Essential thrombocythemia is a myeloproliferative neoplasm characterized by an increased risk of both arterial and venous thrombosis. The findings of this study show that patients with this disorder have elevated levels of platelet-and endothelium-derived microparticles, which may support thrombin generation and play a role in the pathophysiology of thromboembolic complications.
Keywords: microparticles, essential thrombocythemia, E-selectin, thrombin generation, von Willebrand factor
Abstract
Background
Most cell types, including blood - and vascular cells, produce microparticles upon activation. Since cellular microparticles are known to be elevated in thromboembolic diseases, we hypothesized a role for microparticles in the pathogenesis of thrombosis in essential thrombocythemia.
Design and Methods
In plasma samples from 21 patients with essential thrombocythemia and ten healthy subjects, the levels and the cellular origin of microparticles were determined by flowcytometric analysis, while the microparticle-associated procoagulant activity was measured using a thrombin generation assay.
Results
Patients with essential thrombocythemia had significantly higher numbers of circulating annexin V-positive microparticles than controls (median 4500 vs. 2500×106 events/L; p=0.039), including significantly higher numbers of microparticles positive for the platelet marker CD61 (p=0.043), the endothelial markers CD62E (p=0.009) and CD144 (p=0.021), and for tissue factor (p=0.036). CD62E was co-expressed with the platelet marker CD41 on microparticles, suggesting a bilineage origin of such microparticles, which were observed only in patients with risk factors for thrombosis. Patients with essential thrombocythemia had higher plasma levels of mature von Willebrand factor (p=0.045) but similar propeptide levels compared to controls. In thrombin generation analyses, microparticle-rich plasma from patients with essential thrombocythemia had a shorter lag time (p=0.001) and higher peak height (p=0.038) than plasma from controls. Peak height correlated significantly with the total number of microparticles (R=0.634, p<0.001).
Conclusions
Patients with essential thrombocythemia had higher number of circulating microparticles with platelet and endothelial markers, suggesting ongoing platelet and endothelial activation. This was confirmed by an increased level of mature von Willebrand factor, an abnormal mature von Willebrand factor/propeptide ratio, and a hypercoagulable state reflected in thrombin generation. These findings suggest a role for microparticles in thrombosis in essential thrombocythemia.
Introduction
Cellular microparticles are plasma membrane vesicles of <1.5 μm in diameter, mainly composed of lipids and proteins, which are released into the circulation by blood-cells and vascular cells during cellular activation or apoptosis.1 Microparticles are heterogeneous, differing in size, as well as in phospholipid and protein composition. In addition, microparticles display some specific cell surface proteins that indicate their cellular origin. Depending on the cellular process and the cellular origin triggering their formation, the outer surfaces of cellular microparticles may contain phosphatidylserine, which provides a suitable anionic phospholipid surface for assembly of the tenase and prothrombinase complexes, and they may express tissue factor (TF), the primary initiator of coagulation.2 Such phosphatidylserine- and/or TF-bearing microparticles may contribute to the pathogenesis of thrombosis in different diseases, including cancer–associated thrombosis and sepsis.3 Indeed, the numbers and characteristics of circulating microparticles have been found to be altered in many vascular diseases associated with an increased risk of both arterial and venous thrombosis. In particular, elevated numbers of platelet-derived microparticles have been described in diabetes mellitus,4 acute coronary syndrome,5 myocardial infarction6 and disseminated intravascular coagulation.7 Elevated levels of endothelial-derived microparticles have been found in venous thromboembolism8 and in the antiphosholipid syndrome.9 The majority (more than 90%) of microparticles in healthy controls are of platelet origin, whereas less than 10% originate from granulocytes and less than 5% from endothelial cells, red blood cells and monocytes.10
Essential thrombocythemia (ET) is a chronic myeloproliferative disease characterized by an increased risk of both arterial and venous thrombosis. At the time of diagnosis approximately 20% of ET patients have had a major thrombotic event and approximately another 20% will subsequently have an event.11 This makes venous and (more often) arterial thrombosis the leading causes of morbidity and mortality in ET. Established risk factors for thrombosis in ET are older age (over 60 years) and previous thrombotic events.12 Recently, leukocytosis has been identified as an additional risk factor.11,13,14 Numerous mechanisms, including blood hyperviscosity and quantitative/qualitative abnormalities of blood cells, have been advocated to be at the origin of the hypercoagulable state in these patients.15 An increased number of platelets,16 abnormal function of platelets,12 activation of platelets and leukocytes,17,18 their interaction to form platelet-leukocyte aggregates,17 and endothelial activation may all contribute to the increased thrombotic state in ET, which is still not completely understood.
In the present study, we investigated the numbers and the procoagulant potential of circulating microparticles in a group of patients with ET, using flow cytometry and a thrombin generation assay, respectively. In addition, levels of mature von Willebrand factor (vWF) and propeptide, and soluble E-selectin were determined in the same plasma samples as a measure of ongoing endothelial activation.
Design and Methods
Study subjects
Twenty-one consecutive ET patients (8 males and 13 females, median age: 58, range: 34–83 years) were enrolled at the Hematology Department of Bergamo Hospital (Italy), after giving informed consent. Patients were diagnosed as having ET according to the Polycythemia Vera Study Group (PVSG) criteria.19 All investigations were approved by the local ethical committee (Comitato di Bioetica, Ospedali Riuniti, Bergamo, Italy). The patients’ characteristics are shown in Table 1. Nine patients were heterozygous carriers for the JAK2V617F mutation. At the time of the sample collection 18 patients were receiving treatment with aspirin, eight with hydroxyurea and three patients were not receiving any treatment. The sample size was chosen based on a power calculation using data from a preceding unpublished pilot study in which the mean number of microparticles in control subjects was 3400×106/L and that in ET patients was 7500×106/L. With an α of 0.05 this calculation showed that nine controls and 18 ET patients would provide the analysis with sufficient power. In addition, we investigated ten healthy controls (6 males and 4 females, median age 46, range 19–59). None of the healthy controls was on antiplatelet medication at the time of blood collection. Controls were significantly younger than ET patients (p=0.015).
