Previous observations indicate that a lamividune-prophylaxis strategy results in a decrease of hepatitis B virus (HBV) reactivation rates. This report evaluates the benefits from this strategy among lymphoma patients. The findings of this study indicate that extended anti-HBV prophylaxis can improve survival rates by 2.4% in HBsAg-positive lymphoma patients receiving chemotherapy.
Keywords: lymphoma, hepatitis B, reactivation, lamivudine, prophylaxis, mortality
Abstract
Lamivudine prophylaxis is an effective strategy in HbSAg-positive patients receiving cancer chemotherapy. Recent data indicate that a lamividune-prophylaxis strategy results in a decrease of hepatitis B virus (HBV) reactivation rates, though its effect on HBV-mortality remains equivocal. This report evaluates the benefits from this strategy among lymphoma patients and develops a management approach for patients with prolonged immunosuppression. A Medline search was conducted to retrieve published trials on HBsAg-positive lymphoma patients receiving prophylactic lamivudine during chemotherapy. Basic inclusion criterion was to report HBV-reactivation rates with and without lamivudine prophylaxis. A meta-analysis of the risk of HBV-reactivation and HBV-related mortality was conducted, and the pooled effect was calculated as risk ratio (RR). We found that lamivudine prophylaxis is associated with a significant reduction in hepatitis B virus reactivation (RR 0.21, 95%CI 0.13–0.35) and a trend in reducing HBV-related mortality (RR 0.68, 95%CI 0.19–2.49). In order to study the long-term effects of anti-HBV prophylaxis when prolonged immunosuppression is needed, we used our findings to model a decision tree. Overall survival was the main outcome used in the analysis. Rituximab maintenance in B-cell lymphomas was used as a paradigm of prolonged immunosuppression. We found that extended anti-HBV prophylaxis can improve survival rates by 2.4% in HBsAg-positive patients. If 1,000 HBsAg-positive lymphoma patients receive prophylaxis, one will die from hepatitis B virus reactivation versus 25/1,000 if no prophylaxis is administered. This effect is probably mediated through a reduction of hepatitis B virus reactivation and HBV-related mortality. The ideal antiviral agent needs to be determined.
Introduction
Between 350 and 400 million people globally are chronic hepatitis B surface antigen carriers (HbSAg). Complications of HBV infection, including acute liver failure, cirrhosis and hepatocellular carcinoma, are responsible for an estimated 1.2 million deaths per year, placing HBV among the most significant global health burdens. With reactivation of HBV being frequently reported in the setting of concomitant immunosuppression, cytotoxic chemotherapy and transplantation, HBsAg carriers are the focus of considerable investigation including the role of prophylactic antiviral therapy.1–4
Lamivudine, a nucleoside analogue, is an effective suppressor of hepatitis B virus (HBV) replication that reduces HBV DNA in serum and improves liver injury in patients with chronic hepatitis B. Lamividune is well-tolerated and provides an excellent long-term safety profile.5 Although the incidence of resistance due to mutated tyrosine-methionine-aspartate-aspartate motif of the HBV DNA polymerase gene (the YMDD mutation) may rise up to 20% at first year,6,7 it remains a widely used prophylaxis strategy. A recent systematic review of published trials suggested that preventive lamivudine may reduce the risk of HBV reactivation and associated morbidity in HbSAg-positive patients receiving cancer chemotherapy.8 Lamivudine effects were unidirectional in favor of prophylaxis and the lamivudine prophylaxis strategy was also associated with a 70% reduction in reactivation-related mortality.8 Based on level III evidence, the American Association for the study of Liver Diseases recommended in 2004 the continuation of lamivudine prophylaxis for six months after completion of chemotherapy,6 and extended this period to more than six months in the 2007 update, for those patients with high baseline HBV DNA (defined as serum HBV DNA >2×104 copies/mL).9 However, the power of these studies was limited by small sample sizes, heterogeneity of the studied populations and the lack of a valid comparison with newer anti-HBV agents.10 Also, published meta-analyses so far,8,10,11 did not discriminate between the effect of prophylaxis on lymphoma and the effect on solid tumors, nor did they address the question of prolonging such a strategy when maintenance immunosuppressive therapy is indicated. For example, Martyak et al.8 provided a pooled estimate of HBV reactivation risk but in a limited number of hematologic studies. Also, previous studies did not provide an insight on the management of patients receiving prolonged immunosuppression, a strategy that is often used among lymphoma patients.
The aim of this report is to estimate the effect of prophylactic lamivudine on the risk of HBV reactivation and HBV-mortality in HBsAg positive lymphoma patients undergoing chemotherapy or immunotherapy. Bone marrow transplant recipients were excluded. Then we used our findings from the meta-analysis to assess the feasibility of prophylaxis strategy in cases for whom prolonged immunosuppression may be needed (typically during the extended rituximab maintenance of CD20+ B-cell indolent lymphomas).
