Biphenotypic acute leukemia is rare, necessitating large series to provide information on prognosis. In this paper Xu and colleagues review Chinese experience with this conditions. The findings of this study indicate that the prognosis of biphenotypic acute leukemia patients is poor when compared with de novo acute myeloid leukemia or acute lymphoblastic leukemia. See related perspective article on page 891 and related review article on page 984.
Keywords: biphenotypic leukemia, immunophenotype, cytogenetics, prognosis
Abstract
Background
Biphenotypic acute leukemia is a rare disorder that is difficult to diagnose. It displays features of both myeloid and lymphoid lineage. There is still a lack of studies in biphenotypic acute leukemia in a Chinese population. We present here a comprehensive investigation of the clinical and biological characteristics, and outcome of biphenotypic acute leukemia in our hospital in over a seven year period.
Design and Methods
We retrospectively analyzed 452 adult acute leukemia patients diagnosed according to French-American-British (FAB) classification and biphenotypic acute leukemia diagnosed according to European Group for the Immunological Characterization of Leukemias (EGIL) classification, respectively. Biological characteristics, response to treatment, and outcome were examined in biphenotypic acute leukemia patients and compared with that in acute myeloid leukemia and acute lymphoblastic leukemia patients with complete follow-up profiles diagnosed in the same period.
Results
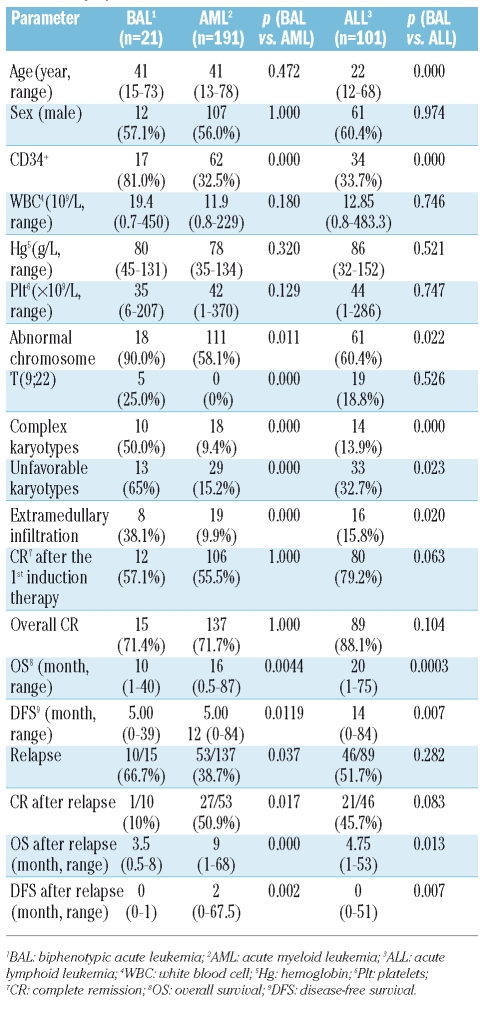
Of 452 acute leukemia patients, 21 cases (4.6%) were diagnosed as biphenotypic acute leukemia. Among them, 14 (66.7%) were B lymphoid and myeloid, 5 (23.8%) were T lymphoid and myeloid, one (4.8%) was T/B lymphoid and one (4.8%) was trilineage differentiation. When compared with acute myeloid leukemia and acute lymphoblastic leukemia, patients with biphenotypic acute leukemia showed significantly higher incidence of CD34 antigen expression, unfavorable karyotypes, and extramedullary infiltration (p<0.05). In this cohort of patients with biphenotypic acute leukemia, t(9;22) was the most common abnormality in chromosome structure. The median disease-free survival and overall survival in biphenotypic acute leukemia patients was five months and ten months, respectively, significantly shorter than those in acute myeloid leukemia and acute lymphoblastic leukemia patients (p<0.05).
Conclusions
The prognosis of biphenotypic acute leukemia patients is poor when compared with de novo acute myeloid leukemia or acute lymphoblastic leukemia. Biphenotypic acute leukemia patients showed a much higher incidence of CD34 antigen expression, complex abnormal karyotype, extramedullary infiltration, relapse, and resistance to therapy after relapse.