Table 1.
Characteristics of study subjects at enrollment into the study. Data are presented as median (range) or number (%).
Blood collection and isolation of microparticles
Blood samples were drawn early in the morning, before any therapy, with a 21-gauge needle after applying a light tourniquet. After discarding the first 3 mL, blood was collected into a 5 mL tube containing 3.2% citrate (BD, Plymouth, UK). Plasma was prepared within 30 min after blood collection by centrifugation for 20 min at 1,550 x g at room temperature, without brake. Aliquots of plasma were snap-frozen in liquid nitrogen, and then stored at −75°C until use. In order to isolate the microparticles, 250 μL of plasma were thawed on ice for 60 min and then centrifuged for 30 min at 17,570 x g at 20°C. Subsequently, 225 μL of supernatant (i.e. microparticle-free plasma) were removed. The remaining 25 μL containing the microparticle pellet were resuspended in 225 μL of phosphate-buffered saline (PBS; 154 mM NaCl, 1.4 mM phosphate, pH 7.4), containing 10.9 mM trisodium citrate to prevent coagulation activation. Samples were centrifuged for 30 min at 17,570 x g at 20 °C; thereafter, 225 μL of supernatant were removed and the microparticle pellet was resuspended in 125 μL of PBS.
Phenotypic analysis of plasma-derived microparticles
Flowcytometric analysis was used to quantify and characterize plasma-derived microparticles, as previously described.20 Briefly, 5 μL of microparticle sample were diluted in 35 μL PBS containing 2.5 mM CaCl2 (pH 7.4). The samples were then incubated for 30 min at room temperature in the dark with 5 μL annexin V-allophyco-cyanin (Caltag Laboratories, Burlingame, CA, USA) and/or 5 μL fluorescein isothiocyanate (FITC)-, phycoery-thrin (PE)-, peridinin-chlorophyll-protein complex (PerCP)- labeled anti-human monoclonal antibodies, or 5 μL isotype-matched control monoclonal antibodies.
For the phenotypic characterization of microparticles the following cell-specific monoclonal antibodies were used: anti-CD8-PE (SK1, IgG1), anti-CD14-PE (MΦP9, IgG2b), anti-CD15-PE (HI98, IgM), anti-CD20-PE (L27, IgG1), anti-CD45-PerCP (H130, IgGλ, κ), anti-CD61-FITC (VI-PL2, IgG1), anti-CD63-FITC (H5C6, IgG1), anti-CD146-PE (P1H12, IgG1), labeled isotype controls IgG1 (X40) and IgG2a (X39), all from Becton Dickinson (San Jose, USA); IgG2b-PE, anti-glycophorin A-FITC (JC159, IgG1) and anti-CD41-FITC (5B12, IgG1) from DAKO (Glostrup, Denmark); anti-CD144-FITC (BMS158FI, IgG1) from Bender MedSystems (Vienna, Austria); anti-CD106-FITC (1.G11B1, IgG1) from Calbiochem (Darmstadt, Germany); anti-CD54-PE (K562, IgG1), anti-CD66b-FITC (80H3, IgG1κ) and anti-CD62P-PE (CLB-Thromb/6, IgG1) from Immunotech (Marseille, France); anti-CD62E-PE (HAE-1f, IgG1) from Kordia (Leiden, The Netherlands); anti-CD4-PE (CLB-T4/2, IgG1) and anti-CD66acde-PE (CLB-gran/10, IH4Fc, IgG1) from Sanquin (Amsterdam, The Netherlands).
For TF measurement on microparticles, anti-TF-FITC from American Diagnostics (VD8, IgG1, Stamford, CT, USA) was used in the same experimental conditions.
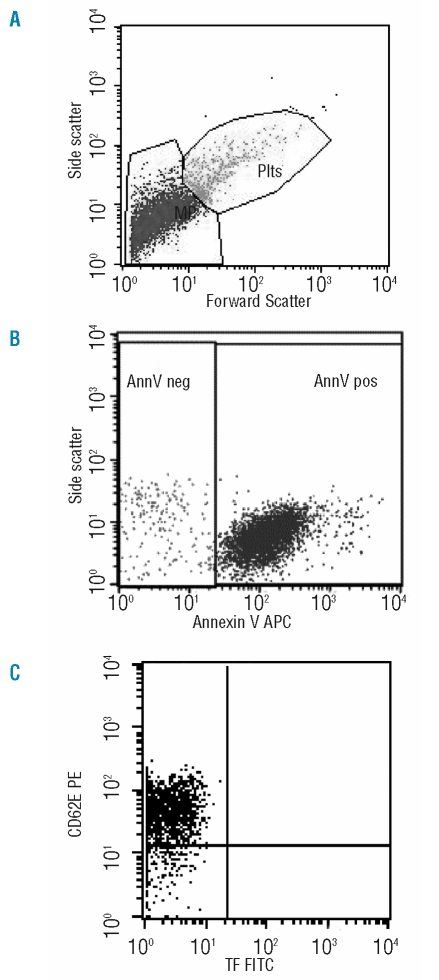
After incubation, 760 μL PBS/calcium buffer were added and the samples were analyzed on a FACS Calibur using Worklist Manager (BD) for 1 min during which the flowcytometer analyzed approximately 55 μL of the suspension. Forward scatter (FSC) and sideward scatter (SSC) were set at logarithmic gain. To distinguish microparticles from events due to noise, microparticles were identified on the basis of their specific FSC and SSC characteristics, with gates set using in vitro platelet activation and microparticle generation data (results not shown), and by annexin V positivity (Figure 1A and 1B).21 To identify annexin V-positive events, a threshold was set in a microparticle sample prepared without calcium. The number of microparticles per liter of plasma was calculated as previously described # MPsper minute *(Volume(V) tube/Vminute) *(Vend/ Vstart) * (1000/ Vlabeled)= # events/mL.7 Data were analyzed with CellQuest-pro software (Becton Dickinson).
