Abstract
Cellular events for neural progenitor cells, such as proliferation and differentiation, are regulated by multiple intrinsic and extrinsic cell signals. Folate plays a central role in central nervous system development, so folate, as an extrinsic signal, may affect neural stem cell (NSC) proliferation and differentiation. In the present study, we investigated the effects of folate deficiency on the cell proliferation, cell apoptosis and homocysteine concentrations in NSCs. NSCs were isolated from fetal rats and identified as NSCs by their expression of immunoreactive nestin. Cell proliferation was quantitated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Apoptotic cells were detected and confirmed by flow cytometric analysis. We measured homocysteine concentrations in NSCs by high performance liquid chromatography and detected the expression of caspase-3 by western blot method. Folate deficiency not only decreased cell proliferation, but also increased the apoptotic rate of NSCs as demonstrated by the increased expression of early apoptotic markers such as caspase-3, compared to control group (p<0.05). Furthermore, There was a statistically significant increase in homocysteine concentration during folate deficiency in NSCs (p<0.05). These data suggest that folate affects the cell proliferation, apoptosis and homocysteine generation in NSC cells.
Keywords: folate, neural stem cells, homocysteine, apoptosis
Introduction
Folate is a nutrient that is best known as a vitamin that prevents neural tube defects during fetal development. Recently, it is becoming apparent that folate deficiency also contributes to many neurological and psychological disorders including dementia, impaired cognition, depression, psychosis, and Alzheimer’s disease (AD) [1–3]. e.g. diminished folate levels are associated with a twofold increased risk in developing AD [4]. The full range of mechanisms by which deficiencies in folate may contribute to these certain nervous system diseases is unclear. However, one likely major impact of folate deprivation is increased in homocysteine (Hcy), since folate is a necessary cofactor for the enzyme (5,10-methyenetetralhydrofolate reductase) that mediates conversion of Hcy to methionine. Elevated Hcy levels are also associated with neuropsychiatric disorders including AD. Hcy is toxic to cultured neurons and neuronal cells and potentiates neurotoxicity induced by amyloid-β. Folate deficiency has been shown to elevate Hcy, compromise neuronal homesostasis, and render neurons more sensitive to amyloid toxicity in mouse models of AD and Parkinson’s disease [5, 6]. To address more fully the neurotoxic effects of folate deprivation, we examined the consequences of folate deficiency on neural stem cells (NSCs) in culture.
Materials and Methods
Reagents
Folate-free Dulbecco’s Modified Eagle’s Medium (DMEM), N2, B27 supplement, fetal bovine serum (FBS), basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), dimethyl sulphoxide (DMSO) were obtained from Gibco (Carlsbad, CA). During the course of the experiments we have also used the following chemicals: tris-(2-carboxyl-ethyl)-phosphine, SBD-F (7-fluorobenzo-2-oxa-1,3-diazole-4-sulphonic acid), folate, d,l-homocysteine, propidium iodide (PI), cystamine, ethylendiaminetetraacetic acid (EDTA) and Rnase. All these reagents were purchased from Sigma (St. Louis, MO). All chemicals and solvents were of analytical reagent grade. Monoclonal anti-nestin for immunocytochemistry and polyclonal antibodies against either caspase-3 or β-actin for western blot analysis were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Fluorescent secondary antibody was obtained from Zhongshan Goldbridge Biotechnology (Beijing, China).
Primary culture of NSCs
Brain tissue was isolated from fetal rats and washed 3 times with DMEM (control group, liquid media contained 4 mg/L folate). The tissue was cut into small pieces and then dissociated by incubation with 0.25% parenzyme and 0.02% EDTA. This step was followed by agitation, centrifugation and resuspension of the cells in DMEM that was supplemented with 2% B27 supplement, 20 ng/mL EGF, 20 ng/mL bFGF, 2 mmol/L L-glutamine, 100 U/mL penicillin and phytomycin. The resulting cell suspension was transferred to a culture flask and grown at 37°C in a humidified atmosphere containing 95% air 5% CO2. The culture medium was changed every 2 days.