Design and Methods
In order to identify studies, we performed a Medline search up to December 2008 and a cross-reference manual search of abstracts and relevant reviews to identify additional relevant studies. Hepatitis B virus, reactivation and lymphoma were used as search terms. HBV reactivation rates and HBV related deaths were extracted. Two authors (PDZ, PK) independently performed the literature review and assessed all potentially relevant publications. All relevant publications were retrieved in full text. Any emerging discrepancies were resolved by consensus between the authors. The study was performed according to the QUOROM statement12 and the checklist was provided to the editors during the review process.
The pooled effect of lamivudine prophylaxis in lymphoma patients can be calculated as Risk Ratio (RR) by summarizing studies reporting reactivation rates with or without lamivudine prophylaxis using the predefined criteria of Loomba et al.10 In brief, these criteria included randomized, controlled trials or cohort studies that allowed assessment of HBV-reactivation rate and HBV-related death after the start of chemotherapy with and without lamivudine prophylaxis. Trials were excluded if relevant data could not be extracted or if they reported non-lymphoma patients (such as studies on post-transplantation patients, patients with rheumatological diseases or HIV co-infection).
Pooled RR was calculated according to the Mantel-Haenszel method for fixed effects13,14 and DerSimonian & Laird for random effects.15 Statistical heterogeneity was measured using the χ2 Q test (p<0.10 is considered representative of significant statistical heterogeneity) and the I2 statistic, as previously described.16,17 Although the selection of a random- vs. fixed-effects model remains controversial, the use of the former in the calculation of CIs results in wider intervals and thus, a more conservative estimate of effects. Whenever heterogeneity is limited, a fixed-effects model appears more appropriate.
The potential benefit of extending anti-HBV prophylaxis during the maintenance phase of lymphoma treatment can be assessed using methods for decision analysis.18 The decision tree was built to estimate the long-term impact of anti-HBV prophylaxis in terms of 3-year overall survival (3yrOS) in lymphoma patients receiving extended 2-year rituximab maintenance. The two competing strategies were to prolong anti-HBV prophylaxis during that phase versus no prophylaxis. The clinical benefit of the two strategies was calculated and graphically represented. One-way and two-way sensitivity analyse were performed to ensure the validity of the results.
In our model, the basic assumptions were that: (a) there is no significant mortality related to prophylaxis regimen (as we confirmed by the results detailed in Table 1), (b) the range of probabilities for reactivation and HBV-associated mortality during the maintenance phase lies within the limits reported in our meta-analysis data and, (c) patients with no HBV reactivation and survivors of HBV reactivation are able to achieve the expected OS in lymphoma. RevMan 5 module (The Nordic Cochrane Centre, The Cochrane Collaboration, 2008) and TreeAgePro 2008 (TreeAge Software, Inc.) were used for statistical analysis and graphical representations.
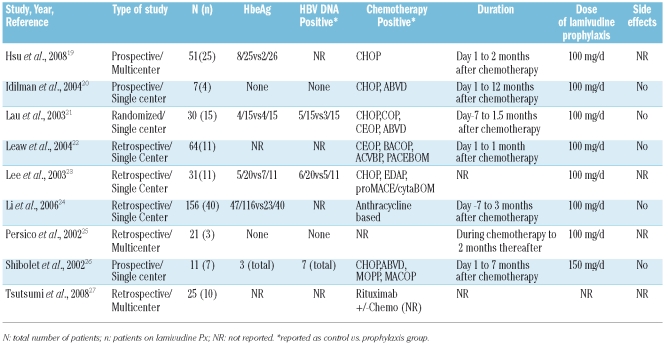
Table 1.
Trials of prophylactic lamivudine in lymphoma patients for the prevention of hepatitis B reactivation.
Results
Studies evaluated in this report
The initial Medline search resulted in 95 potentially relevant publications. Forty-four case reports, 8 review papers, 3 studies in non-lymphoma neoplasias and one study in non-humans were discarded. Of the 39 remaining publications, 23 did not report the prophylactic use of lamivudine and were eliminated. Of the 16 remaining studies, HBV-reactivation data could be extracted in 9.19–27 The review articles added no further studies (Online Supplementary Appendix, flow chart).
Lamivudine prophylaxis in lymphomas: where do we stand?
The 9 eligible studies are summarized in Table 1 and included a total of 396 participants; 127 patients in the prophylactic lamivudine arm and 269 patients in the control group. The cumulative prevalence of HBV reactivation in the prophylaxis group was 8.6% (11/127) versus 50.6% (136/269) in the control group (Figure 1A).
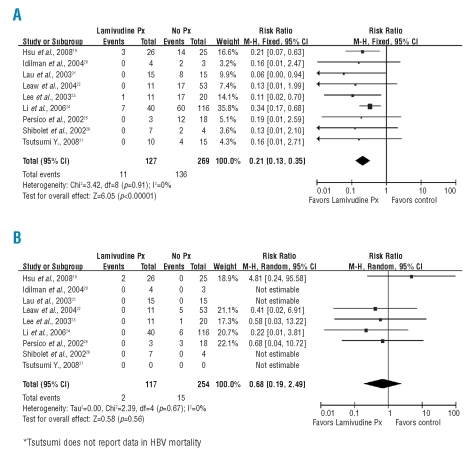
Figure 1.
