Abstract
Hypochlorous acid (HOCl) is toxic and causes cell death. However, this effect is inhibited by reaction with taurine, which generates taurine chloramine (TauCl), thereby protecting the cells from HOCl-generated toxicity. TauCl has been shown to inhibit the production of inflammatory mediators like O2•−, H2O2 and NO. In this study, RAW 264.7 macrophages treated with TauCl were protected from death caused by H2O2. TauCl increased the expression of peroxiredoxin-1, thioredoxin-1 and heme oxygenase (HO)-1, the anti-oxidant enzymes normally induced by activation of NF-E2-related factor-2 (Nrf2). TauCl increased nuclear translocation of Nrf2 and binding to the anti-oxidant response element. These data suggest that TauCl produced abundantly in the activated neutrophils and released to surrounding cells in the inflamed tissues may induce the expression of cytoprotective anti-oxidant enzymes. Elevation of HO activity via induction of HO-1 expression within neighboring cells may provide protection from cytotoxicity caused by inflammatory oxidants like H2O2.
Keywords: taurine chloramine, hydrogen peroxide, heme oxygenase-1, cell death, nuclear factor-erythroid 2-related factor 2
Introduction
Taurine, a decarboxylation product of cysteine, is one of the most abundant free amino acids present in mammalian tissues and blood cells [1, 2]. Taurine protects the cells from inflammatory injury by attenuating the toxicity of hypochlorous acid (HOCl/OCl−) produced by the myeloperoxidase (MPO) system present abundantly in activated neutrophils [3]. Taurine reacts readily with HOCl/OCl− to form less toxic and more stable taurine chloramine (TauCl) and protects neutrophils from direct toxicity of HOCl. TauCl is released from neutrophils to the surrounding inflammatory tissues as the exudated neutrophils undergo apoptotic death after clearing the invaded bacteria. TauCl is transported into surrounding cells in a Na+ and Cl−-dependent manner [4, 5] In murine macrophages, TauCl has been demonstrated to modulate the production of many pro-inflammatory mediators such as superoxide anion (O2•−), nitric oxide (NO), tumor necrosis factor (TNF-α), interleukins and prostaglandins [6–8]. In particular, TauCl has been shown to inhibit the production of O2•− and NO in a dose-dependent manner without causing significant cytotoxicity to murine and human phagocytes [8–10]. TauCl has also been shown to protect tissues from inflammatory cytotoxicity caused by excessive production of reactive oxygen species (ROS) like hydrogen peroxide (H2O2).
Hydrogen peroxide is a toxic product of aerobic metabolism and is produced abundantly in inflammatory tissues. High dose of H2O2 is used as a disinfectant but this often leads to host cell damage [11, 12]. H2O2 diffuses readily across cellular membranes and is converted into a highly reactive and toxic hydroxyl radical via the heme-catalyzed Fenton reaction. Hydroxyl radical causes cell death via peroxidation of lipids, oxidation of thiols in proteins and enzymes and DNA damage, and is thought to be responsible for the cell death caused by H2O2. Cells respond to this H2O2-induced oxidative stress in part by increasing the expression of cytoprotective anti-oxidant enzymes via activation of several cytoplasmic redox-sensitive transcription factors such as activator protein (AP-1) and nuclear factor-erythroid 2-related factor 2 (Nrf2). Among the many cytoprotective anti-oxidant enzymes whose expression is strongly upregulated by activation and nuclear translocation of Nrf2, heme oxygenase-1 (HO-1), NAD(P)H:quinone oxidoreductase (NQO-1), glutathione-S-transferase (GST), γ-glutamate cysteine ligase (GCL), glutathione peroxidase (GPx), peroxiredoxin (Prx) and thioredoxin (Trx) are most prominent. The upregulation of these Nrf2-regulated enzymes are involved in protection against cytotoxicity caused by inflammatory mediators and ROS.
