Abstract
Cartilage growth may involve alterations in the balance between the swelling tendency of proteoglycans and the restraining function of the collagen network. Growth factors, including IGF-I, TGF-β1, BMP-7, and PDGF-AB, regulate chondrocyte metabolism and, consequently, may regulate cartilage growth. Immature bovine articular cartilage explants from the superficial and middle zones were incubated for 13 days in basal medium or medium supplemented with serum, IGF-I, TGF-β1, BMP-7, or PDGF-AB. Variations in tissue size, accumulation of proteoglycan and collagen, and tensile properties were assessed. The inclusion of serum, IGF-I, or BMP-7 resulted in expansive tissue growth, stimulation of proteoglycan deposition but not of collagen, and a diminution of tensile integrity. The regulation of cartilage metabolism by TGF-β1 resulted in tissue homeostasis, with maintenance of size, composition, and function. Incubation in basal medium or with PDGF-AB resulted in small volumetric and compositional changes, but a marked decrease in tensile integrity. These results demonstrate that the phenotype of cartilage growth, and the associated balance between proteoglycan content and integrity of the collagen network, is regulated differentially by certain growth factors.
Introduction
Articular cartilage is a layer of connective tissue located on the ends of long bones (Buckwalter and Mankin 1997) that normally functions as a low friction, wear-resistant, load-bearing material, facilitating joint motion (Maroudas 1979; Mow and Ratcliffe 1997). The ability of cartilage to withstand compressive, tensile, and shear forces depends on the composition and structure of the extracellular matrix (Maroudas 1979; Grodzinsky 1983; Mow and Ratcliffe 1997). The proteoglycan constituent of the extracellular matrix provides the tissue with a fixed negative charge that increases the tissue’s propensity to swell and to resist compressive loading (Lai et al. 1991; Basser et al. 1998). The crosslinked collagen network resists the swelling tendency of the proteoglycan molecules and provides the tissue with tensile and shear stiffness and strength (Woo et al. 1976; Venn and Maroudas 1977; Mow and Ratcliffe 1997). Chondrocytes in cartilage normally maintain a functional matrix by modulating synthesis and degradation of the matrix components.
Growth and remodeling are biological processes that, together, transform cartilage tissue in vivo from an immature to a mature state. Growth is generally defined as an increase in size due to accretion of material similar to that already present, while remodeling is defined as a change in the endogenous or the overall material, and, concomitantly, a change in mechanical properties (Taber 2001; Klisch et al. 2003). Biological tissues can be viewed as composite materials that grow and remodel due to changes in the quantity and/or structure of tissue components such as cells and constituents of the hydrated extracellular matrix. Two distinct mechanisms of tissue growth have been recognized: appositional growth, or growth at a surface of tissue, and interstitial growth, or growth within a volume of tissue (Cowin 2004). While it is possible that tissues can grow appositionally in the absence of remodeling, interstitial tissue growth must involve both growth and remodeling since accretion of a single tissue component will change the overall tissue structure and mechanical properties. Since articular cartilage tissue may undergo both appositional and interstitial growth, the term growth is used subsequently in this paper to refer, collectively, to both growth and remodeling.
Alterations of cartilage function, structure, and composition during growth in vivo and during serum-supplemented culture in vitro appear to depend on the metabolic balance between proteoglycan molecules and the components of the collagen network. Fetal and postnatal growth of articular cartilage normally involves a net deposition of collagen that is greater than that of proteoglycan, as well as an increase in mechanical integrity. During maturation of bovine articular cartilage, from the fetal stage, through the newborn calf, and to the skeletally mature adult, there is an increase in the collagen and pyridinoline crosslink densities, but little or no change in the content of glycosaminoglycan (GAG) (Pal et al. 1981; Thonar and Sweet 1981; Wong et al. 2000; Williamson et al. 2001). These biochemical changes are accompanied by an increase in the tensile modulus and strength, and each of these biomechanical properties is positively correlated with the collagen and pyridinoline crosslink densities (Williamson et al. 2003). In contrast to this type of in vivo growth, growth of immature cartilage tissue in vitro in serum-supplemented medium results in a net deposition of proteoglycan that is greater than that of collagen and a decrease in mechanical integrity. For cartilage explants from bovine fetus and calf, and neonatal rat, incubation in serum-supplemented medium results in an increase in tissue size, maintenance of proteoglycan concentration and a decrease in the concentrations of collagen and pyridinoline crosslinks (Sah et al. 1994; Garcia and Gray 1995; Williamson et al. 2003). These changes in composition are associated with a decrease in tensile modulus and strength (Williamson et al. 2003).
A number of growth factors appear to play important roles in regulation of development through the embryonic stages, as well as during pre-natal and post-natal growth. Insulin-like growth factor-I (IGF-I), transforming growth factor-β1 (TGF-β1), bone morphogenic protein-7 (BMP-7, also known as osteogenic protein-1 (OP-1)), and platelet derived growth factor (PDGF) are localized to certain regions of a developing limb in a specific temporal pattern (Heine et al. 1987; Ralphs et al. 1990; Orr-Urtreger and Lonai 1992; Francis-West et al. 1995; Laufer et al. 1997; Ren et al. 1997; Ataliotis 2000). Deficiencies in either IGF-I or IGF-I receptor cause intrauterine growth retardation and postnatal growth failure (Liu et al. 1993; Woods et al. 1996; Abuzzahab et al. 2003), while deficiencies in BMP-7 result in skeletal abnormalities and death at birth (Luo et al. 1995). The levels of IGF-I in serum and of TGF-β1 in cartilage are low in a neonate and rise during post-natal growth (Clemmons and Van Wyk 1984; Moroco et al. 1997; Fortier et al. 2005). IGF-I, TGF-β1, and BMP-7 continue to be expressed in immature and adult articular cartilages (Ellingsworth et al. 1986; Luyten et al. 1988; Morales et al. 1991; Schneiderman et al. 1995; Fukumura et al. 1998; Anderson et al. 2000; Chubinskaya et al. 2000; Iqbal et al. 2000; Muehleman et al. 2002) and reach concentrations of 1–50 ng/g cartilage tissue (Luyten et al. 1988; Schneiderman et al. 1995), 0.02–0.2 ng/ml synovial fluid (Wei and Messner 1998), 1–20 ng/g cartilage tissue (Chubinskaya et al. 2002), respectively. Pathological cartilage/bone growth, such as that seen during the formation of osteophytes, also involves activities of growth factors such as TGF-β and PDGF (Horner et al. 1996; Horner et al. 1998).
In vitro studies on articular cartilage explants have delineated the ability of certain growth factors to regulate cartilage metabolism and mechanical integrity. Various investigations point to IGF-I as the major factor in fetal bovine serum (FBS) (McQuillan et al. 1986; Luyten et al. 1988; Barone-Varelas et al. 1991; Luyten et al. 1992; Tesch et al. 1992; Sah et al. 1994) that stimulates proteoglycan and collagen synthesis in immature and adult cartilage (Sah et al. 1994), and results in a decreased rate of proteoglycan loss in adult tissue (Sah et al. 1994). Overall, IGF-I results in the net deposition of proteoglycan (Sah et al. 1994) and maintenance of tissue mechanical function in confined compression (Sah et al. 1996). In explant culture of calf cartilage, TGF-β1 has similar effects on proteoglycan metabolism as IGF-I. TGF-β1 increased proteoglycan synthesis and decreased the rate of proteoglycan catabolism, but did not affect the content of collagen (Morales and Roberts 1988). While addition of BMP-7 to explant culture of immature cartilage enhanced the net accumulation of proteoglycan through stimulation of proteoglycan synthesis and diminution of proteoglycan release (Lietman et al. 1997; Nishida et al. 2000), inhibition of autocrine BMP-7 decreased the accumulation of proteoglycan in cartilage matrix (Soder et al. 2005). In immature and adult bovine cartilage explants, PDGF-AB stimulated proteoglycan synthesis and decreased the rate of proteoglycan catabolism (Schafer et al. 1993).
Cartilage hydration and the load-bearing biomechanical function are influenced by the balance between the swelling propensity of proteoglycan molecules and the restraining function of the collagen network. This idea was proposed by Maroudas (Maroudas 1976) and has been supported by theoretical models (Eisenberg and Grodzinsky 1988; Lai et al. 1991; Buschmann and Grodzinsky 1995; Basser et al. 1998). The increased hydration and loss of mechanical integrity of osteoarthritic cartilage, compared with normal cartilage, is due to a weakening of the collagen network and an associated swelling of the tissue (Maroudas and Venn 1977; Akizuki et al. 1986; Bank et al. 2000). Analogously, in the context of growth, a low or reduced restraining function of the collagen network, due to either variations in network composition or structure or due to excessive swelling pressure imposed by the newly synthesized proteoglycan, is predicted to allow tissue swelling and growth (Klisch et al. 2003). Thus, we hypothesized that cartilage growth results from a dynamic imbalance between the swelling pressure of endogenous and newly synthesized GAG and the restraining function of the collagen network.
Since growth factors, such as IGF-I, TGF-β1, BMP-7, and PDGF-AB, regulate chondrocyte metabolism, they may consequently regulate cartilage growth. Thus, the objectives of this study were to examine the effects of IGF-I, TGF-β1, BMP-7, and PDGF-AB on in vitro growth of immature bovine articular cartilage explants in terms of culture-associated variations in tissue size, accumulation of GAG and collagen, and tensile mechanical properties. Additional objectives were to assess, by correlative analysis, whether tissue size is related to the accumulation of GAG in the tissue and the swelling-resistant properties of the collagen network and whether tensile mechanical properties are related to the biochemical composition of the tissue.
Methods
Sample Preparation and Culture
Articular cartilage was harvested from the patellofemoral groove of 3 newborn (1–3 weeks old) bovine calves. Blocks, 9 × 3 × ~0.4 mm3 (length × width × thickness), were prepared using a sledge microtome to either include the intact articular surface (S) or to include the middle zone, starting at a distance of ~0.6 mm from the articular surface (M). The long axis of the blocks was in the anterior-posterior direction and, thus, approximately perpendicular to the split line direction. Blocks were weighed wet (WWi) under sterile conditions.