Introduction
Most cases of acute leukemia (AL) can be classified as myeloid (AML) or lymphoid (ALL) using the FAB classification1 and a panel of immunological markers. However, even with morphology, cytochemistry, and immunological phenotyping it is still difficult to differentiate in some patients whether or not the leukemia cells are derived from myeloid or lymphoid lineage. These cases were classified as acute leukemias of ambiguous lineage according to World Health Organization (WHO) classification of hemopoietic malignancies,2 including acute undifferentiated leukemia, bilineal acute leukemia, and biphenotypic acute leukemia, which account for 5% of total AL. Biphenotypic and bilineal acute leukemia are also known as mixed acute leukemia, in which both myeloid and lymphoid cells are involved. By definition, biphenotypic acute leukemia means one leukemia cell lineage but expresses both lymphoid and myeloid markers. On the other hand, bilineal acute leukemia means that there are two or more subtypes of cells expressing lymphoid or myeloid markers. Dual markers are necessary to distinguish between biphenotypic and bilineal acute leukemia, while in clinical practice, some physicians3,4 didn’t differentiate between biphenotypic and bilineal acute leukemia. They can be classified together as biphenotypic acute leukemia (BAL). There are several classification4,5,6 criteria for BAL diagnoses. The most commonly used is the European Group for the Immunological Characterization of Leukemias (EGIL, 1995)4. In the present study, we retrospectively analyzed the clinical manifestations, treatment, prognosis, and biological features of 21 patients with BAL diagnosed in our hospital during the last seven years.
Design and Methods
Patients
Four hundred and fifty-two patients diagnosed with acute leukemia in our hospital between January 2001 and June 2007 were retrospectively analyzed. This study was approved by the institutional ethics committee of the Second Military Medical University and the procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 2000. All patients (or legal guardians) enrolled in this study signed informed consent according to approved protocols. Diagnoses of AML or ALL were performed according to FAB classification and BAL according to EGIL classification.
Immunophenotyping
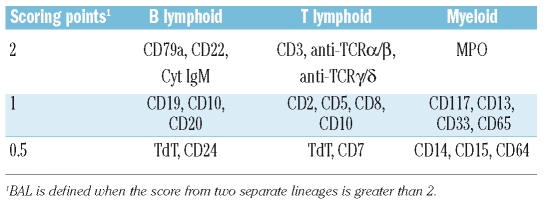
Flow cytometry immunophenotyping was performed on fresh bone marrow or blood specimens. Single-cell suspensions were incubated with combinations of monoclonal antibodies in two or four-color immunofluorescence using concentrations titrated for optimal staining. Antibodies used in the analysis recognized stem cell and panleukocyte antigens including CD45, CD34, CD38, TdT, and HLA-DR; myeloid-associated antigens including myeloperoxidase (MPO), CD117, CD33, CD13, CD14, CD15, and CD64; and lymphoid-associated antigens, including surface and cytoplasmic CD3, CD5, CD7, CD2, CD4, CD8, CD10, CD19, CD20, CD22, CD9, CD79a, and CD56. The myeloid or B/T lymphoid makers were considered to be positive if they were expressed in >20% of blasts.
Morphology and cytochemical analysis
Bone marrow aspiration occurred under Wright Geimsa staining, and 200 cells were analyzed, along with myeloperoxidase, non-specific esterase stain, sodium fluoride inhibition tests, and periodic acid Schiff reactions for cytochemical assay. One hundred Giemsa-stained peripheral blood cells were analyzed.
Cytogenetic analysis
Direct and short-term culture methods were applied in preparation of bone marrow specimens. Chromosome banding was carried out by heating using the Giemsa (RHG) method, with an average of 20 metaphase cells analyzed in each case. Karyotype was determined according to the International System for Human Cytogenic Nomenclature (ISCN, 1995). Unfavorable karyotypes were defined as t(9,22) or abnormalities of chromosomes 5 or 7, abnormalities of chromosome 11q23, and complex abnormalities (≥3 types).
Treatment
Induction
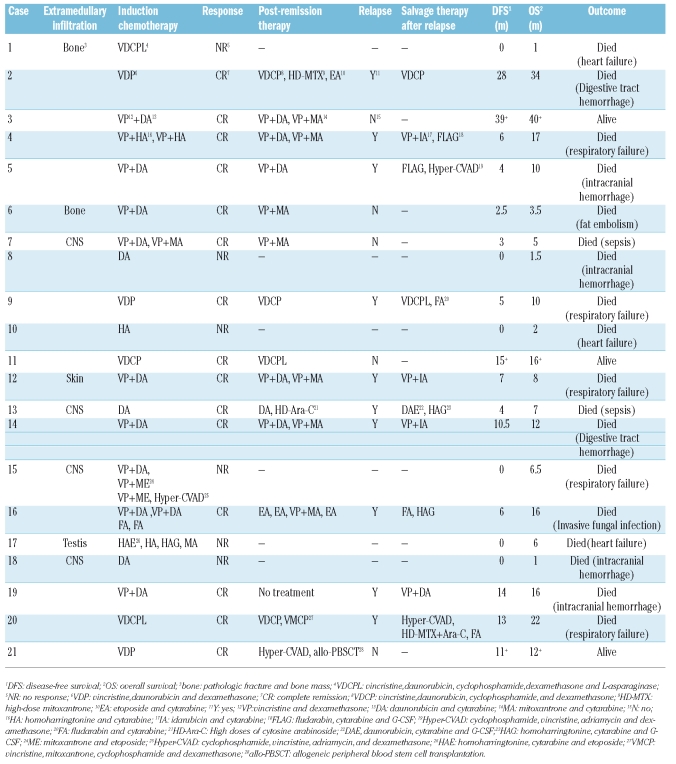
Induction therapy included the DA (daunomycin 45 mg/m2 d 1–3, cytarabine 150 mg/m2 d 1–7) protocol for most AML patients and VDCPL (vincristine 1.5 mg/m2d,1,8,15,22 daunorubicin 45 mg/m2 d,1-3,15–17 cyclophosphamide 0.8 g/m2 d,1,15 prednisolone 40 mg/m2 d,1–28 and asparaginase 6000 units/m2 d 19–28) protocol for most ALL patients. The induction protocol for patients with BAL is listed in Table 1. Three different regimens were adopted in our study. Five cases received DA protocol for myeloid lineage. Six patients received VDCPL protocol. The other 10 patients received VPDA protocol for both myeloid and lymphoid lineages.