(A) Forest plot of the included studies assessing HBV reactivation in lymphoma patients under treatment. The pooled effect of lamivudine prophylaxis justifies its preemptive use for the risk of HBV reactivation. (B) Forest plot of the included studies assessing HBV-associated mortality in lymphoma patients under treatment. Lamivudine prophylaxis is associated with an insignificant decline in mortality rates.
The reactivation rates in the current analysis did not differ from those previously reported in a number of small, uncontrolled studies in lymphoma patients. Reactivation rates for patients not receiving prophylaxis ranged from 54.5% to 100%,28–31 whereas reactivation rates in prophylaxis trials did not exceed 5%.32,33 Notably, in the largest prospective, randomized trial of lamivudine prophylaxis for chemotherapy-associated reactivation in non-Hodgkin’s lymphoma (NHL) patients, the only factor that independently predicted HBV reactivation in multivariable analysis was lamivudine prophylaxis (odds ratio 0.04; 95% CI, 0.005–0.344). Interestingly, although HBV reactivation did occur during chemotherapy, both the incidence and the severity of hepatitis flare were significantly reduced.19
The pooled effect of lamivudine prophylaxis on HBV reactivation (Figure 1A) remained significant under the fixed effects model (RR 0.21, 95%CI 0.13–0.35) with no evidence of statistical heterogeneity between studies (I2=0) despite the documented clinical heterogeneity. In terms of HBV-related mortality, however, (Figure 1B), the pooled effect of 8 studies reporting relevant clinical data displays an insignificant decline (Table 2) from 5.9% (15/254) in the control group to 1.7% (2/117) in the prophylaxis group (RR 0.68, 95%CI 0.19–2.49).
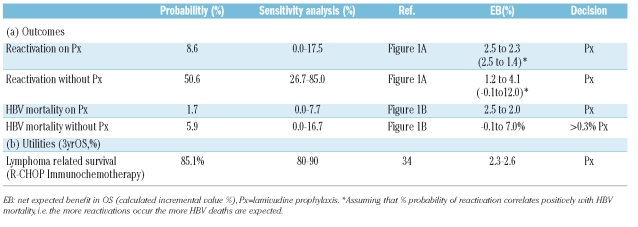
Table 2.
Outcomes and utilities assigned to the decision-tree.
The role of HBV-prevention strategy during maintenance therapy for B-non Hodgkin’s lymphomas
We evaluated if a more prolonged prophylaxis, extending up to two years, is necessary in the case of CD20+ B-non Hodgkin’s lymphomas (B-NHLs). In order to clarify the potential effect of antiviral prophylaxis, we performed a decision analysis (Figure 2) to compare the two competing strategies (the prolonged anti-HBV treatment during maintenance phase with rituximab vs. no prophylaxis). The main outcome measure was overall survival at three years (3yrOS). All outcomes were based on published series and current data (Table 2). A patient entering the prophylaxis arm has an estimated risk of 8.6% (range 0.0–17.5%) to reactivate HBV-infection and 1.7% (range 0.0–7.7%) to die from disease flare. Estimated probabilities in the no-prophylaxis arm are 50.6% (range 26.7%–85.0%) for reactivation and 5.9% (0.0–16.7%) for HBV mortality, respectively. Unless HBV death occurs, all patients are to achieve the estimated 3yrOS (85.1%).34 The expected benefit (EB) was calculated as the percent difference (incremental value) between the averaged-out outcomes of the two arms, and expressed the expected improvement in 3 yrOS.
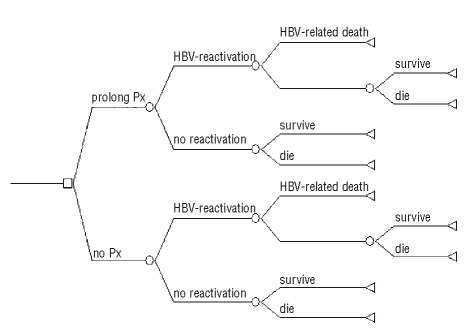
Figure 2.
Decision Tree: a clinical decision tree is a schematic display of the temporal and logical structure of a clinical situation in which one or more decisions must be made. Squares represent decision nodes from which two competing strategies originate. They denote a point in time in which we can elect one of several alternative courses of actions. Circles are chance nodes from which a study leads to a particular outcome, beyond the control of our decision. Terminal nodes (endpoints) represent the final outcome (3yrOS) of a particular pathway. A path (or scenario) in a decision tree is a particular sequence of actions beginning with a particular choice at the initial decision node (Prolong Prophylaxis vs. No Prophylaxis) and following a particular event or choice from left to right. The decision tree compares the strategy of prolonging prophylaxis with that of no prophylaxis. At baseline, the prophylaxis strategy results in 2.4% net benefit in 3yrOS compared to no prophylaxis, a positive benefit preserved for a wide range of probabilities in sensitivity analysis.