Identification of cultured cells
Nestin is an intermediate filament protein in NSCs and other progenitor cells. This protein has been the most extensively used marker to identify NSCs in the developing nervous system as well as in cultured cells in vitro [7]. Therefore, in the present study, cultured cells were identified as NSCs by their expression of immunoreactive nestin. On the sixth day in culture, immunofluorescence analysis was performed. The cells were fixed in 4% paraformaldehydein for 30 min at room temperature and incubated with blocking buffer for 20 min, then permeabilized with 0.1% Triton X-100 in PBS for 5 min at room temperature. After incubation with 2N HCl for 30 min at 37°C and then washing with boracic acid at room temperature, the cells were incubated overnight at 4°C with 1:100 rabbit anti-rat monoclonal anti-nestin antibody. Subsequently, cells were incubated for 2 h at room temperature with TRITC (tetramethylrhodamine isothiocyanate)-conjugated goat anti-rabbit IgG.
Assay of cell proliferation
Cultures of NSCs were assigned to one of three treatment groups: folate deficiency (Folate-D, liquid media contained 0 mg/L folate), normal medium condition group (control group, liquid media contained 4 mg/L folate), and folate supplementation group (Folate-S, liquid media contained 40 mg/L folate). Cell proliferation was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, aliquots containing 5 × 105 cells/mL were transferred to one of 8 wells in a 96-well plate. The cells were incubated at 37°C within 72, or 96-h period. MTT (0.5 mg) was added to each well at 4 h before the end of the incubation period, when the MTT reaction was stopped by addition of 10% SDS-0.1 mol/L HCl. The cell’s formazan crystals were dissolved in DMSO, and then absorbance at 490 nm was measured in a Microplate reader (Bio-Tek ELX800uv, Bio-Tek Instrument Inc, Winooski, VT).
Apoptosis analyses
Apoptotic cells were identified as the percentage by flow cytometry. After 4 days of cell culture as described above, cells were harvested and treated with RNase and centrifuged at 1000 r/min for 10 min at 4°C. The cell pellet was gently resuspended in 1 mL PI solution at a concentration of 50 µg/mL in PBS containing 1% Triton-X 100 and 20 µg/mL RNase and incubated at 4°C in the dark for 30 min. A minimum of 104 cells/sample was analyzed by using a FACScan flow cytometry (Becton Dickinson, Mountain View, CA).
Western blot analysis
Cells were washed with ice-cold PBS and then lysed with radioimmunoprecipitation assay buffer. Proteins were separated on SDS-12% polyacrylamide gel and then transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% non-fat milk and incubated with primary antibody (either rabbit anti-caspase-3 antibody diluted 1:1,000 or anti-β-actin antibody diluted 1:5,000) overnight at 4°C and then with a secondary antibody for l h at room temperature. Proteins were detected by chemiluminescence assay. Quantitation of proteins was done by densitometric analysis using NIH Image software (version 1.61).
Determination of Hcy levels
Hcy was monitored in proliferated NSCs by HPLC (high performance liquid chromatography) using a modified version of the methodology of Araki and Sako (1987) [8]. Cultures were rinsed 2× with TBS (Tris buffer saline), scraped from the plate in a small volume of TBS, and homogenized on ice. Aliquots of lysates (50 µL) were combined with 25 µL of 20 µmol/L cystamine (as an internal standard) and 25 µL of phosphate buffered saline (PBS, pH 7.4). Samples were vortexed, incubated with 10 µL of 100 g/L tris-(2-carboxyl-ethyl)-phosphine for 30 min at room temperature. 90 µL of 100 g/L trichloroacetic acid containing 1 mmol/L EDTA was added for deproteinization. After the sample was centrifuged for 10 min at 10,000 g, 50 µL of the supernatant was added to an autosampler vial containing 10 µL of 1.55 mol/L NaOH; 125 µL of 0.125 mol/L borate buffer containing 4 mmol/L EDTA, pH 9.5; and 50 µL of 1 g/L SBD-F in the borate buffer (0.125 mol/L pH 9.5). The mixture was then incubated for 60 min at 60°C and then cooled on ice for subsequent HPLC analysis. A 10 µL aliquot was later injected into the Waters 2795 HPLC system (Waters, Milford, MA). The mobile phase was 1 mol/L acidacetate buffer, pH 5.0, containing 30 mL/L methanol at a flow rate 0.7 mL/L.