Table 1.
Induction therapy, post-remission therapy, and salvage therapy after relapse, overall survival, and disease free survival of patients with biphe-notypic acute leukemia.
Post-remission therapy
AML patients received DA, IA (idarubicin 8 mg/m2 d 1–3 and cytarabine 150 mg/m2 d 1–7), HA (homoharringtonine 2 mg/m2 d 1–7 and cytarabine 150 mg/m2d 1–7) and MA (mitoxantrone 8 mg/m2 d 1–3 and cytarabine 150 mg/m2 d 1–7) in turn and at least 4 courses of high-dose cytarabine (1.5-2 g/m2 twice daily d 1–3). Fifty-three patients received allogeneic stem cell transplantation (AHSCT) after complete remission (CR). The post-remission therapy for BAL patients is listed in Table 1. Salvage therapy after relapse: patients received original induction protocols. If resistant to the original induction protocol, AML patients received either FLAG (fludara-bin 50 mg/m2 d 1–5, cytarabine 1–2 g/m2 d 1–5, and G-CSF 5 μg/kg d 0 until the count returned to normal) or HAG (homoharringtonine 1 mg d 1–14, cytarabine 50 mg d 1–14, and G-CSF 5 μg/kg d 0 until the count returned to normal). Patients with ALL received either FLAG or Hyper-CVAD (cyclophosphamide 0.3 g/m2 twice daily d 1–3, vincristine 1.5 mg/m2 d 4, adriamycin 50 mg/m2 d 4, and dexamethasone 20 mg/m2 d)1–4,11–14 . The treatment of BAL patients is listed in Table 1.
Follow-up
All patients were followed-up from diagnosis of the disease until the end of May 2008. The median follow-up period was 37 months (range, 12-85), 45 months (range, 12–89), and 46.5 months (range, 15.5–89) for BAL, AML, and ALL, respectively.
Statistical methods
Mann-Whitney U tests were used for comparison of numerical values. χ2 or Fisher’s exact tests were used for categorical comparison of small expected values. Kaplan-Meier survival curves were used to compare survival rates. Differences between the curves were examined statistically using the log rank test and derivatives. All data show as median (range) and differences were considered significant at the p<0.05 level. Statistical analysis was performed using SPSS 11.0.
Results
Incidence and immunophenotyping
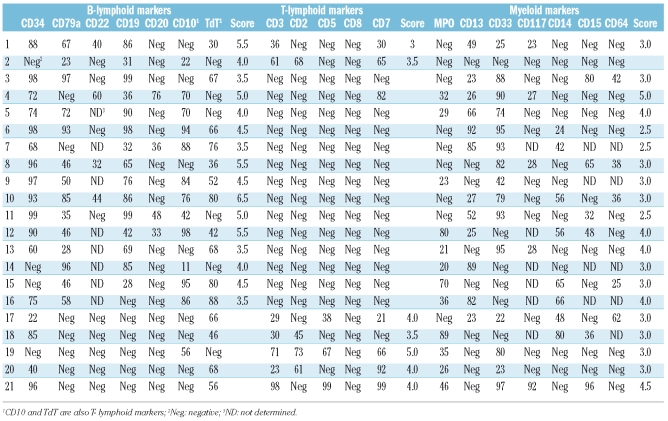
From a large group of 452 acute leukemia patients, 21 patients were diagnosed with BAL using EGIL (Table 2); the overall incidence was 4.6%. BAL immunophenotyping is shown in Table 3. One case was myeloid, T and B lymphoid triphenotype (4.8%), one B and T lymphoid (4.8%), 14 B lymphoid and myeloid biphenotype (66.7%) and 5 T lymphoid and myeloid (23.8%). The rate of CD34 positive was 81.0% (17/21).
Table 2.
Scoring system for the definition of biphenotypic acute leukemia.
Table 3.
Immunological markers of 21 patients with biphenotypic acute leukemia.
