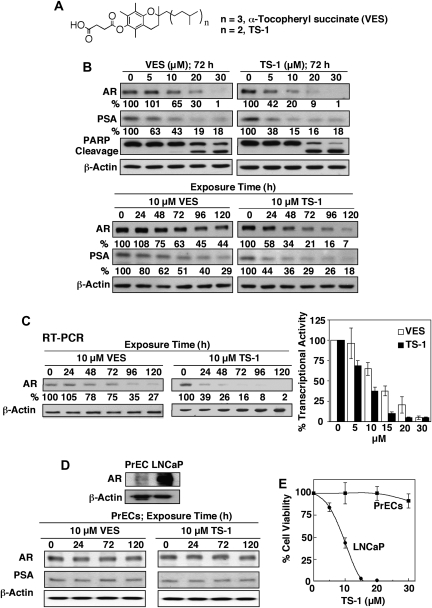
In the course of our investigation of the inhibitory effect of VES on Bcl-xL/Bcl-2 function, we developed a structurally optimized derivative, TS-1 (Figure 1A), of which the phytyl side chain was shortened by one isopranyl unit relative to that of VES (16). In this study, western blot analysis indicates that this side chain truncation also led to higher potency in suppressing the expression of AR and its target gene product prostate-specific antigen in LNCaP cells (Figure 1B). For example, TS-1 at 5 μM effectively reduced the expression of these biomarkers by 50% after 72 h of incubation, whereas VES required at least 10 μM to achieve the same extent of suppression (Figure 1B). The abilities of VES and TS-1 to repress AR correlated with the respective potencies in inducing apoptosis, as manifest by the extents of poly (adenosine diphosphate-ribose) polymerase cleavage (Figure 1B). Furthermore, two lines of evidence reveal that this decrease in AR protein expression was attributed to the transcriptional inhibition of AR gene expression. First, reverse transcription–PCR analysis of the messenger RNA transcript of the AR gene in LNCaP cells showed a time-dependent reduction paralleling that of AR protein in response to 10 μM VES or TS-1 (Figure 1C, left panel). Second, the AR promoter-luciferase reporter assay confirmed that these agents were able to inhibit AR gene transcription in a dose-dependent manner after 72 h of exposure (Figure 1C, right panel). Together, these data indicate that VES and TS-1 mediated the inhibition of AR messenger RNA expression by targeting the transcriptional regulation of the AR promoter.
Fig. 1.
Effect of VES and TS-1 on the transcriptional regulation of AR expression in LNCaP cells versus PrECs. (A) Structures of VES and TS-1. (B) Western blot analysis of the dose- (upper panel) and time-dependent (lower panel) effects of VES and TS-1 on the expression of AR and its target gene product prostate-specific antigen (PSA) in LNCaP cells in 2.5% FBS-supplemented medium. Values in percentage denote the relative intensity of protein bands of drug-treated samples to that of the respective DMSO vehicle-treated control after normalization to the respective internal reference β-actin. Each value represents the average of two independent experiments. (C) Left panel, reverse transcription (RT)–PCR analysis of the time-dependent suppressive effect of 10 μM VES or TS-1 on AR messenger RNA levels in LNCaP cells after 72 h incubation in 2.5% FBS-supplemented medium. Values in percentage denote the relative intensity of messenger RNA bands of drug-treated samples to that of the respective DMSO vehicle-treated control after normalization to the respective internal reference β-actin. Each value represents the average of two independent experiments. Right panel, dose-dependent inhibitory effect of VES and TS-1 on luciferase reporter activity in hAR-Luc-transfected LNCaP cells after 72 h incubation in 2.5% FBS-supplemented medium. Columns indicate mean; barsindicate SD (n = 6). (D) Upper panel, differential expression of AR in PrECs versus LNCaP cells. Lower panel, western blot analysis of the time-dependent effect of 10 μM VES or TS-1 on the expression of AR and PSA in PrECs in 2.5% FBS-supplemented medium. Cells were exposed to 10 μM VES or TS-1 for the indicated time intervals, and the expression levels of AR and PSA were analyzed by western blot analysis. (E) Selective dose-dependent suppression of the viability of PrECs versus LNCaP cells by TS-1 after 72 h incubation in 2.5% FBS-supplemented prostate epithelial growth and RPMI 1640 media, respectively, as determined by 3-(4,5-dimethylthiazole-2-yl)-2,5-biphenyl tetrazolium bromide assays. Each data point represents mean ± SD (n = 6).