Some blocks were (a) analyzed immediately. Other blocks were incubated in medium (DMEM supplemented with 100 μg/mL of ascorbate, 0.1 mM nonessential amino acids, 0.4 mM L-proline, 2 mM L-glutamine, 10 mM HEPES, 100 U/ml of penicillin, 100 μg/mL of streptomycin, and 0.25 μg/mL of amphotericin B) (DiMicco et al. 2002) at 37°C in a humidified 5% CO2 - 95% air incubator with modifications as noted: (b) 0.01% cell culture tested bovine serum albumin (BSA, basal medium) (Sigma, St. Louis, MO), (c) 20% FBS, or 0.01% BSA with either (d) 50 ng/mL recombinant human (rh) IGF-I, (e) 10 ng/mL rhTGF-β1, (f) 50 ng/mL rhBMP-7, or (g) 50 ng/mL rhPDGF-AB. All growth factors were from PeproTech, Inc. (Rocky Hill, NJ), except rhBMP-7 that was from R&D Systems Inc. (Minneapolis, MN). Non-tissue culture treated plates were used for incubation to minimize cell adhesion to the plate and cell outgrowth from the explants. Medium (0.5 mL/block) was changed every other day, and, during the first 12 days of culture, supplemented with 10 μCi/mL [3H]proline and 2 μCi/mL [35S]sulfate. To remove unincorporated isotopes, blocks were then washed (6 times over 1.5 hr., 37°C), transferred to a new culture plate, and incubated for an additional day in medium without radiolabel. At termination, blocks were weighed wet (WWf) and punched to form a tapered tensile test strip and residual cartilage.
Biochemical Analysis
Residual cartilage and the failed portions of the corresponding tensile strip were analyzed together to quantify the biochemical composition of the fresh and cultured samples. Samples were lyophilized, weighed dry, and solubilized with proteinase K (Schinagl 1997). Portions of the tissue digest were analyzed to quantify the content of DNA (McGowan et al. 2002), GAG (Farndale et al. 1986), and hydroxyproline (Woessner 1961). DNA was converted to cell number by using a conversion constant of 7.7 pg of DNA per cell (Kim et al. 1988). Hydroxyproline content was converted to collagen content by assuming a mass ratio of collagen to hydroxyproline equal to 7.25 (Herbage et al. 1977; Pal et al. 1981). A portion of spent medium collected over 13 days of culture was analyzed for the content of GAG (Farndale et al. 1986) to asses the loss of this matrix component from the tissue. The net change in GAG during culture was calculated for each explant as the difference between the sum of GAG content in the tissue on day 13 and GAG released into the medium over 13 days and GAG content in fresh cartilage of the same layer. This represented GAG synthesis as measured by the DMB dye-binding assay (Farndale et al. 1986). Total GAG content was calculated as the sum of GAG content in the tissue and GAG content released into the medium over 13 days. Biochemical parameters were normalized to initial wet weight of the tissue (WWi) to represent constituent content and to final wet weight of the tissue (WWf) to represent constituent concentration.
Analysis of Matrix Metabolism
Other portions of the solubilized tissue and portions of the medium were analyzed for the incorporated radioactivity to assess matrix metabolism. [35S] and [3H] radioactivity was determined as indices of sulfated GAG and protein synthesis respectively. To more specifically assess collagen synthesis, portions of tissue digests were pooled for each layer/experimental condition and analyzed for [3H]hydroxyproline residues (Stern et al. 1963; Sah et al. 1991). A portion of spent medium was analyzed for the content of [35S]GAG using the Alcian Blue precipitation method (Masuda et al. 1994). The rates of incorporation of [35S]sulfate and [3H]proline were used to estimate the absolute rates of sulfate and proline incorporation based on the specific activity of [35S]sulfate and [3H]proline in the medium and an assumed equivalence between the specific activity of the intracellular biosynthetic precursor pools and of the medium. The sulfate concentration in the medium was about 0.86 mM, assuming the sulfate concentration in serum of 0.32 mM (Cole and Evrovski 1997) and proline concentration in the medium was about 0.42mM, with serum proline concentration of 0.16mM (HyClone, Logan, UT). Total sulfate incorporation was calculated as the sum of the content of sulfate incorporation in the tissue and medium and represented GAG synthesis as measured by [35S]sulfate incorporation.
Biomechanical Analysis
Tapered tensile strips were analyzed to determine mechanical properties as described previously (Chen et al. 2002). From each cartilage block, a tapered strip (Kempson 1982) with a gage region of 4 mm × 0.80 mm was prepared using a punch. The thickness of each tensile strip was measured at three locations in the gage region, using a contact-sensing micrometer, and the average was used for cross-sectional area calculations. Tapered specimens were then secured in clamps (4.0 mm apart) of a mechanical tester and elongated at a constant extension rate (5 mm/min) until failure. Structural tensile parameters were obtained from the load-displacement curves. Structural tensile strength was determined as the maximum load sustained at failure. Ramp stiffness was calculated as the slope of the linear regression of the load-displacement curve from 25–75% of the maximum load. Load and displacement were converted to stress (defined as load normalized to the cross-sectional area of the gage region) and strain (defined as the elongation distance normalized to the initial clamp to clamp distance) to obtain material tensile parameters of tensile strength, strain at failure, and ramp modulus. The strain at failure was the strain at which maximum stress was attained. The failed portions of each tensile strip, resulting from the tensile test, were saved for biochemical analysis (described above) in addition to the adjacent cartilage samples obtained during preparation of the tensile strips.
Statistical Analysis
For each layer (S and M), the effects of experimental conditions (a–g) were assessed by analysis of variance (ANOVA) with experimental condition as fixed factor and donor animal as a random factor. Where significant (p<0.05) differences were detected, Tukey post-hoc testing was performed. To analyze the effect of 13 days of incubation on wet weight, repeated measures ANOVA was performed for each layer and experimental condition with wet weight (WWi and WWf) as a repeated factor. All correlative relationships were analyzed by univariate linear regression. A significance criterion of 0.05 was used when assessing regression slopes. To determine whether the level of correlative relationships in the S and M layers were similar, the regression slopes for these groups of samples were compared by t test. Data are expressed as mean ± SEM. Statistical analysis was performed using Systat 10.2 (Systat Software, Inc., Richmond, CA).
Results
Assessment of Volumetric Tissue Growth
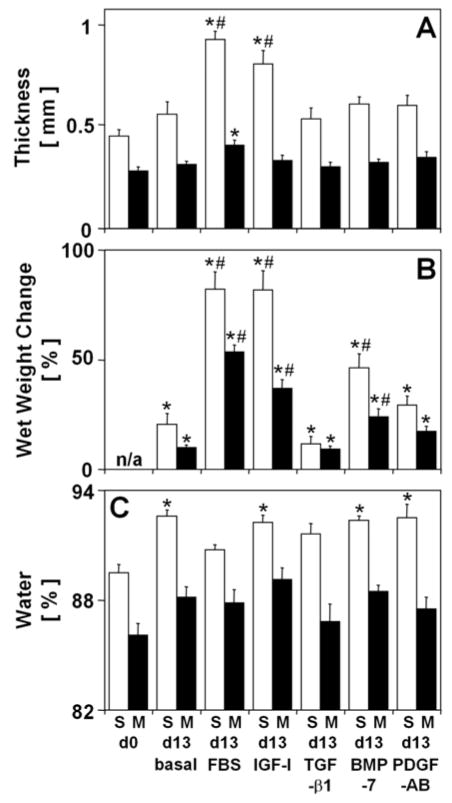
The extent of in vitro volumetric growth of articular cartilage blocks was markedly affected by experimental conditions as assessed by changes in thickness (p<0.05 in each layer) (Fig. 1A), wet weight (p<0.001 in each layer) (Fig. 1B), and water content (Fig. 1C). The thickness increased during culture with FBS (104%, p<0.001 in S, 45%, p<0.05 in M) and IGF-I (78%, p<0.001 in S) and, in the S layer, was larger than the thickness of explants cultured in basal medium (66%, p<0.001 for FBS; 44%, p<0.05 for IGF-I). Similarly, large extents of wet weight change occurred during culture with FBS (82% in S, 54% in M), IGF-I (82% in S, 37% in M), and BMP-7 (46% in S, 24% in M) (all p<0.001) and exceeded the extent of wet weight change during culture in basal medium (62% in S, 44% in M for FBS; 61% in S, 27% in M for IGF-I, p<0.001; 26% in S, 14% in M for BMP-7, p<0.05). In contrast, incubation in basal medium or with TGF-β1 or PDGF-AB did not affect the thickness (all p>0.38); consistent with this, the change in wet weight of these explants was small (21%, p<0.05 in S and 10%, p<0.001 in M for basal; 12%, p<0.05 in S and 9%, p<0.001 in M for TGF-β1; 29%, p<0.01 in S and 17%, p<0.01 in M for PDGF-AB). The volumetric growth of all explants appeared to be predominantly axial, as changes in wet weight can mostly be accounted for by changes in thickness alone. The content of water varied slightly in the S layer (p<0.001), increasing during basal culture or culture with IGF-I, BMP-7, or PDGF-AB (3%, p<0.01 each). Since water content varied slightly, changes in wet weight were largely due to changes in tissue volume and not density and, thus, along with changes in thickness, represent volumetric growth of tissue samples.
Figure 1.
Effect of experimental conditions on general indices of in vitro growth of calf articular cartilage explants from the superficial (S) and middle (M) layers. Blocks were analyzed on day 0 (d0), or incubated for 13 days (d13) in basal medium (0.01% BSA) or medium supplemented with either 20% FBS, 50 ng/ml IGF-I, 10 ng/ml TGF-β1, 50 ng/ml BMP-7, or 50 ng/ml PDGF-AB. (A) Thickness, (B) change in wet weight, and (C) percent water of cartilage blocks. Data are mean ± SEM, n = 8–9 blocks from 3 animals. * = p<0.05 vs. d0, # = p<0.05 vs. basal condition.