Figure 1.
Flow cytometric analysis of microparticles. A representative set of scattergrams from flowcytometric microparticle analysis in a sample from an ET patient is shown to illustrate microparticle and subpopulation definition. Panel A: Forward and side scatter were used to define the microparticle (MP) and platelet (Plts) gates as shown. Panel B: Events defined as microparticles were then selected for their annexin V binding, determined by positivity for annexin V-allophycocyanin fluorescence (on the x-axis). Panel C: Annexin V-positive microparticles were further examined for expression of other antigens by co-labeling with PE - and FITC- labeled antibodies as is shown here for CD62E-PE and TF-FITC binding.
Thrombin generation measurements
Thrombin generation in platelet-poor but microparticle-rich plasma, as prepared for microparticle isolation (described above), was measured with the calibrated automated thrombogram method (Thrombinoscope BV, Maastricht, The Netherlands).22,23 Thrombin generation was triggered in 80 μL of plasma by different conditions; 1 pM of TF and 4 μM of phospholipids, 4 μM of phospholipids alone, and buffer (no exogenous TF or phospholipids added), using reagents obtained from Thrombinoscope BV. Thrombin generation was measured as fluorescence, read in a Fluoroskan Ascent reader (Thermo Labsystems OY, Helsinki, Finland) equipped with a 390/460 filter set and thrombin generation curves were calculated with the Thrombinoscope software (Thrombinoscope BV). Three parameters were derived from the thrombin generation curves: lag time (initiation phase of coagulation), endogenous thrombin potential, and peak height. Lag time was defined as the time to reach 1/6 of the peak height. Validation of the calibrated automated thrombogram method showed normalization of non-time-dependent parameters to be mandatory to obtain acceptable inter-assay variations.23 Intra-assay variations for normalized parameters are typically below 6%, and inter-assay variations below 8%.23 Therefore, each thrombin generation measurement includes normal pooled plasma and both the endogenous thrombin potential and peak height values are expressed as the ratio of patient’s value to the value in normal pooled plasma, expressed in percentages.
Plasma markers of endothelial activation
Plasma concentrations of soluble E-selectin were determined by a commercially available enzyme-linked immunosorbent assay (ELISA), according to the manufacturer’s instructions (Diaclone, Cedex, France). Mature vWF and propeptide plasma levels were measured by ELISA as described previously.24 The half-life of mature vWF is four times that of the propeptide half-life, and due to this difference in half-life their relative concentration is a distinctive indicator of ongoing chronic as opposed to acute endothelial activation.25
Statistical analysis
We established the statistical significance of differences in microparticle numbers between groups with the non-parametric Mann-Whitney U and Kruskal-Wallis tests, where appropriate. Bivariate correlations were estimated by Spearman’s rank correlation (R). All tests for statistical significance were two-tailed and p values less than 0.05 were considered statistically significant. Analyses were performed using SPSS 15.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Number and phenotypic characterization of microparticles
The total number of microparticles is significantly higher in ET patients (median: 4500×106/L) than in controls (2500×106/L, p=0.039). For most study subjects, more than 90% of circulating microparticles bound to annexin-V, indicating the presence of phosphatidylserine on their membrane (Figure 1A and 1B).
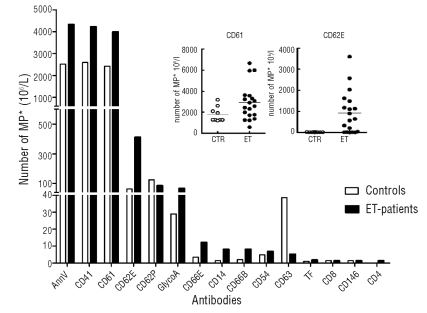
The phenotypic characterization of microparticles depicted in Figure 2 showed that the subset composition of the microparticle population (i.e. of platelet -, leukocyte -, endothelial cell or erythrocyte origin) is similar in ET and controls. The large majority of microparticles is of platelet origin in both groups of subjects, as determined by positivity for the platelet markers CD41 and/or CD61 (CD41 ET vs. controls: median±SD 95±0.34% vs. 95.7±0.7%; p=0.15). Accordingly, the number of platelet-derived microparticles was greater in ET patients than in controls (CD61 median 4000 vs. 2400×106/L; p=0.043). The levels of the two platelet activation markers CD62P and CD63 were lower on microparticles from ET patients than on those from controls, and this difference was statistically significant for CD63 (median 5.5 vs. 40×106/L, p<0.001).
Figure 2.