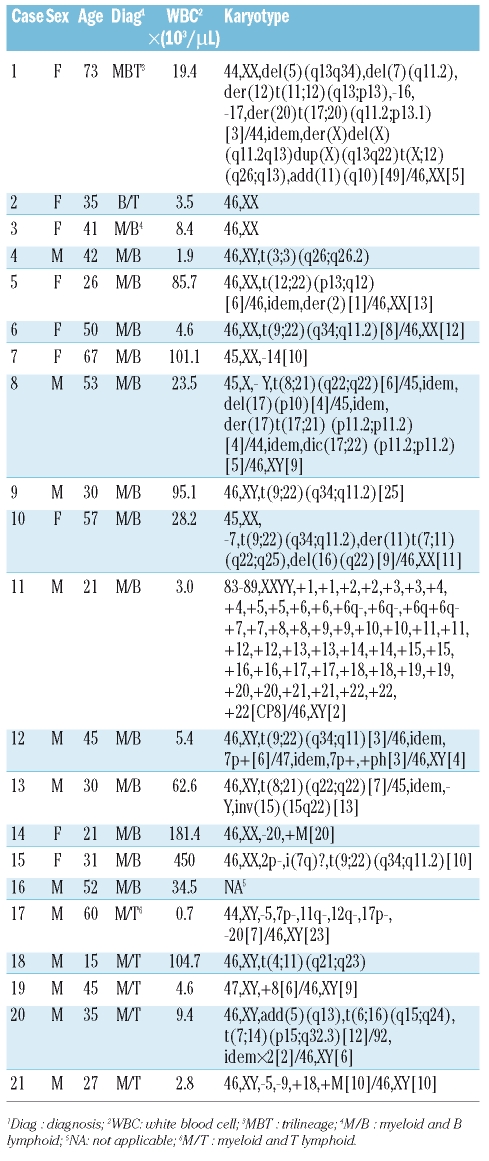
General information, peripheral blood cell count, and karyotype
Patients’ general information, peripheral blood cell count, and karyotype are shown in Table 4. In this study, 12 males and 9 females were diagnosed with BAL. Median age was 41 years (range, 15–73). The median counts for WBC, Hg, and Plt were 19.4 (0.7–450)×109/L, 80 (45–131) g/L, and 35 (6–207)×109/L, respectively. The median prevalence of blasts was 86% (53–98%). Among 20 patients with valid chromosome profiles, a normal karyotype was found in only 2 cases (10%), while clonal chromosome aberrations were observed in 18 patients (90%). The most common karyotype was t(9;22) (q34;q11.2) (25%, 5/20). Two patients had t(8;21) (q22;q22), both with complex chromsome abnormalities. Ten patients had complex karyotypes, in which 6 patients showed abnormalities in chromosome 7; 5 patients showed abnormalities in chromosome 5, and 3 patients had −17/17p-.
Table 4.
General information, peripheral white blood cell count, and karyotype of patients with biphenotypic acute leukemia.
Treatment and follow-up
Induction and consolidation regimens, overall survival (OS), and disease-free survival (DFS) are shown in Table 1. The CR rate of BAL was 71.4% (15/21). Among 21 patients, 12 achieved CR after the first course of induction. Four of the 6 non-remission patients died during the first induction. The other 2 patients were given different protocols; however, no remission occurred, and they died six months later due to primary disease. Fifteen CR patients received consolidation regimens after remission except for one case (Table 1). Ten cases developed relapse during follow-up and were resistant to original induction therapy, although one case achieved hematologic remission but relapsed again one month later. All relapsed cases died of primary disease or complications. Two CR patients died of chemotherapy complications (patients 6 and 7). Three cases survived until the end of follow-up. Among them, one patient received allogeneic hemopoietic stem cell transplantation ten months after diagnosis and had a good general status without severe transplantation complications. Eight patients had extramedullary infiltration, which most commonly affected the central nervous system (excluding the liver, spleen, and lymph node).
Survival analysis
Univariate analysis of OS including clinical manifestations and laboratory tests showed that age, WBC count, extramedullary infiltration, primary induction treatment, and whether or not patients achieved CR after induction, were significantly related to patients’ prognosis.
Comparison of biphenotypic acute leukemia and acute myeloid leukemia in clinical and biological features
Comparison of BAL and 191 AML patients was carried out as shown in Table 5, excluding patients lost to follow-up and M3 subtype because of its particular biological features. Significant differences were noted in CD34 positive rates, incidence of karyotype abnormalities, t(9;22), complex karyotypes, unfavorable karyotypes and extramedullary infiltration (p<0.05).
Table 5.
The overall review of the clinical features and outcome of patients with biphenotypic acute leukemia, acute myeloid leukemia, and acute lymphoid leukemia.