Statistical analysis
The data were expressed as mean ± SD values and analyzed by statistical software SPSS13.0 (SPSS Inc., Chicago, IL). One-way analysis of variance (ANOVA) followed by Student-Newman-Keuls (SNK) test for multiple comparisons were used to determine significant differences among the experimental groups. The criterion for statistical significance was p<0.05.
Results
Cell identification
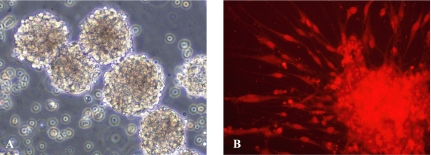
Undifferentiated NSCs formed round neurospheres that were suspended in the culture medium (Fig. 1A). Almost all the cells in neurospheres expressed nestin protein, which was detected as intense red staining of cytoplasm in the immunofluorescence assay (Fig. 1B).
Fig. 1.
Photomicrographs of cells in neurospheres. (A) Phase contrast photomicrograph of neurospheres (×200). (B) Immunofluorescence staining of cells for nestin (red, ×200).
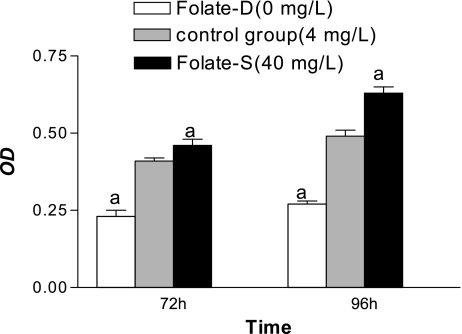
Effect of folate on NSC proliferation
Significant differences in cell proliferation were detected between control and Folate-D groups (p<0.05) (Fig. 2). The effect of folate was concentration dependent, because proliferation differed significantly between control and Folate-S (p<0.05) (Fig. 2).
Fig. 2.
Effect of folate on NSC proliferation determined by MTT assay. Shown are the mean ± SD values from 8 experiments. ap<0.05 vs control group.
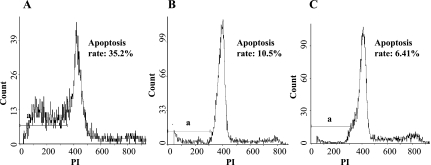
Determination of apoptosis rate with flow cytometry
The quantitative assessment of sub-G1 cells by flow cytometry was used to estimate the number of apoptotic cells. Fig. 3 shows that NSC apoptosis was induced in folate deficiency group that the apoptosis rate was 35.4% ± 1.3%, whereas 6.5% ± 0.33% of the cells became apoptotic after treatment with 40 mg/L folate. There was a significant difference between control and Folate-D groups (p<0.05). Furthermore, Obvious differences in apoptosis rate between control and Folate-S groups were also visible (p<0.05).
Fig. 3.
Measurement of NSC apoptosis rate by flow cytometry. (A) the cells in folate deficiency group with a large apoptosis peak. Measuring the volume of apoptosis peak. A = 0.35; (B) (C) the cells in control and Folate-S groups with a small apoptosis peak. Analysis of NSCs treated with 4 mg/L and 40 mg/L folate for 48 h. A = 0.11 and 0.06. a: apoptosis peak.
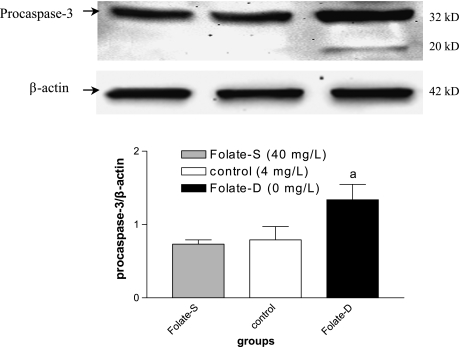
Folate deprivation activaties caspase-3
Caspases are crucial mediators of apoptosis. Among them, caspase-3 is a frequently activated death protease, catalyzing the specific cleavage of many key cellular proteins [9]. Therefore upregulation of caspase-3 expression is an important phenomenon for induction of apoptosis. In the present investigation, we analyzed the expession level of caspase-3 by western blot method. A significant increase in procaspase-3 protein level was detected in folate deprivation group, compared to control group (p<0.05, Fig. 4). Furthermore, the results also indicated that folate deprivation resulted in the activation of caspase-3 as evidenced by the appearance of caspase-active form (20 kDa). This finding was associated with a significant increase in NSC apoptosis after folate deprivation treatment by PI staining.
