In vitro depletion of alloreactive T cells using the proteasome inhibitor bortezomib is a promising approach to prevent graft-versus-host disease (GVHD) after allogeneic stem cell transplantation. The findings of this study strengthen the idea of using bortezomib in the prevention of GVHD, not only because of its selective cytotoxic effect on activated T cells, but also due to its ability to preserve and/or generate regulatory T cells.
Keywords: bortezomib, regulatory T cells, graft-versus-host disease, prevention
Abstract
Background
In vitro depletion of alloreactive T cells using the proteasome inhibitor bortezomib is a promising approach to prevent graft-versus-host disease after allogeneic stem cell transplantation. We have previously described the ability of bortezomib to selectively eliminate alloreactive T cells in a mixed leukocyte culture, preserving non-activated T cells. Due to the role of regulatory T cells in the control of graft versus host disease, in the current manuscript we have analyzed the effect of bortezomib in regulatory T cells.
Design and Methods
Conventional or regulatory CD4+ T cells were isolated with immunomagnetic microbeads based on the expression of CD4 and CD25. The effect of bortezomib on T-cell viability was analyzed by flow cytometry using 7-amino-actinomycin D staining. To investigate the possibility of obtaining an enriched regulatory T-cell population in vitro with the use of bortezomib, CD4+ T cells were cultured during four weeks in the presence of anti-CD3 and anti-CD28 antibodies, IL-2 and bortezomib. The phenotype of these long-term cultured cells was studied, analyzing the expression of CD25, CD127 and FOXP3 by flow cytometry, and mRNA levels were determined by RT-PCR. Their suppressive capacity was assessed in co-culture experiments, analyzing proliferation and IFN-γ and CD40L expression of stimulated responder T cells by flow cytometry.
Results
We observed that naturally occurring CD4+CD25+ regulatory T cells are resistant to the pro-apoptotic effect of bortezomib. Furthermore, we found that long-term culture of CD4+ T cells in the presence of bortezomib promotes the emergence of a regulatory T-cell population that significantly inhibits proliferation, IFN-γ production and CD40L expression among stimulated effector T cells.
Conclusions
These results reinforce the proposal of using bortezomib in the prevention of graft versus host disease and, moreover, in the generation of regulatory T-cell populations, that could be used in the treatment of multiple T-cell mediated diseases.
Introduction
The ability of regulatory T (Treg) cells of suppressing immune responses has raised a deep interest for their potential therapeutic use in T-cell mediated diseases, including autoimmune disorders, allograft rejection or graft-versus-host disease (GVHD). Different Treg-cell subsets have been described.1 The best studied are naturally occurring CD4+CD25+ regulatory T (nTreg) cells. nTreg cells develop in the thymus and then migrate to the periphery, where they play a key role in self-tolerance.2 Phenotypically, they are characterized by the constitutive expression of high levels of CD25 (IL-2 receptor α chain),2 the transcription factor forkhead box P3 (FOXP3)3 and other markers such as GITR (glucocorticoid induced TNF receptor family-related protein)4 CD62L5 or CTLA-4.6 They also express low levels or no CD127.7 However, none of these markers is uniquely associated with nTreg cells and activated conventional T cells acquire a very similar phenotype.8 Therefore, it is difficult to obtain pure nTreg populations without activated non-regulatory T-cell contamination.
Several experimental protocols have been described for the extrathymic generation of suppressor T cells. These adaptive or inducible regulatory T (iTreg) cells can be generated in the periphery in vivo under various tolerogenic conditions, such as oral tolerance induction9 or injection of tolerogenic dendritic cells.10 In vitro, repetitive stimulation with immature dendritic cells11,12 or the presence of suppressive cytokines such as IL-1013 or TGF-β in the culture14 can induce iTreg cells. It is also possible to generate or expand Treg cells ex vivo by treating T cells with pharmacological immunosuppressants such as vitamin D3 and dexamethasone15,16 or rapamycin, which has been reported to promote selective expansion of naturally occurring Treg cells.17
We have previously shown that the proteasome inhibitor bortezomib selectively induces apoptosis of activated T cells while preserving viability of resting T cells.18 This property turns bortezomib into a potential therapeutic tool against GVHD, raising the possibility of in vitro purging alloreactive T cells while preserving effector T cells with other specificities. Due to the suggested importance of nTreg cells in the control of GVHD,19–22 in the current manuscript we have analyzed the effect of bortezomib on nTreg-cell viability. Our results demonstrate that the addition of bortezomib to activated CD4+ T-cell cultures allows survival of nTreg cells and, moreover, promotes the emergence of a distinct suppressor CD4+ T-cell population (bTreg) that strongly inhibits the activation of in vitro stimulated T cells.