Size of circulating microparticle (MP) subpopulations in ET patients and controls. Bar graph: Number of microparticles with specific cellular origins as defined by marker positivity in plasma from ET patients (ET) and controls (CTR). Data are presented as medians. Numbers of microparticles positive for CD15, CD20, CD45, CD106 and CD144 were too low to be shown adequately in this graph. Inset: dotplot of the same data presented individually for the platelet marker CD61 and the endothelial marker CD62E.
With regard to endothelium-derived microparticles, the number of microparticles expressing the endothelial marker CD62E was significantly higher in ET patients than in controls (median 875 vs. 14×106/L; p=0.007) (Figure 1C) as was the number expressing CD144 (p=0.021). Microparticles of granulocyte (CD66b and CD66acde) and of monocyte (CD14) origin were present in low but significantly higher numbers in ET patients. Microparticles derived from T cells (CD4 or CD8), B cells (CD20), intracellular adhesion molecule (ICAM)-positive cells (CD54) and vascular cell adhesion molecule (VCAM)-positive cells (CD106) account for less than 1% of all microparticles and their numbers were not different between ET patients and controls (data not shown).
Relation of microparticles with the presence of the JAK2V617F mutation or pharmaceutical treatment
Neither JAK2V617F mutation status nor the treatment given (hydroxyurea or aspirin) affected the number or the cellular origin of microparticles, including TF-positive microparticles in ET patients, (data not shown).
CD41- and CD62E-positive microparticles
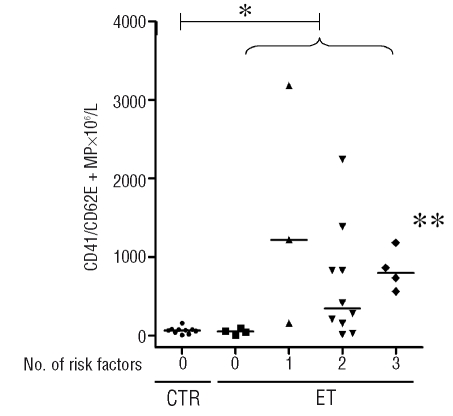
Since more than 95% of microparticles were positive for CD41 and CD61, we suspected that the CD62E-positive microparticles, constituting 27% of all microparticles in ET patients, but 1% in controls, could also co-express a platelet marker. We, therefore, analyzed the microparticles with a combination of CD41-FITC/CD62E-PE monoclonal antibodies. Indeed, in both groups 90% of the CD62E positive microparticles also expressed CD41. The CD62E-positive microparticles constituted 24% of the CD41-positive microparticles in ET patients and 1% in controls. When patients with ET were classified according to a risk score, allocating one point for a history of thrombosis, age over 60 years, platelet count over 1000×109/L, and the presence of a cardiovascular risk factor (for example hypertension or diabetes),26 microparticles with combined expression of CD41/CD62E were increased only in patients with one or more risk factors (Figure 3, p=0.045). This correlation was not observed for microparticles originating from other cells.
Figure 3.
CD41/CD62E-positive microparticles and risk factors for thrombosis. Dotplot of the number and the median of CD41/CD62E-positive microparticles in controls and in ET patients categorized for number of risk factors (age older than 60, previous thrombotic event, platelets > 1000x109/L, presence of a cardiovascular risk factor:26 0=no risk factor, 1=one risk factor, 2= two risk factors, etc. *ET patients have significantly more CD41/CD62E-positive microparticles than controls (p=0.01), and **ET patients with one or more risk factors have higher numbers of CD41/ CD62E-positive microparticles compared to ET patients without risk factors (p=0.034).
Plasma markers of endothelial cell activation
Since ET patients had higher levels of CD41/CD62E-positive microparticles, which suggests endothelial activation, we investigated the activation status of endothelium by measuring plasma levels of soluble E-selectin, mature vWF and propeptide.
No significant differences in the plasma levels of E-selectin were observed between ET patients (20 ng/mL, range, 5–40) and controls (14 ng/mL; range, 5–41; p=0.52). The removal of microparticles from plasma by centrifugation did not affect the levels of E-selectin (data not shown), indicating that most of the soluble E-selectin was not bound to these microparticles. Furthermore, no correlation was found between soluble E-selectin levels and the number of CD41/CD62E-positive microparticles (data not shown).
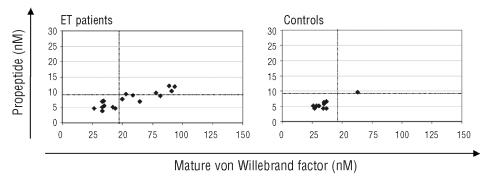
ET patients had significantly higher concentrations of mature vWF in plasma than did controls (median 50 vs. 35 nM, p=0.045) but similar concentrations of propeptide (7 vs. 5 nM, p=0.07). The mature vWF and propeptide pattern was, therefore, significantly different in patients and controls, resulting in a higher mature vWF: propeptide ratio, a pattern previously shown to indicate a state of chronic endothelial activation25 (Figure 4). No correlation was found between mature vWF, propeptide and CD41/CD62E-positive microparticles.
Figure 4.
Relation between mature vWF and propeptide levels in patients with ET and controls. Dotted lines represent the upper limit of the 95% confidence interval of mature vWF and propeptide levels of the control group. ET patients have higher levels of mature vWF (p=0.045), and similar levels of propeptide as compared to levels in controls.
Tissue factor-positive microparticles
TF-positive microparticles accounted for less than 1% of all microparticles in both patients and controls. TF was expressed on microparticles also expressing platelet markers and CD62E. The number of microparticles carrying TF was significantly higher (p=0.036) in ET patients (median 1.8×106/L) than in controls (median 0.9×106/L). No correlation was found between a history of thromboembolic events or positive risk score for thrombosis and TF-positive microparticles.