As compared with LNCaP cells, normal PrECs, which exhibited low abundance of AR, were resistant to the repressive effect of drug on AR expression (Figure 1D). This selectivity might, in part, account for the differential sensitivity of normal versus malignant cells to the ability of TS-1 to suppress cell viability (Figure 1E).
VES and TS-1 target Sp1 to downregulate AR gene transcription
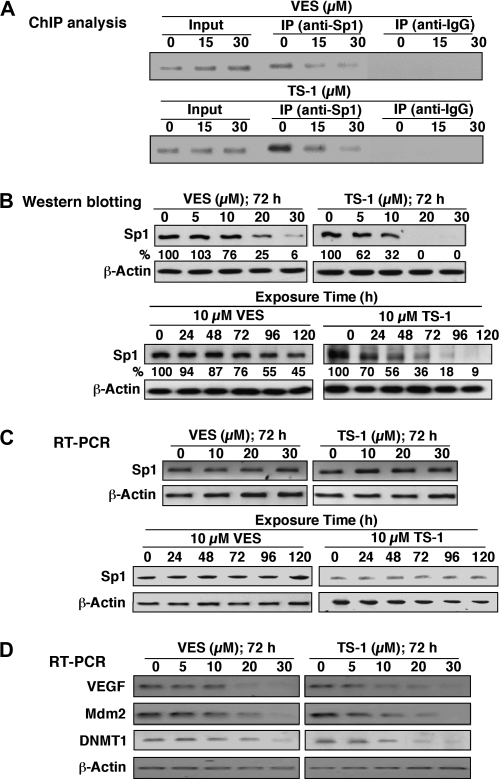
In our previous study of the effect of thiazolidinediones on modulating AR expression in LNCaP cells, we demonstrated a mechanistic link between drug-mediated AR ablation and the downregulation of Sp-1 expression (26). To investigate this putative link in VES- and TS-1-induced AR repression, ChIP assays were performed to detect the binding of Sp1 to AR promoter in LNCaP cells treated with various doses of VES or TS-1 for 72 h. After formaldehyde treatment of cells, antibodies against Sp1 or immunoglobulin G were used to immunoprecipitate Sp1-bound genomic DNA fragments, followed by PCR analysis with a pair of primers spanning the AR promoter. As shown in Figure 2A, VES and TS-1 diminished the Sp1 binding to AR promoter in a dose-dependent manner. Based on western blot analysis, this reduced binding was attributed to decreases in Sp1 expression in drug-treated cells (Figure 2B). Moreover, this repression occurred at the posttranslational level since Sp1 messenger RNA expression remained unaltered even after treatment of LNCaP cells with high doses of VES and TS-1 (Figure 2C). The ability of VES and TS-1 to reduce Sp1 levels was confirmed by the dose-dependent transcriptional repression of a series of Sp1 downstream target genes, including those encoding vascular endothelial growth factor, the negative p53 regulator Mdm2 and DNA methyltransferase 1 (Figure 2D), all of which play important roles in prostate tumorigenesis and cancer progression (32–34).
Fig. 2.
Suppression of Sp1 expression by VES and TS-1 at the posttranslational level in LNCaP cells. (A) ChIP analysis of the inhibitory effect of VES and TS-1 on Sp1 recruitment to the AR promoter in LNCaP cells treated for 72 h. (B) Western blot analysis of the dose- (upper panel) and time-dependent (lower panel) inhibitory effects of VES and TS-1 on Sp1 expression in LNCaP cells in 2.5% FBS-supplemented medium. Values in percentage denote the relative intensity of protein bands of drug-treated samples to that of the respective DMSO vehicle-treated control after normalization to the respective internal reference β-actin. Each value represents the average of two independent experiments. (C) Reverse transcription (RT)–PCR analysis of the dose- (upper panel) and time-dependent (lower panel) suppressive effects of VES and TS-1 on Sp1 messenger RNA levels in LNCaP cells in 2.5% FBS-supplemented medium. (D) RT–PCR analysis of the dose-dependent repressive effects of VES and TS-1 on the transcription of Sp1 downstream target genes, including vascular endothelial growth factor (VEGF), Mdm2 and DNA methyltransferase 1 (DNMT1) in LNCaP cells after 72 h incubation in 2.5% FBS-supplemented medium.