Sensitivity analysis and decision making
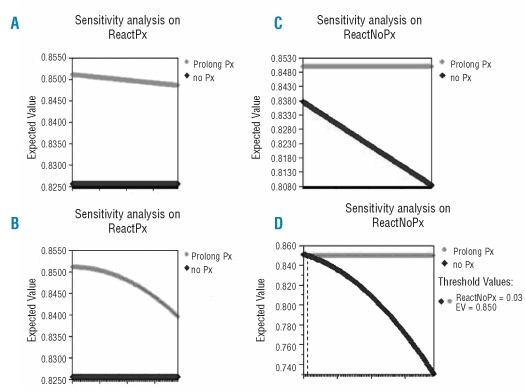
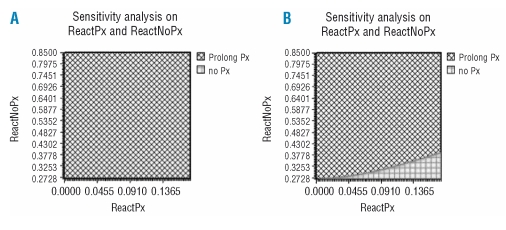
We performed one-way and two-way sensitivity analyses to reinforce the validity of the assumptions and robustness of the results. Initially, threshold analysis was completed on each variable to determine whether the decision altered within the probability range specified in Table 2. At baseline the expected 3yrOS for prolonging prophylaxis is estimated at 85.0% versus 82.6% for the arm without antiviral prophylaxis. The net expected benefit is therefore 2.4%, favoring the prophylaxis strategy. The optimal path is to continue prophylaxis as long as immunotherapy maintenance is administered. One-way sensitivity analysis always favors the prophylaxis arm, with an expected benefit in 3yrOS depending upon the assumed probabilities presented in Table 2. The preemptive strategy appears to be justified even when we assume that the risk of reactivation in both arms positively correlates with the HBV-mortality rates (Figure 3A–D). Importantly, we also performed two-way sensitivity analysis evaluating the risk of HBV-associated death in both arms, with the prophylaxis arm being the optimal strategy in all possible scenarios. In other terms, provided that HBV-mortality in both arms varies within the limits specified (Table 2), prophylaxis will always yield a clinical benefit in overall survival. Finally, two-way analysis on probabilities of reactivation with or without correlation with HBV-mortality risks, displayed the superiority of the prophylaxis arm (Figure 4 A and B).
Figure 3.
One way sensitivity analysis on probability of reactivation under prophylaxis (ReactPx) and without anti-HBV prophylaxis (ReactNoPx). (A, C) assume no correlation with HBV-related deaths. (B, D) assume a positive correlation.
Figure 4.
Two-way sensitivity analysis, strategy graph. (A) assumes no correlation of reactivation probability with HBV-related death, (B) assumes a positive correlation. For a wide range of probablities of HBV reactivation, the prophylaxis strategy is the optimal choice in terms of expected survival in HBsAg positive lymphoma patients.
Discussion
We evaluated the effect of prophylactic lamivudine on the risk of HBV reactivation and HBV-mortality in HBsAg positive lymphoma patients undergoing chemotherapy or immunotherapy. The first part of this report focused on the protective effect of lamivudine prophylaxis and demonstrated a significant decline in HBV reactivation and a trend in HBV-related deaths. The second part of the study focused on the decision making process, and demonstrated that the prophylaxis strategy during chemotherapy extended throughout the maintenance phase is the optimal strategy in terms of long-term outcome.
A previous decision analysis model also indicated that lamivudine prophylaxis was the preferred strategy for most clinical event ranges, assuming a high cost for end-stage treatment and a low cost for lamivudine. Prophylaxis was effective in reducing both liver and lymphoma related deaths with an incremental cost-effectiveness ratio (ICER) of $33,514 per life year, and therefore considered optimal compared with starting lamivudine on evidence of overt hepatitis.35 It is reasonable to assume that prolonging immunossupression during the maintenance phase may inevitably expose lymphoma patients to the risk of HBV-reactivation. Especially rituximab maintenance prolongs the disease-free survival in patients with indolent lymphomas after successful initial cytoreduction.34 The addition of rituximab to anthracycline based chemotherapy was a milestone in the development of front-line therapy for CD20-positive B-cell lymphomas, virtually improving all aspects of long-term prognosis, including disease-free, progression-free and overall survival. Notably, a clinical benefit of rituximab maintenance has also been observed after different induction treatments such as rituximab single agent therapy or polychemotherapy. Current data indicate that rituximab maintenance can be safely administered for up to two years, although assessment of long-term safety requires longer follow-up.36 A clinical benefit from maintenance strategies in diffuse large B-cell lymphoma is less evident.37
However, data on HBV reactivation during rituximab maintenance are lacking. None of the previously analyzed studies (Table 1, Figure 1) evaluated the risk of rituximab alone vs. the risk of chemotherapy in terms of HBV reactivation. This is not surprising since most of these studies refer to CHOP or CHOP-like regimens as standard protocols without concomitant immunotherapy. In a recent series of patients with diffuse large B-cell lymphoma treated with CHOP or R-CHOP regimens, the use of rituximab was predictive of HBV reactivation,38 confirming a previous report27 that HBV reactivation occurs in patients treated with both rituximab monotherapy and rituximab-containing chemotherapy. Moreover, rituximab has been associated with serious viral infections including cytomegalovirus infection, varicella-zoster infection and HBV infection, the latter being the most frequent in a recent incidence-case review,39 leading to a fatal outcome due to hepatic failure in half of the affected individuals.