Comparison of biphenotypic acute leukemia and acute lymphocytic leukemia in clinical and biological features
Comparison of BAL and 101 ALL patients was carried out as shown in Table 5, excluding patients lost to follow-up. Significant differences were noted in age, CD34 positive rates, incidence of karyotype abnormalities, complex karyotypes, unfavorable karyotypes and extramedullary infiltration (p<0.05).
Comparison of the response to treatment and outcomes between acute myeloid leukemia, acute lymphocytic leukemia, and biphenotypic acute leukemia
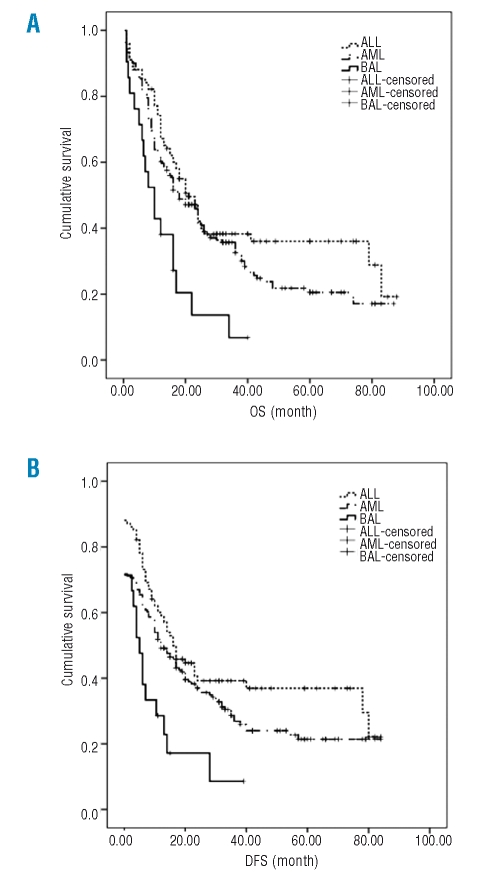
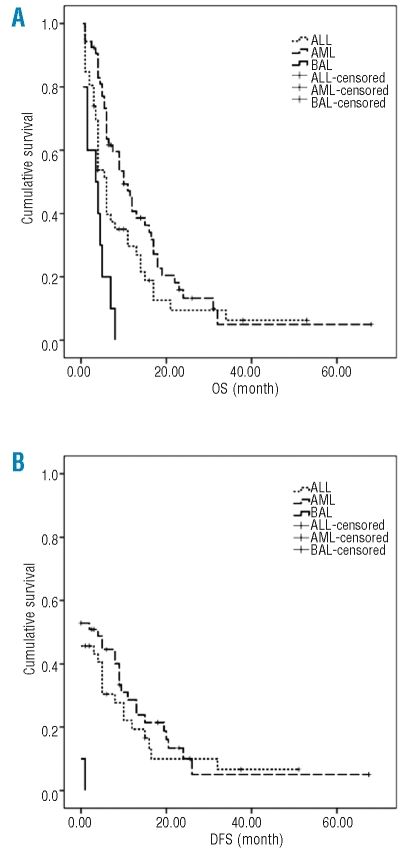
Responses to treatment and outcomes in BAL patients were compared with those of AML and ALL (Table 5) diagnosed in the same period. No statistical difference was noted in CR rate after the first induction and overall CR rate (p>0.05). The incidence of relapse in BAL patients was significantly higher than that of patients with AML, while CR rates after relapse were lower in BAL patients than those with AML (p<0.05). A significant worse OS and DFS for BAL when compared with that of ALL (p=0.0003, p=0.007)and AML (p=0.0044, p=0.0119) is denoted by the Kaplan-Meier curve (Figure 1). ALL and AML patients had better outcome than BAL patients (Figure 1). The OS (p=0.000 and p=0.013, respectively) as well as DFS (p=0.002 and p=0.007, respectively) of patients with AML and ALL was significantly higher than that of BAL after relapse (Figure 2).
Figure 1.
(A) The overall survival of patients with BAL (solid line), AML (dashed line) and ALL (dotted line). The survival time was analyzed by Kaplan-Meier curve. (B) The disease free survival of patients with BAL, AML and ALL. The survival time was analyzed by Kaplan-Meier curve. The x-axes are the survival time and y-axes are cumulative survival.
Figure 2.
(A) The overall survival of patients with BAL (solid line), AML (dashed line) and ALL (dotted line) after relapse. The survival time was analyzed by Kaplan-Meier curve. (B) The disease free survival of patients with BAL, AML and ALL after relapse. The survival time was analyzed by Kaplan-Meier curve. The x-axes are the survival time and y-axes are cumulative survival.