Biochemical Analysis
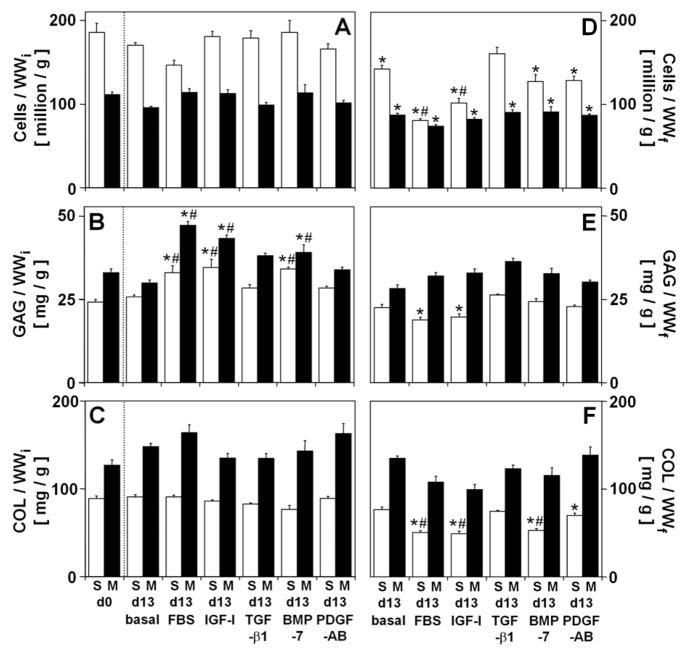
The content of cells (Cells/WWi, Fig. 2A) did not vary with experimental conditions and, when normalized to WWf to give a measure of concentration of cells (Cells/WWf, Fig. 2D), reflected the changes in wet weight (p<0.001 in each layer). During culture, Cells/WWf decreased in explants cultured in basal medium (−24% in S, −22% in M), with FBS (−57% in S, −34% in M), IGF-I (−45% in S, −26% in M), BMP-7 (−31% in S, −19% in M), and PDGF-AB (−31% in S, −22% in M) (all p<0.01). In contrast, during culture with TGF-β1 Cells/WWf did not change (p=0.44 in S) or decreased slightly (−19%, p<0.01 in M).
Figure 2.
Effect of experimental conditions on biochemical composition of calf articular cartilage explants from the S and M layers. (A, D) Cells, (B, E) glycosaminoglycan (GAG), and (C, F) collagen (COL) normalized to initial wet weight (WWi) to represent constituent content (A–C) and final wet weight (WWf) to represent constituent concentration (D–F). The dotted line separates explants analyzed on day 0, where WWf = WWi. Data are mean ± SEM, n = 8–9 blocks from 3 animals. * = p<0.05 vs. d0, # = p<0.05 vs. basal condition.
The extent of volumetric growth was generally paralleled by variations in the tissue content of GAG but not collagen (COL). GAG content (GAG/WWi, Fig. 2B) varied with experimental conditions (p<0.001 in each layer), while the content of COL (COL/WWi, Fig. 2C) did not vary. GAG/WWi increased during culture with FBS (36%, p<0.01 in S, 43%, p<0.001 in M), IGF-I (43%, p<0.001 in S, 31%, p<0.001 in M), and BMP-7 (41%, p<0.01 in S, 19%, p=0.09 in M) and exceeded GAG/WWi in explants cultured in basal medium (28%, p<0.05 in S, 58%, p<0.001 in M for FBS; 34%, p<0.01 in S, 45%, p<0.001 in M for IGF-I; 32%, p<0.05 in S, 31%, p<0.01 in M for BMP-7). In contrast, GAG/WWi did not change during basal culture or culture with TGF-β1 or PDGF-AB (all p>0.24).
When normalized to WWf to give an index of concentration in the tissue, the concentrations of extracellular matrix components reflected changes in the content of the components and in the wet weight of the tissue during culture. The concentrations of GAG (GAG/WWf, Fig. 2E) and COL (COL/WWf, Fig. 2F) varied with experimental conditions in the S layer (p<0.01) but did not change during culture of explants from the M layer. In the S layer, GAG/WWf did not change (p>0.51 for basal, TGF-β1, BMP-7, and PDGF-AB) or decreased slightly (−21–25%, p<0.01 for FBS and IGF-I), while COL/WWf decreased markedly during culture with FBS (−43%, p<0.001), IGF-I (−45%, p<0.001), BMP-7 (−40%, p<0.001), and PDGF-AB (−31%, p<0.05), but not with TGF-β1 or during basal culture (p>0.27).
Analysis of Matrix Metabolism
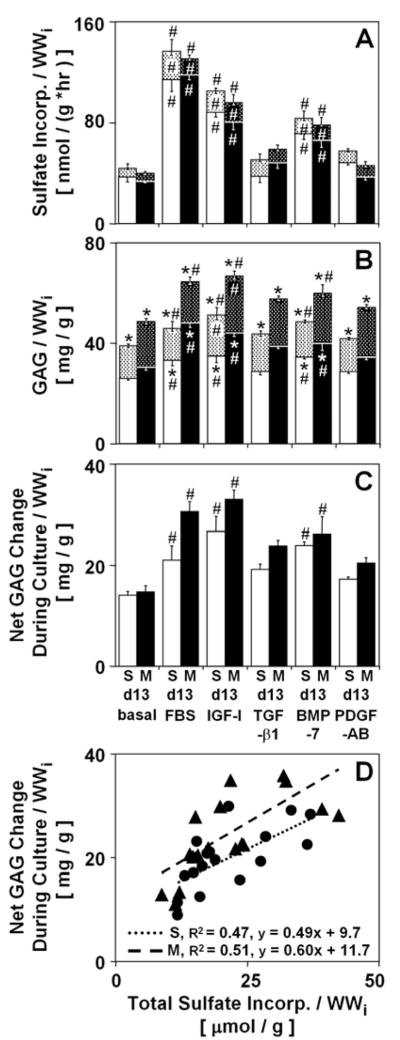
Changes in GAG content (Fig. 3B) were generally paralleled by similar variations in the incorporation of sulfate (Fig. 3A). The experimental conditions had only a minor effect on GAG released into the medium, with greater release during incubation with IGF-I than during incubation in basal medium (27%, p=0.08 in S; 26%, p<0.05 in M) or with FBS (31%, p<0.05 in S; 39%, p<0.001 in M). While the total GAG content increased in all cultures (44–57% for basal; 85–91% for FBS; 98–107% for IGF-I; 71–77% for TGF-β1; 78–96% for BMP-7; 61–69% for PDGF-AB, all p<0.001), only incubation with FBS, IGF-I, and BMP-7 resulted in a higher total GAG content than that in explants incubated in basal medium (18%, p=0.08 in S and 33%, p<0.001 in M for FBS; 32–38%, p<0.001 for IGF-I; 24–25%, p<0.01 for BMP-7). Consistent with this, the net GAG change during culture with FBS, IGF-I, and BMP-7 was higher than that during incubation in basal medium (50%, p=0.08 in S and 107%, p<0.001 in M for FBS; 90–123%, p<0.01 for IGF-I; 70–77%, p<0.05 for BMP-7). Similarly, incubation with FBS, IGF-I, and BMP-7 stimulated the total sulfate incorporation and incorporation of sulfate into the tissue and medium above the levels in explants incubated in basal medium (187–255%, p<0.001 for FBS; 135–142%, p<0.01 for IGF-I; 89–95%, p<0.05 for BMP-7). A correlation analysis indicated a positive correlation (p<0.01, R2 = 0.47 in S; p<0.001, R2 = 0.51 in M) between the total sulfate incorporation, an indicator of GAG synthesis measured by [35S]sulfate incorporation, and the net GAG change during culture, an indicator of GAG synthesis measured by the DMB dye-binding assay (Fig. 3D). Assuming one mole of sulfate per disaccharide, the regression slopes should yield the molecular weight of GAG and, indeed, approach 502.4 g/mol in both the S (490 g/mol) and M (600 g/mol) layers. Thus, the metabolism of [35S] can be used to quantitatively assess the amount of sulfated GAG deposition.
Figure 3.
Effect of experimental conditions on GAG balance of calf articular cartilage explants from S and M layers. The dotted regions (above) represent (A) sulfate and (B) GAG released into the culture medium, while the solid color regions (below) represent sulfate incorporation (A) and GAG in the tissue (B). The overall bar height represents the total (A) incorporated sulfate and (B) GAG content. (C) The net change in GAG during culture, and (D) relationship between the net change in GAG during culture and total incorporated sulfate. Regression lines, corresponding R2 values and equations are shown for the S (●) and M (▲) layers, where the statistical significance p<0.05. Data are mean ± SEM, n = 8–9 blocks from 3 animals. * = p<0.05 vs. d0, # = p<0.05 vs. basal condition.
Variations in the incorporation of sulfate (Fig. 3A) were paralleled by variations in proline incorporation (not shown) (all p<0.05) and hydroxyproline formation (not shown). Incubation with FBS, IGF-I, and BMP-7 enhanced incorporation of proline into the tissue above the levels in explants incubated in basal medium (247% in S, 461% in M for FBS; 124% in S, 155% in M for IGF-I; 75% in S, 95% in M for BMP-7, all p<0.01). In the absence of FBS, the percent of hydroxyproline residues did not vary and reached 27–31%, while in explants incubated with FBS, the percent of hydroxyproline residues reached 36–38%. The resultant variations in hydroxyproline formation paralleled variations in proline incorporation.