Design and Methods
Cell purification and culture
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats of volunteer healthy donors by density gradient centrifugation using Ficoll-Paque solution (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). All donors provided written informed consent in accordance with the Declaration of Helsinki. CD4+ T cells were purified by negative selection using the Untouched CD4+ T Cell Isolation Kit II (Miltenyi Biotec, Bergisch Gladbach, Germany). Isolation of CD4+CD25+ T cells was performed by using the CD4+CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotec). To obtain the CD4+CD25− population, CD25+ cells were depleted from isolated CD4+ T cells using CD25 Microbeads (Miltenyi Biotec). All the magnetic separations were performed with the Automacs™ Separator, following the manufacturer’s instructions. The purity of isolated populations was routinely >95%. Cells were cultured in RPMI 1640 medium supplemented with L-glutamine 2 mM, penicillin 100 U/mL, streptomycin 100 mg/mL (all from GIBCO, Grand Island, NY, USA) and 10% human AB serum (Sigma, St. Louis, MO, USA).
Viability assays
CD4+CD25+ T cells were isolated from CD4+ T cells. CD4+CD25+ cells were then stained with the green fluorescent dye PKH-67 (Sigma) following the manufacturer’s instructions and mixed back with CD4+CD25−PKH− T cells. The cells were then cultured in the absence of any stimulus or stimulated with either allogeneic irradiated (15 Gy) PBMCs or with plate bound anti-CD3 (10 μg/mL) and soluble anti-CD28 (1 μg/mL) mAbs (BD Biosciences, San Jose, CA, USA). Different concentrations (0, 100, 500 or 1000 nM) of bortezomib (kindly provided by Millenium Pharmaceuticals Inc, Cambridge, MA, USA) were added to every culture condition. After five days of culture, cells were collected, stained with CD25-PE, 7 amino-actinomycin D (7-AAD) and CD4-APC (all from BD Biosciences), and acquired in a FACSCalibur flow cytometer (BD Biosciences). Paint-A-Gate software was used to calculate the percentage of viable cells (7-AAD-) in every subpopulation: regulatory T cells (PKH+CD25+), resting conventional T cells (PKH−CD25−) and activated conventional T cells (PKH−CD25+).
In another set of experiments, CD4+CD25+ and CD4+CD25− T cells were isolated and separately stimulated for five days with anti-CD3 and anti-CD28 mAbs. Different concentrations (0, 100, 500 or 1000nM) of bortezomib were added to every culture condition. Viability of each subpopulation was analyzed by flow cytometry as described above.
CD4+ T-cell long-term culture and immunophenotypic analysis
Purified total CD4+ or CD4+CD25− T cells were stimulated with plate bound anti-CD3 (10 μg/mL) and soluble anti-CD28 (1 μg/mL) mAbs (BD Biosciences). After 48 h, medium or bortezomib 500 nM was added. Three rounds of stimulation of seven days each were performed. IL-2 (R&D Systems, Minneapolis, MN, USA) was added starting from the second round of stimulation at 50 U/mL. IL-2 and medium/bortezomib were added every two or three days. Cells were left resting for one more week in the presence of IL-2, but in the absence of anti-CD3/anti-CD28 and bortezomib. After four weeks of culture, cells were collected and stained with CD25-FITC/CD127-PE-/CD4-PerCP-Cy5.5/FOXP3-APC antibodies (all from BD Biosciences, except FOXP3 from eBiosciences, San Diego, CA, USA). Briefly, for surface staining, 100 μl of sample per tube were incubated with the corresponding monoclonal antibodies for 15 min at room temperature in the dark. Cells were washed in PBS and then fixed and permeabilized with FoxP3 Staining Buffer Set (eBiosciences) for FOXP3 staining.
Quantification of FOXP3 mRNA expression
Total RNA was obtained from T cells using the QiaGen AllPrep DNA/RNA Microkit (Valencia, CA, USA) according to the manufacturer’s instructions. Reverse transcription was performed as previously described.23 FOXP3 RNA levels were quantified using the 7900 HT Fast Real-Time PCR System and TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. The assay IDs were: ABL1, Hs00245445_m1, and FOXP3, Hs01085835_m1. The cycle number at which the reaction crossed an arbitrarily placed threshold (Ct) was determined and the relative expression of FOXP3 regarding ABL1 was described using the equation 2-ΔCt where ΔCT = CtFOXP3 − CtABL1.24
Functional studies: suppression assays
PBMCs from healthy donors were stained with PKH-67 and stimulated with plate bound anti-CD3 (10 μg/mL) and soluble anti-CD28 (1 μg/mL) mAbs. CD4+ T cells cultured during four weeks as previously described were added at a 1:1 responder cells:long-term cultured cells ratio. After four days, cells were collected, stained with CD25-PE, 7-AAD and CD3-APC mAbs and analyzed by flow cytometry. Paint-A-Gate software was used to calculate the percentage of proliferating activated T cells (PKHdimCD25+), non-divided activated T cells (PKHhighCD25+) and non-activated T cells (PKHhighCD25−) among viable responder T cells. ModFit software was also used to calculate the percentage of resting and proliferating cells.