Discussion
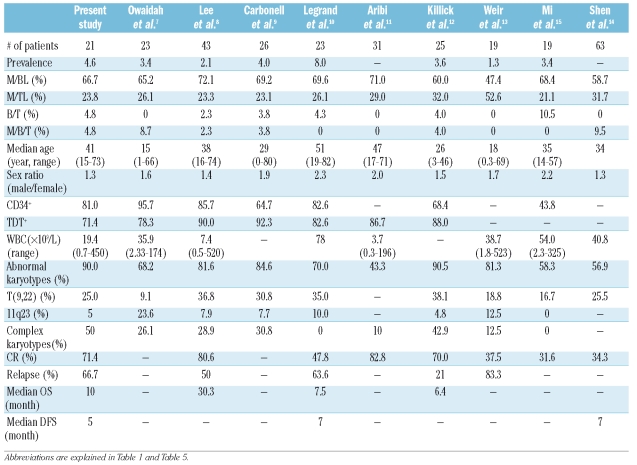
Unlike commonly seen acute leukemias classified as B or T lymphoid or myeloid lineage, BAL is a type of acute leukemia with uncommon biological and clinical features. We summarized several reports about the clinical and biological features of BAL in recent years (Table 6). To further understand the characteristics and prognosis of BAL in the Chinese population, we adopted EGIL classification criteria to retrospectively analyze 452 adult patients with de novo acute leukemia admitted to our hospital. Among them, 21 (4.6%) were diagnosed with BAL, which is consistent with previous reports ranging from 1.3% to 8.0% (Table 6). Katsura16 proposed the myeloid-based model of hematopoiesis, in which the stem cell initially generates common myeloerythroid progenitors and common myelolymphoid progenitors. T-cell and B-cell progenitors subsequently arise from common myelolymphoid progenitors through myeloid-T and myeloid-B stages, respectively. Most recently, Wada et al.17 and Bell et al.18 demonstrated that a substantial number of early T-cell precursors in the thymus have myeloid potential. These results provided evidence to support the supposition that BAL arises from a pluripotent progenitor cell, which then can differentiate into both myeloid and lymphoid lineages during the development of acute leukemia. In our series, CD34 positive was predominant in BAL (81%) patients compared to AML and ALL patients in the same period (p<0.05). Other researchers have found that the percentage of CD34 and TdT positive was more than 80%.8,10 These results support the supposition that BAL may originate from stem/progenitor cells.
Table 6.
Clinical and biological features of patients with biphenotypic acute leukemia in different studies.
Abnormal karyotypes were detected in 90% of 20 BAL patients with valid analysis. This was higher than in AML and ALL patients during the same period (p<0.05). Notably, patients with normal karyotypes (patients 2 and 3) had the longest survival period in this study. Whether BAL patients with normal karyotypes would take an advantage from normal karyotype needs further observation in a larger scale clinical study. Karyotype analysis showed that chromosome abnormality was highly heterogeneous in BAL patients. In our study, t(9;22) was the most common abnormality in chromosome structure, occurring in 25% of patients (5 out of 20) without the history of chronic myeloid leukemia. These 5 patients showed B lymphoid and myeloid lineage antigen co-expression. The median survival time of these 5 patients was 6.5 months, which is shorter than that of Ph negative patients with B lymphoid and myeloid lineage antigen co-expression (6.5 months vs. 12 months, p<0.05). This suggests that the Ph chromosome might be a poor prognosis indicator for BAL patients. Structural abnormalities of chromosome 11 were observed in 4 patients (20%). Only one showed 11q23 rearrangement and corresponded to an adolescent (the youngest in this cohort of patients) with a T cell and myeloid phenotype. The incidence of 11q23 rearrangement in our group of patients was lower than that in Owaidah’s7 report because of the younger patients in their study, suggesting that the frequency of an 11q23 rearrangement occurs more in infants than in adults. Similar to other reports, we found that the incidence of Ph chromosome and 11q23 rearrangement was relatively high. This supports the proposal that BAL closely relates to the Ph and MLL genes, as previously proposed by Jenning19 and Thirman.20
Additionally, we also reported 2 cases with t(8;21). AML with t(8;21) translocation is classified as a unique category (recurrent cytogenetic translocation) according to WHO classification (2001). Although the 2 patients with t(8;21) translocation in our study were diagnosed as AML-M2 based on pathological morphology, the myeloid and B-lymphoid markers were all positive in immunophenotyping. So we included these 2 patients in BAL. He et al.21 also reported 6 cases of B-lymphoid and myeloid lineages BAL with t(8;21)(q22;q22). By analyzing the characteristics of morphology, immune phenotype, chromosome karyotype and clinical manifestations of these 6 cases, they considered that BAL with t(8; 21)(q22;q22) might be a new subgroup of BAL. Miyamoto’s findings suggested that the acquisition of the t(8;21) occurs at the level of stem cell, capable of differentiating into B cells as well as all myeloid lineages.22 The survival times of 2 patients with t(8;21) in our series were 1.5 and 7 months, which were shorter than those of AML patients with t(8;21) (median survival time 22 months, p<0.05). We hypothesized that: (i) the particular biological and clinical features of BAL should be taken into consideration, i.e., t(8;21) is not a favorable indication of good prognosis in BAL, and (ii) these 2 patients also had other abnormal chromosome structures, which were classified as complex abnormalities.