Heme oxygenase catalyzes the oxidative degradation of free-heme to generate Fe++, carbon monoxide (CO) gas and biliverdin/bilirubin [13, 14]. As the Fe++ released from heme gets stored quickly in ferritin and does not participate in Fenton reaction, production of hydroxyl radical from H2O2 is halted by the action of HO. Also, since the produced biliverdin/bilirubin is a strong scavenger of hydroxyl radical, it serves as intracellular cytoprotective anti-oxidant. Furthermore, cells are protected from additional oxidative stress because CO produced upon heme oxidation inhibits additional production of O2•− and NO by its tight binding to heme-containing enzymes like NADPH oxidases (NOX) and nitric oxide synthases (NOS). Between the two isoforms of HOs present in most mammals, HO-1 is strongly induced by many chemicals that cause oxidative stress and also directly by the free-heme released from several heme-containing enzymes during oxidative stress. Thus, cells with upregulated HO-1 and elevated HO activity could survive from oxidative injury caused by inflammation [15–19].
In the present study, we reported that TauCl increased expression of HO-1, Prx-1 and Trx-1, the Nrf2-regulated cytoprotective anti-oxidant enzymes, and protected cells from necrotic death caused by H2O2. In particular, upregulation of HO-1 expression and elevation of HO activity induced by TauCl may serve as the key mechanism of cytoprotection and survival during the resolution phase of inflammation. Our data suggest that TauCl, produced and released normally by neutrophils in the inflamed tissue, can protect the surrounding cells from oxidative damage caused by inflammatory products like H2O2.
Materials and Methods
Antibodies and reagents
Monoclonal antibody against HO-1 was purchased from Stressgen (Victoria, Canada). Rabbit polyclonal antibodies against Prx-1 and Trx-1 were purchased from AbFrontier (Seoul, Korea). Horseradish peroxidase-conjugated mouse- and rabbit-IgG antibodies were obtained from BD Pharmingen (San Diego, CA). Oligonucleotide primers were bought from Bioneer (Daejeon, Korea). Fetal bovine serum (FBS), penicillin and streptomycin were purchased from Hyclone (Logan, UT). Other chemicals, not specifically indicated, were purchased form Sigma (St, Louis, MO). TauCl was synthesized on the day of use by adding equimolar amounts of NaOCl (Aldrich Chemical, Milwaukee, MI) to taurine. The authenticity of TauCl formation and its concentration were monitored by UV absorption [20].
RAW 264.7 cell culture and viability assays
The murine macrophage, RAW 264.7 cells (ATCC, Manassas, VA) were grown in Dulbecco’s modified eagle medium (DMEM) supplemented with 100 units/ml of penicillin, 100 µg/ml of streptomycin and 10% FBS at 37°C in 5% CO2.
Cell viability was measured using the conventional thiazolyl blue tetraxolium bromide (MTT) reduction assay. To distinguish the proportion of cells undergoing necrotic and apoptotic deaths, propidium iodide (PI) and annexin V staining assays were employed [9, 21]. Cells stained with PI and annexin V, were analyzed on FACScan cytometer (BD Bioscience, San Jose, CA) using CellQuest software (BD Bioscience).
Reverse transcriptase-polymerase chain reaction
Total RNA was extracted using TRI reagent (MRC, Cincinnati, OH) according to the manufacturer’s instructions. PCR amplification of HO-1 mRNA was carried out with the following primers, 5'-TGA AGG AGG CCA CCA AGG AGG-3' (forward) and 5'-AGA GGT CAT CCA GGT AGC GGG-3' (reverse). The mRNA of glyceraldehyde-3-phosphate dehydrogenase (GADPH) served as the loading control.
Western blot analysis
Cell lysates were prepared as described previously [9, 22], and 20~30 µg of total proteins were electrophoresis on 10~12.5% SDS-PAGE. Separated proteins were transferred onto PVDF membrane (Millipore, Bedford, MA) and the protein bands were probed with specific antibodies and developed using the ECL method (Amersham, Arlington Heights, IL). Integrated densitometry was used to determine the intensity of scanned films using the ImageJ software (NIH, Bethesda, MD).