CD40L and IFN-γ production of responder and long-term cultured CD4+ T cells were also analyzed by flow cytometry after staining with CD25-FITC/IFN-γ-PE/CD4-PerCP-Cy5.5/CD40L-APC. The IntraStain kit (Dako Cytomation, Denmark) was used following the manufacturer’s instructions.
Statistical analysis
Mean values and their SD as well as the median and range were calculated for each variable using the SPSS software program (SPSS 15.0, Chicago, IL, USA). Comparison between groups was made by analysis of variance (post hoc Scheffé and Tukey tests were performed to confirm differences between groups). A two-way Measurement of Repeated Multiple Analysis (MR-MANOVA two ways) was performed to compare the effect of the different doses of the drug within the different types of culture. p values less than 0.05 were considered statistically significant.
Results
Bortezomib does not affect CD4+CD25+ regulatory T-cell viability
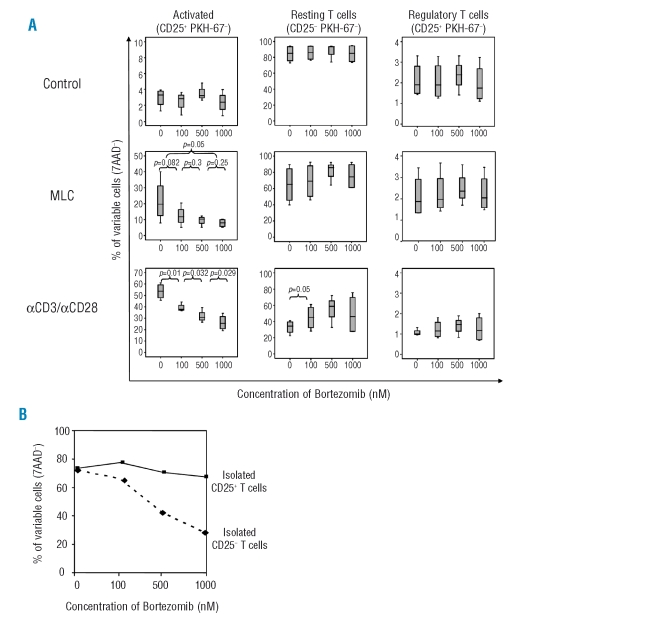
To investigate whether bortezomib affects nTreg cell viability, we isolated CD4+ T cells and subsequently separated the CD4+CD25+ nTreg cell population. As conventional activated T cells showed a similar phenotype to nTreg cells after in vitro expansion (data not shown), we stained CD4+CD25+ T cells with the green fluorescent dye PKH-67 to specifically identify nTreg cells after culture, and mixed them again with CD4+CD25− (PKH-67−) cells. We cultured CD4+ T cells in the absence of stimulus (control) or stimulated either with allogeneic leukocytes (mixed leukocyte culture, MLC) or with anti-CD3 and anti-CD28 monoclonal antibodies. Different concentrations of bortezomib (0, 100, 500, 1000 nM) were added to each culture condition. Five days later, we analyzed by flow cytometry the viability of the different types of CD4+ T cells: regulatory (CD25+ PKH+), resting conventional (CD25− PKH−) and activated conventional (CD25+ PKH−) T cells, assessed by the percentage of 7AAD− cells of each subpopulation (Online Supplementary Figure S1).
In un-stimulated samples, neither viability of regulatory nor conventional T cells was affected. As previously shown by our group,18 when stimulated in MLC, or with anti-CD3/anti-CD28 antibodies, viability of activated T cells decreased in a dose-dependent manner, whereas resting T cell viability barely changed with the addition of bortezomib. Interestingly, viability of regulatory T cells in stimulated cultures remained essentially constant at the different doses of bortezomib (Figure 1A).
Figure 1.
(A) High doses of bortezomib (1000 nM) did not decrease viability among Treg cells (PKH-67+CD25+ population) or resting T cells (PKH-67−CD25− population) in either un-stimulated (control), MLC or αCD3/αCD28 stimulated CD4+ T-cell cultures. Conversely, percentages of viable activated T cells (PKH-67−CD25+ population) significantly decreased in a dose dependent manner in stimulated cultures. (B) Viability of isolated CD4+CD25− cells versus CD4+CD25+ cells after exposure to increasing concentrations of bortezomib.
In addition, nTreg cells were selected based on the expression of CD25 as previously specified and the effect of the drug was evaluated on isolated non-Treg versus nTreg cells. Again, nTreg cells were not significantly affected in terms of viability by increasing the doses of the drug (Figure 1B).
It is worth mentioning that the phenotypic characteristics of isolated CD4+CD25+ T cells were analyzed by flow cytometry (Online Supplementary Figure S2), confirming that they showed the typical phenotype of naturally occurring regulatory T cells: CD4+CD25+FOXP3+CD127−/low.
