Abstract
The purpose of this study was to examine the effects of γ-aminobutyric acid (GABA) in fermented drinking water prepared from sodium glutamate, vinegar, and dried bonito (FDWG) compared with placebo [vinegar and dried bonito without GABA (FDW)] and its safety in normotensive and mildly or moderately hypertensive volunteers. A double-blind, placebo-controlled, randomized study was conducted involving volunteers with normal (group-N) and mildly or moderately high (group-H) blood pressure (BP). After a pretreatment period of 2 weeks (weeks –2), the subjects received FDWG or FDW for 12 weeks followed by 4 weeks of no intake (weeks 16). In group-H, both FDWG and FDW significantly decreased systolic (SBP, −7.6 ± 4.0 and −5.5 ± 1.5 mmHg, p<0.05, respectively) and diastolic (DBP, −10.6 ± 4.0 and −7.6 ± 1.7 mmHg, p<0.01, respectively) BP compared to the baseline (0-week) value at 12 weeks, respectively. There were no abnormal changes in hematological or blood chemistry variables, urinalysis, heart rate, or body weight in the study groups. These findings indicated that vinegar and dried bonito with or without GABA might have an effect on BP in mildly or moderately hypertensive patients.
Keywords: γ-aminobutyric acid, vinegar, dried bonito, hypertension, human
Introduction
Hypertension is considered a major risk factor for the progression of cerebrovascular and cardiovascular disorders. The development of hypertension is caused by several factors, including poor dietary and lifestyle habits. It is essential in mild or moderate hypertension for patients to receive dietary therapy before beginning a course of medication [1].
The blood pressure (BP)-lowering effect of vinegar and peptides from dried bonito has been shown in earlier reports [2–4]. γ-Aminobutyric acid (GABA) is an amino acid that is widely distributed in mammals, crustacea, microorganisms, and many plants such as tea [5] and radish greens [6], as well as mushrooms [7]. It is well-known that GABA is one of the major inhibitory neurotransmitters and is present at a high concentration in the central nervous system [8], but it also exists in peripheral tissues [9]. Several animal experiments have demonstrated the hypotensive effect of GABA [10–12]. Furthermore, the safety and hypotensive actions of some food products containing GABA were shown in hypertensive patients [13–15]. Therefore, the hypotensive effect of drinking water containing vinegar and dried bonito with may be stronger than that without GABA. However, the overall impact of the food products containing GABA on BP remains unclear.
Fermented drinking water containing GABA is prepared from sodium glutamate, vinegar, and dried bonito (FDWG), and GABA is transformed from sodium glutamate by decarboxylase with the use of lactobacilli [16]. We have performed preliminary experiments in rats using FDWG at varying concentrations. As a result, there were problems in regarding the predictability of the hypotensive effect, such as transient hypotension or rebound hypertension at a high concentration of GABA (600 mg/90 ml) (data not shown). The above experiments were also conducted in humans, showing that there were no problems at a low concentration of GABA (200 mg/90 ml or less) for a short period (data not shown). The BP-lowering effect of fermented milk containing GABA at a concentration of 10–12 mg/100 ml has also been reported by Inoue et al. [15]. We have chosen FDWG at a concentration of 70 mg/90 ml (approximately 35% of 200 mg of GABA), which would be expected to have a greater effect on BP than fermented milk containing GABA (approximately 7-fold higher than that in the above study [15]) and higher-level safety.
Thus, the purpose of this study was to examine the effects of GABA in FDWG, compared with drinking water containing vinegar and dried bonito without GABA (FDW), and its safety in normotensive and mildly or moderately hypertensive volunteers.