Biomechanical Analysis
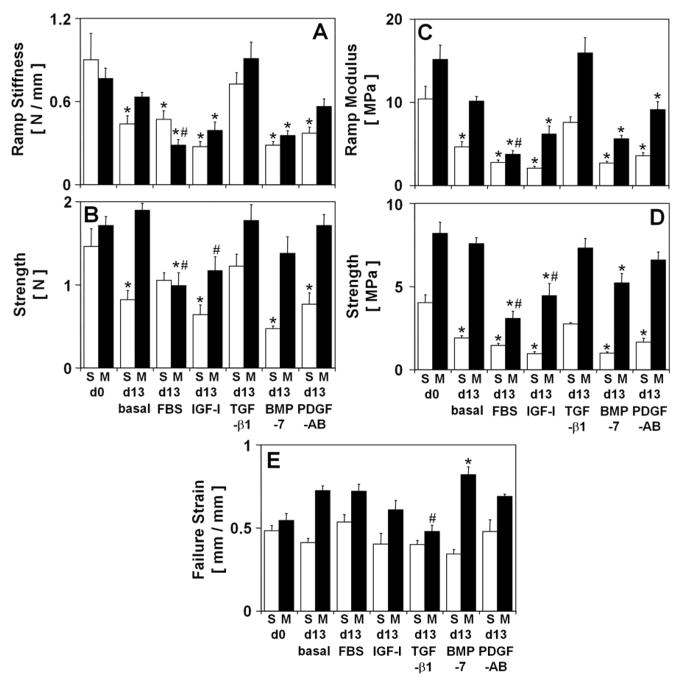
Structural biomechanical properties of tensile ramp stiffness (Fig. 4A) and strength (Fig. 4B) varied with experimental conditions (all p<0.001) and the variations were consistent with the variations in the material biomechanical properties of tensile ramp modulus (Fig. 4C) and strength (Fig. 4D), which are described below. Ramp modulus decreased during culture in basal medium (−55%, p<0.001 in S), with FBS (−73%, p<0.001 in S, −75%, p<0.001 in M), IGF-I (−80%, p<0.001 in S, −59% p<0.01 in M), BMP-7 (−74%, p<0.001 in S, −63%, p<0.001 in M), and PDGF-AB (−66%, p<0.001 in S, −40% p=0.08 in M). Similar to variations in ramp modulus, strength decreased during culture in basal medium (−53%, p<0.001 in S), with FBS (−64%, p<0.001 in S, −62%, p<0.001 in M), IGF-I (−76%, p<0.001 in S, −46% p<0.01 in M), BMP-7 (−75%, p<0.001 in S, −36%, p<0.05 in M), and PDGF-AB (−59%, p<0.001 in S). In contrast, after culture with TGF-β1 both ramp modulus and strength remained unchanged (all p>0.24). Strain at failure (Fig. 5C) did not vary with experimental conditions in the S layer but varied slightly in the M layer (p<0.001). Strain at failure increased during culture with BMP-7 (51%, p<0.01) and, although not significantly, increased (12–33%) after incubation in basal medium, with FBS, IGF-I, and PDGF-AB. In explants incubated with TGF-β1, strain at failure tended to decrease (−12%) and was lower than that of explants incubated in basal medium (−34%, p<0.05).
Figure 4.
Effect of experimental conditions on structural and material tensile mechanical properties of calf articular cartilage explants from the S and M layers. Structural properties: (A) Ramp modulus, and (B) strength. Material properties: (C) ramp modulus, (D) strength, and (E) strain at failure. Data are mean ± SEM, n = 8–9 blocks from 3 animals. * = p<0.05 vs. d0, # = p<0.05 vs. basal condition.
Figure 5.
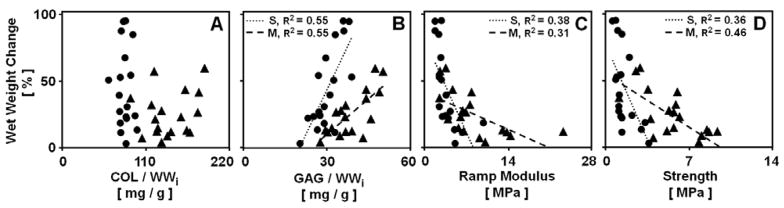
Relationships between the change in wet weight during culture and constituent content and tensile mechanical properties. Data are for cartilage from the S (●) and M (▲) layers for blocks analyzed on day 0 and incubated in medium. Regression lines, and corresponding R2 values are shown where the statistical significance p<0.05.
Correlative Analysis
The correlations between certain parameters were examined to assess factors involved in volumetric growth (Fig. 5). The change in wet weight correlated positively with GAG/WWi in the tissue (p<0.001 for each layer), reaching R2 values of 0.55 (Fig. 5B), while it did not correlate to COL/WWi (p>0.35, R2 <0.06) (Fig. 5A). The tensile mechanical properties were used in the correlative analysis as they reflect the swelling-resistant properties of the collagen network. Both ramp modulus and strength showed a negative correlation with the change in wet weight (all p<0.01) with R2 values of 0.31–0.46 (Fig. 5C–D). The level of dependency of the change in wet weight on GAG/WWi and mechanical properties was more pronounced in the S layer (3–5 fold, all p<0.05).
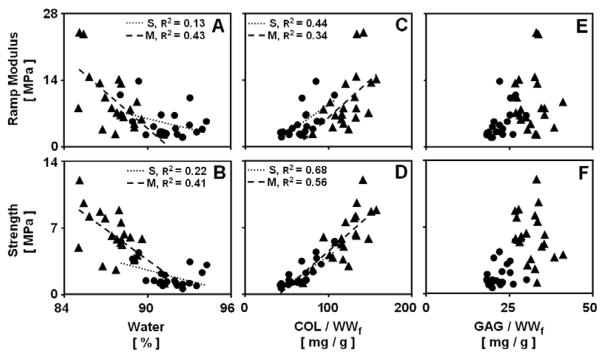
Tensile mechanical properties were correlated with the concentration of several matrix components (Fig. 6). In each layer, ramp modulus and strength showed a negative correlation with water content (each p<0.05, Fig. 6A–B), and a strong positive correlation with COL/WWf (each p<0.01, Fig. 6C–D). The correlation coefficients were highest for strength, reaching R2 values of 0.56–0.68 for COL/WWf (Fig. 6D) and 0.22–0.41 for water (Fig. 6B). The level of dependency of mechanical properties on COL/WWf were similar in each layer, while the level of dependency of mechanical properties on water was more pronounced in the S layer (3–4 fold, each p<0.05). The tensile mechanical properties did not correlate with GAG/WWf (p>0.16, R2 <0.27) (Fig. 6E–F). Strain at failure did not correlate with the biochemical constituents measured (p>0.16, R2 <0.18).
Figure 6.
Relationships between cartilage tensile mechanical properties and constituent concentrations. Data are for cartilage from the S (●) and M (▲) layers for blocks analyzed on day 0 and incubated in medium. Regression lines, and corresponding R2 values are shown where the statistical significance p<0.05.
Discussion
The data presented here demonstrate that in vitro growth of immature articular cartilage explants, as indicated by changes in tissue size, content of matrix components, and the integrity of the collagen network, is regulated differentially by certain growth factors. Incubation with FBS, IGF-I, or BMP-7 resulted in expansive cartilage growth, characterized by an increase in tissue size (Fig. 1A–B), stimulation of GAG synthesis and deposition (Fig. 3A–C), and loss in the concentration of matrix components (Fig. 2D–F) and the tensile mechanical integrity (Fig. 4A–D). Many of the effects of serum on cartilage growth were similar to those reported previously (Williamson et al. 2003) and appear attributable to IGF-I. Incubation in basal medium or with PDGF-AB also resulted in growth with some, but not all, of the characteristics of expansive growth. Under these conditions, tissue volume and composition changed slightly (Fig. 1–2), but were accompanied by a marked decrease in mechanical integrity (Fig. 4A–D). In contrast, the regulation of cartilage metabolism by TGF-β1 induced a state of homeostasis. Volumetric growth and biosynthetic rates were minimal under these conditions (Fig. 1A–B, Fig. 3A) and content and concentration of GAG and collagen were maintained (Fig. 2). Notably, these changes were accompanied by maintenance of the tensile mechanical integrity (Fig. 4A–D). These findings suggest that remodeling and reorganization of the tissue matrix that takes place during culture in basal medium or culture with serum, IGF-I, BMP-7, and PDGF-AB facilitates a relatively loose and weak collagen network that allows tissue expansion, while TGF-β1-supplemented culture induces conditions that restrict volumetric growth while maintaining the integrity of the collagen network.
The assessment of cartilage growth in response to growth factors required consideration of certain experimental and theoretical issues. The overall culture duration was chosen based on pilot and previous studies (Williamson et al. 2003) which indicated that changes in tissue size, biochemical content and mechanical integrity become apparent after ~2 weeks. Further analysis could address the time course of these changes. The selection of growth factors and their concentrations was based on their ability to stimulate chondrocytes in explant culture and the intent of testing physiologic concentrations. Although the dose response studies were not performed, it is likely that the concentrations of growth factors used here achieved maximal or near maximal stimulation. Analysis of articular cartilage explants incubated with 30 or 300 ng/ml of IGF-I indicated that these doses of IGF-I produced relatively similar effects on the contents of proteoglycan and collagen, and mechanical properties in compression (Sah et al. 1994; Sah et al. 1996). Stimulation of proteoglycan synthesis in cartilage explants with TGF-β1 saturated at around 10 ng/ml (Morales and Roberts 1988). Incubation with 30 or 100 ng/ml of BMP-7 showed no detectable dose-dependence of sulfate incorporation (Lietman et al. 1997). As a result, incubation with IGF-I or with TGF-β1, as well as incubation with serum, have served as control conditions for interpreting the stimulatory effects of other biochemical factors (Luyten et al. 1992; Tesch et al. 1992; Rayan and Hardingham 1994; Sah et al. 1994; Hills et al. 2005). Other growth factors (Luyten et al. 1992; Sah et al. 1994; Hills et al. 2005), such as basic fibroblast growth factor (bFGF), BMP-2, 3, 4, 9, and 13, and growth factor combinations (Trippel 1995; Loeser et al. 2005), such as IGF-I and BMP-7, which stimulate proteoglycan synthesis of calf articular cartilage explants, may also regulate cartilage growth and could be examined in future studies.