Long-term culture of CD4+ T cells in the presence of bortezomib gives rise to a regulatory T-cell population
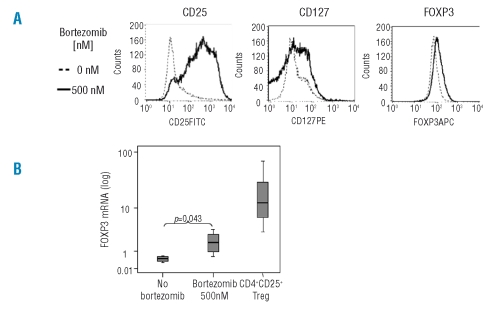
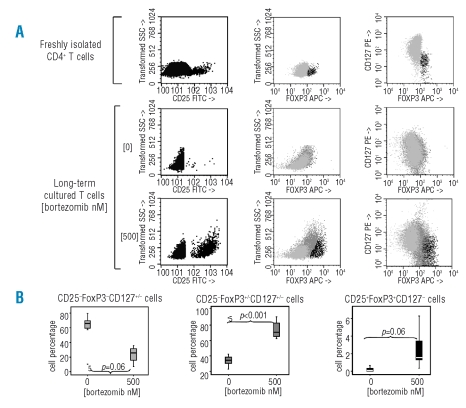
Since bortezomib did not affect nTreg cell viability, we next investigated whether we could obtain a purified regulatory T-cell population after long-term culture of CD4+ T cells in the presence of bortezomib, as has been described with other immunosuppressants. For this purpose, we cultured CD4+ T cells for three weeks with anti-CD3/anti-CD28 and exogenously added IL-2, in the presence or in the absence of 500nM bortezomib, and one more week in the presence of IL-2, but in the absence of stimulus or bortezomib. At the end of the culture we analyzed the phenotypic characteristics of these cells. As shown in Figure 2A, bortezomib induced a significant increase of CD25 expression (p<0.001), although no significant differences were observed by flow cytometry in the expression of FOXP3 or CD127 between untreated and bortezomib-treated cells. However, RT-PCR analysis showed a significant increase of FOXP3 RNA levels in cells long-term cultured in the presence of 500nM bortezomib. Nevertheless, this FOXP3 mRNA level was much lower as compared to naturally occurring nTreg (Figure 2B). A more detailed analysis (shown in Figure 3A) of these long-term cultured cells showed that at least three subpopulations of CD4+ T cells appeared: 1) a CD25−FOXP3−CD127+/− population; 2) a CD25+FoxP3+/−CD127+/− population and 3) a CD25+FoxP3+CD127− population. Interestingly, we found that in those cultures performed with the drug, the percentage of CD25− T cells significantly decreased, while both the CD25+FOXP3+/−CD127+/− and the CD25+FOXP3+CD127−populations significantly increased (Figure 3A and B).
Figure 2.
Phenotypic analysis of long-term cultured CD4+ T cells in the presence or in the absence of 500nM bortezomib. (A) Histograms representing the expression of CD25, FOXP3 and CD127; one of five similar experiments is shown. Mean fluorescence intensity ratio (MFIR) (MFI cells cultured with 500nM bortezomib/MFI cells cultured with 0nM bortezomib) were 4.9 for CD25, 1.4 for FoxP3 and 0.95 for CD127 expression. (B) Mean (SD) of FOXP3 mRNA level was 0.45 (0.17), 1.95 (1.1) and 28.5 (36.1) for bortezomib untreated and bortezomib treated long-term cultured T cells, and for freshly isolated CD4+CD25+ T cells, respectively.
Figure 3.
CD4+ T-cell sub-populations obtained after long-term culture in the presence of bortezomib. (A) Immunophenotypic analysis allowed the identification of CD25−FOXP3−CD127+/− cells, CD25+FOXP3+CD127+/− cells and CD25−FOXP3−CD127−cells among freshly isolated or long-term cultured (bortezomib 0 or 500 nM) CD4+ T cells. One representative experiment of five is presented. (B) Percentages of each of the three subpopulations obtained after CD4+ T-cell long-term culture in the presence or in the absence of borteozmib 500 nM.