Materials and Methods
Subjects
The study population comprised 15 subjects with a normal BP and 21 with mild or moderate hypertension [systolic BP (SBP) is from 130 to 159 mmHg, and diastolic BP (DBP) is from 85 to 99 mmHg] (JSH2004 grade) [1]. The criteria for patient selection were: being aged between 20 and 65 years; having no history of antihypertensive medication; and having no other abnormal symptoms, diagnostic opinions, hematological or blood chemistry variables, or physical exam results. The criteria for subject exclusion were: those with food allergies; those who took medicine within seven days of the start of the study; those medicated with other novel drugs within four months of the start of the study; those requiring emergent antihypertensive therapy; those with symptoms of cerebrovascular disorder, heart failure and/or a previous history of myocardial infarction, atrial fibrillation and/or serious arrhythmia, renal dysfunction (a serum creatinine level of more than 2.0 mg/dl), severe liver disorder, diabetes, anemia (a hemoglobin concentration of less than 9.5 g/dl); and those pregnant or lactating. Subjects provided written informed consent before participation. This study was approved by the ethics committee of the Bio Research Center of Niigata City in Japan.
Study protocol
This study was carried out using a placebo-controlled, double-blind design and consisted of a two-week preobservation term (weeks –2) and a 12-week daily-intake phase, which was followed by 4 weeks with no intake (weeks 16) [13]. Subjects were classified into those with a normal BP (group-N) and those with moderate or mild hypertension (group-H) according to JSH2004 on 2 weeks before the first intake. Each group was randomly split into two subgroups by investigator. One subgroup of subjects took 90 ml (one bottle) of FDWG daily for 12 weeks. The other subgroup took FDW as a placebo. We measured the BP and heart rate (HR) of seated subjects at weeks −2, 0, 2, 4, 8, 10, 12, 14, and 16. After BP measurement, we obtained blood and urine samples for the assessment of hematological and blood chemistry variables and urinalysis.
Test products
FDWG was prepared by Yasuda Yogurt Co., Ltd. (Niigata Japan), and the method of preparation was as follows: dried bonito powder was added to a mixture of liquid sugar from rice and water, and, after decoction and cooling, protease treatment was performed. Then, sodium glutamate and a culture of lactic acid bacteria were added to the above solution and fermented. The resultant preparation was heated, sterilized, and filtered. Water, liquid sugar from rice, vinegar, and malic acid were added to the above solution; thus, FDWG was prepared. Ninety ml of FDWG contains 2.07 g of vinegar, 0.15 g of dried bonito, and 70 mg of GABA. FDW (the placebo not containing GABA) was obtained by performing the same operation without adding sodium glutamate. Ninety ml of FDW contains 2.07 g of vinegar and 0.15 g of dried bonito, in which GABA was undetectable. The doses of vinegar and dried bonito used in the study were determined considering earlier reports [3, 17], each ingredients in FDWG which would be expected to have hypotensive effect, and taste of test drinks.
Measurements
BP and HR were measured between 9:00 and 10:00 a.m. at the Niigata Association of Occupational Health, Inc. BP measurements were performed using a mercury sphygmomanometer while the subjects were seated after they had rested for 15 min. Hematological and blood chemistry variables were measured, and urinalysis was performed at the laboratory of SRL Corporation in Japan. The GABA concentration in FDWG and FDW was measured using ion-exchange chromatography (Shimadzu Corporation, Japan) and postcolumn fluorometric detection.
Statistical analysis
Data are presented as the mean ± standard error of the mean. Analysis of variance and paired t tests were used to analyze changes between the postintake and baseline levels [18]. Differences between the FDWG- and FDW-intake values were analyzed using unpaired t tests, which were also employed to compare group-N with group-H. Significance was established at p<0.05.
Results
Subject characteristics and side effects
All 36 subjects completed the study. Table 1 presents the baseline demographics of all subjects. None of the subjects developed any side effects during the study.
Table 1.
