This study on patients with acute lymphoblastic leukemia (ALL) shows that overexpression of CD123 is an aberrant phenotype present in a subset of precursor-B ALL with hyperdiploid genotype, and represents an additional marker of good prognosis in pediatric precursor-B ALL.
Keywords: overexpression, CD123, hyperdiploid genotype
Abstract
We evaluated CD123 expression in 95 pediatric and 24 adult ALL patients and compared the results with the CD123 expression in normal B-cell precursors. Early B-cell precursors were negative while intermediate precursors and mature B cells showed weak CD123 expression. Leukemic blasts in 31% of precursor-B ALL samples exhibited strong expression of CD123, 61% had moderate CD123 expression and 8% were negative; 81.5% of ALL with hyperdiploid karyotype (≥ 52 chromosomes) showed strong CD123 overexpression. In contrast, cases with ETV6/RUNX1 rearrangement had weak CD123 expression. Our study suggests that overexpression of CD123 is an aberrant phenotype present in a subset of precursor-B ALL with hyperdiploid genotype, and represents an additional marker of good prognosis in pediatric precursor-B ALL. Moreover, aberrant CD123 expression in ALL is a good marker for monitoring of minimal residual disease.
Introduction
CD123 is an α subunit of a heterodimer IL-3 receptor, a member of the cytokine receptor superfamily.1,2 It is a 70 kDa protein, which binds IL-3 with high specificity but with low affinity.3 CD123 is encoded by the IL-3a gene, located in the pseudo-autosomal region (PAR) at the ends of the short arms of the X and Y chromosomes (Xp22.3 and Yp11.3).4 B-lymphoid blasts from acute lymphoblastic leukemia (ALL) have been shown to express functional CD123 and CD40 ligand stimulates proliferation of precursor-B (pre-B)ALL by upregulating CD123 expression.5,6 However, the prevalence of CD123 expression in pre-B ALL is not clear: one study of 13 B-ALL cases reported CD123 expression in all cases as significantly higher than normal B-cell precursors.7 Another study (25 pre-B ALL cases) found 40% of pre-B ALL to have high CD123 expression in comparison to human myeloid and lymphoid progenitors.8
Our goal was to characterize the expression of CD123 in different maturational stages of normal B-cell precursors in the bone marrow by comparison to leukemic blasts of pre-B ALL, using multiparameter flow cytometry (FC). We also sought to establish if the level of CD123 expression in ALL correlated with any recurrent genetic abnormality. Finally, we investigated whether the overexpression of CD123 on residual leukemic blasts can be applied for follow-up of minimal residual disease (MRD).
Design and Methods
A total of 95 consecutive diagnostic samples of childhood ALL (89 newly diagnosed, 6 at relapse, mean age six years, range 0–18) were collected from December 2001 to October 2004. Samples from 24 adults with newly diagnosed ALL (mean age 48 years, range 21–78) were collected between December 2002 and March 2005. The diagnosis of ALL was established according to WHO 2001 criteria.9 Immunophenotype was classified according to EGIL:10 among pediatric ALL cases there were 5 BI, 60 BII, 20 BIII and 10 T-ALL, and among adults 2 BI, 14 BII, 5 BIII and 3 TALL.
All children were karyotyped, and in most cases, additional genetic analyses, including interphase FISH, SKY, or PCR for BCR/ABL1, ETV6/RUNX1, PBX1/TCF3 and MLL rearrangements, were made as part of the routine investigations.11 Genetic abnormalities in childhood pre-B-ALL cases included 26 cases with hyperdiploid karyotype, 18 with ETV6/RUNX1, 2 with BCR/ABL1, 2 with PBX1/TCF3, 4 with MLL gene rearrangement, and 11 with normal karyotype; 18 cases showed abnormal karyotype with non-recurrent cytogenetic abnormalities. Karyotypes were available for 13 of 21 adult patients with pre-B ALL: 3 with BCR/ABL1, 1 with MLL gene rearrangement, 1 hyperdiploid, 3 with normal karyotype and 5 cases with non-recurrent genetic abnormalities.