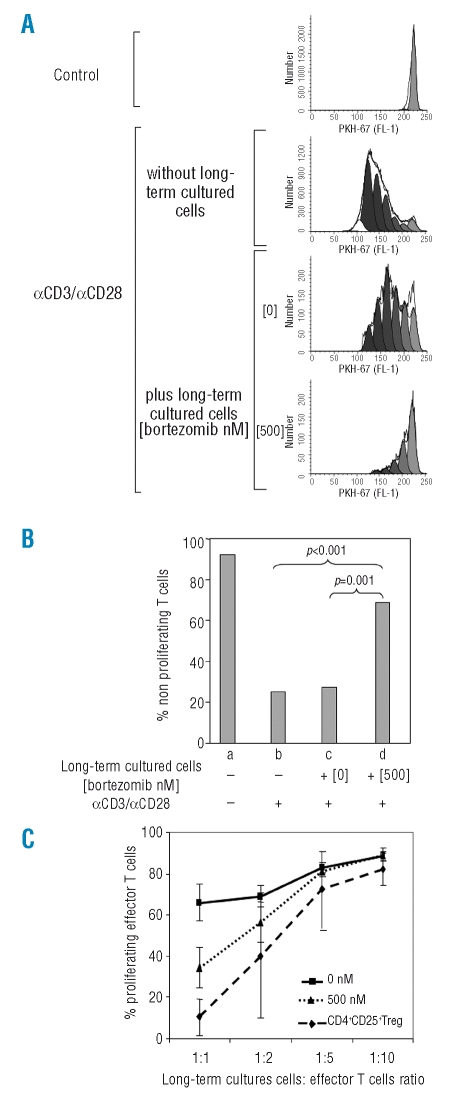
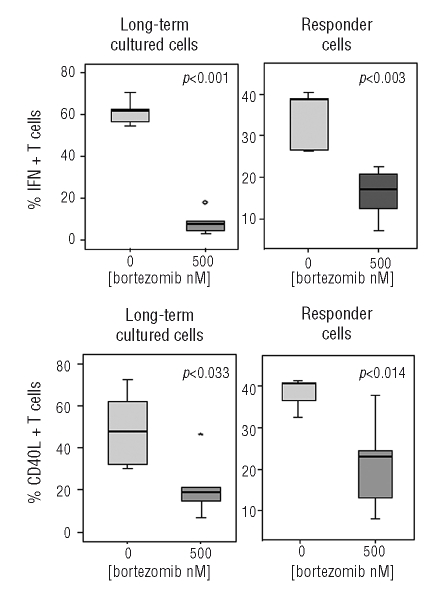
Next, we investigated whether these bortezomib long-term cultured T cells showed suppressive capacity using functional assays. For this purpose, freshly isolated PKH-67 stained T cells (responder T cells) were stimulated with anti-CD3 and anti-CD28 antibodies in the absence of bortezomib and co-cultured with CD4+ T cells long-term cultured with or without the drug (Online Supplementary Figure S3). As controls, responder T cells were cultured alone, either unstimulated or stimulated with anti-CD3/anti-CD28. After four days, we analyzed the proliferation and activation of responder T cells. The expression of CD25 slightly decreased in responder T cells co-incubated with bortezomib-treated cells. However, the number of proliferating cells, as assessed by PKH fluorescence diminution, significantly decreased when co-incubated with cells previously treated for three weeks with high doses of bortezomib (Figure 4A and B). Escalating ratios of long-term cultured:effector T cells were performed in order to evaluate the suppressive capacity of these cells. Freshly isolated CD4+CD25+ nTreg cells were used as controls. Results are shown in Figure 4C. We also analyzed IFN-γ production and CD40L expression by both responder and long-term cultured populations in these suppression assays. The percentage of IFN-γ positive responder T cells significantly decreased when stimulated in the presence of 500nM bortezomib-treated cells, compared to responder cells co-incubated with bortezomib-untreated T cells (Online Supplementary Figure S4). Regarding the long-term cultured populations, 60.9% of the cells cultured in the absence of bortezomib were IFN-γ positive, versus 8% among T cells treated with 500nM bortezomib. Likewise, CD40L positive cells decreased from 48.9% for untreated cells to 21.56% for bortezmib-treated cells (Figure 5).
Figure 4.
Suppression assays: proliferation. (A) proliferation, monitored by PKH-67 dilution of control or αCD3/αCD28 stimulated responder T cells, co-incubated or not with cells long-term cultured in the presence or in the absence of 500 nM bortezomib (1:1 ratio); one of five similar experiments is shown. (B) Mean (SD) number of non-proliferating cells among responding T lymphocytes were 92 (18.3), 25 (8.4), 27.3 (7.3), and 68.7 (9.6) for (a) unstimulated T cells (negative control), (b) T cells stimulated with aCD3/aCD28 (positive control) and T cells incubated with αCD3/αCD28 plus T lymphocytes long-term cultured (c) without bortezomib or (d) with 500nM bortezomib. (C) Titration assays showing the suppressive capacity of T cells long-term cultured at 500nM bortezomib (bTreg) at increasing ratios of long-term cultured versus effector T cells. Naturally occurring CD4+CD25+ Treg as well as CD4+ T cells long-term cultured in the absence of bortezomib (shown as 0nM) were used as controls.
Figure 5.
Suppression assays: IFN-γ and CD40L intracytoplasmic production after co-culture of responder plus long-term cultured T cells; expression by both responder and long-term cultured T cells is shown.
A regulatory T-cell population can also be obtained after long-term culture of CD4+CD25− T cells in the presence of bortezomib
These results suggested that long-term culture of CD4+ T cells in the presence of bortezomib increases the percentage of naturally occurring CD4+CD25+ regulatory T cells but, most importantly, gives rise to a distinct population of suppressor or regulatory T lymphocytes (bTreg) (CD25+FOXP3+/−CD127+/− cells). In order to confirm that this bTreg population was not derived from CD4+CD25+ nTreg cells, but a different Treg population, we used the same approach starting the experiment from CD4+ T cells depleted of CD25+ Treg cells. Upon analyzing the different T-cell populations obtained after culture, again a significant decrease of the CD25−FOXP3−CD127+/− population was observed, while the percentage of CD25+FOXP3+/−CD127+/−cells (bTreg) significantly increased. Likewise, a significant increase in the CD25+FOXP3+CD127− cells was observed (Figure 6A and B).