Baseline clinical demographics of the four study groups
| Group-N |
Group-H |
|||
|---|---|---|---|---|
| FDWG | FDW | FDWG | FDW | |
| Number of subjects | 8 | 7 | 10 | 11 |
| Men/women | 7/1 | 3/4 | 5/5 | 10/1 |
| Age (year) | 45.1 ± 3.3 | 49.3 ± 10.5 | 45.7 ± 9.8 | 46.9 ± 3.2 |
| Body weight (kg) | 65.3 ± 8.0 | 66.7 ± 3.9 | 62.2 ± 5.3 | 62.5 ± 9.5 |
| Height (cm) | 167.9 ± 2.8 | 161.3 ± 3.6 | 165.7 ± 3.7 | 168.5 ± 1.9 |
| Body mass index (kg/m2) | 23.1 ± 0.7 | 22.3 ± 1.1 | 25.5 ± 0.9 | 21.9 ± 0.7 |
| Systolic blood pressure (mmHg) | 116.0 ± 2.8 | 115.7 ± 3.4 | 136.0 ± 1.9 | 130.2 ± 1.4 |
| Diastolic blood pressure (mmHg) | 76.0 ± 1.6 | 74.9 ± 2.5 | 92.2 ± 2.3 | 89.3 ± 2.4 |
| Heart rate (beats/min) | 68.0 ± 4.5 | 62.6 ± 1.2 | 66.9 ± 2.7 | 68.7 ± 3.7 |
The FDWG subgroup did not differ significantly (p<0.05; unpaired t test) with respect to any characteristic compared with the FDW subgroup in group-N and group-H. Where appropriate, data are presented as the mean ± standard error of the mean for the 8 subjects who received FDWG or the 7 subjects who received FDW in group-N, and the 10 subjects who received FDWG or the 11 subjects who received FDW in group-H. FDWG: fermented drinking water containing vinegar and dried bonito with GABA. FDW: fermented drinking water containing vinegar and dried bonito without GABA.
Blood pressure
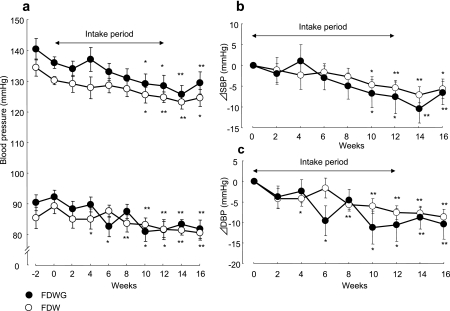
In group-H, the time-courses of the SBP and DBP during the two weeks of the preobservation, 12-week daily intake, and the 4 weeks of the postintake observation term without ingestion are shown in Fig. 1a. Both FDWG and FDW lowered SBP and DBP modestly. A significant decrease in SBP was noted from 10 weeks onwards in the FDWG subgroup (−6.8 ± 3.3 mmHg, p<0.05) and in the FDW subgroup (−4.7 ± 2.0 mmHg, p<0.05). DBP was significantly decreased at 6, 10, 12, 14, and 16 weeks in the FDWG subgroup (−9.6 ± 3.7 mmHg, p<0.05; −11.2 ± 4.0 mmHg, p<0.05; −10.6 ± 4.0 mmHg, p<0.05; −8.8 ± 2.8 mmHg, p<0.01; and −10.4 ± 3.6 mmHg, p<0.01, respectively) and at 4, 8, 10, 12, 14, and 16 weeks in the FDW subgroup (−4.2 ± 1.8 mmHg, p<0.05; −5.6 ± 1.1 mmHg, p<0.01; −6.0 ± 1.8 mmHg, p<0.01; −7.6 ± 1.7 mmHg, p<0.01; −7.8 ± 1.5 mmHg, p<0.01; and −8.7 ± 0.9 mmHg, p<0.01, respectively). The changes in SBP and DBP from the baseline values after FDWG or FDW ingestion are shown in Fig. 1b and 1c, respectively. The changes in SBP in the FDWG subgroup tended to be lower than those in the FDW subgroup at 8, 10, 12, and 14 weeks, and the decreases of DBP in the FDWG subgroup tended to be lower than those in the FDW subgroup at 6, 10, and 12 weeks, between which there were no significant differences. Two weeks after test-solution ingestion had stopped (14 weeks), SBP continued decreasing in both the FDWG and FDW subgroups. At 4 weeks after test-solution ingestion had stopped (16 weeks), SBP was increased but remained lower than the baseline level in both the FDWG and FDW subgroups.
Fig. 1.
Time course of blood pressure (a) during the preobservation term, the intake period of FDWG (n = 10, closed circles) or FDW (n = 11, open circles), and after finishing intake in group-H, and changes (relative to baseline values) in systolic (SBP) (b) and diastolic (DBP) (c) blood pressure after the intake of FDWG or FDW. Each point represents the mean ± standard error. *p<0.05 and ** p<0.01 (paired t test) compared to the 0-week value.