Nuclear protein extraction and electrophoretic mobility gel shift assay (EMSA)
Treated cells were lysed by incubation at 4°C for 10 min in buffer A [10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, 0.2 mM phenylmethylsulfonyl fluoride (PMSF)]. The cell lysates were centrifuged, and the resulting pellets (nuclear fraction) were resuspended in ice-cold buffer B [20 mM HEPES, pH 7.9, 20% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 0.2 mM PMSF], followed by incubation on ice for 20 min. After centrifugation at 14,500 × g for 6 min the supernatant (nuclear extract) was collected. Protein concentration was determined using the Bradford method (Bio-Rad, Hercules, CA). EMSA was performed using a DNA-protein binding kit (Qiagen, Hilden, Germany). Nrf2 oligonucleotide (5'-TGG GGA ACC TGT GCT GAG TCA CTG GAG -3'; Santa Cruz, Santa Cruz, CA) was labeled with [γ-32P]ATP using T4 polynucleotide kinase (Takara, Shinga, Japan) and purified from unincorporated [γ-32P]ATP by gel filtration using a nick spin column (Qiagen). The samples were electrophoresed through a 5% non-denaturing polyacrylamide gel at 150 V for 1 h as reported previously [9].
Measurement of heme oxygenase activity
Heme oxygenase activity was determined by employing the conventional bilirubin production assay as previously described [23]. Briefly, microsomal suspension obtained from RAW 264.7 macrophages was added to the reaction mixture containing 0.8 mM NADPH, 2 mM glucose-6-phosphate, 0.2 U glucose-6-phosphate dehydrogenase, 20 µM hemin and 2 mg of rat-liver cytosol in 100 mM potassium phosphate buffer, pH 7.4. The reaction mixture (1.0 ml) was incubated at 37°C for 1 h in the dark and then placed on ice for 2 min to terminate the reaction. The production of bilirubin was quantified by calculating from the difference in absorbance between 464 and 530 nm (extinction coefficient, 40 mM−1 cm−1 for bilirubin). HO activity was expressed as nmoles of bilirubin formed from per mg of microsomal protein per hour.
Statistical analysis
The two-tailed Student’s t test (un-paired) was performed using Microsoft Excel software (Redmond, WA). Data are expressed as mean ± SD, and a p value<0.05 was considered significant.
Results and Discussion
H2O2 induces cell death of RAW 264.7 cells and TauCl protects the cells from H2O2 toxicity
When bacteria invade tissues, intravascular neutrophils respond to bacterial chemokines and exit capillaries to phagocytose the bacteria. During phagocytosis, neutrophils and macrophages are activated by the bacterial wall products like lipopolysaccharide (LPS) and undergo immediate oxidative burst producing large amounts of O2•−. This O2•− is converted readily to H2O2 for utilization toward the killing of engulfed bacteria within phagosomes. Thus, in the tissues invaded by bacteria and undergoing inflammation, a large amount of H2O2 is produced and this H2O2 can also cause deaths of host cells like the phagocytes themselves as well as the surrounding cells.
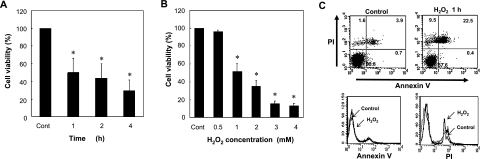
Within phagocytes, H2O2 is converted readily to HOCl by the action of MPO present abundantly. Phagocytes use this highly toxic HOCl to kill the engulfed bacteria within phagosomes. However, when this HOCl is released into cytoplasm, cytosolic contents like GSH and thiol-containing enzyme proteins are oxidized and HOCl can cause cytotoxicity to phagocytes themselves. In support of previous reports, RAW 264.7 mouse macrophage cells treated with various concentrations of H2O2 died in a time- and concentration-dependent manner (Fig. 1) [11, 12]. Upon 1 h exposure to 1 mM H2O2, nearly 50% of cells died and over 30% of cells died of necrosis, as shown by the results of MTT assay and PI-Annexin V staining, respectively.