Hospital control (HC) BM samples for studies of non-leukemic B-cell precursors were obtained from 28 patients: 15 children (median age seven years, range 0–18) and 13 adults (median age 63 years, range 20–84) with normal patterns of B-cell differentiation in the bone marrow. HC patients were investigated due to anemia, leukopenia, reactive lymphocytosis, staging of malignant lymphoma, suspect but not confirmed myelodysplastic syndrome or myeloma, and included children who were at least six months post-maintenance therapy for leukemia with no evidence of disease recurrence. In addition, the expression of CD123 on regenerating B-precursors was analyzed in 42 BM samples taken during follow-up of patients who were a part of the childhood ALL cohort, but as shown by the extensive FC analysis (see below) did not have any MRD after induction therapy (8 follow-up samples were taken at day 50 post-induction therapy, 29 samples were taken at day 103, 2 samples were taken six months post-induction and 3 samples were taken 12 months post-induction therapy. Non-leukemic B-cell progenitors were categorized as early, intermediate, and mature B cells by expression of CD10, CD20 and/or CD34 on CD19-gated cells as previously described.12
Immunophenotypic studies were performed by standard four-color FC13 and included the combination: CD66c-FITC/CD123-PE/CD19-PerCP/CD34-APC.
All studied patients were uniformly treated by NOPHO 2000 protocol and followed by FC for detection of MRD.13 MRD follow-up panels were individually tailored depending on aberrant findings at diagnosis.
The most commonly applied antibody combinations with CD123 were: CD66c/CD123/CD19/CD45, CD34/CD123/CD19/CD10, CD19/CD123/CD20/CD33, TdT/CD123/CD19/CD10, CD56/CD123/CD19/CD45 and CD19/CD123/HLADR/CD10. MRD was defined as a cluster of at least 10-20 events with aberrant phenotype.
The clustered events had forward and side scatter characteristics identical to those of leukemic blasts at diagnosis.
Overall, samples for immunophenotypic studies included bone marrow (BM) aspirate (n=189), peripheral blood (n=16), and cell suspension from lymph node biopsy or fine needle aspirate (n=3). The CD123 expression was quantified by mean fluorescence intensity (MFI) in each gated cell population. A significant change in CD123 and other antigen expression in gated leukemic cells in follow-up samples were defined as a 1 SD increase or decrease of MFI by comparison to the MFI at diagnosis as calculated by the analysis software (Paint-a-Gate Pro, BD).
Descriptive statistical values including arithmetic mean, median, SD and range were used. Sensitivity and specificity were calculated according to the formula shown at http://www.cebm.utoronto.ca/glossary/spsn.htm). Comparisons of different cell populations were done by the Mann-Whitney U test and two-tailed Student’s t-test. All reported p values are two-sided. All calculations were performed using SPSS 12.0 (SPSS, Chicago, IL, USA) software.
The study was approved by the Ethics Committee of Karolinska University Hospital in accordance with institutional research guidelines.
Results and Discussion
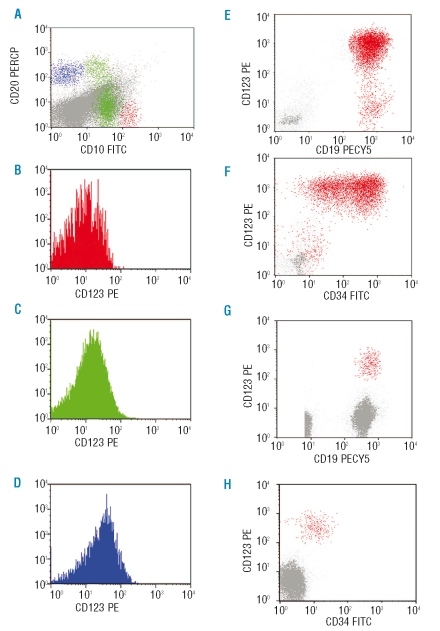
Hospital control samples (n=28) showed median values of 0.23% (range 0.01–1.03), 1.00% (range 0.04–20.50) and 1.72% (range 0.26–17.27) of early, intermediate and mature B cells in the bone marrow, respectively (representative pattern in a child is shown in Figure 1 A–D). The CD123 expression in normal B cells was low and showed median MFI values of 8 (range 4–26), 18 (range 5–36), and 9 (range 3–48), in three stages of maturation, respectively. CD34-positive B-cell precursors had a median CD123 MFI of 10 (range 4–39), while CD34-negative B-cell precursors had a median CD123 MFI of 13 (range 3–65). There was no significant difference in CD123 expression between studied subpopulations of B cells in HC BM samples and in regenerating (post-chemotherapy) B cells.