Proteasomal degradation of Sp1
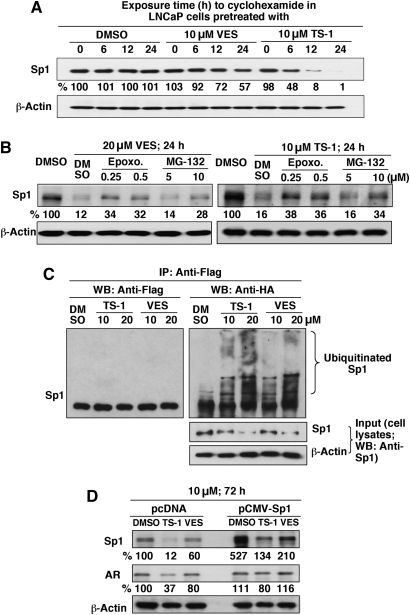
The ability of VES and TS-1 to modulate the stability of Sp1 protein was confirmed by its shortened half-life in drug-treated LNCaP cells relative to the DMSO control, which was more prominent after TS-1 treatment (Figure 3A). Moreover, pharmacological inhibition of proteasomal degradation by epoxomicin and MG-132 protected Sp1 from VES- and TS-1-facilitated ablation (Figure 3B). Because proteasome-facilitated proteolysis is preceded by ubiquitination, we examined the formation of ubiquinated Sp1 in response to different doses of VES and TS-1 in LNCaP cells expressing ectopic HA-ubiquitin and Flag-Sp1. After drug treatment for 48 h, cell lysates were immunoblotted with Sp1 antibodies (input) or immunoprecipitated by anti-Flag antibody–agarose conjugates. Equivalent amounts of the immunoprecipitated proteins were subjected to immunoblotting with Flag or HA antibodies (Figure 3C). As shown, both TS-1 and VES increased the extent of Sp1 ubiquitination as indicated by a complex ladder of ubiquitinated Sp1 bands (immunoprecipitation, anti-Flag; western blot, anti-HA; Figure 3C, right).
Fig. 3.
VES and TS-1 facilitate the ubiquitin-dependent proteasomal degradation of Sp1. (A) Differential effect of VES and TS-1 on shortening the half-life of Sp1 in LNCaP cells. LNCaP cells were pretreated with the VES or TS-1 (each at 10 μM) for 24 h, followed by exposure to cyclohexamide (50 μg/ml) for the indicated durations. Levels of Sp1 protein were determined by western blotting. Values in percentage denote the relative intensity of protein bands of drug-treated samples to that of the respective DMSO vehicle-treated control after normalization to the respective internal reference β-actin. Each value represents the average of two independent experiments. This definition also applies to those in panels (B) and (D). (B) Dose-dependent effect of the proteasome inhibitors MG132 and epoxomicin on rescuing Sp1 from degradation after treatment of LNCaP cells with 20 μM VES or 10 μM TS-1 for 24 h. (C) VES and TS-1 facilitate Sp1 ubiquitination. LNCaP cells cotransfected with plasmids encoding HA-ubiquitin and Sp1-Flag were treated with VES or TS-1 at the indicated concentrations in 2.5% FBS-supplemented medium for 48 h. Equal amounts of cell lysates were probed with anti-Sp1 antibodies (input) or immunoprecipitated with anti-Flag affinity gels followed by immunoblotting with anti-HA and anti-Flag antibodies. (D) ectopic expression of Sp1 attenuates the suppressive effect of VES or TS-1 (10 μM) on AR expression. LNCaP cells were transiently transfected with the pcDNA3.1 or pCMVSp1 plasmid and exposed to individual agents in 2.5% FBS-supplemented RPMI 1640 for 72 h.