In clinical terms, under baseline assessment for HBV-reactivation rates and HBV-related mortality without prophylaxis, anti-HBV prophylaxis will effectively reduce the number of patients who suffer HBV-reactivation and succumb to this complication. Practically, if 1,000 HBsAg-positive lymphoma patients receive prophylaxis only one will die from HBV reactivation (851/1,000 expected OS for lymphoma minus 850/1,000 in the prophylaxis arm) versus 25/1,000 if no prophylaxis is administered (851/1,000 expected OS minus 826/1,000 expected survival without prophylaxis). The positive impact on HBV-mortality is mediated in a bimodal fashion, by minimizing the absolute risk of HBV-reactivation as well as by reducing the clinical severity of HBV flare.
Our analysis indicates that the beneficial effect of prophylaxis peaks when the probability of reactivation without prophylaxis maximizes, or when HBV-reactivation under prophylaxis approaches zero. The ideal scenario of zero HBV reactivation under prophylaxis combined with maximal risk of reactivation without prophylaxis results in a net benefit of 4.3% in survival (85.1% expected OS for prophylaxis minus 80.8% expected OS without prophylaxis) and 12.1% (85.1% expected OS minus 73.0% expected OS) if reactivation risks correlate with HBV-related mortality. In the latter assumption, however, the no-prophylaxis arm would be a viable option in the unlikely scenario that reactivation rates were both minimal (Figure 4B).
As noted above, lamivudine was the first oral agent for this indication and most studies reporting data on escape mutants are from before the release of newer potent antiviral agents. The Achilles heel of prophylactic lamivudine and an area of substantial controversy, is the development of lamivudine-resistant mutant strains of HBV, which can cause hepatitis flare.40 Initial reports in case series of HBV infection reported a mutation range, specifically within the YMDD motif, from 20% at first year up to 67% for longer duration. Importantly, despite emergence of mutant strains, the clinical outcomes were equivalent among chronic HBV carriers with and without YMDD mutant HBV.41,42 Regarding the incidence of lamivudine-resistant YMDD mutations among chronic HbSAg carriers who receive lamivudine prophylaxis, data are conflicting. For hematologic malignancies, the reported rates vary from 3.1% to 7.7%.19,43 Among case series of patients receiving liver or stem cell transplants, emergence of YMMD mutants is signicantly increased, reaching up to 21% at first year and 34% at two years.44,45 The reasons for development of resistance are complex and include viral load and patient compliance in particular.
It may be the case for many patients with lymphoma that lamivudine will remain extremely effective as long as their viral loads are low, and the patient is compliant with the medication. The survival advantage could be maximized by selecting an antiviral agent with a similar safety profile to lamivudine, but with negligible risk of resistance in long-term administration. Recently, The European Association for the Study of The Liver (EASL) has recommended the prophylactic use of drugs with low resistance rate as a prophylactic treatment in HBsAg patients with high level of HBV DNA at baseline. Lamivudine prophylaxis could still be recommended in cases of low HBV DNA.46 A similar strategy could be effective in lymphoma patients combined with careful on-treatment monitoring and the prompt modification of therapy in cases of reactivation.47,48
Although the results of our research synthesis provide significant support for the use of preventive lamivudine during chemotherapy, our report is limited by the quality of data of the primary studies. First, in our analysis of HBV-related mortality we included data from 2 patients who died after discontinuation of lamivudine. In this study lamivudine was interrupted only two months after completion of therapy for lymphoma. Importantly, the authors of the primary study as well as relevant reviews included this outcome19,10 in the prophylactic arm of lamivudine. It is considered that these 2 patients had been exposed to the prophylactic effect of lamivudine during chemotherapy (inevitably, their risk for reactivation is expected to be different from those in the control group). Based on these previous reports, we included these figures in the final analysis to defer selection bias. Second, reactivation rates in patients treated with rituximab remain largely unknown. Tsutsumi et al.27 in a small cohort study, reported a cumulative 16%, with figures varying from 20% for rituximab alone versus 100% for those receiving rituximab plus corticosteroids. Given the lack of definite evidence for rituximab, we adopted the ranges reported for HBV reactivation during protocol treatments in our 9 analyzed studies. Also, the small sample sizes did not allow us to perform subgroup analysis and covariate adjustment because of lack of stratification and standardization of outcomes based on important baseline characteristics, such as HBV DNA, serum ALT and HbeAg status (Table 1). Risk stratification according to baseline serum ALT, HBV DNA, or HbeAg status can not be made at this point. This remains an important shortcoming of these studies and future trials should perform stratification of baseline characteristics.