The univariate analysis of prognosis in our study showed that the prognosis of patients with BAL was significantly related to age, peripheral WBC count, extramedullary infiltration and whether or not patients achieved CR after induction. But due to the small numbers of cases, it’s still difficult for us to draw any conclusion from our series. A multi-center and perspective clinical study will be critical to have a wider picture of this rare disease.
Treatment of BAL is complicated and problematic. In our series, the overall CR rate was 71.4%. There was no statistical difference when comparing with AML and ALL (p>0.05). However, the relapse rate in BAL patients was significantly higher than that of AML patients, while the CR rate after relapse was lower than that of AML patients. We also observed a low CR rate after relapse in BAL patients when compared with ALL patients, although no statistical difference was noted (p=0.083). Survival time and DFS after relapse in BAL patients were also shorter than those in AML or ALL patients. This may suggest that cross resistance more likely developed in BAL patients than in AML or ALL patients, causing a poor response to treatment and short survival period. Nakagawa et al.23 found that the overall expression levels of inhibitor of apoptosis protein (IAP)-family proteins in BAL bone marrow cells were higher than those in control, AML, and ALL cells. These results partly supported our observations.
Overall, the DFS and OS in our study were five and ten months, respectively. These were much shorter than those of AML and ALL patients in the same period (p<0.05). The OS and DFS of BAL patients were also less than eight months in other reports (Table 6). This might be due to: (i) common abnormal karyotype in BAL, high incidence of Ph chromosome, complex abnormal karyotype, and high incidence of abnormal chromosomes 5 and 7; (ii) high incidence of CD34 positive in BAL; (iii) high incidence of extramedullary infiltration; (iv) lack of optimized guidelines for induction therapy; and (v) high incidence of relapse after CR and resistance to therapy after relapse. Ottmann et al.24 reported that imatinib can improve CR rates in patients with refractory and relapse Ph positive ALL. For BAL patients with Ph chromosomes, imatinib added in treatment regimens might increase remission rates.
In summary, BAL is a particular kind of AL because of its rare incidence, difficulties in proper diagnosis and rational treatment. Thus, it is necessary to carry out multi-center co-operative studies in both clinical and basic research to further characterize the features of BAL.
Footnotes
Authorship and Disclosures
JMW was the principal investigator and takes primary responsibility for the paper. XQX and JMW designed the study. XQX, SQL, LC, JMY, WPZ, XMS, JH, and XN recruited the patients. HYQ performed the laboratory work for this study. XQX and JMW wrote the paper. The authors reported no potential conflicts of interest.
Funding: this work was supported by grants from Science and Technology Commission of Shanghai Municipality (8JC1406500 and 5DZ19327) and from Ministry of Public Health, P.R China (KJ2007-3-001) to JMW.
References
- 1.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposed revised criteria for the classification of acute myeloid leukemia: A report of the French-American-British Cooperative group. Ann Intern Med. 1985;103:620–5. doi: 10.7326/0003-4819-103-4-620. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe ES, Harris NL, Stein H, Vardiman JW. Pathology and Genetics, Tumor of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon: 2001. [Google Scholar]
- 3.Matutes E, Morilla R, Farahat N, Carbonell F, Swansbury J, Dyer M, et al. Definition of acute biphenotypic leukemia. Haematologica. 1997;82:64–6. [PubMed] [Google Scholar]
- 4.Bene MC, Castoldi G, Knapp W, Ludwig WD, Matutes E, Orfao A, van't Veer MB. Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL) Leukemia. 1995;9:1783–6. [PubMed] [Google Scholar]
- 5.Kantarjian HM, Hirsch-Ginsberg C, Yee G, Huh Y, Freireich EJ, Stass S. Mixed-lineage leukemia revisited: Acute lymphocytic leukemia with myeloperoxidasepositive blasts by electron microscopy. Blood. 1990;76:808–13. [PubMed] [Google Scholar]