Figure 6.
Analysis of long-term cultured CD4+CD25− T cells in the presence or in the absence of 500 nM bortezomib. (A) Dot plots representing the subpopulations obtained; one of five similar experiments is shown. (B) Box-plots showing cell percentages of the three subpopulations obtained: C D 2 5 − F O X P 3 − C D 1 2 7 +/−, CD25+FOXP3+/−CD127+/− and CD25+FOXP3+CD127−. (C) Suppression of proliferation of responder T cells by long-term cultured CD4+CD25− T cells. The mean (SD) number of non-proliferating cells among responding T lymphocytes was 98.4 (0.7), 13.4 (1.63), 17.9 (5.9), and 72 (9.3) for (a) un-stimulated T cells (negative control), (b) T cells stimulated with αCD3/αCD28 (positive control) and T cells incubated with αCD3/αCD28 plus (c) untreated versus (d) 500nM bortezomib-treated long-term cultured T lymphocytes.
Functional assays were again performed in order to evaluate the suppressive effect of long-term cultured CD4+CD25− cells on the proliferation of responder T cells. Interestingly, as shown in Figure 6C, T cells long-term cultured in the presence of bortezomib significantly decreased the proliferation of responder T cells, the mean percentage (SD) of resting cells among responding T lymphocytes being 17.9% (5.9) versus 72% (9.3) upon culture with untreated versus bortezomib-treated T lymphocytes (p<0.001). Thus, bTreg with suppressive capacity were also obtained after culture of CD4+CD25− selected T cells in the presence of bortezomib.
Finally, we compared percentage of the three subpopulations obtained after long-term culture of total CD4+ cells versus CD4+CD25− selected T cells. Although there were no statistically significant differences, long-term cultured total CD4+ T cells showed a higher percentage of bTreg and nTreg cells than CD4+CD25− cells (data not shown). Upon comparing functional effect of both type of cells, those bTreg obtained after culture of total CD4+ cells displayed a higher suppressive effect, although these differences were not statistically significant, the mean percentage (SD) of non-proliferating effector T cells in suppression assays being 78.5 (8.9) versus 72 (9.3) for responder T cells co-cultured with total CD4+ or CD4+CD25− T cells, respectively, p=0.27.
Discussion
We have previously proposed the use of the proteasome inhibitor bortezomib for in vitro depletion of allore-active T cells in order to prevent GVHD. To this extent, we have demonstrated that, in a mixed leukocyte culture, bortezomib selectively kills alloreactive T cells.18 Moreover, preliminary data using this approach in the clinical setting have already been reported showing promising results.25 But another important issue remained to be considered. Accumulating evidence suggests an important role of Treg cells in the control of GVHD. Several studies have found a positive correlation between high numbers of circulating CD4+CD25+FoxP3+ Treg cells and reduced GVHD22,26,27 after hematopoietic stem cell transplantation. Likewise, it has been suggested that approaches of selective depletion of alloreactive T cells based on CD25+ T-cell depletion could exacerbate GVHD due to the elimination of CD4+CD25+ Treg cells.28 Thus, it would be desirable to develop new procedures of GVHD prophylaxis which allow the preservation or even expansion of regulatory T cells. In the current study we have addressed the effect of bortezomib on the viability of naturally occurring CD4+CD25+ regulatory T cells. Our results indicate that bortezomib does not affect nTreg viability, both in resting condition or in a mixed leukocyte culture in which some T cells will presumably recognize alloantigens and become activated. Moreover, even after polyclonal activation with anti-CD3 and anti-CD28 antibodies, the viability of nTreg cells remained unaffected.
On the other hand, the resistance of nTreg cells to the pro-apoptotic effect of bortezomib prompted us to explore the possibility of obtaining a cell population highly enriched on regulatory T cells using bortezomib, as previously reported with immunosuppressive drugs such as the combination vitamin D3-dexamethasone or rapamycin.15–17 For that purpose, we stimulated CD4+ T cells for three weeks in the presence or in the absence of bortezomib, and left them resting one more week in the absence of the drug. The analysis of CD4+ T cells at the end of the culture reveals the existence of at least three subpopulations: a CD25−FOXP3−CD127+/− population, most likely cells that have returned to the resting state; a CD25+FoxP3+CD127− population, presumably naturally occurring Treg cells and a CD25+FoxP3+/− population (bTreg), expressing variable levels of CD127. The nTreg cell population significantly augmented with the addition of bortezomib, although the percentage remained rather modest. These data suggest that treatment with bortezomib, even during long periods of time and at high doses, preserves Treg cells but does not promote a great expansion of this cell subset. Nevertheless, the analysis of the suppressive capacity of this long-term cultured CD4+ cells showed that T cells cultured in the presence of bortezomib significantly reduced the proliferation, IFN-γ production and CD40L expression of anti-CD3/anti-CD28 stimulated responder T cells, while the addition of bortezomib-untreated cells to the cultures barely changed these parameters among responder T cells. It’s unlikely that this marked suppressor effect is only due to the modest increase in the percentage of nTreg cells, but most probably to the CD25+FOXP3+/− (bTreg) population which significantly increases upon culture with high doses of bortezomib.