Thrombin generation
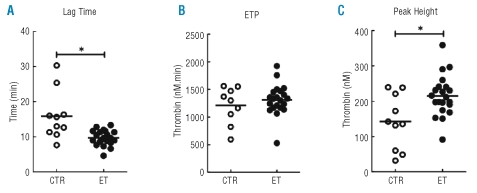
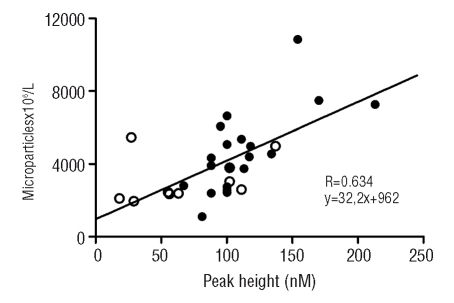
Thrombin generation triggered with 1 pM of TF and 4 μM of phopholipids was increased in microparticle-rich plasma from ET patients as indicated by increased peak height [ET vs. controls: 411 nM (95% CI: 358–465) and 279 nM (95% CI: 196–361), respectively, p=0.01[. No differences were found in lag time and endogenous thrombin potential, suggesting attenuated inhibition rather than altered activation or stimulation under these conditions. Since plasma-, possibly microparticle-derived TF may contribute to the initiation of coagulation, the thrombin generation assay was repeated in the absence of additional TF and only 4 μM of phospholipids were added. Again, the peak height was higher for ET patients (325 nM; 95% CI: 289–360) than for controls (171 nM; 95% CI: 94–249; p=0.001). Furthermore, the lag times were on average 4 min shorter for ET patients (12.0 min; 95% CI: 10.7–13.4) than for controls (15.8 min; 95% CI: 12.0–19.7; p=0.04). This latter observation is suggestive of the presence of more TF in plasma, and indeed maybe on microparticles, from ET patients than from controls. To further characterize the procoagulant potential of plasma and microparticles from patients with ET, the thrombin generation assay was performed in the absence of both TF and phospholipids (Figure 5). Using these conditions, ET patients had a shorter lag time (9.7 min; 95% CI: 8.7–10.7 versus 15.9 min; 95% CI: 10.9–20.9; p=0.001), and an increased peak height (215 nM; 95% CI: 189–241 versus 142 nM; 95% CI: 87–189; p=0.038), indicating endogenous presence of procoagulant phospholipids and TF, possibly provided by microparticles. Indeed, a negative correlation was found between the total number of microparticles and lag time (R= −0.379, p=0.039) and a positive correlation between the total numer of microparticles and peak height (R=0.634, p<0.001) (Figure 6). Finally, after removal of microparticles by centrifugation there was no activation of thrombin generation (data not shown), which is compatible with the absence of microparticle-derived TF and phospholipids.
Figure 5.
Thrombin generation in the absence of exogenous TF and phospholipids in platelet–poor, microparticle-rich plasma. The results of the parameters lag time (A), endogenous thrombin potential (ETP) (B) and peak height (C) are shown for ET patients (•) and controls (CTR) (○). Lines represent the median value. ET patients have a significantly shorter lag time and a higher peak height compared to controls. *p<0.05.
Figure 6.
Correlation between the peak height of thrombin generation in the absence of exogenous TF and phospholipids, and the total number of microparticles. Data are shown for microparticle-rich plasma of ET patients (•) and controls (○). R=0.634, p<0.001.
Discussion
This is the first extensive analysis of microparticles and their cellular origin in patients with ET. Like patients with other thromboembolic diseases, ET patients show higher levels of platelet-derived microparticles than healthy subjects.4,5,27 This is not necessarily attributable to higher numbers of platelets because neither in ET patients nor in controls platelet did microparticle numbers correlate with platelet numbers. This lack of correlation was also observed in an earlier study,28 suggesting that microparticle formation may be a regulated rather than a constitutive process. In spite of the large proportion of platelet-derived microparticles in ET patients, the actual number of these microparticles with markers of platelet activation (CD62P and CD63) was not increased. This could be because most ET patients were on anti-thrombotic drugs at the time of blood sampling, which may have affected markers of platelet activation; aspirin inhibits the expression of CD62P and CD63 on platelets.29
Remarkably, half of the ET patients showed a large increase in CD62E-positive microparticles. These CD62E-positive microparticles were not normal endothelial microparticles since they co-expressed CD41, a platelet marker, a finding that we did not observe in other conditions characterized by endothelial perturbation, including diabetes mellitus20 and renal failure (data not shown). Chirinos et al.8 described marked elevations of CD62E-positive microparticles in patients with venous thromboembolism, but co-expression with platelet markers was not investigated. CD62E, or E-selectin, is an adhesion molecule that mediates contact between endothelial cells and other cells, including platelets. Normal resting endothelial cells do not express E-selectin, but a soluble form of this molecule is released from activated cells.30 The presence of CD62E-positive microparticles suggest endothelial activation, a finding substantiated by the higher levels of mature vWF in ET. The observation that this was not accompanied by a rise in propeptide levels suggests that the endothelial activation is chronic rather than acute in nature.25
However, these CD62E-positive microparticles co-express CD41, a platelet marker. CD62E was not observed on platelets from controls or ET patients (data not shown). An explanation for this double positivity could be an interaction between platelets (or platelet fragments) and endothelial cells resulting in cellular activation and generation of microparticles of bilineage origin. Circulating microparticles with characteristics of two distinct cell populations have been described, and substantiated by confocal immunofluorescence microscopy.10 Membrane transfer from microparticles to cells resulting in expression of cell lineage-unrelated receptors is also a recognized phenomenon.31,32 In these cases microparticles express antibodies of both original cell types, thereby showing that they are the result of direct or indirect cell-cell contact, in this case endothelial cell/platelet activation. It is also conceivable that microparticles acquire CD62E during their formation via expression by the microparticle source, the activated platelet. Passive adherence of CD62E was considered as an alternative explanation. However, we consider this unlikely since there was no relation between microparticle CD62E expression and soluble E-selectin levels and removal of the microparticles from plasma by centrifugation did not affect soluble E-selectin levels, indicating a very low quantity of CD62E on microparticles as compared to soluble E-selectin in the plasma of patients as well as of controls. A low quantity of CD62E on microparticles as compared to soluble E-selectin in the plasma was shown previously for septic patients.33
Increased numbers of CD41/CD62E-positive micro-particles may be of pathophysiological significance since they appear to be related to risk factors for thrombosis in ET. A relation between these microparticles and thrombosis was not apparent in our present study, but the study was not designed to detect such a relation, and had a limited sample size and short follow-up.