Fig. 4.
Western blot analysis of caspase-3 expression in folate-untreated and treated neural stem cells. After a 48 h folate treatment, cell proteins were probed with polyclonal antibodies against caspase-3 and β-actin (figure top). Markers indicate caspase-3 precursor (32 kDa) and the processed form of caspase-3 (20 kDa). A densitometric analysis of immunoblots (figure bottom) was also carried out after normalization against β-actin. Results were similar in three separate experiments. ap<0.05 vs control.
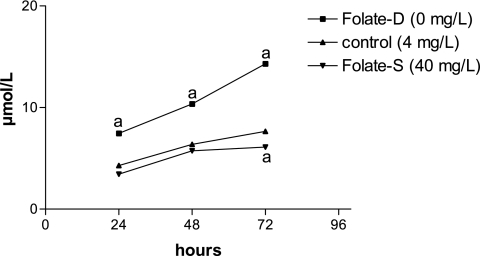
Hcy formation following folate deprivation
One likely major impact of folate deprivation is increased Hcy production [10]. We confirmed that folate deprivation indeed increased Hcy production by HPLC analyses in medium of neural stem cell culture. In accordance with prior studies that demonstrate that increased Hcy formation increases its export rather than intracellular accumulation [11]. In folate-D group, Hcy levels within medium of cells cultured for 3 d increased 2.3-fold compared with folate-S group (Fig. 5). Cells were treated with folate, which inhibited Hcy formation.
Fig. 5.
Effect of folate on Hcy concentration in NSCs detected by HPLC. Shown are the mean ± SD values from 8 experiments. ap<0.05 vs control.
Discussion
The mammalian central nervous system is organized by a variety of cells such as neurons and glial cells. These cells are generated from a common progenitor, NSCs. The fate of NSC in brain may be regulated by growth factors, neurotransmitters and nutrients [12]. Folate is an essential co-factor for the de novo biosynthesis of purines and thymidylate and hence plays an important role in DNA and RNA synthesis for the production of new cells [13]. In addition, clinical trials show that a significant proportion of NTD recurrences or first occurrence can be prevented by folic acid supplementation in the periconceptional period. Reduced levels of folate or vitamin B12 in maternal plasma are risk factors for NTD. Clinical studies also suggest a relationship between folate deficiency and neurological and disorders including Alzheimer’s disease (AD) [1–4]. The basis for this association has not been clearly defined. It is likely that the beneficial neurological effects of folate involve stimulation of NSC proliferation. To investigate mechanisms underlying this association, we examined the consequences of folate deprivation and supplementation on NSC cultures. Our findings demonstrated that folate deprivation ultimated apoptosis, whereas folate supplementation increased NSC proliferation. This finding is consistent with folate’s well known role as a nutrient essential for the normal development and functioning of the nervous system.
Dietary folate has a major impact on homocysteine levels, with an inverse relationship between plasma folate and Hcy levels. Epidemiological studies have linked folate deficiency and hyperhomocysteinemia to neurodegenerative and neuropsychiatric disease, including Alzheimer and Parkinson disease, depression, etc. Homocysteine is a neurotoxic non-proteinogenic amino acid, an abnormal increase of which in plasma has been implicated in many pathological conditions including cardiovascular diseases, neural tube defects and is now recognized and Alzheimer’s disease [14, 15]. Hcy elimination is regulated by the transmethylation and the transsulfuration pathways and is modulated by folate. Metabolic product of folate, 5 methyltetrahydrofolate, provides a methyl group that is used to reconvert Hcy back to methionine through the transmethylation pathway. In addition, the efficiency of folate metabolism has an impact on the availability of S-adenosylmethionine (SAM), a compound that is known to activate Hcy flux through the transsulfuration pathway. Some studies in recent years investigated the role of Hcy as a cause of brain damage. Numerous neurotoxic effects of Hcy can be blocked by folate. Folate deficiency can cause decreased SAM. On the other hand, increased concentration of Hcy is associated with increased production of SAH via the reverible reaction mediated by SAH-hydrolase [16, 17]. A lower ratio of SAM/SAH causes DNA damage and thereby apoptosis, which is one important explanation for Hcy neurotoxicity.