In order to confirm that this suppressor effect was mainly due to a population different from nTreg, either selected or generated by the treatment with bortezomib, we studied if we could obtain the same suppressor population starting from CD4+CD25− T cells. With this aim, we performed similar experiments with CD4+ T cells depleted of nTreg cells. Interestingly, for all the parameters analyzed, we obtained similar results to those achieved when the starting population contained nTreg cells. It is of note that small percentages of cells with nTreg phenotype appeared in Treg depleted cells cultured with bortezomib. These cells may correspond to contaminating Treg cells that remained after CD25+ T-cell depletion (<0.5%) the percentage of which has increased because of their proliferative advantage over conventional T cells in the presence of bortezomib. Likewise, bortezomib long-term cultured cells, both total CD4+ or CD4+CD25− cells, showed a marked reduced production of IFN-γ, and significantly lower expression of CD40L, while FOXP3 expression did not reach the levels observed among nTreg cells. Interestingly, it has been demonstrated that FOXP3 may not be essential for the function of ex vivo generated regulatory T cells, as FOXP3− Treg cells induced by several approaches have been shown to efficiently inhibit proliferation of T cells similarly to CD4+CD25+ nTreg cells.11,16 Therefore, on one hand, the culture of CD4+ T cells in the presence of bortezomib does not affect nTreg cell viability and, on the other hand, long-term culture of CD4+ T cells in the presence of bortezomib, irrespective of whether they contain CD25+ T cells or not, gives rise to a regulatory population, apparently different from naturally occurring Treg cells. Both properties may be of clinical relevance, not only for the treatment of GVHD, but also for situations in which activation of T cells is undesirable, such as autoimmune disorders or solid organ transplantation. Pre-clinical studies have clearly shown protection of GVHD after nTreg cells transfer in murine models of bone marrow transplantation.29–31 The inconvenience of this approach is that the nTreg population represents a small percentage of peripheral blood CD4+ T cells (5–10%) so that expansion procedures are required. This expansion can be achieved by polyclonal stimulation with anti-CD3 and anti-CD28 antibodies in the presence of IL-2. However, these culture conditions are highly favorable for the expansion of conventional T cells, which can contaminate the purified nTreg-cell population and overgrow nTreg after prolonged culturing in vitro.32 As it has been pointed out,33 the risk of co-expanding potentially harmful cells represents a major concern for the clinical use of expanded nTreg cells. In order to minimize this problem, Battaglia et al. have proposed the addition of rapamycin to the culture media, resulting in a significant reduction of the undesired expansion of effector T cells and allowing the proliferation of nTreg cells.17 In a similar way, we have found that bortezomib reduces the expansion of conventional T cells, allowing both the survival of naturally occurring nTreg cells and the appearance of a distinct suppressor T-cell population, limiting the expansion of undesired effector T cells. In this regard, our results resemble more those obtained by Valmori et al., who postulate that rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T-cell cultures is not due to the selective expansion of nTreg cells, but to the induction of regulatory functions in conventional CD4+ T cells.34 Accordingly, our treatment gives rise to a population of suppressor T cells which differ from nTreg cells. But whether these cells were committed or not to be regulatory, that is, if they are selected or newly induced with this approach, should be addressed.
In summary, the promising results obtained in this study strengthen our idea of using bortezomib in the prevention of GVHD, not only because of its selective cytotoxic effect on activated T cells, but also due to its ability to preserve and/or generate regulatory T cells.
Footnotes
The online version of this article contains a supplementary appendix.
Authorship and Disclosures
BB performed data analysis and wrote the manuscript; JAP-S designed the research project; LIS-A performed viability assays; TC-V performed functional assays; SG-C performed Treg expansion; PH-C performed functional assays; MD-C performed cytometry assays; ST performed viability assays; CR-S performed Treg expansion; CS performed molecular studies; FMS-G critically reviewed the manuscript; CdC critically reviewed the manuscript; JSM supervised the research project.
The authors reported no potential conflicts on interest.
Funding: José A Pérez-Simón was supported by a fellowship from the European Hematology Association; Belén Blanco was supported by a fellowship from the Fondo de Investigación Sanitaria.