Moreover, the studies that addressed the issue of preventive lamivudine administration in lymphoproliferative disorders and were included in our study were heterogeneous and included different histological subtypes, various pharmacological therapies, different duration of treatment and small data sets. Finally, development of alternative prophylactic strategies is hampered by the lack of a valid comparison of lamivudine with newer antiviral agents. It is of note that there is also accumulating evidence that patients who are negative for HBsAg but positive for anti-HBc are still at risk for reactivation of latent hepatitis B during and after chemotherapy, and may be considered for prophylaxis.49–51 Although this issue was not part of the focus of our manuscript, it may soon arise as an important issue in future studies, given that the addition of rituximab in standard chemotherapy may further increase the risk of reactivation in this group of patients.38
Despite its limitations, decision analysis might be helpful in clinical situations where there are legitimate treatment options and quality outcome data and point out the best strategy, especially when the conclusion remains stable over a wide range of estimates around our baseline assessment. Variation among reported studies due to design, population, location and incidence of hepatitis B, and date of completion results in a significant clinical heterogeneity between studies. This heterogeneity is reflected in a wide range of reported probabilities for analyzed outcomes, the most important being probabilities of reactivation with and without lamivudine prophylaxis. Sensitivity analysis, however, attenuated these differences and displayed that prophylaxis is the optimal choice in terms of long-term survival. Our analysis at the current level of evidence supports the practice that all patients diagnosed with lymphoma should be screened for their HBV status. All HbSAg carriers should receive pre-emptive antiviral treatment during immunochemotherapy, extending to include maintenance phase. The strategy of prolonged prophylaxis can further increase the survival rate in this patient population, through a decrease in HBV reactivation rates and HBV-related mortality.
Supplementary Material
Footnotes
Authorship and Disclosures
PDZ collected the studies, performed the statistical analysis, interpreted data and drafted the manuscript. PK collected the studies, interpreted data and drafted the manuscript. EM critically interpreted data, drafted the manuscript, and finalized the submitted version. All authors have read and approved the final version of the manuscript.
All authors declare no conflicts of interest regarding this manuscript.
References
- 1.Kane M. Global programme for control of hepatitis B infection. Vaccine. 1995;13(Suppl 1):S47–9. doi: 10.1016/0264-410x(95)80050-n. [DOI] [PubMed] [Google Scholar]
- 2.Kane M. Epidemiology of hepatitis B infection in North America. Vaccine. 1995;13(Suppl 1):S16–7. doi: 10.1016/0264-410x(95)80040-k. [DOI] [PubMed] [Google Scholar]
- 3.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepatol. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733–45. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 5.de Franchis R, Hadengue A, Lau G, Lavanchy D, Lok A, McIntyre N, et al. EASL Jury. J Hepatol; EASL International Consensus Conference on Hepatitis B; 13–14 September, 2002; Geneva, Switzerland. 2003. pp. S3–25. Consensus statement (long version) [PubMed] [Google Scholar]
- 6.Lok AS, McMahon BJ. Practice Guidelines Committee, American Association for the Study of Liver Diseases (AASLD). Chronic hepatitis B: update of recommendations. Hepatology. 2004;39:857–61. doi: 10.1002/hep.20110. [DOI] [PubMed] [Google Scholar]
- 7.Gauthier J, Bourne EJ, Lutz MW, Crowther LM, Dienstag JL, Brown NA, et al. Quantitation of hepatitis B viremia and emergence of YMDD variants in patients with chronic hepatitis B treated with lamivudine. J Infect Dis. 1999;180:1757–62. doi: 10.1086/315147. [DOI] [PubMed] [Google Scholar]
- 8.Martyak LA, Taqavi E, Saab S. Lamivudine prophylaxis is effective in reducing hepatitis B reactivation and reactivation-related mortality in chemotherapy patients: a meta-analysis. Liver Int. 2008;28:28–38. doi: 10.1111/j.1478-3231.2007.01618.x. [DOI] [PubMed] [Google Scholar]