Some studies showed folate deficiency induces neurotoxicity by multiple routes, including increasing cytosolic calcium and oxidative stress via increasing levels of the neurotoxin homocysteine, and inducing mitochondrial and DNA damage [18–20]. One major mechanism by which folate deprivation is likely to promote neurotoxicity is by extracellular accumulation of Hcy. As shown herein, folate deprivation induced Hcy accumulation within medium, these findings confirm and extend the indication that increased Hcy mediates a portion of the neurotoxicity resulting from folate deprivation. Importantly, the activation of caspase-3 has been shown to be essential in the process of Hcy-induced apoptotic cell death [21, 22]. Here, we observed that increased Hcy concentrations from folate deprivation triggerd the activation of caspase 3, the early protein marker of apoptosis onset in NSCs and may lead to NSC apoptosis.
Conclusions
In summary, the present study showed that the proliferation of NSCs could be significantly inceased by folate in concentration-dependent manner, consistent with the results that folate could inhibit NSC apoptosis. Folate may decrease Hcy concentration to reduce NSC toxicity.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (No. 30571563; No. 30771797) and by the grant from Tianjin Education Commission, China (No. 20070208).
References
- 1.Mattson M.P., Shea T.B. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003;26:137–146. doi: 10.1016/S0166-2236(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 2.Ramos M.I., Allen L.H., Mungas D.M., Jagust W.J., Haan M.N., Green R., Miller J.W. Low folate status is associated with impaired cognitive function and dementia in the Sacramento Area Latino Study on Aging American. J. Clin. Nutr. 2005;82:1346–1352. doi: 10.1093/ajcn/82.6.1346. [DOI] [PubMed] [Google Scholar]
- 3.Nilsson K., Gustafson L., Faldt R., Anderson A., Hultberg B. Plasma homocysteine in relation to serum cobalamin and blood folate in a psychogeriatric population. Eur. J. Clin. Invest. 1994;24:600–606. doi: 10.1111/j.1365-2362.1994.tb01111.x. [DOI] [PubMed] [Google Scholar]
- 4.Wang H-X., Wahlin A., Basun H., Fastom J., Winblad B., Fratiglioni L. Vitamin B12 and folate in relation to the development of Alzheimer’s disease. Neurology. 2001;56:1188–1194. doi: 10.1212/wnl.56.9.1188. [DOI] [PubMed] [Google Scholar]
- 5.Kruman I.I., Kumaravel T.S., Lohani A., Pederson W.A., Cutler R.G., Kruman Y., Haughey N., Lee J., Evans M., Mattson M.P. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer’s disease. J. Neurosci. 2002;22:1752–1762. doi: 10.1523/JNEUROSCI.22-05-01752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duan W., Ladenheim B., Cutler R.G., Kruman I.I., Cadet J.L., Mattson M.P. Dietary folate defeciency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson’s disease. J. Neurochem. 2002;80:101–110. doi: 10.1046/j.0022-3042.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- 7.Rietze R.L., Valcanis H., Brooker G.F., Thomas T., Voss A.K., Bartlett P.F. Purification of a pluripotent neural stem cell from the adult mouse brain. Nature. 2001;412:736–739. doi: 10.1038/35089085. [DOI] [PubMed] [Google Scholar]
- 8.Atsushi A., Yoshiyasu S. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. 1987;422:43–52. doi: 10.1016/0378-4347(87)80438-3. [DOI] [PubMed] [Google Scholar]