Fig. 1.
H2O2 causes cell death in RAW 264.7 macrophages. (A, B) Exposure to 1 mM H2O2 induces cell death in about 50% of cells by 1 h when determined by the MTT reduction assay (n = 6), *p<0.01. At higher doses of H2O2, a greater proportion of cells died. (C) Exposure to 1 mM H2O2 for 1 h caused around 30% of necrotic cell death without showing significant apoptotic death (PI-Annexin V staining assay) (n = 5).
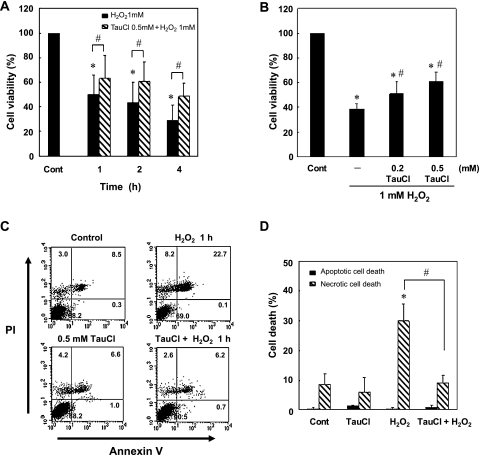
Under physiological conditions, professional phagocytes like neutrophils and macrophages protect themselves from the toxicity of HOCl by storing large amounts of taurine, up to 50 mM, in the cytoplasm as a free amino acid. Taurine is a decarboxylation product of cysteine and reacts readily with HOCl. Taurine inactivates HOCl by generating TauCl, previously considered to be a detoxified end-product of HOCl ready to be disposed and excreted in urine. At the inflammatory tissue, the exudated and activated neutrophils die of apoptosis after clearing the invading bacteria and release their contents like TauCl and H2O2. Thus, to examine whether the released TauCl could ameliorate the toxicity of H2O2, RAW 264.7 cells were initially treated with 1 mM H2O2 and 0.5 mM TauCl together. In the presence of TauCl, the cells appeared to survive better and longer (data not shown). In subsequent experiments, cells were pretreated with various concentrations of TauCl for various durations before exposing them to 1 mM H2O2. In cells exposed to 0.5 mM TauCl, cellular content of ROS increased along with decrease of GSH initially, and then the ROS level decreased along with the return of GSH content which was above the control level by 12 h (data not shown and manuscript in preparation). The cytotoxicity of H2O2 was most effectively prevented by 12 h pretreatment of the cells with TauCl (Fig. 2A and B). These results suggested that pretreatment of cells with TauCl caused enhancement of anti-oxidant capacity and protected cells from necrotic death caused by H2O2 (Fig. 2C and D). Preliminary results indicated that the cellular contents of total GSH was elevated along with induction of γ-GCL, an Nrf2 modulated enzyme involved in GSH biosynthesis, in cells treated with TauCl for 12 h (data not shown). The induction of GCLC and increase of GSH content were observed in association with elevation of HO-1 activity and overproduction of CO gas (data not shown).
Fig. 2.
TauCl pretreatment protects cells from H2O2-induced cell death. RAW 264.7 cells were pretreated with 0.5 mM TauCl for 12 h prior to exposure to 1 mM H2O2. (A) Viability of TauCl pretreated cells was assessed after exposure to 1 mM H2O2 using the MTT reduction assay (n = 6), *p<0.001 compared to control and #p<0.05 compared to H2O2 treated cells. (B) Viability of cells pretreated with various concentrations of TauCl was determined at 1 h after exposure to 1 mM H2O2 (n = 5), *p<0.001 compared to control and #p<0.05 compared to H2O2. (C) Cells were stained with annexin V and PI for 15 min and analyzed by using FACS. Data show representative FACS graphs (n = 5). (D) Bar graphs represent the mean values of FACS data (n = 5), *p<0.05 compared to control and #p<0.01 compared to H2O2.