- 9.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–39. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 10.Loomba R, Rowley A, Wesley R, Liang TJ, Hoofnagle JH, Pucino F, et al. Systematic review: the effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med. 2008;148:519–28. doi: 10.7326/0003-4819-148-7-200804010-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohrt HE, Ouyang DL, Keeffe EB. Systematic review: lamivudine prophylaxis for chemotherapy-induced reactivation of chronic hepatitis B virus infection. Aliment Pharmacol Ther. 2006;24:1003–16. doi: 10.1111/j.1365-2036.2006.03081.x. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896–900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 13.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 14.Greenland S, Robins J. Estimation of a common effect parameter from sparse follow up data. Biometrics. 1985;41:55–68. [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical Trials. Controlled Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29. [Google Scholar]
- 17.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinstein MC, Fineberg HV. Clinical decision analysis. WB Saunders Company. 1980 [Google Scholar]
- 19.Hsu C, Hsiung CA, Su IJ, Hwang WS, Wang MC, Lin SF, et al. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin’s lymphoma: a randomized trial. Hepatology. 2008;47:844–53. doi: 10.1002/hep.22106. [DOI] [PubMed] [Google Scholar]
- 20.Idilman R, Arat M, Soydan E, Törüner M, Soykan I, Akbulut H, et al. Lamivudine prophylaxis for prevention of chemotherapy-induced hepatitis B virus reactivation in hepatitis B virus carriers with malignancies. J Viral Hepatol. 2004;11:141–7. doi: 10.1046/j.1365-2893.2003.00479.x. [DOI] [PubMed] [Google Scholar]
- 21.Lau GK, Yiu HH, Fong DY, Cheng HC, Au WY, Lai LS, et al. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology. 2003;125:1742–9. doi: 10.1053/j.gastro.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 22.Leaw SJ, Yen CJ, Huang WT, Chen TY, Su WC, Tsao CJ. Preemptive use of interferon or lamivudine for hepatitis B reactivation in patients with aggressive lymphoma receiving chemotherapy. Ann Hematol. 2004;83:270–5. doi: 10.1007/s00277-003-0825-8. [DOI] [PubMed] [Google Scholar]
- 23.Lee GW, Ryu MH, Lee JL, Oh S, Kim E, Lee JH, et al. The prophylactic use of lamivudine can maintain dose-intensity of adriamycin in hepatitis-B surface antigen (HBs Ag)-positive patients with Non-Hodgkin’s lymphoma who receive cytotoxic chemotherapy. J Korean Med Sci. 2003;18:849–54. doi: 10.3346/jkms.2003.18.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li YH, He YF, Jiang WQ, Wang FH, Lin XB, Zhang L, et al. Lamivudine prophylaxis reduces the incidence and severity of hepatitis in hepatitis B virus carriers who receive chemotherapy for lymphoma. Cancer. 2006;106:1320–5. doi: 10.1002/cncr.21701. [DOI] [PubMed] [Google Scholar]
- 25.Persico M, De Marino F, Russo GD, Morante A, Rotoli B, Torella R, et al. Efficacy of lamivudine to prevent hepatitis reactivation in hepatitis B virus-infected patients treated for non-Hodgkin lymphoma. Blood. 2002;99:724–5. doi: 10.1182/blood.v99.2.724. [DOI] [PubMed] [Google Scholar]
- 26.Shibolet O, Ilan Y, Gillis S, Hubert A, Shouval D, Safadi R. Lamivudine therapy for prevention of immuno-suppressive-induced hepatitis B virus reactivation in hepatitis B surface antigen carriers. Blood. 2002;100:391–6. doi: 10.1182/blood.v100.2.391. [DOI] [PubMed] [Google Scholar]
- 27.Tsutsumi Y, Shigematsu A, Hashino S, Tanaka J, Chiba K, Masauzi N, et al. Analysis of reactivation of hepatitis B virus in the treatment of B cell non-Hodgkin’s lymphoma in Hokkaido. Ann Hematol. 2009;88:375–7. doi: 10.1007/s00277-008-0585-6. [DOI] [PubMed] [Google Scholar]
- 28.Liao CA, Lee CM, Wu HC, Wang MC, Lu SN, Eng HL. Lamivudine for the treatment of hepatitis B virus reactivation following chemotherapy for non-Hodgkin’s lymphoma. Br J Haematol. 2002;116:166–9. doi: 10.1046/j.1365-2141.2002.03239.x. [DOI] [PubMed] [Google Scholar]
- 29.Ozguroglu M, Bilici A, Turna H, Serdengecti S. Reactivation of hepatitis B virus infection with cytotoxic therapy in non-Hodgkin’s lymphoma. Med Oncol. 2004;21:67–72. doi: 10.1385/MO:21:1:67. [DOI] [PubMed] [Google Scholar]
- 30.Markovic S, Drozina G, Vovk M, Fidler-Jenko M. Reactivation of hepatitis B but not hepatitis C in patients with malignant lymphoma and immunosuppressive therapy. A prospective study in 305 patients. Hepatogastroenterology. 1999;46:2925–30. [PubMed] [Google Scholar]
- 31.Picardi M, Pane F, Quintarelli C, De Renzo A, Del Giudice A, De Divitiis B, et al. Hepatitis B virus reactivation after fludarabine-based regimens for indolent non-Hodgkin’s lymphomas: high prevalence of acquired viral genomic mutations. Haematologica. 2003;88:1296–303. [PubMed] [Google Scholar]
- 32.Rossi G, Pelizzari A, Motta M, Puoti M. Primary prophylaxis with lamivudine of hepatitis B virus reactivation in chronic HbsAg carriers with lymphoid malignancies treated with chemotherapy. Br J Haematol. 2001;115:58–62. doi: 10.1046/j.1365-2141.2001.03099.x. [DOI] [PubMed] [Google Scholar]