Higher numbers of CD66acde and CD66b-positive microparticles in ET are likely to be related to granulocyte activation in this condition.18 The level of TF-positive microparticles was increased in ET patients, but such microparticles accounted for less than 1% of all microparticles in this study, and it is unclear whether this difference is clinically relevant. In this respect, the absence of a correlation between TF-positive microparticles and clinical parameters, such as a history of thromboembolic events or a positive risk score for thrombosis, may be primarily due to a lack of power. TF expression on platelets has been related to the presence of a JAK2V617F mutation,34 but a correlation was not observed in our study between TF-positive microparticles and the presence of the JAK2V617F mutation.
A limitation of this study is that the ages of the control subjects and patients differed significantly. However, we did not find a correlation between older age and microparticles in our study, and such a correlation has not been described in other studies.35
The thrombin generation measurements showed an increased peak height for assays with 1 pM of TF and 4 μM of phospholipids, which is compatible with a hyper-coagulable state in ET patients.36 The shorter lag times observed for ET patients whose assays were conducted without additional TF and phospholipids provide evidence for the presence of a procoagulant factor, possibly on the membrane of microparticles, in the plasma of these patients. We suggest that the differences found in thrombin generation are due to microparticles, since removal of phospholipids abolished thrombin generation. This result is compatible with the observation by Pereira et al.37 that thrombin generation is largely dependent on the number of microparticles in plasma, the only available phospholipid source in such a system. We found a correlation between peak height and the total number of microparticles, as well as for lag time and microparticles under these conditions, suggesting that phospholipids from microparticles and intrinsic or extrinsic (TF) coagulation activators in plasma and/or on the membrane of microparticles do indeed account for the observed differences that were abolished after removal of microparticles by centrifugation. In the light of the previously described evidence of the presence of non-microparticle bound functional TF in human plasma,38 future studies should address the question of whether a particular form of plasma TF contributes to the observed differences between microparticle-rich ET plasma and normal plasma.
In conclusion, ET patients have higher numbers of microparticles expressing platelet and endothelial markers, suggesting ongoing endothelial activation. This is confirmed by a signature of chronic endothelial activation given by an elevated level of mature vWF in the presence of a relatively low level of propeptide. Microparticles from ET patients are associated with increased thrombin generation, shortened lag time and increased peak height. CD41/CD62E-positive microparticles are elevated only in ET patients with risk factors for thrombosis. These findings suggest a role for microparticles in thrombosis in ET and this deserves further prospective studies.
Acknowledgments:
the authors are indebted to C. Eckmann and B. de Laat (Sanquin, Amsterdam, The Netherlands) for the measurements of mature von Willebrand factor and propeptide levels, and to L. Dijksman (Onze Lieve Vrouwe Gasthuis, Amsterdam, The Netherlands) for her assistance with statistical analyses.
Footnotes
Authorship and Disclosures
MCT: collection/analysis of clinical and experimental data, flow cytometry, writing of the paper; MvS: collection/analysis of experimental data, flow cytometry, writing of the paper; MM: thrombin generation experiments, discussion of the data; HS: analysis and interpretation of thrombin generation data; HtC: discussion of the data; AL, WT: study design, data analysis, writing of the paper; AF: study design, data analysis, clinical care of the patients.
The authors reported no potential conflict of interest.
Funding: this study was supported in part by grants from the ‘Annadal Foundation’ to HtC, from the ‘Stichting Teaching Hospital OLVG’ to Al and WT, and from the ‘Associazione Italiana per la Ricerca sul Cancro (AIRC)’ to AF.