TauCl activates nuclear translocation of Nrf2 and induces the expression of several anti-oxidant proteins
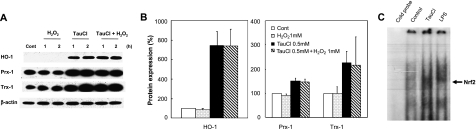
Anti-oxidant proteins like peroxiredoxin (Prx) and thioredoxin (Trx) protect cells from oxidative cytotoxicity caused by ROS like O2•−, H2O2 and HOCl. These anti-oxidant proteins provide potent immunomodulatory activity during the resolution phase of inflammation and are induced as a normal adaptive response to oxidative stimuli and proinflammatory cytokines. Prx reduces hydroperoxides using the electrons provided from Trx; Trx on its own facilitates the reduction of oxidized proteins by catalyzing the cysteine thiol-disulfide exchange reaction. Another anti-oxidant enzyme commonly induced in response to oxidative stimuli is HO-1. Induction of these anti-oxidant proteins occurs in response to the nuclear translocation of a key redox-sensitive transcription factor Nrf2 from the cytoplasm to nucleus and binding to the anti-oxidant response element (ARE) sequences localized in the promoter region of genes that encode several anti-oxidant proteins. Induction of these anti-oxidant proteins is thus essential for the survival of cells in inflammatory tissues from the oxidative stress caused by ROS like H2O2. When the RAW 264.7 cells were exposed to various nontoxic doses of TauCl, cellular levels of Nrf2 modulated proteins like HO-1, Prx-1 and Trx-1 were markedly increased. The levels of these anti-oxidant enzymes increased markedly and rapidly in cells exposed to 0.5 mM TauCl for 12 h (Fig. 3A and B). In support of these observations, the level of Nrf2 translocated into nucleus and bound to ARE were higher than that obtained in cells treated with LPS (Fig. 3C). Although moderate doses of H2O2 have been known to induce the expression of several anti-oxidant enzymes, we could not detect upregulation of HO-1, Prx-1 and Trx-1 levels in RAW 264.7 cells treated with 1 mM H2O2 (Fig. 3A). These results suggest that TauCl stimulates the activation of Nrf2 and enhances its nuclear translocation to promote the synthesis of several key anti-oxidant enzymes and allows the survival against the cell death caused by excess H2O2.
Fig. 3.
TauCl increases the Nrf2/ARE binding in nucleus and upregulates the expression of Nrf2-regulated anti-oxidant enzymes. RAW 264.7 cells were pretreated with 0.5 mM TauCl for 12 h prior to 1 mM H2O2 treatment. (A) Levels of HO-1, Prx-1 and Trx-1 expression were determined at 1 and 2 h after H2O2 exposure. β-actin levels were measured to ensure equal protein loading. Representative blots obtained from three independent experiments are shown. (B) Bar graphs show relative levels of proteins expressed at 1 h after H2O2 exposure and they were estimated by densitometry of immunoblots normalized to β-actin (n = 3). (C) TauCl induced nuclear translocation of Nrf2 and its ARE binding. Representative radiogram of EMSA is shown (n = 3).