- 33.He YF, Li YH, Wang FH, Jiang WQ, Xu RH, Sun XF, et al. The effectiveness of lamivudine in preventing hepatitis B viral reactivation in ritux-imab-containing regimen for lymphoma. Ann Hematol. 2008;87:481–5. doi: 10.1007/s00277-008-0454-3. [DOI] [PubMed] [Google Scholar]
- 34.Buske C, Hiddemann W. Rituximab maintenance therapy in indolent NHL: a clinical review. Leuk Res. 2006;30:S11–5. doi: 10.1016/s0145-2126(06)80003-2. [DOI] [PubMed] [Google Scholar]
- 35.Saab S, Dong MH, Joseph TA, Tong MJ. Hepatitis B prophylaxis in patients undergoing chemotherapy for lymphoma: a decision analysis model. Hepatology. 2007;46:1049–56. doi: 10.1002/hep.21783. [DOI] [PubMed] [Google Scholar]
- 36.van Oers MH. Rituximab maintenance in indolent lymphoma: indications and controversies. Curr Oncol Rep. 2007;9:378–83. doi: 10.1007/s11912-007-0051-y. [DOI] [PubMed] [Google Scholar]
- 37.Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, et al. Rituximab-CHOP versus CHOP alone or with maintenance ritux-imab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–7. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 38.Yeo W, Chan TC, Leung NW, Lam WY, Mo FK, Chu MT, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol. 2009;27:605–11. doi: 10.1200/JCO.2008.18.0182. [DOI] [PubMed] [Google Scholar]
- 39.Aksoy S, Harputluoglu H, Kilickap S, Dede DS, Dizdar O, Altundag K, et al. Rituximab-related viral infections in lymphoma patients. Leuk Lymphoma. 2007;48:1307–12. doi: 10.1080/10428190701411441. [DOI] [PubMed] [Google Scholar]
- 40.Gutfreund KS, Williams M, George R, Bain VG, Ma MM, Yoshida EM, et al. Genotypic succession of mutations of the hepatitis B virus polymerase associated with lamivudine resistance. J Hepatol. 2000;33:469–75. doi: 10.1016/s0168-8278(00)80284-6. [DOI] [PubMed] [Google Scholar]
- 41.Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61–8. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 42.Chang TT, Lai CL, Chien RN, Guan R, Lim SG, Lee CM, et al. Four years of lamivudine treatment in Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol. 2004;19:1276–82. doi: 10.1111/j.1440-1746.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- 43.Pelizzari AM, Motta M, Cariani E, Turconi P, Borlenghi E, Rossi G. Frequency of hepatitis B virus mutant in asymptomatic hepatitis B virus carriers receiving prophylactic lamivudine during chemotherapy for hematologic malignancies. Hematol J. 2004;5:325–8. doi: 10.1038/sj.thj.6200396. [DOI] [PubMed] [Google Scholar]
- 44.Chan HL, Chui AK, Lau WY, Chan FK, Hui AY, Rao AR, et al. Outcome of lamivudine resistant hepatitis B virus mutant post-liver transplantation on lamivudine monoprophylaxis. Clin Transplant. 2004;18:295–300. doi: 10.1111/j.1399-0012.2004.00163.x. [DOI] [PubMed] [Google Scholar]
- 45.Chen PM, Yao NS, Wu CM, Yang MH, Lin YC, Hsiao LT, et al. Detection of reactivation and genetic mutations of the hepatitis B virus in patients with chronic hepatitis B infections receiving hematopoietic stem cell transplantation. Transplantation. 2002;74:182–8. doi: 10.1097/00007890-200207270-00007. [DOI] [PubMed] [Google Scholar]
- 46.European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: Management of chronic hepatitis B. J Hepatol. 2009;50:227–42. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 47.Papatheodoridis GV, Manolako-poulos S, Archimandritis AJ. Current treatment indications and strategies in chronic hepatitis B virus infection. World J Gastroenterol. 2008;14:6902–10. doi: 10.3748/wjg.14.6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol. 2008;6:1315–41. doi: 10.1016/j.cgh.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 49.Orlando R, Tosone G, Tiseo D, Piazza M, Portella G, Ciancia R, et al. Severe reactivation of hepatitis B virus infection in a patient with hairy cell leukemia: Should lamivudine prophylaxis be recommended to HBsAg-negative, anti-HBc-positive patients? Infection. 2006;34:282–4. doi: 10.1007/s15010-006-4150-8. [DOI] [PubMed] [Google Scholar]
- 50.Sarrecchia C, Cappelli A, Aiello P. HBV reactivation with fatal fulminating hepatitis during rituximab treatment in a subject negative for HBsAg and positive for HBsAb and HBcAb. J Infect Chemother. 2005;11:189–91. doi: 10.1007/s10156-005-0385-z. [DOI] [PubMed] [Google Scholar]
- 51.Law JK, Ho JK, Hoskins PJ, Erb SR, Steinbrecher UP, Yoshida EM. Fatal reactivation of hepatitis B post-chemotherapy for lymphoma in a hepatitis B surface antigen-negative, hepatitis B core antibody-positive patient: potential implications for future prophylaxis recommendations. Leuk Lymphoma. 2005;46:1085–9. doi: 10.1080/10428190500062932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.