Figure 1.
CD123 expression in non-leukemic B-precursors and precursor-B ALL in a bone marrow sample from a 5-year old child from the Hospital Control group (investigated for low-grade fever and lymphadenopathy, and found not to have any aberrant lymphoid population). (A) CD10 vs. CD20 expression in CD19 cells: CD19+ cells with high CD10 and no CD20 expression are early B-cell precursors (red); CD19+ cells with moderate CD10 and variable CD20 expression are intermediate B cell precursors (green); CD19+ cells with no CD10 and high CD20 expression are mature B cells (blue). (B–D) histograms showing CD123 expression by early precursors (red), intermediate precursors (green) and mature B cells (blue). (E–F) diagnostic sample from a patient with B-precursor ALL shows high expression of CD123 on CD19+/CD34+ leukemic B-cells; CD19+ B-cells are painted red. (G–H) high CD123 expression identifies the residual leukemic population which shows reduced CD34 expression in day 15 bone marrow sample.
CD123 expression showed considerable variability in childhood preprecursor-B ALL. Six samples (7%) had no CD123 expression (MFI <10), 50 (59%) had intermediate expression (MFI 10–100), and 29 (34%) had strong expression of CD123 (MFI> 100), (Figure 1 E and F). Adult preprecursor-B ALL showed similar heterogeneity: 2 samples (10%) had no CD123 expression, 15 (71%) had intermediate expression, and 4 (19%) had a high CD123 expression (MFI>100). There was no correlation between expression of CD123 and other myeloid-associated markers, such as CD13 or CD33. Only a single case of T-precursor ALL showed CD123 expression with MFI=33 (7.7%). The immunophenotypic characteristics of this case were consistent with the most immature T-ALL recently shown to express CD123.14
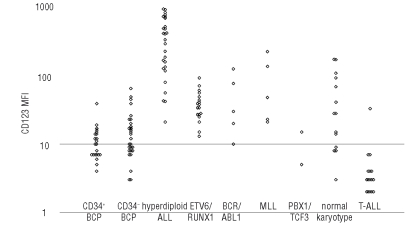
When EGIL categories of B-precursor ALL were considered, the highest CD123 expression was found in EGIL B-II category (sc. common ALL) (p>0.001). This was probably due to a strong correlation found between high CD123 expression and hyperdiploid genotype. The hyperdiploid precursor-B ALL showed significantly higher CD123 expression than other major subcategories including ETV6/RUNX1, BCR/ABL1, MLL, PBX/TCF3 and B-precursor ALL with normal karyotype (Figure 2, p<0.0001). Twenty-two of 27 samples from patients with hyper-diploid ALL displayed CD123 MFI >100, but only 6 of 71 non-hyperdiploid precursor-B ALL showed similar high expression. The calculated sensitivity and specificity of high CD123 expression for hyperdiploid genotype are 81.5% and 91.5%, respectively, with a positive predictive value of 78.6%. Of interest, all cases with ETV6/RUNX1 showed uniformly low expression of CD123. However, some precursor-B ALL genetic categories (such as BCR/ABL1 and MLL rearrangements) were not frequent in our material and require further studies.
Figure 2.
CD123 Expression in B-cell precursors, B-ALL and T-ALL. Dots for CD34+ and CD34– B cells demonstrate CD123 MFI for the two populations of precursors in non-leukemic (HC) samples. Dots for B-ALL genetic subtypes and for T-ALL represent CD123 MFI of the leukemic population. Thirteen (of total 21) adult leukemia cases for which karyotypes were available are plotted.