Ectopic Sp1 expression confers resistance to the effect of VES and TS-1 on AR transcriptional repression
To validate the link between the drug-induced AR repression and Sp1 downregulation, we assessed the ability of ectopic Sp1 expression to protect AR from VES- and TS-1-induced repression. Transient transfection of LNCaP cells with the pCMVSp1 plasmid resulted in a higher expression level of Sp1 than that of the pcDNA-transfected cells (Figure 3D). Although treatment of pCMVSp1-transfected cells with 10 μM TS-1 or VES caused differential reduction in Sp1 expression, the respective Sp1 levels were still higher than that of untreated pcDNA-transfected cells. As a consequence, the expression level of AR remained virtually unchanged after drug treatment, indicating the protective effect of ectopic Sp1.
VES and TS-1 mediate Sp1 degradation through a JNK-dependent pathway
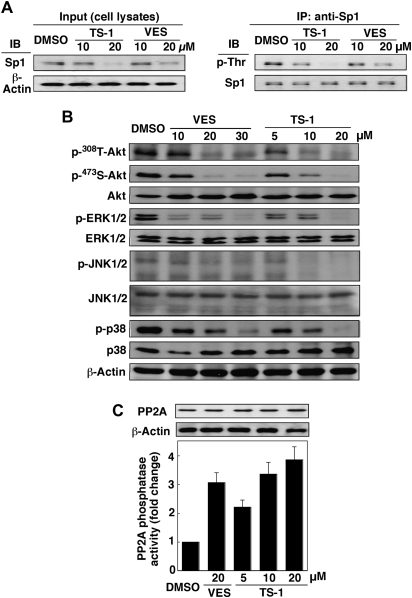
Despite recent advances in understanding Sp1′s biological functions, the mechanism governing the turnover of this transcription factor remains unclear (35,36). Our data indicate that VES- and TS-1-facilitated Sp1 degradation was accompanied by concomitant reduction in its phosphorylation level (Figure 4A). In light of a recent report that JNKs were involved in maintaining the stability of Sp1 (35), this finding suggests a putative role of JNK in mediating the drug-induced Sp1 proteolysis. To corroborate this premise, we examined the effect of VES and TS-1 on the phosphorylation status of JNKs and other kinases including Akt, ERK and p38 in LNCaP cells. As shown, treatment of LNCaP cells with VES and, to a greater extent, TS-1 led to a dose-dependent reduction in the phosphorylation levels of all four kinases examined (Figure 4B), which was also noted in PC-3 cells (data not shown). As these kinases are known PP2A substrates, their concomitant dephosphorylation raised a possible link with PP2A activation in drug-treated cells. This causal relationship was supported by the ability of VES and TS-1 to increase PP2A phosphatase activity (Figure 4C, lower panel). This enhancement in PP2A activity, however, was not due to increases in PP2A protein levels after drug treatment (upper panel).
Fig. 4.
Effects of VES and TS-1 on the phosphorylation states of Sp1 and various signaling kinases, as well as the phosphatase activity of PP2A. (A) VES and TS-1 decrease Sp1 phosphorylation levels in LNCaP cells in 2.5% FBS-supplemented medium after 48 h of incubation. Cell lysates were immunoblotted with anti-Sp1 antibody (input) or immunoprecipitated with anti-Sp1 affinity gel. The immunoprecipitates were analyzed by western blotting with anti-phospho-threonine antibodies with Sp1 protein as internal reference. (B) Dose-dependent downregulation of the phosphorylation levels of Akt, ERKs, JNKs and p38 by VES and TS-1 in LNCaP cells in 2.5% FBS-supplemented medium after 72 h of exposure. (C) Effect of VES and TS-1 on PP2A expression and phosphatase activity in LNCaP cells in 2.5% FBS-supplemented medium after 12 h of treatment.