- 6.Catovsky D, Matutes E, Buccheri V, Shetty V, Hanslip J, Yoshida N, et al. A classification of acute leukaemia for the 1990s. Ann Hematol. 1991;62:16–21. doi: 10.1007/BF01714978. [DOI] [PubMed] [Google Scholar]
- 7.Owaidah TM, Al Beihany A, Iqbal MA, Elkum N, Roberts GT. Cytogenetics, molecular and ultra-structural characteristics of biphenotypic acute leukemia identified by the EGIL scoring system. Leukemia. 2006;20:620–6. doi: 10.1038/sj.leu.2404128. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Min YH, Chung CW, Kim BK, Yoon HJ, Jo DY, et al. Korean Society of Hematology AML/MDS Working Party. Prognostic implications of the immunophenotype in biphenotypic acute leukemia. Leuk Lymphoma. 2008;49:700–9. doi: 10.1080/10428190701843247. [DOI] [PubMed] [Google Scholar]
- 9.Carbonell F, Swansbury J, Min T, Matutes E, Farahat N, Buccheri V, et al. Cytogenetic findings in acute biphenotypic leukaemia. Leukemia. 1996;10:1283–7. [PubMed] [Google Scholar]
- 10.Legrand O, Perrot JY, Simonin G, Baudard M, Cadiou M, Blanc C, et al. Adult biphenotypic acute leukemia: an entity with poor prognosis which is related to unfavourable cytogenetics and P-glycoprotein over-expression. Br J Haematol. 1998;100:147–55. doi: 10.1046/j.1365-2141.1998.00523.x. [DOI] [PubMed] [Google Scholar]
- 11.Aribi A, Bueso-Ramos C, Estey E, Estrov Z, O’Brien S, Giles F, et al. Biphenotypic acute leukaemia: a case series. Br J Haematol. 2007;138:213–6. doi: 10.1111/j.1365-2141.2007.06634.x. [DOI] [PubMed] [Google Scholar]
- 12.Killick S, Matutes E, Powles RL, Hamblin M, Swansbury J, Treleaven JG, et al. Outcome of biphenotypic acute leukemia. Haematologica. 1999;84:699–706. [PubMed] [Google Scholar]
- 13.Weir EG, Ali Ansari-Lari M, Batista DA, Griffin CA, Fuller S, Smith BD, et al. Acute bilineal leukemia: a rare disease with poor outcome. Leukemia. 2007;21:2264–70. doi: 10.1038/sj.leu.2404848. [DOI] [PubMed] [Google Scholar]
- 14.Shen Y, Li J, Xue Y, Zhu M, Lu D, Geng M, et al. Acute biphenotypic leukemia in the adults. Zhonghua Zhong Liu Za Zhi. 2002;24:375–7. [PubMed] [Google Scholar]
- 15.Mi Y, Bian S, Meng Q, Xue Y, Yu M, Chen G, et al. Study on the clinical characteristics of biphenotypic acute leukemia. Zhonghua Xue Ye Xue Za Zhi. 2000;21:352–4. [PubMed] [Google Scholar]
- 16.Katsura Y. Redefinition of lymphoid progenitors. Nature Rev Immunol. 2002;2:127–32. doi: 10.1038/nri721. [DOI] [PubMed] [Google Scholar]
- 17.Wada H, Masuda K, Satoh R, Kakugawa K, Ikawa T, Katsura Y, et al. Adult T-cell progenitors retain myeloid potential. Nature. 2008;452:768–72. doi: 10.1038/nature06839. [DOI] [PubMed] [Google Scholar]
- 18.Bell JJ, Bhandoola A. The earliest thymic progenitors for T cells possess myeloid lineage potential. Nature. 2008;452:764–7. doi: 10.1038/nature06840. [DOI] [PubMed] [Google Scholar]
- 19.Jennings CD, Foon KA. Recent advances in flow cytometry: application to the diagnosis of hematologic malignancy. Blood. 1997;90:2863–92. [PubMed] [Google Scholar]
- 20.Thirman MJ, Gill HJ, Burnett BC, Mbangkollo D, McCabe NR, Koba-yashi H, et al. Rearrangement of the MLL gene in acute lymphoblastic and acute myeloid leukemias with 11q23 chrosomosal translocatoin. N Engl J Med. 1993;329:909–14. doi: 10.1056/NEJM199309233291302. [DOI] [PubMed] [Google Scholar]
- 21.He G, Wu D, Sun A, Xue Y, Jin Z, Qiu H, et al. B-Lymphoid and myeloid lineages biphenotypic acute leukemia with t(8;21) (q22;q22) Int J Hematol. 2008;87:132–6. doi: 10.1007/s12185-008-0029-z. [DOI] [PubMed] [Google Scholar]
- 22.Miyamoto T, Weissman IL, Akashi K. AML/ETO1-expressing non-leukemia stem cells in acute myelogenous leukemia with t(8;21) chromosomal translocation. Proc Natl Acad Sci USA. 2000;97:7521–6. doi: 10.1073/pnas.97.13.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakagawa Y, Hasegawa M, Kurata M, Yamamoto K, Abe S, Inoue M, et al. Expression of IAP-family proteins in adult acute mixed lineage leukemia (AMLL) Am J Hematology. 2005;78:173–80. doi: 10.1002/ajh.20285. [DOI] [PubMed] [Google Scholar]
- 24.Ottmann OG, Druker Bj, Sawyers CL, Goldman JM, Reiffers J, Silver RT, et al. A phase II trial of imatinib in patients with relapsed or refractory Philadelphia chromosome positive acute lymphoid leukemias. Blood. 2002;100:1965–71. doi: 10.1182/blood-2001-12-0181. [DOI] [PubMed] [Google Scholar]