The overall CD123 expression in precursor-B ALL was significantly higher than in normal B cells (p<0.0001) of any maturation stage (Figure 2). Therefore, high expression of CD123 in preprecursor-B ALL can be defined as an aberrant or leukemia-related phenotype. CD123 overexpression could be used as leukemia-related aberrant phenotype for MRD follow-up in 60 of 106 (57%) of pre-B ALL patients in our cohort. High CD123 expression was one of the aberrant features confirming residual leukemia found in 22 samples (from 21 patients) taken at day 15 and/or day 29 post-therapy (see Figure 1 G and H). The CD123 showed stable overexpression in 77% samples with residual leukemia. By comparison, MFI of CD34 and TdT was stable in 32% and 31% of samples, respectively, as previously described by Gaipa et al.15
In conclusion, we found that CD123 expression is generally low in B-cell precursors and mature B cells both in HC BM samples and in post-chemotherapy regenerating B cells. The CD123 overexpression in B-precursor ALL correlated with hyperdiploid genotype, the second most common genetic abnormality in childhood ALL. In contrast, B-ALL samples with ETV6/RUNX1 rearrangement showed expression similar to normal B-cell precursors. The biological significance of CD123 expression in early precursor-B ALL with hyperdiploid genotype remains unclear. Analogous to the situation in acute myeloid leukemia, it may provide a proliferative advantage to leukemic blasts.16 The mechanism of overexpression is not known. However, 88% of children with high hyperdiploid karyotype have an extra X chromosome, on which the IL-3Rα gene is located.17 Whether a gene dosage effect may be responsible for the overexpression of this gene is not clear. The gene dosage hypothesis may be supported by a recent study of gene expression profiles in acute lymphoblastic leukemia, where Kuchinskaya et al. found that high expression of the IL-3Rα mRNA was one of the ten significant predictors of hyperdiploid phenotype (predictive strength 8.3). Besides the IL-3Rα gene, three other genes in this group were located on chromosome X.11
A remarkably stable expression of CD123 in residual leukemic cells during early therapy confirmed its reliability as a marker of MRD. It showed much less variation of expression than other commonly used markers for MRD including CD34, TdT, and CD10.
The high expression of CD123 in a subset of ALL may provide an important therapeutic target as has recently been suggested by Lhermitte et al.14 Diphtheria toxin fused to human interleukin-3 was shown to induce cytotoxicity in acute myeloid leukemia with CD123 expression.18 Our data suggest that CD123 expression should be evaluated in acute lymphoblastic leukemia for (i) its prognostic value as a predictor of hyperdiploid genotype, (ii) monitoring minimal residual disease at follow-up and (iii) to prospectively select patients who may benefit from the novel therapies.
Footnotes
Authorship and Disclosures
All authors contributed to conception, design, analysis and interpretation of data, drafting and critically revising the article for important intellectual content, and final approval of the version to be published.
The authors reported no potential conflicts of interest.
References
- 1.Blalock WL, Weinstein-Oppenheimer C, Chang F, Hoyle PE, Wang XY, Algate PA, et al. Signal transduction, cell cycle regulatory, and anti-apoptotic pathways regulated by IL-3 in hematopoietic cells: possible sites for intervention with antineoplastic drugs. Leukemia. 1999;13:1109–66. doi: 10.1038/sj.leu.2401493. [DOI] [PubMed] [Google Scholar]
- 2.Miyajima A, Kitamura T, Harada N, Yokota T, Arai K. Cytokine receptors and signal transduction. Annu Rev Immunol. 1992;10:295–31. doi: 10.1146/annurev.iy.10.040192.001455. [DOI] [PubMed] [Google Scholar]
- 3.Rapoport AP, Luhowskyj S, Doshi P, DiPersio JF. Mutational analysis of the α subunit of the human interleukin-3 receptor. Blood. 1996;87:112–22. [PubMed] [Google Scholar]