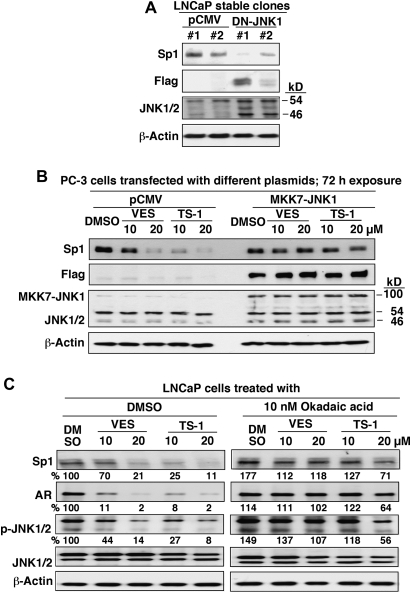
Furthermore, the mechanistic link between JNK inactivation and Sp1 degradation was borne out by two lines of evidence. First, stable transfection of LNCaP cells with a dominant-negative mutant of JNK1 mimicked the effect of VES and TS-1 on attenuating Sp1 expression (Figure 5A). Second, we used PC-3 cells as a model to demonstrate that the constitutively active fusion protein MKK7-JNK1 conferred protection against VES- and TS-1-induced Sp1 degradation (Figure 5B). Relative to PC-3 cells, LNCaP cells were vulnerable to the upregulation of this stress kinase as transient transfection of LNCaP cells with MKK7-JNK1 plasmids resulted in apoptotic death in nearly all transfected cells. Equally important, the PP2A inhibitor okadaic acid could protect cells from the suppressive effect of VES and TS-1 on the phosphorylation or expression of JNK, Sp1 and AR, confirming that VES and TS-1 facilitated the transcriptional repression of AR by targeting the PP2A-JNK-Sp1-signaling axis.
Fig. 5.
Evidence that VES- and TS-1-induced Sp1 degradation is attributable to JNK deactivation. (A) Dominant-negative JNK mimicked the suppressive effect of VES and TS-1 on Sp1 expression. LNCaP cells were stably transfected with the dominant-negative Flag-JNK1a1 or pCMV plasmid and were incubated in 10% FBS-supplemented RPMI 1640. (B) Constitutively active form of JNK (MKK7-JNK1) protected PC-3 cells from VES- or TS-1-facilitated Sp1 repression. PC-3 cells were transiently transfected with the pCMV or MKK7-JNK1 plasmid, incubated for 48 h and exposed to VES or TS-1 at the indicated concentrations in 2.5% FBS-supplemented RPMI 1640 for 72 h. Cell lysates were subjected to western blot analysis with anti-Sp1, anti-Flag and anti-JNK antibodies. (C) Okadaic acid protected LNCaP cells from the suppressive effect of VES and TS-1 on the phosphorylation or expression of JNKs, Sp1 and AR at the indicated concentrations in 2.5% FBS-supplemented RPMI 1640 for 72 h. Values in percentage denote the relative intensity of protein bands of drug-treated samples to that of the DMSO vehicle-treated control after normalization to the respective internal reference β-actin (Sp1 and AR) or JNK1/2 (p-JNK1/2). Each value represents the average of two independent experiments.
Discussion
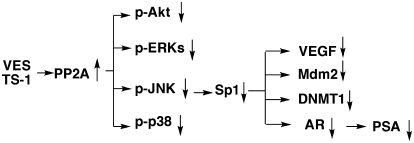
In light of the therapeutic relevance of targeting AR in prostate cancer, we investigated the mechanism by which VES and its truncated derivative TS-1 suppress AR gene transcription. Our data demonstrate that the effect of VES- and TS-1 on facilitating AR transcriptional repression was attributable to their ability to promote Sp1 degradation, which, in turn, was mediated through PP2A-mediated JNK inactivation. Equally important, relative to malignant cells, PrECs were resistant to the antiproliferative effects of VES and TS-1. From a mechanistic perspective, the function of VES and TS-1 to activate PP2A activity underscores their pleiotropic effects on targeting multiple signaling pathways. This study indicates that these mechanisms included, but were not limited to, those mediated by Akt, ERKs, JNKs, p38, Sp1, AR and the respective downstream targets (Figure 6), all of which are clinically relevant to prostate carcinogenesis and tumor progression. Based on the ubiquitous action of PP2A in a growing list of phosphoproteins and signaling pathways, PP2A has been recognized as a tumor suppressor protein (37). A recent study demonstrated that suppression of PP2A activity cooperates with other oncogenic changes to cause neoplastic transformation of multiple cell types (38). Thus, the effect of VES and TS-1 to activate PP2A phosphatase activity is of translational value to develop novel PP2A-activating agents for prostate cancer therapy and prevention.
Fig. 6.
A working model for the effects of VES and TS-1 on the PP2A-JNK-Sp1-AR-signaling axis.