TauCl increases the expression of HO-1 and elevates HO activity
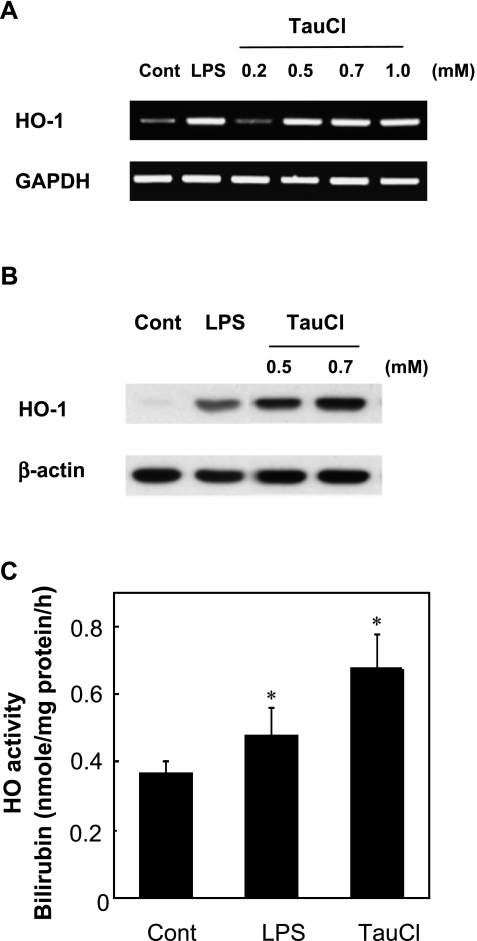
HO catalyzes the first and rate-limiting step in the oxidative degradation of free-heme released from heme-containing proteins during oxidative stress. As the released free-heme catalyzes the production of hydroxyl radical from H2O2 by Fenton reaction, rapid elimination of free-heme via elevation of HO activity appears to be essential for the survival of cells exposed to H2O2 in the inflammatory tissue. The release of TauCl from neutrophils undergoing apoptotic death in the inflamed tissue, or added exogenously to macrophages showed marked upregulation of HO-1 expression and elevation of HO activity (Fig. 4). TauCl appeared to stimulate the HO-1 expression to an even greater extent and at a faster rate than that stimulated by 1 µg/ml LPS (Fig. 4A and B). Although the results obtained at early time points are not shown, TauCl increased HO-1 mRNA expression beginning at 2 h and reached maximum at 4 to 6 h and this increased level was maintained throughout 24 h. In contrast, LPS-treated cells showed a significant increase of HO-1 expression at 6 h and reached a maximum by 12 h. TauCl (0.5 mM) also increased HO activity to a greater level than that induced by 1 µg/ml LPS (Fig. 4C). Elevation of HO activity may thus serve several purposes to protect cells from the cytotoxicity of ROS; (1) stops additional production of hydroxyl radical by eliminating the free-heme to limit the Fenton reaction, (2) provides intracellular anti-oxidants like biliverdin and bilirubin, and (3) stops additional production of O2•− and NO by generating CO gas that inhibits NADPH oxidase and iNOS activation [16]. Furthermore, the CO produced by the elevated HO activity has been shown to stimulate the rate of GSH biosynthesis via activation of the Nrf2/ARE pathway and upregulation of GCLC expression [18].
Fig. 4.
TauCl increases HO-1 expression and elevates HO activity. (A) Levels of HO-1 mRNA expressed at 12 h after exposure to varying doses of TauCl and 1 µg/ml LPS. TauCl increased HO-1 expression in a dose-dependent manner. GAPDH mRNA expression level was used to ensure equal loading (n = 5). (B) The HO-1 protein expression increased by 0.5 mM TauCl was greater than that induced by 1 µg/ml LPS. HO-1 protein expression was determined at 12 h compared to the β-actin level (n = 5). (C) TauCl increases HO activity to a greater level than LPS did. Cells were treated with 0.5 mM TauCl for 12 h and HO activity was determined by employing the bilirubin production assay (n = 3), *p<0.01 compared to control. LPS (1 µg/ml) was used as a positive control for HO-1 expression and HO activity.