- 9.Sleeper E., Tamm C., Frisén J., Zhivotovsky B., Orrenius S., Ceccatelli S. Cell death in adult neural stem cells. Cell Death Differ. 2002;9:1377–1378. doi: 10.1038/sj.cdd.4401127. [DOI] [PubMed] [Google Scholar]
- 10.Fiskerstrand T., Ueland P.M., Refsum H. Folate depletion induced by methotrexate affects methionine synthase activity and its susceptibility to inactivation by nitrous oxide. J. Pharmacol. Exp. Ther. 1997;282:1305–1311. [PubMed] [Google Scholar]
- 11.Blom H.J. Consequences of homocysteine export and oxidation in vascular system. Semin. Thromb. Hemost. 2000;26:227–232. doi: 10.1055/s-2000-8467. [DOI] [PubMed] [Google Scholar]
- 12.Hu S., Cheeran M.C.J., Sheng W.S., Ni H.T., Lokensgard J.R., Peterson P.K. Cocaine alters proliferation, migration, and differentiation of human fetal brain-derived neural precursor cells. J. Pharmaco. Exp. Ther. 2006;318:1280–1286. doi: 10.1124/jpet.106.103853. [DOI] [PubMed] [Google Scholar]
- 13.Duthie S.J., Narayanan S., Brand G.M., Pirie L., Grant G. Impact of folate deficiency on DNA stability. J. Nutr. 2002;132:S2444–S2449. doi: 10.1093/jn/132.8.2444S. [DOI] [PubMed] [Google Scholar]
- 14.Seshadri S., Beiser A., Selhub J., Jacques P.F., Rosenberg I.H., D’Agostino R.B., Wilson P.W., Wolf P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 15.Shea T.B., Lyons-Weiler J., Rogers E. Homocysteine, folate deprivation and Alzheimer neuropathology. J. Alzheimers Dis. 2002;4:261–267. doi: 10.3233/jad-2002-4401. [DOI] [PubMed] [Google Scholar]
- 16.Fiskerstrand T., Ueland P.M., Refsum H. Folate depletion induced by methotrexate affects methionine synthase activity and its susceptibility to inactivation by nitrous oxide. J. Pharmacol. Exp. Ther. 1997;282:1305–1311. [PubMed] [Google Scholar]
- 17.Pietrzik K., Bronstrup A. Vitamins B12, B6 and folate as determinants of homocysteine concentration in the healthy population. Eur. J. Pediatr. 1998;157:S135–S138. doi: 10.1007/pl00014298. [DOI] [PubMed] [Google Scholar]
- 18.Obeid R., Herrmann W. Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS. Lett. 2006;580:2994–3005. doi: 10.1016/j.febslet.2006.04.088. [DOI] [PubMed] [Google Scholar]
- 19.Tjiattas L., Ortiz D.O., Dhivant S., Mitton K., Rogers E., Shea T.B. Folate deficiency and homocysteine induce toxicity in cultured dorsal root ganglion neurons via cytosolic calcium accumulation. Aging Cell. 2004;3:71–76. doi: 10.1111/j.1474-9728.2004.00086.x. [DOI] [PubMed] [Google Scholar]
- 20.Ho P.I., Ortiz D., Rogers E., Shea T.B. Multiple aspects of homocysteine neurotoxicity: Glutamate excitotoxicity, kinase hyperactivation and DNA damage. J. Neurosci. Res. 2002;70:694–702. doi: 10.1002/jnr.10416. [DOI] [PubMed] [Google Scholar]
- 21.Austin R.C., Lentz S.R., Werstuck G.H. Role of hyperhomocysteinemia in endothelial dysfunction and atherothrombotic disease. Cell Death Differ. 2004;11:S56–S64. doi: 10.1038/sj.cdd.4401451. [DOI] [PubMed] [Google Scholar]
- 22.Levrand S., Pacher P., Pesse B., Rolli J., Feihl F., Waeber B., Liaudet L. Homocysteine induces cell death in H9C2 cardiomyocytes through the generation of peroxynitrite. Biochem. Biophys. Res. Commun. 2007;359:445–450. doi: 10.1016/j.bbrc.2007.05.147. [DOI] [PMC free article] [PubMed] [Google Scholar]