The PP2A-mediated downregulation of mitogen-activated protein kinases in VES/TS-1-treated prostate cancer cells, however, contrasts with recent reports that VES induced differentiation and/or apoptosis in breast and gastric cancer cells by activating ERKs and JNK (3,4,9,21). This discrepancy might be caused by differences in the regulation of the respective signaling networks in different cancer types. At present, the mechanism underlying the effect of VES and TS-1 on activating PP2A phosphatase activity remains unclear. We hypothesize that this PP2A activation might be attributed to increased intracellular levels of ceramide, a known PP2A activator, in drug-treated cells since VES has been reported to stimulate ceramide production (13,23). The ability of VES and TS-1 to mediate ceramide-induced PP2A activation is currently under investigation.
In summary, in the course of investigating the mechanism underlying VES- and TS-1-mediated suppression of AR gene transcription, we demonstrated the ability of these small-molecule agents to modulate the PP2A-JNK-Sp1-signaling axis, of which the significance is multifold. First, this signaling axis provides a molecular basis to account for the broad spectrum of activities of VES on multiple signaling targets. This pleiotropic effect in conjunction with low toxicity is of clinical relevance to cancer therapy/prevention. Second, the higher potency of TS-1 relative to VES in modulating the PP2A-Sp1-AR-signaling pathway provides a proof of principle that these agents could be structurally optimized to develop potent PP2A-targeted agents for prostate cancer therapy.
Funding
National Institutes of Health/National Cancer Institute (CA112250); Department of Defense Prostate Cancer Research Program (W81XWH-09-1-0198).
Acknowledgments
HA-ubiquitin plasmid and the constitutively active JNK plasmid Flag-MKK7-JNK1 were kind gifts from Dr Hung-Wen Chen (Academia Sinica, Taiwan) and Dr Roger Davis (University of Massachusetts Medical School), respectively.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AEBSF
4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride
- AR
androgen receptor
- ChIP
chromatin immunoprecipitation
- DMSO
dimethyl sulfoxide
- EDTA
ethylenediaminetetraacetic acid
- ERK
extracellular signal-regulated kinase
- FBS
fetal bovine serum
- HA
hemagglutinin
- JNK
Jun N-terminal kinase
- PCR
polymerase chain reaction
- PrEC
prostate epithelial cell
- PP2A
protein phosphatase 2A
- SDS
sodium dodecyl sulfate
- TBST
Tris-buffered saline containing 0.1% Tween 20
- VES
vitamin E succinate
References
- 1.Wang XF, et al. Vitamin E analogues as anticancer agents: lessons from studies with alpha-tocopheryl succinate. Mol. Nutr. Food Res. 2006;50:675–685. doi: 10.1002/mnfr.200500267. [DOI] [PubMed] [Google Scholar]
- 2.Neuzil J, et al. Vitamin E analogs, a novel group of “mitocans,” as anticancer agents: the importance of being redox-silent. Mol. Pharmacol. 2007;71:1185–1199. doi: 10.1124/mol.106.030122. [DOI] [PubMed] [Google Scholar]
- 3.You H, et al. RRR-alpha-tocopheryl succinate induces MDA-MB-435 and MCF-7 human breast cancer cells to undergo differentiation. Cell Growth Differ. 2001;12:471–480. [PubMed] [Google Scholar]
- 4.Yu W, et al. Activation of extracellular signal-regulated kinase and c-Jun-NH(2)-terminal kinase but not p38 mitogen-activated protein kinases is required for RRR-alpha-tocopheryl succinate-induced apoptosis of human breast cancer cells. Cancer Res. 2001;61:6569–6576. [PubMed] [Google Scholar]
- 5.Bang OS, et al. Activation of PKC but not of ERK is required for vitamin E-succinate-induced apoptosis of HL-60 cells. Biochem. Biophys. Res. Commun. 2001;288:789–797. doi: 10.1006/bbrc.2001.5839. [DOI] [PubMed] [Google Scholar]
- 6.Barnett KT, et al. Vitamin E succinate inhibits colon cancer liver metastases. J. Surg. Res. 2002;106:292–298. doi: 10.1006/jsre.2002.6466. [DOI] [PubMed] [Google Scholar]