References
- 1.Jiang S, Lechler RI, He XS, Huang JF. Regulatory T cells and transplantation tolerance. Hum Immunol. 2006;67:765–76. doi: 10.1016/j.humimm.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 3.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 4.McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, et al. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–23. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–42. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 6.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cozzo C, Larkin J, III, Caton AJ. Cutting edge: self-peptides drive the peripheral expansion of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:5678–82. doi: 10.4049/jimmunol.171.11.5678. [DOI] [PubMed] [Google Scholar]
- 8.Gregori S, Bacchetta R, Hauben E, Battaglia M, Roncarolo MG. Regulatory T cells: prospective for clinical application in hematopoietic stem cell transplantation. Curr Opin Hematol. 2005;12:451–6. doi: 10.1097/01.moh.0000177826.41262.0a. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 10.Li M, Zhang X, Zheng X, Lian D, Zhang ZX, Sun H, et al. Tolerogenic dendritic cells transferring hyporesponsiveness and synergizing T regulatory cells in transplant tolerance. Int Immunol. 2008;20:285–93. doi: 10.1093/intimm/dxm142. [DOI] [PubMed] [Google Scholar]
- 11.Levings MK, Gregori S, Tresoldi E, Cazzaniga S, Bonini C, Roncarolo MG. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood. 2005;105:1162–9. doi: 10.1182/blood-2004-03-1211. [DOI] [PubMed] [Google Scholar]
- 12.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of inter-leukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–22. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 14.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor FoxP3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, et al. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)-and Th2-inducing cytokines. J Exp Med. 2002;195:603–16. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vieira PL, Christensen JR, Minaee S, O’Neill EJ, Barrat FJ, Boonstra A, et al. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5986–93. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 17.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338–47. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 18.Blanco B, Perez-Simon JA, Sanchez-Abarca LI, Carvajal-Vergara X, Mateos J, Vidriales B, et al. Bortezomib induces selective depletion of alloreactive T lymphocytes and decreases the production of Th1 cytokines. Blood. 2006;107:3575–83. doi: 10.1182/blood-2005-05-2118. [DOI] [PubMed] [Google Scholar]
- 19.Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–50. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 20.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–9. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 21.Rieger K, Loddenkemper C, Maul J, Fietz T, Wolff D, Terpe H, et al. Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood. 2006;107:1717–23. doi: 10.1182/blood-2005-06-2529. [DOI] [PubMed] [Google Scholar]
- 22.Zorn E, Kim HT, Lee SJ, Floyd BH, Litsa D, Arumugarajah S, et al. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106:2903–11. doi: 10.1182/blood-2005-03-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santamaria C, Chillon MC, Fernandez C, Martin-Jimenez P, Balanzategui A, Garcia SR, et al. Using quantification of the PML-RARalpha transcript to stratify the risk of relapse in patients with acute promyelocytic leukemia. Haematologica. 2007;92:315–22. doi: 10.3324/haematol.10734. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔC(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Koreth J, Kim HT, Ho VT, Cutler CS, Soiffer RJ, Antin JH, et al. Bortezomib plus tacrolimus/methotrexate for prophylaxis of acute GVHD after HLA mismatched allogeneic non-myeloablative transplantation: results of a dose finding phase I study. Biology of Blood and Bone Marrow Transplantation. 2008;(suppl # 140) [Google Scholar]
- 26.Miura Y, Thoburn CJ, Bright EC, Phelps ML, Shin T, Matsui EC, et al. Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood. 2004;104:2187–93. doi: 10.1182/blood-2004-03-1040. [DOI] [PubMed] [Google Scholar]
- 27.Rezvani K, Mielke S, Ahmadzadeh M, Kilical Y, Savani BN, Zeilah J, et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108:1291–7. doi: 10.1182/blood-2006-02-003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin PJ, Pei J, Gooley T, Anasetti C, Appelbaum FR, Deeg J, et al. Evaluation of a CD25-specific immunotoxin for prevention of graft-versus-host disease after unrelated marrow transplantation. Biol Blood Marrow Transplant. 2004;10:552–60. doi: 10.1016/j.bbmt.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Hanash AM, Levy RB. Donor CD4+CD25+ T cells promote engraftment and tolerance following MHC-mismatched hematopoietic cell transplantation. Blood. 2005;105:1828–36. doi: 10.1182/blood-2004-08-3213. [DOI] [PubMed] [Google Scholar]
- 30.Joffre O, Gorsse N, Romagnoli P, Hudrisier D, van Meerwijk JP. Induction of antigen-specific tolerance to bone marrow allografts with CD4+CD25+ T lymphocytes. Blood. 2004;103:4216–21. doi: 10.1182/blood-2004-01-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–99. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levings MK, Sangregorio R, Sartirana C, Moschin AL, Battaglia M, Orban PC, et al. Human CD25+ CD4+ T suppressor cell clones produce transforming growth factor beta, but not interleukin 10, and are distinct from type 1 T regulatory cells. J Exp Med. 2002;196:1335–46. doi: 10.1084/jem.20021139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585–98. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 34.Valmori D, Tosello V, Souleimanian NE, Godefroy E, Scotto L, Wang Y, et al. Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J Immunol. 2006;177:944–9. doi: 10.4049/jimmunol.177.2.944. [DOI] [PubMed] [Google Scholar]