References
- 1.Tedgui A, Mallat Z. Apoptosis as a determinant of atherothrombosis. Thromb Haemost. 2001;86:420–6. [PubMed] [Google Scholar]
- 2.Horstman LL, Jy W, Jimenez JJ, Bidot C, Ahn YS. New horizons in the analysis of circulating cell-derived microparticles. Keio J Med. 2004;53:210–30. doi: 10.2302/kjm.53.210. [DOI] [PubMed] [Google Scholar]
- 3.Mackman N. Role of tissue factor in hemostasis and thrombosis. Blood Cells Mol Dis. 2006;36:104–7. doi: 10.1016/j.bcmd.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Nomura S, Suzuki M, Katsura K, Xie GL, Miyazaki Y, Miyake T, et al. Platelet-derived microparticles may influence the development of atherosclerosis in diabetes mellitus. Atherosclerosis. 1995;116:235–40. doi: 10.1016/0021-9150(95)05551-7. [DOI] [PubMed] [Google Scholar]
- 5.Mallat Z, Benamer H, Hugel B, Benessiano J, Steg PG, Freyssinet JM, et al. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation. 2000;101:841–3. doi: 10.1161/01.cir.101.8.841. [DOI] [PubMed] [Google Scholar]
- 6.Morel O, Hugel B, Jesel L, Lanza F, Douchet MP, Zupan M, et al. Sustained elevated amounts of circulating procoagulant membrane microparticles and soluble GPV after acute myocardial infarction in diabetes mellitus. Thromb Haemost. 2004;91:345–53. doi: 10.1160/TH03-05-0294. [DOI] [PubMed] [Google Scholar]
- 7.Nieuwland R, Berckmans RJ, McGregor S, Boing AN, Romijn FP, Westendorp RG, et al. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood. 2000;95:930–5. [PubMed] [Google Scholar]
- 8.Chirinos JA, Heresi GA, Velasquez H, Jy W, Jimenez JJ, Ahn E, et al. Elevation of endothelial microparticles, platelets, and leukocyte activation in patients with venous thromboembolism. J Am Coll Cardiol. 2005;45:1467–71. doi: 10.1016/j.jacc.2004.12.075. [DOI] [PubMed] [Google Scholar]
- 9.Dignat-George F, Camoin-Jau L, Sabatier F, Arnoux D, Anfosso F, Bardin N, et al. Endothelial microparticles: a potential contribution to the thrombotic complications of the antiphospholipid syndrome. Thromb Haemost. 2004;91:667–73. doi: 10.1160/TH03-07-0487. [DOI] [PubMed] [Google Scholar]
- 10.Tesselaar ME, Romijn FP, Van DLI, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2007;5:520–7. doi: 10.1111/j.1538-7836.2007.02369.x. [DOI] [PubMed] [Google Scholar]
- 11.Tefferi A, Elliott M. Thrombosis in myeloproliferative disorders: prevalence, prognostic factors, and the role of leukocytes and JAK2V617F. Semin Thromb Hemost. 2007;33:313–20. doi: 10.1055/s-2007-976165. [DOI] [PubMed] [Google Scholar]
- 12.Vannucchi AM, Barbui T. Thrombocytosis and thrombosis. Hematology Am Soc Hematol Educ Program. 2007;2007:363–70. doi: 10.1182/asheducation-2007.1.363. [DOI] [PubMed] [Google Scholar]
- 13.Carobbio A, Finazzi G, Guerini V, Spinelli O, Delaini F, Marchioli R, et al. Leukocytosis is a risk factor for thrombosis in essential thrombocythemia: interaction with treatment, standard risk factors, and Jak2 mutation status. Blood. 2007;109:2310–3. doi: 10.1182/blood-2006-09-046342. [DOI] [PubMed] [Google Scholar]
- 14.Carobbio A, Antonioli E, Guglielmelli P, Vannucchi AM, Delaini F, Guerini V, et al. Leukocytosis and risk stratification assessment in essential thrombocythemia. J Clin Oncol. 2008;26:2732–6. doi: 10.1200/JCO.2007.15.3569. [DOI] [PubMed] [Google Scholar]
- 15.Elliott MA, Tefferi A. Thrombosis and haemorrhage in polycythaemia vera and essential thrombocythaemia. Br J Haematol. 2005;128:275–90. doi: 10.1111/j.1365-2141.2004.05277.x. [DOI] [PubMed] [Google Scholar]
- 16.Briere JB. Essential thrombocythemia. Orphanet J Rare Dis. 2007;2:3. doi: 10.1186/1750-1172-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villmow T, Kemkes-Matthes B, Matzdorff AC. Markers of platelet activation and platelet-leukocyte interaction in patients with myeloproliferative syndromes. Thromb Res. 2002;108:139–45. doi: 10.1016/s0049-3848(02)00354-7. [DOI] [PubMed] [Google Scholar]
- 18.Falanga A, Marchetti M, Vignoli A, Balducci D, Barbui T. Leukocyte-platelet interaction in patients with essential thrombocythemia and polycythemia vera. Exp Hematol. 2005;33:523–30. doi: 10.1016/j.exphem.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Murphy S, Peterson P, Iland H, Laszlo J. Experience of the Polycythemia Vera Study Group with essential thrombocythemia: a final report on diagnostic criteria, survival, and leukemic transition by treatment. Semin Hematol. 1997;34:29–39. [PubMed] [Google Scholar]
- 20.Sommeijer DW, Joop K, Leyte A, Reitsma PH, ten Cate H. Pravastatin reduces fibrinogen receptor gpIIIa on platelet-derived microparticles in patients with type 2 diabetes. J Thromb Haemost. 2005;3:1168–71. doi: 10.1111/j.1538-7836.2005.01403.x. [DOI] [PubMed] [Google Scholar]