- 7.Malafa MP, et al. Inhibition of angiogenesis and promotion of melanoma dormancy by vitamin E succinate. Ann. Surg. Oncol. 2002;9:1023–1032. doi: 10.1007/BF02574523. [DOI] [PubMed] [Google Scholar]
- 8.Weber T, et al. Vitamin E succinate is a potent novel antineoplastic agent with high selectivity and cooperativity with tumor necrosis factor-related apoptosis-inducing ligand (Apo2 ligand) in vivo. Clin. Cancer Res. 2002;8:863–869. [PubMed] [Google Scholar]
- 9.You H, et al. Role of extracellular signal-regulated kinase pathway in RRR-alpha-tocopheryl succinate-induced differentiation of human MDA-MB-435 breast cancer cells. Mol. Carcinog. 2002;33:228–236. doi: 10.1002/mc.10040. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, et al. Vitamin E succinate inhibits the function of androgen receptor and the expression of prostate-specific antigen in prostate cancer cells. Proc. Natl Acad. Sci. USA. 2002;99:7408–7413. doi: 10.1073/pnas.102014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawson KA, et al. Novel vitamin E analogue decreases syngeneic mouse mammary tumor burden and reduces lung metastasis. Mol. Cancer Ther. 2003;2:437–444. [PubMed] [Google Scholar]
- 12.Ni J, et al. Vitamin E succinate inhibits human prostate cancer cell growth via modulating cell cycle regulatory machinery. Biochem. Biophys. Res. Commun. 2003;300:357–363. doi: 10.1016/s0006-291x(02)02851-6. [DOI] [PubMed] [Google Scholar]
- 13.Weber T, et al. Mitochondria play a central role in apoptosis induced by alpha-tocopheryl succinate, an agent with antineoplastic activity: comparison with receptor-mediated pro-apoptotic signaling. Biochemistry. 2003;42:4277–4291. doi: 10.1021/bi020527j. [DOI] [PubMed] [Google Scholar]
- 14.Birringer M, et al. Vitamin E analogues as inducers of apoptosis: structure-function relation. Br. J. Cancer. 2003;88:1948–1955. doi: 10.1038/sj.bjc.6600981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn T, et al. Dietary administration of the proapoptotic vitamin E analogue alpha-tocopheryloxyacetic acid inhibits metastatic murine breast cancer. Cancer Res. 2006;66:9374–9378. doi: 10.1158/0008-5472.CAN-06-2403. [DOI] [PubMed] [Google Scholar]
- 16.Shiau CW, et al. alpha-Tocopheryl succinate induces apoptosis in prostate cancer cells in part through inhibition of Bcl-xL/Bcl-2 function. J. Biol. Chem. 2006;281:11819–11825. doi: 10.1074/jbc.M511015200. [DOI] [PubMed] [Google Scholar]
- 17.Crispen PL, et al. Vitamin E succinate inhibits NF-kappaB and prevents the development of a metastatic phenotype in prostate cancer cells: implications for chemoprevention. Prostate. 2007;67:582–590. doi: 10.1002/pros.20468. [DOI] [PubMed] [Google Scholar]
- 18.Dong LF, et al. Vitamin E analogues inhibit angiogenesis by selective induction of apoptosis in proliferating endothelial cells: the role of oxidative stress. Cancer Res. 2007;67:11906–11913. doi: 10.1158/0008-5472.CAN-07-3034. [DOI] [PubMed] [Google Scholar]
- 19.Shanker M, et al. Vitamin E succinate in combination with mda-7 results in enhanced human ovarian tumor cell killing through modulation of extrinsic and intrinsic apoptotic pathways. Cancer Lett. 2007;254:217–226. doi: 10.1016/j.canlet.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Wang XF, et al. A peptide conjugate of vitamin E succinate targets breast cancer cells with high ErbB2 expression. Cancer Res. 2007;67:3337–3344. doi: 10.1158/0008-5472.CAN-06-2480. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y, et al. alpha-Tocopheryl succinate-induced apoptosis in human gastric cancer cells is modulated by ERK1/2 and c-Jun N-terminal kinase in a biphasic manner. Cancer Lett. 2007;247:345–352. doi: 10.1016/j.canlet.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Yin Y, et al. The therapeutic and preventive effect of RRR-alpha-vitamin E succinate on prostate cancer via induction of insulin-like growth factor binding protein-3. Clin. Cancer Res. 2007;13:2271–2280. doi: 10.1158/1078-0432.CCR-06-1217. [DOI] [PubMed] [Google Scholar]