Combined, these results suggest that TauCl released from apoptosing neutrophils during resolution phase of inflammation may be essential for the stimulation of several key anti-oxidant enzyme expression (HO-1, Prx-1 and Trx-1) via activation of Nrf2/ARE system in surrounding macrophages. In conclusion, our results demonstrate that TauCl, considered previously as one of the simple taurine-inactivated end-product of HOCl, is released endogenously from neutrophils during resolution phase of inflammation with a purpose to protect cells in the inflammatory tissue from cytotoxicity caused by H2O2 and other ROS.
Acknowledgments
The Korea Research Foundation Grant (KRF-2008-531-C00051) and Nano R&D program through the KOSEF (M10642040001-07N4204-00110) supported this work.
References
- 1.Vinton N.E., Laidlaw S.A., Ament M.E., Kopple J.D. Taurine concentrations in plasma and blood cells of patients undergoing long-term parenteral nutrition. Am. J. Clin. Nutr. 1986;44:398–404. doi: 10.1093/ajcn/44.3.398. [DOI] [PubMed] [Google Scholar]
- 2.Learn D.B., Fried V.A., Thomas E.L. Taurine and hypotaurine content of human leukocytes. J. Leukoc. Biol. 1990;48:174–182. [PubMed] [Google Scholar]
- 3.Thomas E.L., Grisham M.B., Melton D.F., Jefferson M.M. Evidence for a role of taurine in the in vitro oxidative toxicity of neutrophils toward erythrocytes. J. Biol. Chem. 1985;260:3321–3329. [PubMed] [Google Scholar]
- 4.Tallan H.H., Jacobson E., Wright C.E., Schneidman K., Gaull G.E. Taurine uptake by cultured human lymphoblastoid cells. Life Sci. 1983;33:1853–1860. doi: 10.1016/0024-3205(83)90669-0. [DOI] [PubMed] [Google Scholar]
- 5.Kim C., Chung J.K., Jeong J.M., Chang Y.S., Lee Y.J., Kim Y.J., Lee M.C., Koh C.S., Kim B.K. Uptake of taurine and taurine chloramine in murine macrophages and their distribution in mice with experimental inflammation. Adv. Exp. Med. Biol. 1998;442:169–176. doi: 10.1007/978-1-4899-0117-0_22. [DOI] [PubMed] [Google Scholar]
- 6.Park E., Schuller-Levis G., Quinn M.R. Taurine chloramine inhibits production of nitric oxide and TNF-alpha in activated RAW 264.7 cells by mechanisms that involve transcriptional and translational events. J. Immunol. 1995;154:4778–4784. [PubMed] [Google Scholar]
- 7.Marcinkiewicz J., Grabowska A., Bereta J., Stelmaszynska T. Taurine chloramine, a product of activated neutrophils, inhibits in vitro the generation of nitric oxide and other macrophage inflammatory mediators. J. Leukoc. Biol. 1995;58:667–674. doi: 10.1002/jlb.58.6.667. [DOI] [PubMed] [Google Scholar]
- 8.Kim C., Park E., Quinn M.R., Schuller-Levis G. The production of superoxide anion and nitric oxide by cultured murine leukocytes and the accumulation of TNF-alpha in the conditioned media is inhibited by taurine chloramine. Immunopharmacology. 1996;34:89–95. doi: 10.1016/0162-3109(96)00113-0. [DOI] [PubMed] [Google Scholar]
- 9.Kim J.W., Kim C. Inhibition of LPS-induced NO production by taurine chloramine in macrophages is mediated though Ras-ERK-NF-kappaB. Biochem. Pharmacol. 2005;70:1352–1360. doi: 10.1016/j.bcp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Kim C., Choi H.S., Kim J.W. Taurine chloramine inhibits the production of nitric oxide and superoxide anion by modulating specific mitogen-activated protein kinases. Adv. Exp. Med. Biol. 2006;583:493–498. doi: 10.1007/978-0-387-33504-9_55. [DOI] [PubMed] [Google Scholar]