The regulatory effects of growth factors on net cell proliferation, and synthesis and deposition of matrix components within the cartilage tissue, were generally consistent with those reported in previous studies. Maintenance of the content of cells and collagen in explant cultures with serum, IGF-I, TGF-β1, or PDGF-AB is generally consistent with previous studies that investigated the effect of these factors on proliferation (Morales and Roberts 1988; Schafer et al. 1993) and collagen deposition (Morales and Roberts 1988; Luyten et al. 1992; Schafer et al. 1993). While the effects of serum, IGF-I, and BMP-7 on proteoglycan synthesis and deposition also agree with other studies (Barone-Varelas et al. 1991; Luyten et al. 1992; Schafer et al. 1993; Sah et al. 1994; Lietman et al. 1997; Nishida et al. 2000), the effects of TGF-β1 and PDGF-AB on proteoglycan synthesis are somewhat different. In contrast to results obtained here, in cartilage derived from 1–6 month old bovines, TGF-β1 stimulated proteoglycan synthesis (Morales and Roberts 1988; Morales and Hascall 1991). This difference may be due to the age of calf animals that were used, as the effects of TGF-β1 on proteoglycan synthesis appear to be dependent on the stage of development of the source tissue. In cultures of articular cartilage explants from horses aged 3 months to 14 years, stimulation of sulfate incorporation by TGF-β1 was enhanced markedly in the older but not in the immature animals (Iqbal et al. 2000). It also does not appear that the absence of stimulation of proteoglycan synthesis was due to inactivity of TGF-β1, as it did markedly stimulate the secretion of proteoglycan 4 (PRG4) (data not shown) in the explants from the S layer, consistent with the specific effect of TGF-β1 on PRG4 secretion (Schmidt et al. 2005). Results of the present study are also different than those of Schafer and coworkers, where 50 ng/ml of PDGF-AB enhanced proteoglycan synthesis ~2.6 fold over basal cultures in bovine explants derived from full thickness metacarpophalangeal cartilage (Schafer et al. 1993). This difference may be due to site-associated and depth-dependent differences in response of articular cartilage to PDGF-AB. Full-thickness tissue, with high content of chondrocytes derived from deep zones of cartilage, may have a stronger response to PDGF-AB than chondrocytes in the top ~1 mm of tissue. Indeed, although insignificant, the percent increase in the net change of GAG in explants during incubation with PDGF-AB over that during incubation in basal medium was 40% in the M layer and only 23% in the S layer (Fig. 3C). In future studies, it maybe possible to determine if the effects of a certain growth factor involve modulation of other growth factors using neutralizing antibodies (Guerne and Lotz 1991).
The growth rate of cartilage explants in vitro was generally greater than that which occurs naturally in vivo. To estimate the rate of cartilage growth in vivo, measurements of the radius of the femoral condyle of a bovine fetus at a gestational age of 207 days (based on femur and tibia length and an overall gestational duration of 282 days (Pal et al. 1981)) (11 mm), and of a 2 weeks of age bovine calf (16 mm) were used. Growth rate in terms of radial growth (proportional to radius) and volumetric growth (proportional to radius cubed) was calculated as ~54 μm/day and ~2.2 %/day, respectively and is comparable to the radial growth of articular cartilage of the femoral head in neonatal rat (~47 μm/day) (Hashimoto et al. 2002), and the volumetric growth of cartilage from the femoral condyle in immature marsupial Monodelphis domestica (~0.18 %/day) (Hayes et al. 2001). In contrast, growth rate during culture supplemented with serum or IGF-I was ~3.4–8.0 %/day or ~260 μm/day in terms of thickness (calculated based on the rate of change in thickness of the S layer (35 μm/day, Fig. 1A) and by extrapolating the rate of change in thickness of the M layer (10 μm/day, Fig. 1A) for the tissue below the first 400 μm and assuming cartilage thickness of the donor joints of ~6 mm) and ~4.1–6.3 %/day in terms of wet weight (Fig. 1B). Similarly, during a long-term serum-supplemented culture, immature bovine articular cartilage grew ~2.6 %/day in thickness and ~6.4 %/day in wet weight (Williamson et al. 2003) and cartilage from the mandibular condyle of neonatal rat extended ~8.2 %/day along the longitudinal axis (Garcia and Gray 1995). Even higher rates of growth were observed during a short-term serum-free culture of embryonic chick tibiae, where the wet weight of the cartilaginous portion increased by ~24–27 %/day (Bosmann 1968; Fitton-Jackson 1970). In addition, a direct comparison of the rate of cartilage lengthening during in vitro incubation and normal in vivo development of mandibular condyles from age-matched neonatal rats demonstrated rates of extension of up to ~3.7 %/day in vitro and a decrease in the amount of cartilage in vivo (~2.9 %/day) (Copray et al. 1983).
The indices of metabolism of certain matrix molecules were consistent with their role in determining the volumetric growth of cartilage tissue. Cartilage explants grew in volume as the content of GAG in the tissue, an indicator of swelling pressure, increased (Fig. 5B) and as the tensile modulus and strength, indicators of the integrity of the collagen network, decreased (Fig. 5C–D). Inhibition of biosynthesis with cycloheximide or at 4°C halted cartilage growth (changes in tissue size and composition) and remodeling (changes in the mechanical properties) for at least 13 days in serum-supplemented culture (data not shown), suggesting that volumetric expansion of cartilage tissue during culture is indicative of active growth and remodeling mediated by chondrocytes and is not a passive swelling process. Thus, it appears that factors that lead to growth of immature cartilage explants in vitro involve a shift in the balance between the swelling pressure of the proteoglycan molecules and the restraining ability of the collagen network, toward an overall expansive effect resulting from the swelling pressure. Studies to investigate the spatial distribution of newly deposited GAG molecules and other matrix components may provide additional insights into the mechanisms of cartilage growth.
Large extents of volumetric cartilage growth in vitro appeared to be due to deposition of proteoglycans that exceeded the deposition of collagen. Normal rate of volumetric growth of bovine cartilage from fetus to calf in vivo, involves deposition of collagen (~1.7 %/day) that exceeds that of GAG (which exhibits a ~steady content) and an increase in the tensile mechanical integrity (~5.7 %/day) (Williamson et al. 2003). A notably higher rate of volumetric growth during serum or IGF-I-supplemented growth in vitro was associated with GAG deposition (~3.3 %/day, Fig. 2B) that exceeded that of collagen (Fig. 2C) and with a decrease in the tensile mechanical integrity of the tissue (~6 %/day, Fig. 4C–D). Similarly, in comparison with articular cartilage, a higher tendency to grow is expected in cartilage of the growth plate where the proportion of GAG is higher and tensile strength and modulus are lower than those of articular cartilage (Cohen et al. 1992; Nakano and Sim 1995). Indeed, a relatively high rate of axial growth is observed in the growth plate in vivo (up to ~400 μm/day) (Wilsman et al. 1996), as well as in vitro where larger increases in length were observed in cartilages containing one or more osteogenic zones as compared to that of entirely cartilaginous explants (Copray et al. 1986).
An additional determinant of volumetric growth of cartilage tissue appeared to be the structural organization of the collagen network and its resultant restraining function. Different extents of volumetric growth in the S and M layers for a given increase in GAG content and a given decrease in mechanical integrity, as indicated by steeper regression slopes for the S layer (Fig. 5), may be associated with the depth-associated variations in the structural organization of the collagen network. It is possible that the parallel orientation of collagen fibrils that exists in the superficial zone of cartilage (Benninghoff 1925) allows the swelling pressure of the newly synthesized GAG to be dissipated between the collagen fibers, resulting in, for the S layer, growth with minimal remodeling of the collagen network. In contrast, randomly oriented collagen fibrils in the middle zone of cartilage are necessitated to bear the swelling pressure, leading to, in the M layer, growth that is accompanied by major remodeling of the collagen network and, concomitantly, a marked tensile softening and weakening.
The relationship between tensile mechanical properties and collagen concentration was generally maintained for cartilage explants from both the S and M layers at explant, as well as after incubation in vitro. During growth, the tensile material properties of ramp modulus and strength, diminished as did the concentration of collagen. The dependence of properties was similar to that observed previously (Williamson et al. 2003; Williamson et al. 2003) and agrees with the general notion that tensile properties of cartilage are primarily dependent on the collagen network. However, these relationships may be nonlinear, in that the decrease in tensile material properties was somewhat more than the decrease in concentration of collagen. The residual variation in tensile properties may be due to a variety of factors, including components of the cartilage tissue and the structural organization of tissue components. One of these major factors may be the remodeling of the collagen network that changes its stress-free state. Similar determinants can also contribute to the nonlinearity in the relationship between the change in wet weight and tissue GAG content.
Expansive cartilage growth shows many attributes of a distinct immature and growing phenotype, rather than signs of progression to a more mature state. The presence of serum, IGF-I or BMP-7 resulted in deposition of GAG that exceeded the deposition of collagen, creating swelling pressures that were excessive relative to the restraining ability of the collagen network. This metabolic imbalance facilitated a relatively loose and weak collagen network that allowed volumetric expansion. While expansive growth is also observed during incubation in basal medium and with PDGF-AB, it occurs in the absence of changes in biochemical composition. It appears that growth under these conditions may be driven by alterations in the structural organization of the collagen network itself that render it weak and malleable. Such remodeling may involve induction of various proteinases such as collagenase, which can be stimulated by PDGF (Bauer et al. 1985).
Remodeling and reorganization of cartilage tissue that occurs during incubation with TGF-β1 allows maintenance of tissue composition and function resulting in a state of homeostasis. Preservation of collagen network integrity may result from low swelling pressures, as a result of ~steady GAG content, as well as from modulation of remodeling through suppression of proteinase synthesis and stimulation of production of protease inhibitors. TGF-β has the ability to decrease the activity of various extracellular proteinases found in connective tissues, including collagenase, stromelysin, and plasminogen activator (Roberts et al. 1988; Ballock et al. 1993; Hui et al. 2003). In addition, TGF-β1 can induce secretion of protease inhibitors such as tissue inhibitors of metalloproteinase (TIMPs) (Gunther et al. 1994; Su et al. 1996; Hui et al. 2003).
These findings may facilitate the development of growth factor-based strategies for treatment and prevention of articular cartilage pathology associated with aging, injury, and disease. A decline in the concentrations of anabolic growth factors with increasing age (Florini and Roberts 1980; Clemmons and Van Wyk 1984; Yamamoto et al. 1991; Wei and Messner 1998; Iqbal et al. 2000; Chubinskaya et al. 2002) may contribute to aging-related changes in articular cartilage, while the apparent increase in the expression of certain anabolic growth factors in states of injury or disease (Soma et al. 1992; Schneiderman et al. 1995; Chambers et al. 1997; Green et al. 1997; Wei and Messner 1998; Koepp et al. 1999; Muehleman et al. 2002) may stimulate chondrocytes to repair the damaged tissue matrix. Thus, specific alteration of the growth factor environment that can shift chondrocyte metabolism towards matrix deposition (as with serum, IGF-I, or BMP-7) and stabilization (as with TGF-β1) may be used to treat or prevent conditions associated with aging, disease, and injury.