- 21.Jy W, Horstman LL, Jimenez JJ, Ahn YS, Biro E, Nieuwland R, et al. Measuring circulating cell-derived microparticles. J Thromb Haemost. 2004;2:1842–51. doi: 10.1111/j.1538-7836.2004.00936.x. [DOI] [PubMed] [Google Scholar]
- 22.Dielis AW, Castoldi E, Spronk HM, van Oerle R, Hamulyak K, ten Cate H, et al. Coagulation factors and the protein C system as determinants of thrombin generation in a normal population. J Thromb Haemost. 2008;6:125–31. doi: 10.1111/j.1538-7836.2007.02824.x. [DOI] [PubMed] [Google Scholar]
- 23.Spronk HM, Dielis AW, de Smedt E, van Oerle R, Fens D, Prins MH, et al. Assessment of thrombin generation II: validation of the calibrated automated thrombogram in platelet-poor plasma in a clinical laboratory. Thromb Haemost. 2008;100:362–4. [PubMed] [Google Scholar]
- 24.Borchiellini A, Fijnvandraat K, ten Cate JW, Pajkrt D, van Deventer SJ, Pasterkamp G, et al. Quantitative analysis of von Willebrand factor propeptide release in vivo: effect of experimental endotoxemia and administration of 1-deamino-8-D-arginine vasopressin in humans. Blood. 1996;88:2951–8. [PubMed] [Google Scholar]
- 25.van Mourik JA, Boertjes R, Huisveld IA, Fijnvandraat K, Pajkrt D, van Genderen PJ, et al. von Willebrand factor propeptide in vascular disorders: a tool to distinguish between acute and chronic endothelial cell perturbation. Blood. 1999;94:179–85. [PubMed] [Google Scholar]
- 26.Harrison CN, Campbell PJ, Buck G, Wheatley K, East CL, Bareford D, et al. Hydroxyurea compared with ana-grelide in high-risk essential thrombocythemia. N Engl J Med. 2005;353:33–45. doi: 10.1056/NEJMoa043800. [DOI] [PubMed] [Google Scholar]
- 27.Nieuwland R, Berckmans RJ, Rotteveel-Eijkman RC, Maquelin KN, Roozendaal KJ, Jansen PG, et al. Cell-derived microparticles generated in patients during cardiopulmonary bypass are highly procoagulant. Circulation. 1997;96:3534–41. doi: 10.1161/01.cir.96.10.3534. [DOI] [PubMed] [Google Scholar]
- 28.Mairuhu A, Joop K, Setiati T, Koraka P, Soemantri A, Osterhaus A, et al. Decreased number of microparticles in severe dengue virus infections: possible involvement in disease pathogenesis Studies on clinical and pathophysiological aspects of dengue virus infection. Submitted for publication. [Google Scholar]
- 29.McKenzie ME, Malinin AI, Bell CR, Dzhanashvili A, Horowitz ED, Oshrine BR, et al. Aspirin inhibits surface glycoprotein IIb/IIIa, P-selectin, CD63, and CD107a receptor expression on human platelets. Blood Coagul Fibrinolysis. 2003;14:249–53. doi: 10.1097/01.mbc.0000046182.72384.ab. [DOI] [PubMed] [Google Scholar]
- 30.Roldan V, Marin F, Lip GY, Blann AD. Soluble E-selectin in cardiovascular disease and its risk factors. A review of the literature. Thromb Haemost. 2003;90:1007–20. doi: 10.1160/TH02-09-0083. [DOI] [PubMed] [Google Scholar]
- 31.Rozmyslowicz T, Majka M, Kijowski J, Murphy SL, Conover DO, Poncz M, et al. Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. AIDS. 2003;17:33–42. doi: 10.1097/00002030-200301030-00006. [DOI] [PubMed] [Google Scholar]
- 32.Mack M, Kleinschmidt A, Bruhl H, Klier C, Nelson PJ, Cihak J, et al. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat Med. 2000;6:769–75. doi: 10.1038/77498. [DOI] [PubMed] [Google Scholar]
- 33.Osmanovic N, Romijn FP, Joop K, Sturk A, Nieuwland R. Soluble selectins in sepsis: microparticle-associated, but only to a minor degree. Thromb Haemost. 2000;84:731–2. [PubMed] [Google Scholar]
- 34.Falanga A, Marchetti M, Vignoli A, Balducci D, Russo L, Guerini V, et al. V617F JAK-2 mutation in patients with essential thrombocythemia: relation to platelet, granulocyte, and plasma hemostatic and inflammatory molecules. Exp Hematol. 2007;35:702–11. doi: 10.1016/j.exphem.2007.01.053. [DOI] [PubMed] [Google Scholar]
- 35.Proulle V, Hugel B, Guillet B, Grunebaum L, Lambert T, Freyssinet JM, et al. Circulating microparticles are elevated in haemophiliacs and non-haemophilic individuals aged <18 years. Br J Haematol. 2005;131:487–9. doi: 10.1111/j.1365-2141.2005.05792.x. [DOI] [PubMed] [Google Scholar]
- 36.Marchetti M, Castoldi E, Spronk HM, van Oerle R, Balducci D, Barbui T, et al. Thrombin generation and activated protein C resistance in patients with essential thrombocythemia and polycythemia vera. Blood. 2008;112:4061–8. doi: 10.1182/blood-2008-06-164087. [DOI] [PubMed] [Google Scholar]
- 37.Pereira J, Alfaro G, Goycoolea M, Quiroga T, Ocqueteau M, Massardo L, et al. Circulating platelet-derived microparticles in systemic lupus ery-thematosus. Association with increased thrombin generation and procoagulant state. Thromb Haemost. 2006;95:94–9. [PubMed] [Google Scholar]
- 38.Livnat T, Zivelin A, Martinowitz U, Salomon O, Seligsohn U, et al. Prerequisites for recombinant factor VIIa-induced thrombin generation in plasmas deficient in factors VIII, IX or XI. J Thromb Haemost. 2006;4:192–200. doi: 10.1111/j.1538-7836.2005.01683.x. [DOI] [PubMed] [Google Scholar]