- 4.Kremer E, Baker E, D’Andrea RJ, Slim R, Phillips H, Moretti PA, et al. A cytokine receptor gene cluster in the X-Y pseudoautosomal region? Blood. 1993;82:22–8. [PubMed] [Google Scholar]
- 5.Uckun FM, Gesner TG, Song CW, Myers DE, Mufson A. Leukemic B-cell precursors express functional receptors for human interleukin-3. Blood. 1989;73:533–42. [PubMed] [Google Scholar]
- 6.Zhou M, Gu L, Holden J, Yeager AM, Findley HW. CD40 ligand upregulates expression of the IL-3 receptor and stimulates proliferation of B-lineage acute lymphoblastic leukemia cells in the presence of IL-3. Leukemia. 2000;14:403–11. doi: 10.1038/sj.leu.2401682. [DOI] [PubMed] [Google Scholar]
- 7.Munoz L, Nomdedeu JF, Lopez O, Carnicer MJ, Bellido M, Aventin A, et al. Interleukin-3 receptor alpha chain (CD123) is widely expressed in hematologic malignancies. Haematologica. 2001;86:1261–9. [PubMed] [Google Scholar]
- 8.Testa U, Torelli GF, Riccioni R, Muta AO, Militi S, Annino L, et al. Human acute stem cell leukemia with multi-lineage differentiation potential via cascade activation of growth factor receptors. Blood. 2002;99:4634–7. doi: 10.1182/blood.v99.12.4634. [DOI] [PubMed] [Google Scholar]
- 9.Brunning R. B precursor leukemia/lymphoma. In: Jaffe E, Harris N, Stein H, Vardiman J, editors. Pathology and Genetics: Tumors of Haematopoietic system. Lyon, France: IARC Press; 2001. pp. 111–4. [Google Scholar]
- 10.Bene MC. Immunophenotyping of acute leukaemias. Immunol Lett. 2005;98:9–21. doi: 10.1016/j.imlet.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Kuchinskaya E, Heyman M, Grander D, Linderholm M, Soderhall S, Zaritskey A, et al. Children and adults with acute lymphoblastic leukaemia have similar gene expression profiles. Eur J Haematol. 2005;74:466–80. doi: 10.1111/j.1600-0609.2005.00433.x. [DOI] [PubMed] [Google Scholar]
- 12.Lucio P, Parreira A, van den Beemd MW, van Lochem EG, van Wering ER, Baars E, et al. Flow cytometric analysis of normal B cell differentiation: a frame of reference for the detection of minimal residual disease in precursor-B-ALL. Leukemia. 1999;13:419–27. doi: 10.1038/sj.leu.2401279. [DOI] [PubMed] [Google Scholar]
- 13.Bjorklund E, Mazur J, Soderhall S, Porwit-MacDonald A. Flow cytometric follow-up of minimal residual disease in bone marrow gives prognostic information in children with acute lymphoblastic leukemia. Leukemia. 2003;17:138–48. doi: 10.1038/sj.leu.2402736. [DOI] [PubMed] [Google Scholar]
- 14.Lhermitte L, de LA, Dupret C, Lapillonne H, Millien C, Landman-Parker J, et al. Most immature TALLs express Ra-IL3 (CD123): possible target for DT-IL3 therapy. Leukemia. 2006;20:1908–10. doi: 10.1038/sj.leu.2404349. [DOI] [PubMed] [Google Scholar]
- 15.Gaipa G, Basso G, Maglia O, Leoni V, Faini A, Cazzaniga G, et al. I-BFM-ALL-FCM-MRD Study Group. Drug-induced immunophenotypic modulation in childhood ALL: implications for minimal residual disease detection. Leukemia. 2005;19:49–56. doi: 10.1038/sj.leu.2403559. [DOI] [PubMed] [Google Scholar]
- 16.Testa U, Riccioni R, Diverio D, Rossini A, Lo CF, Peschle C. Interleukin-3 receptor in acute leukemia. Leukemia. 2004;18:219–26. doi: 10.1038/sj.leu.2403224. [DOI] [PubMed] [Google Scholar]
- 17.Heinonen K, Mahlamäki E, Riikonen P, Meltoranta RL, Rahiala J, Perkkiö M. Acquired X-chromosome aneuploidy in children with acute lymphoblastic leukemia. Med Pediatr Oncol. 1999;32:360–5. doi: 10.1002/(sici)1096-911x(199905)32:5<360::aid-mpo9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Testa U, Riccioni R, Biffoni M, Diverio D, Lo-Coco F, Foa R, et al. Diphtheria toxin fused to variant human interleukin-3 induces cytotoxicity of blasts from patients with acute myeloid leukemia according to the level of interleukin-3 receptor expression. Blood. 2005;106:2527–9. doi: 10.1182/blood-2005-02-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]