These findings may also have practical utility for tissue engineering of cartilaginous tissue for the purposes of repairing articular cartilage defects. Specific in vitro conditions may aid in designing tissue engineered constructs that attain the geometry of an in vivo defect and the structural and functional properties of native cartilage surrounding the defect. For example, the expansive growth phenotype may be useful to produce a tissue construct quickly but with characteristics typical of immature cartilage. Subsequently, methods, such as mechanical stimulation, may be used to enhance maturation of the immature cartilaginous tissue (Hung et al. 2004). The homeostasis phenotype may then be used to maintain a tissue construct or graft until implantation with a steady-state maintenance of biochemical and biomechanical characteristics.
Acknowledgments
This work was supported by the National Institute of Health and National Science Foundation.
References
- Abuzzahab MJ, Schneider A, Goddard A, Grigorescu F, Lautier C, Keller E, Kiess W, Klammt J, Kratzsch J, Osgood D, Pfaffle R, Raile K, Seidel B, Smith RJ, Chernausek SD. IGF-I receptor mutations resulting in intrauterine and postnatal growth retardation. N Engl J Med. 2003;349:2211–2222. doi: 10.1056/NEJMoa010107. [DOI] [PubMed] [Google Scholar]
- Akizuki S, Mow VC, Muller F, Pita JC, Howell DS, Manicourt DH. Tensile properties of human knee joint cartilage: I. influence of ionic conditions, weight bearing, and fibrillation on the tensile modulus. J Orthop Res. 1986;4:379–392. doi: 10.1002/jor.1100040401. [DOI] [PubMed] [Google Scholar]
- Anderson HC, Hodges PT, Aguilera XM, Missana L, Moylan PE. Bone morphogenetic protein (BMP) localization in developing human and rat growth plate, metaphysis, epiphysis, and articular cartilage. J Histochem Cytochem. 2000;48:1493–1502. doi: 10.1177/002215540004801106. [DOI] [PubMed] [Google Scholar]
- Ataliotis P. Platelet-derived growth factor A modulates limb chondrogenesis both in vivo and in vitro. Mech Dev. 2000;94:13–24. doi: 10.1016/s0925-4773(00)00321-x. [DOI] [PubMed] [Google Scholar]
- Ballock RT, Heydemann A, Wakefield LM, Flanders KC, Roberts AB, Sporn MB. TGF-beta 1 prevents hypertrophy of epiphyseal chondrocytes: regulation of gene expression for cartilage matrix proteins and metalloproteases. Dev Biol. 1993;158:414–429. doi: 10.1006/dbio.1993.1200. [DOI] [PubMed] [Google Scholar]
- Bank RA, Soudry M, Maroudas A, Mizrahi J, TeKoppele JM. The increased swelling and instantaneous deformation of osteoarthritic cartilage is highly correlated with collagen degradation. Arthritis Rheum. 2000;43:2202–2210. doi: 10.1002/1529-0131(200010)43:10<2202::AID-ANR7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Barone-Varelas J, Schnitzer TJ, Meng Q, Otten L, Thonar E. Age-related differences in the metabolism of proteoglycans in bovine articular cartilage explants maintained in the presence of insulin-like growth factor-1. Connect Tissue Res. 1991;26:101–120. doi: 10.3109/03008209109152167. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Schneiderman R, Bank RA, Wachtel E, Maroudas A. Mechanical properties of the collagen network in human articular cartilage as measured by osmotic stress technique. Arch Biochem Biophys. 1998;351:207–219. doi: 10.1006/abbi.1997.0507. [DOI] [PubMed] [Google Scholar]
- Bauer EA, Cooper TW, Huang JS, Altman J, Deuel TF. Stimulation of in vitro human skin collagenase expression by platelet-derived growth factor. Proc Natl Acad Sci U S A. 1985;82:4132–4136. doi: 10.1073/pnas.82.12.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninghoff A. Form und bau der gelenkknorpel in ihren beziehungen zur funktion. Zweiter teil: der aufbau des gelenkknorpels in seinen beziehungen zur funktion. Z Zellforsch. 1925;2:783–862. [Google Scholar]
- Bosmann HB. Cellular control of macromolecular synthesis: rates of synthesis of extracellular macromolecules during and after depletion by papain. Proc Roy Soc Lond B. 1968;169:399–425. doi: 10.1098/rspb.1968.0017. [DOI] [PubMed] [Google Scholar]
- Buckwalter JA, Mankin HJ. Articular cartilage. Part I: tissue design and chondrocyte-matrix interactions. J Bone Joint Surg Am. 1997;79-A:600–611. [PubMed] [Google Scholar]
- Buschmann MD, Grodzinsky AJ. A molecular model of proteoglycan-associated electrostatic forces in cartilage mechanics. J Biomech Eng. 1995;117:179–192. doi: 10.1115/1.2796000. [DOI] [PubMed] [Google Scholar]
- Chambers MG, Bayliss MT, Mason RM. Chondrocyte cytokine and growth factor expression in murine osteoarthritis. Osteoarthritis Cartilage. 1997;5:301–308. doi: 10.1016/s1063-4584(97)80034-9. [DOI] [PubMed] [Google Scholar]
- Chen AC, Temple MM, Ng DM, DeGroot J, Verzijl N, TeKoppele JM, Sah RL. Induction of advanced glycation endproducts alters tensile properties of articular cartilage. Arthritis Rheum. 2002;46:3212–3217. doi: 10.1002/art.10627. [DOI] [PubMed] [Google Scholar]
- Chubinskaya S, Kumar B, Merrihew C, Heretis K, Rueger DC, Kuettner KE. Age-related changes in cartilage endogenous osteogenic protein-1 (OP-1) Biochim Biophys Acta. 2002;1588:126–134. doi: 10.1016/s0925-4439(02)00158-8. [DOI] [PubMed] [Google Scholar]
- Chubinskaya S, Merrihew C, Cs-Szabo G, Mollenhauer J, McCartney J, Rueger DC, Kuettner KE. Human articular chondrocytes express osteogenic protein-1. J Histochem Cytochem. 2000;48:239–250. doi: 10.1177/002215540004800209. [DOI] [PubMed] [Google Scholar]
- Clemmons DR, Van Wyk JJ. Factors controlling blood concentration of somatomedin C. Clin Endocrinol Metab. 1984;13:113–143. doi: 10.1016/s0300-595x(84)80011-0. [DOI] [PubMed] [Google Scholar]
- Cohen B, Chorney GS, Phillips DP, Dick HM, Buckwalter JA, Ratcliffe A, Mow VC. The microstructural tensile properties and biochemical composition of the bovine distal femoral growth plate. J Orthop Res. 1992;10:263–275. doi: 10.1002/jor.1100100214. [DOI] [PubMed] [Google Scholar]
- Cole DE, Evrovski J. Quantitation of sulfate and thiosulfate in clinical samples by ion chromatography. J Chromatogr A. 1997;789:221–232. doi: 10.1016/s0021-9673(97)00821-2. [DOI] [PubMed] [Google Scholar]
- Copray JC, Jansen HW, Duterloo HS. Growth of the mandibular condylar cartilage of the rat in serum-free organ culture. Arch Oral Biol. 1983;28:967–974. doi: 10.1016/0003-9969(83)90095-x. [DOI] [PubMed] [Google Scholar]
- Copray JC, Jansen HW, Duterloo HS. Growth and growth pressure of mandibular condylar and some primary cartilages of the rat in vitro. Am J Orthod Dentofacial Orthop. 1986;90:19–28. doi: 10.1016/0889-5406(86)90023-5. [DOI] [PubMed] [Google Scholar]
- Cowin SC. Tissue growth and remodeling. Annu Rev Biomed Eng. 2004;6:77–107. doi: 10.1146/annurev.bioeng.6.040803.140250. [DOI] [PubMed] [Google Scholar]
- DiMicco MA, Waters SN, Akeson WH, Sah RL. Integrative articular cartilage repair: dependence on developmental stage and collagen metabolism. Osteoarthritis Cartilage. 2002;10:218–225. doi: 10.1053/joca.2001.0502. [DOI] [PubMed] [Google Scholar]
- Eisenberg SR, Grodzinsky AJ. Electrokinetic micromodel of extracellular matrix and other polyelectrolyte networks. Physicochemical Hydrodynamics. 1988;10:517–539. [Google Scholar]
- Ellingsworth LR, Brennan JE, Fok K, Rosen DM, Bentz H, Piez KA, Seyedin SM. Antibodies to the N-terminal portion of cartilage-inducing factor A and transforming growth factor beta. Immunohistochemical localization and association with differentiating cells. J Biol Chem. 1986;261:12362–12367. [PubMed] [Google Scholar]
- Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- Fitton-Jackson S. Environmental control of macromolecular synthesis in cartilage and bone: morphogenetic response to hyaluronidase. Proc Roy Soc Lond B. 1970;175:405–453. doi: 10.1098/rspb.1970.0029. [DOI] [PubMed] [Google Scholar]
- Florini JR, Roberts SB. Effect of rat age on blood levels of somatomedin-like growth factors. J Gerontol. 1980;35:23–30. doi: 10.1093/geronj/35.1.23. [DOI] [PubMed] [Google Scholar]
- Fortier LA, Kornatowski MA, Mohammed HO, Jordan MT, O’Cain LC, Stevens WB. Age-related changes in serum insulin-like growth factor-I, insulin-like growth factor-I binding protein-3 and articular cartilage structure in Thoroughbred horses. Equine Vet J. 2005;37:37–42. doi: 10.2746/0425164054406838. [DOI] [PubMed] [Google Scholar]
- Francis-West PH, Robertson KE, Ede DA, Rodriguez C, Izpisua-Belmonte JC, Houston B, Burt DW, Gribbin C, Brickell PM, Tickle C. Expression of genes encoding bone morphogenetic proteins and sonic hedgehog in talpid (ta3) limb buds: their relationships in the signalling cascade involved in limb patterning. Dev Dyn. 1995;203:187–197. doi: 10.1002/aja.1002030207. [DOI] [PubMed] [Google Scholar]