- 23.Gu X, et al. Vitamin E succinate induces ceramide-mediated apoptosis in head and neck squamous cell carcinoma in vitro and in vivo. Clin. Cancer Res. 2008;14:1840–1848. doi: 10.1158/1078-0432.CCR-07-1811. [DOI] [PubMed] [Google Scholar]
- 24.Dalen H, et al. Alpha-tocopheryl succinate sensitises a T lymphoma cell line to TRAIL-induced apoptosis by suppressing NF-kappaB activation. Br. J. Cancer. 2003;88:153–158. doi: 10.1038/sj.bjc.6600683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neuzil J, et al. Induction of cancer cell apoptosis by alpha-tocopheryl succinate: molecular pathways and structural requirements. FASEB J. 2001;15:403–415. doi: 10.1096/fj.00-0251com. [DOI] [PubMed] [Google Scholar]
- 26.Yang CC, et al. Peroxisome proliferator-activated receptor gamma-independent suppression of androgen receptor expression by troglitazone mechanism and pharmacologic exploitation. Cancer Res. 2007;67:3229–3238. doi: 10.1158/0008-5472.CAN-06-2759. [DOI] [PubMed] [Google Scholar]
- 27.Lei K, et al. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH(2)-terminal kinase. Mol. Cell. Biol. 2002;22:4929–4942. doi: 10.1128/MCB.22.13.4929-4942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pore N, et al. Sp1 is involved in Akt-mediated induction of VEGF expression through an HIF-1-independent mechanism. Mol. Biol. Cell. 2004;15:4841–4853. doi: 10.1091/mbc.E04-05-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hobom U, et al. E1B-55-kilodalton protein is not required to block p53-induced transcription during adenovirus infection. J. Virol. 2004;78:7685–7697. doi: 10.1128/JVI.78.14.7685-7697.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egger G, et al. Identification of DNMT1 (DNA methyltransferase 1) hypomorphs in somatic knockouts suggests an essential role for DNMT1 in cell survival. Proc. Natl Acad. Sci. USA. 2006;103:14080–14085. doi: 10.1073/pnas.0604602103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang LG, et al. Repression of androgen receptor in prostate cancer cells by phenethyl isothiocyanate. Carcinogenesis. 2006;27:2124–2132. doi: 10.1093/carcin/bgl075. [DOI] [PubMed] [Google Scholar]
- 32.Kitagawa Y, et al. Vascular endothelial growth factor contributes to prostate cancer-mediated osteoblastic activity. Cancer Res. 2005;65:10921–10929. doi: 10.1158/0008-5472.CAN-05-1809. [DOI] [PubMed] [Google Scholar]
- 33.Khor LY, et al. MDM2 as a predictor of prostate carcinoma outcome: an analysis of Radiation Therapy Oncology Group Protocol 8610. Cancer. 2005;104:962–967. doi: 10.1002/cncr.21261. [DOI] [PubMed] [Google Scholar]
- 34.McCabe MT, et al. Inhibition of DNA methyltransferase activity prevents tumorigenesis in a mouse model of prostate cancer. Cancer Res. 2006;66:385–392. doi: 10.1158/0008-5472.CAN-05-2020. [DOI] [PubMed] [Google Scholar]
- 35.Chuang JY, et al. Phosphorylation by c-Jun NH2-terminal kinase 1 regulates the stability of transcription factor Sp1 during mitosis. Mol. Biol. Cell. 2008;19:1139–1151. doi: 10.1091/mbc.E07-09-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang YT, et al. Sumoylation of specificity protein 1 augments its degradation by changing the localization and increasing the specificity protein 1 proteolytic process. J. Mol. Biol. 2008;380:869–885. doi: 10.1016/j.jmb.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 37.Mumby M. PP2A: unveiling a reluctant tumor suppressor. Cell. 2007;130:21–24. doi: 10.1016/j.cell.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 38.Junttila MR, et al. CIP2A inhibits PP2A in human malignancies. Cell. 2007;130:51–62. doi: 10.1016/j.cell.2007.04.044. [DOI] [PubMed] [Google Scholar]