- 11.Yoshioka Y., Kitao T., Kishino T., Yamamuro A., Maeda S. Nitric oxide protects macrophages from hydrogen peroxide-induced apoptosis by inducing the formation of catalase. J. Immunol. 2006;176:4675–4681. doi: 10.4049/jimmunol.176.8.4675. [DOI] [PubMed] [Google Scholar]
- 12.Chow J.M., Shen S.C., Huan S.K., Lin H.Y., Chen Y.C. Quercetin, but not rutin and quercitrin, prevention of H2O2-induced apoptosis via anti-oxidant activity and heme oxygenase 1 gene expression in macrophages. Biochem. Pharmacol. 2005;69:1839–1851. doi: 10.1016/j.bcp.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Maines M.D., Trakshel G.M., Kutty R.K. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J. Biol. Chem. 1986;261:411–419. [PubMed] [Google Scholar]
- 14.McCoubrey W.K. Jr., Huang T.J., Maines M.D. Heme oxygenase-2 is a hemoprotein and binds heme through heme regulatory motifs that are not involved in heme catalysis. J. Biol. Chem. 1997;272:12568–12574. doi: 10.1074/jbc.272.19.12568. [DOI] [PubMed] [Google Scholar]
- 15.Otterbein L.E., Bach F.H., Alam J., Soares M., Tao Lu H., Wysk M., Davis R.J., Flavell R.A., Choi A.M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 16.Srisook K., Han S.S., Choi H.S., Li M.H., Ueda H., Kim C., Cha Y.N. CO from enhanced HO activity or from CORM-2 inhibits both O2•− and NO production and downregulates HO-1 expression in LPS-stimulated macrophages. Biochem. Pharmacol. 2006;71:307–318. doi: 10.1016/j.bcp.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 17.Brouard S., Otterbein L.E., Anrather J., Tobiasch E., Bach F.H., Choi A.M., Soares M.P. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J. Exp. Med. 2000;192:1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M.H., Jang J.H., Na H.K., Cha Y.N., Surh Y.J. Carbon monoxide produced by heme oxygenase-1 in response to nitrosative stress induces expression of glutamate-cysteine ligase in PC12 cells via activation of phosphatidylinositol 3-kinase and Nrf2 signaling. J. Biol. Chem. 2007;282:28577–28586. doi: 10.1074/jbc.M701916200. [DOI] [PubMed] [Google Scholar]
- 19.Ryter S.W., Choi A.M. Heme oxygenase-1: molecular mechanisms of gene expression in oxygen-related stress. Antioxid. Redox Signal. 2002;4:625–632. doi: 10.1089/15230860260220120. [DOI] [PubMed] [Google Scholar]
- 20.Thomas E.L., Grisham M.B., Jefferson M.M. Preparation and characterization of chloramines. Methods Enzymol. 1986;132:569–585. doi: 10.1016/s0076-6879(86)32042-1. [DOI] [PubMed] [Google Scholar]
- 21.Choi H.S., Cha Y.N., Kim C. Taurine chloramine inhibits PMA-stimulated superoxide production in human neutrophils perhaps by inhibiting phosphorylation and translocation of p47(phox) Int. Immunopharmacol. 2006;6:1431–1440. doi: 10.1016/j.intimp.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Kim C., Dinauer M.C. Rac2 is an essential regulator of neutrophil nicotinamide adenine dinucleotide phosphate oxidase activation in response to specific signaling pathways. J. Immunol. 2001;166:1223–1232. doi: 10.4049/jimmunol.166.2.1223. [DOI] [PubMed] [Google Scholar]
- 23.Srisook K., Cha Y.N. Biphasic induction of heme oxygenase-1 expression in macrophages stimulated with lipopolysaccharide. Biochem. Pharmacol. 2004;68:1709–1720. doi: 10.1016/j.bcp.2004.07.001. [DOI] [PubMed] [Google Scholar]