- Fukumura K, Matsunaga S, Yamamoto T, Nagamine T, Ishidou Y, Sakou T. Immunolocalization of transforming growth factor-beta s and type I and type II receptors in rat articular cartilage. Anticancer Res. 1998;18:4189–4193. [PubMed] [Google Scholar]
- Garcia AM, Gray ML. Dimensional growth and extracellular matrix accumulation by neonatal rat mandibular condyles in long-term culture. J Orthop Res. 1995;13:208–219. doi: 10.1002/jor.1100130209. [DOI] [PubMed] [Google Scholar]
- Green RJ, Usui ML, Hart CE, Ammons WF, Narayanan AS. Immunolocalization of platelet-derived growth factor A and B chains and PDGF-alpha and beta receptors in human gingival wounds. J Periodontal Res. 1997;32:209–214. doi: 10.1111/j.1600-0765.1997.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Grodzinsky AJ. Electromechanical and physicochemical properties of connective tissue. CRC Crit Rev Bioeng. 1983;9:133–199. [PubMed] [Google Scholar]
- Guerne PA, Lotz M. Interleukin-6 and transforming growth factor-beta synergistically stimulate chondrosarcoma cell proliferation. J Cell Physiol. 1991;149:117–124. doi: 10.1002/jcp.1041490115. [DOI] [PubMed] [Google Scholar]
- Gunther M, Haubeck HD, van de Leur E, Blaser J, Bender S, Gutgemann I, Fischer DC, Tschesche H, Greiling H, Heinrich PC, Graeve L. Transforming growth factor beta 1 regulates tissue inhibitor of metalloproteinases-1 expression in differentiated human articular chondrocytes. Arthritis Rheum. 1994;37:395–405. doi: 10.1002/art.1780370314. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Kihara I, Otani H. Perinatal development of the rat hip joint with restrained fetal movement. Congenit Anom (Kyoto) 2002;42:135–142. doi: 10.1111/j.1741-4520.2002.tb00863.x. [DOI] [PubMed] [Google Scholar]
- Hayes AJ, MacPherson S, Morrison H, Dowthwaite G, Archer CW. The development of articular cartilage: evidence for an appositional growth mechanism. Anat Embryol (Berl) 2001;203:469–479. doi: 10.1007/s004290100178. [DOI] [PubMed] [Google Scholar]
- Heine U, Munoz EF, Flanders KC, Ellingsworth LR, Lam HY, Thompson NL, Roberts AB, Sporn MB. Role of transforming growth factor-beta in the development of the mouse embryo. J Cell Biol. 1987;105:2861–2876. doi: 10.1083/jcb.105.6.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbage D, Bouillet J, Bernengo J-C. Biochemical and physicochemical characterization of pepsin-solubilized type-II collagen from bovine articular cartilage. Biochem J. 1977;161:303–312. doi: 10.1042/bj1610303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills RL, Belanger LM, Morris EA. Bone morphogenetic protein 9 is a potent anabolic factor for juvenile bovine cartilage, but not adult cartilage. J Orthop Res. 2005;23:611–617. doi: 10.1016/j.orthres.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Horner A, Bord S, Kemp P, Grainger D, Compston JE. Distribution of platelet-derived growth factor (PDGF) A chain mRNA, protein, and PDGF-alpha receptor in rapidly forming human bone. Bone. 1996;19:353–362. doi: 10.1016/s8756-3282(96)00217-7. [DOI] [PubMed] [Google Scholar]
- Horner A, Kemp P, Summers C, Bord S, Bishop NJ, Kelsall AW, Coleman N, Compston JE. Expression and distribution of transforming growth factor-beta isoforms and their signaling receptors in growing human bone. Bone. 1998;23:95–102. doi: 10.1016/s8756-3282(98)00080-5. [DOI] [PubMed] [Google Scholar]
- Hui W, Cawston T, Rowan AD. Transforming growth factor beta 1 and insulin-like growth factor 1 block collagen degradation induced by oncostatin M in combination with tumour necrosis factor alpha from bovine cartilage. Ann Rheum Dis. 2003;62:172–174. doi: 10.1136/ard.62.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung CT, Mauck RL, Wang CC, Lima EG, Ateshian GA. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng. 2004;32:35–49. doi: 10.1023/b:abme.0000007789.99565.42. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Dudhia J, Bird JL, Bayliss MT. Age-related effects of TGF-beta on proteoglycan synthesis in equine articular cartilage. Biochem Biophys Res Commun. 2000;274:467–471. doi: 10.1006/bbrc.2000.3167. [DOI] [PubMed] [Google Scholar]
- Kempson GE. Relationship between the tensile properties of articular cartilage from the human knee and age. Ann Rheum Dis. 1982;41:508–511. doi: 10.1136/ard.41.5.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Sah RLY, Doong JYH, Grodzinsky AJ. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174:168–176. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- Klisch SM, Chen SS, Sah RL, Hoger A. A growth mixture theory for cartilage with applications to growth-related experiments on cartilage explants. J Biomech Eng. 2003;125:169–179. doi: 10.1115/1.1560144. [DOI] [PubMed] [Google Scholar]
- Koepp HE, Sampath KT, Kuettner KE, Homandberg GA. Osteogenic protein-1 (OP-1) blocks cartilage damage caused by fibronectin fragments and promotes repair by enhancing proteoglycan synthesis. Inflamm Res. 1999;48:199–204. doi: 10.1007/s000110050446. [DOI] [PubMed] [Google Scholar]
- Lai WM, Hou JS, Mow VC. A triphasic theory for the swelling and deformation behaviors of articular cartilage. J Biomech Eng. 1991;113:245–258. doi: 10.1115/1.2894880. [DOI] [PubMed] [Google Scholar]
- Laufer E, Pizette S, Zou H, Orozco OE, Niswander L. BMP expression in duck interdigital webbing: a reanalysis. Science. 1997;278:305. doi: 10.1126/science.278.5336.305. [DOI] [PubMed] [Google Scholar]
- Lietman SA, Yanagishita M, Sampath TK, Reddi AH. Stimulation of proteoglycan synthesis in explants of porcine articular cartilage by recombinant osteogenic protein-1 (bone morphogenetic protein-7) J Bone Joint Surg Am. 1997;79:1132–1137. doi: 10.2106/00004623-199708000-00003. [DOI] [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- Loeser RF, Chubinskaya S, Pacione C, Im HJ. Basic fibroblast growth factor inhibits the anabolic activity of insulin-like growth factor 1 and osteogenic protein 1 in adult human articular chondrocytes. Arthritis Rheum. 2005;52:3910–3917. doi: 10.1002/art.21472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 1995;9:2808–2820. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- Luyten FP, Hascall VC, Nissley SP, Morales TI, Reddi AH. Insulin-like growth factors maintain steady-state metabolism of proteoglycans in bovine articular cartilage explants. Arch Biochem Biophys. 1988;267:416–425. doi: 10.1016/0003-9861(88)90047-1. [DOI] [PubMed] [Google Scholar]
- Luyten FP, Yu YM, Yanagishita M, Vukicevic S, Hammonds RG, Reddi AH. Natural bovine osteogenin and recombinant human bone morphogenetic protein-2B are equipotent in the maintenance of proteoglycans in bovine articular cartilage explant cultures. J Biol Chem. 1992;267:3691–3695. [PubMed] [Google Scholar]
- Maroudas A. Balance between swelling pressure and collagen tension in normal and degenerate cartilage. Nature. 1976;260:808–809. doi: 10.1038/260808a0. [DOI] [PubMed] [Google Scholar]
- Maroudas A. Physico-chemical properties of articular cartilage. In: Freeman MAR, editor. Adult Articular Cartilage. Pitman Medical; Tunbridge Wells, England: 1979. [Google Scholar]
- Maroudas A, Venn M. Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage. II. Swelling. Ann Rheum Dis. 1977;36:399–406. doi: 10.1136/ard.36.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda K, Shirota H, Thonar EJ-MA. Quantification of 35S-labeled proteoglycans complexed to alcian blue by rapid filtration in multiwell plates. Anal Biochem. 1994;217:167–175. doi: 10.1006/abio.1994.1105. [DOI] [PubMed] [Google Scholar]
- McGowan KB, Kurtis MS, Lottman LM, Watson D, Sah RL. Biochemical quantification of DNA in human articular and septal cartilage using PicoGreen and Hoechst 33258. Osteoarthritis Cartilage. 2002;10:580–587. doi: 10.1053/joca.2002.0794. [DOI] [PubMed] [Google Scholar]
- McQuillan DJ, Handley CJ, Campbell MA, Bolis S, Milway VE, Herington AC. Stimulation of proteoglycan biosynthesis by serum and insulin-like growth factor-I in cultured bovine articular cartilage. Biochem J. 1986;240:423–430. doi: 10.1042/bj2400423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales TI, Hascall VC. Transforming growth factor-β1 stimulates synthesis of proteoglycan aggregates in calf articular organ cultures. Arch Biochem Biophys. 1991;286:99–106. doi: 10.1016/0003-9861(91)90013-9. [DOI] [PubMed] [Google Scholar]
- Morales TI, Joyce ME, Soble ME, Danielpour D, Roberts AB. Transforming growth factor-β in calf articular cartilage organ cultures: synthesis and distribution. Arch Biochem Biophys. 1991;288:397–405. doi: 10.1016/0003-9861(91)90212-2. [DOI] [PubMed] [Google Scholar]
- Morales TI, Roberts AB. Transforming growth factor-β regulates the metabolism of proteoglycans in bovine cartilage organ cultures. J Biol Chem. 1988;263:12828–12831. [PubMed] [Google Scholar]
- Moroco JR, Hinton R, Buschang P, Milam SB, Iacopino AM. Type II collagen and TGF-betas in developing and aging porcine mandibular condylar cartilage: immunohistochemical studies. Cell Tissue Res. 1997;289:119–124. doi: 10.1007/s004410050857. [DOI] [PubMed] [Google Scholar]
- Mow VC, Ratcliffe A. Structure and function of articular cartilage and meniscus. In: Mow VC, Hayes WC, editors. Basic Orthopaedic Biomechanics. Raven Press; New York: 1997. [Google Scholar]
- Muehleman C, Kuettner KE, Rueger DC, Ten Dijke P, Chubinskaya S. Immunohistochemical localization of osteogenetic protein (OP-1) and its receptors in rabbit articular cartilage. J Histochem Cytochem. 2002;50:1341–1350. doi: 10.1177/002215540205001007. [DOI] [PubMed] [Google Scholar]
- Nakano T, Sim JS. A study of the chemical composition of the proximal tibial articular cartilage and growth plate of broiler chickens. Poult Sci. 1995;74:538–550. doi: 10.3382/ps.0740538. [DOI] [PubMed] [Google Scholar]
- Nishida Y, Knudson CB, Kuettner KE, Knudson W. Osteogenic protein-1 promotes the synthesis and retention of extracellualr matrix within bovine articular cartilage and chondrocyte cultures. Osteoarthritis Cartilage. 2000;8:127–136. doi: 10.1053/joca.1999.0281. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A, Lonai P. Platelet-derived growth factor-A and its receptor are expressed in separate, but adjacent cell layers of the mouse embryo. Development. 1992;115:1045–1058. doi: 10.1242/dev.115.4.1045. [DOI] [PubMed] [Google Scholar]
- Pal S, Tang L-H, Choi H, Habermann E, Rosenberg L, Roughley P, Poole AR. Structural changes during development in bovine fetal epiphyseal cartilage. Collagen Rel Res. 1981;1:151–176. doi: 10.1016/s0174-173x(81)80017-9. [DOI] [PubMed] [Google Scholar]
- Ralphs JR, Wylie L, Hill DJ. Distribution of insulin-like growth factor peptides in the developing chick embryo. Development. 1990;109:51–58. doi: 10.1242/dev.109.1.51. [DOI] [PubMed] [Google Scholar]
- Rayan V, Hardingham T. The recover of articular cartilage in explant culture from interleukin-1α: effects on proteoglycan synthesis and degradation. Matrix Biol. 1994;14:263–271. doi: 10.1016/0945-053x(94)90190-2. [DOI] [PubMed] [Google Scholar]
- Ren P, Rowland GN, 3rd, Halper J. Expression of growth factors in chicken growth plate with special reference to tibial dyschondroplasia. J Comp Pathol. 1997;116:303–320. doi: 10.1016/s0021-9975(97)80005-9. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Flanders KC, Kondaiah P, Thompson NL, Van Obberghen-Schilling E, Wakefield L, Rossi P, de Crombrugghe B, Heine U, Sporn MB. Transforming growth factor beta: biochemistry and roles in embryogenesis, tissue repair and remodeling, and carcinogenesis. Recent Prog Horm Res. 1988;44:157–197. doi: 10.1016/b978-0-12-571144-9.50010-7. [DOI] [PubMed] [Google Scholar]
- Sah RL, Chen AC, Grodzinsky AJ, Trippel SB. Differential effects of bFGF and IGF-I on matrix metabolism in calf and adult bovine cartilage explants. Arch Biochem Biophys. 1994;308:137–147. doi: 10.1006/abbi.1994.1020. [DOI] [PubMed] [Google Scholar]
- Sah RL, Doong JYH, Grodzinsky AJ, Plaas AHK, Sandy JD. Effects of compression on the loss of newly synthesized proteoglycans and proteins from cartilage explants. Arch Biochem Biophys. 1991;286:20–29. doi: 10.1016/0003-9861(91)90004-3. [DOI] [PubMed] [Google Scholar]
- Sah RL, Trippel SB, Grodzinsky AJ. Differential effects of serum, insulin-like growth factor-I, and fibroblast growth factor-2 on the maintenance of cartilage physical properties during long-term culture. J Orthop Res. 1996;14:44–52. doi: 10.1002/jor.1100140109. [DOI] [PubMed] [Google Scholar]
- Schafer SJ, Luyten FP, Yanagishita M, Reddi AH. Proteoglycan metabolism is age related and modulated by isoforms of platelet-derived growth factor in bovine articular cartilage explant cultures. Arch Biochem Biophys. 1993;302:431–438. doi: 10.1006/abbi.1993.1236. [DOI] [PubMed] [Google Scholar]
- Schinagl RM. Depth-Dependent Mechanical Properties and Chondrocyte Adhesion to Articular Cartilage. University of California; San Diego, La Jolla: 1997. [Google Scholar]
- Schmidt TA, Schumacher BL, Han EH, Klein TJ, Voegtline MS, Sah RL. Chemo-mechanical coupling in articular cartilage: IL-1a and TGF-β1 regulate chondrocyte synthesis and secretion of proteoglycan 4. In: Aaron RK, Bolander ME, editors. Physical Regulation of Skeletal Repair. American Academy of Orthopaedic Surgeons; Chicago: 2005. [Google Scholar]
- Schneiderman R, Rosenberg N, Hiss J, Lee P, Liu F, Hintz RL, Maroudas A. Concentration and size distribution of insulin-like growth factor-I in human normal and osteoarthritic synovial fluid and cartilage. Arch Biochem Biophys. 1995;324:173–188. doi: 10.1006/abbi.1995.9913. [DOI] [PubMed] [Google Scholar]
- Soder S, Hakimiyan A, Rueger DC, Kuettner KE, Aigner T, Chubinskaya S. Antisense inhibition of osteogenic protein 1 disturbs human articular cartilage integrity. Arthritis Rheum. 2005;52:468–478. doi: 10.1002/art.20856. [DOI] [PubMed] [Google Scholar]
- Soma Y, Dvonch V, Grotendorst GR. Platelet-derived growth factor AA homodimer is the predominant isoform in human platelets and acute human wound fluid. Faseb J. 1992;6:2996–3001. doi: 10.1096/fasebj.6.11.1644262. [DOI] [PubMed] [Google Scholar]
- Stern BD, Mechanic GL, Glimcher MJ. The resorption of bone collagen in tissue culture. Biochem Biophys Res Commun. 1963;13:137–143. [Google Scholar]
- Su S, Dehnade F, Zafarullah M. Regulation of tissue inhibitor of metalloproteinases-3 gene expression by transforming growth factor-beta and dexamethasone in bovine and human articular chondrocytes. DNA Cell Biol. 1996;15:1039–1048. doi: 10.1089/dna.1996.15.1039. [DOI] [PubMed] [Google Scholar]
- Taber LA. Biomechanics of cardiovascular development. Annu Rev Biomed Eng. 2001;3:1–25. doi: 10.1146/annurev.bioeng.3.1.1. [DOI] [PubMed] [Google Scholar]
- Tesch GH, Handley CJ, Cornell HJ, Herington AC. Effects of free and bound insulin-like growth factors on proteoglycan metabolism in articular cartilage explants. J Orthop Res. 1992;10:14–22. doi: 10.1002/jor.1100100103. [DOI] [PubMed] [Google Scholar]
- Thonar EJ-M, Sweet MBE. Maturation-related changes in proteoglycans of fetal articular cartilage. Arch Biochem Biophys. 1981;208:535–547. doi: 10.1016/0003-9861(81)90542-7. [DOI] [PubMed] [Google Scholar]
- Trippel SB. Growth factor actions on articular cartilage. J Rheumatol. 1995;43S:129–132. [PubMed] [Google Scholar]
- Venn MF, Maroudas A. Chemical composition and swelling of normal and osteoarthritic femoral head cartilage. I. Chemical composition. Ann Rheum Dis. 1977;36:121–129. doi: 10.1136/ard.36.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Messner K. Age- and injury-dependent concentrations of transforming growth factor-beta 1 and proteoglycan fragments in rabbit knee joint fluid. Osteoarthritis Cartilage. 1998;6:10–18. doi: 10.1053/joca.1997.0087. [DOI] [PubMed] [Google Scholar]
- Williamson AK, Chen AC, Masuda K, Thonar EJ-MA, Sah RL. Tensile mechanical properties of bovine articular cartilage: variations with growth and relationships to collagen network components. J Orthop Res. 2003;21:872–880. doi: 10.1016/S0736-0266(03)00030-5. [DOI] [PubMed] [Google Scholar]
- Williamson AK, Chen AC, Sah RL. Compressive properties and function-composition relationships of developing bovine articular cartilage. J Orthop Res. 2001;19:1113–1121. doi: 10.1016/S0736-0266(01)00052-3. [DOI] [PubMed] [Google Scholar]
- Williamson AK, Masuda K, Thonar EJ-MA, Sah RL. Growth of immature articular cartilage in vitro: correlated variation in tensile biomechanical and collagen network properties. Tissue Eng. 2003;9:625–634. doi: 10.1089/107632703768247322. [DOI] [PubMed] [Google Scholar]
- Wilsman NJ, Farnum CE, Leiferman EM, Fry M, Barreto C. Differential growth by growth plates as a function of multiple parameters of chondrocytic kinetics. J Orthop Res. 1996;14:927–936. doi: 10.1002/jor.1100140613. [DOI] [PubMed] [Google Scholar]
- Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Wong M, Ponticiello M, Kovanen V, Jurvelin JS. Volumetric changes of articular cartilage during stress relaxation in unconfined compression. J Biomech. 2000;33:1049–1054. doi: 10.1016/s0021-9290(00)00084-1. [DOI] [PubMed] [Google Scholar]
- Woo SL-Y, Akeson WH, Jemmott GF. Measurements of nonhomogeneous directional mechanical properties of articular cartilage in tension. J Biomech. 1976;9:785–791. doi: 10.1016/0021-9290(76)90186-x. [DOI] [PubMed] [Google Scholar]
- Woods KA, Camacho-Hubner C, Savage MO, Clark AJ. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med. 1996;335:1363–1367. doi: 10.1056/NEJM199610313351805. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Sohmiya M, Oka N, Kato Y. Effects of aging and sex on plasma insulin-like growth factor I (IGF-I) levels in normal adults. Acta Endocrinol (Copenh) 1991;124:497–500. doi: 10.1530/acta.0.1240497. [DOI] [PubMed] [Google Scholar]