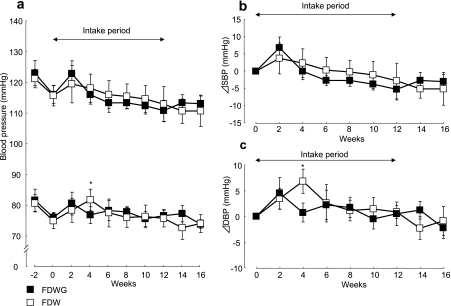
No significant decreases of SBP and DBP in group-N were confirmed during FDWG or FDW ingestion (Figs. 2a, b, and 3c).
Fig. 2.
Time course of blood pressure (a) during the preobservation term, the intake period of FDWG (n = 8, closed squares) or FDW (n = 7, open squares), and after finishing intake in group-N, and changes (relative to baseline values) in systolic (SBP) (b) and diastolic (DBP) (c) blood pressure after the intake of FDWG or FDW. Each point represents the mean ± standard error. *p<0.05 (paired t test) compared to the 0-week value.
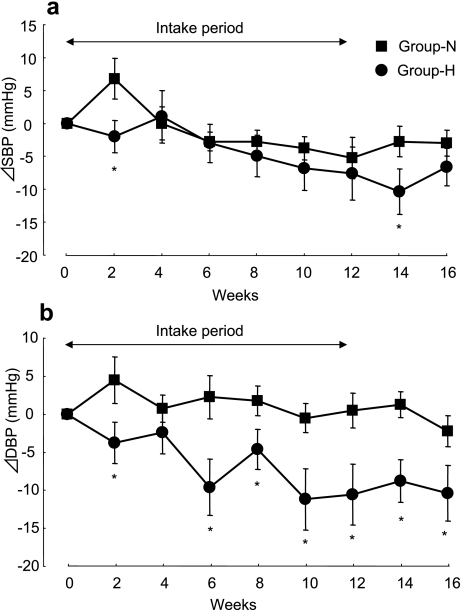
Fig. 3.
Changes (relative to baseline values) in systolic (SBP) (a) and diastolic (DBP) (b) blood pressure after the intake of FDWG in group-N (n = 8, squares) and group-H (n = 10, circles). Each point represents the mean ± standard error. *p<0.05 (paired t test) compared to the week-matched value in group-N.
Fig. 3 presents a comparison between group-N and group-H regarding changes in SBP and DBP from baseline levels after the intake of FDWG. SBP was significantly decreased at 2 and 14 weeks (all p<0.05) in group-H compared with group-N. DBP was significantly decreased at 2, 6, 8, 10, 12, 14, and 16 weeks (all p<0.05) in group-H compared with group-N.
In all subjects (group-N + group-H), SBP was significantly decreased from 8 weeks after FDWG intake (−4.0 ± 1.9 mmHg, p<0.05) and from 12 weeks after FDW intake (−4.4 ± 2.1 mmHg, p<0.05) compared to the 0-week value. DBP was significantly decreased from 10 weeks in the FDWG subgroup (−6.4 ± 2.7 mmHg, p<0.05) and from 8 weeks in the FDW subgroup (−3.0 ± 1.3 mmHg, p<0.05) compared to the 0-week value.
Heart rate, body weight, and body mass index
HR did not change significantly in any group. The baseline HR was 68.0 ± 4.5 beats/min in group-N and 66.9 ± 2.7 beats/min in group-H before the intake of FDWG, and 62.6 ± 1.2 beats/min in group-N and 68.7 ± 3.7 beats/min in group-H before the intake of FDW. After 12 weeks of FDWG intake, HR was 65.0 ± 2.9 beats/min in group-N and 67.2 ± 2.0 beats/min in group-H. After 12 weeks of FDW intake, HR was 64.3 ± 1.7 beats/min in group-N and 66.4 ± 2.1 beats/min in group-H.
No significant change in body weight or the body mass index was seen until FDWG or FDW intake in all subjects.
Hematological and blood chemistry variables, and urinalysis
Table 2 presents the results of hematology, blood chemistry studies, and urinalysis before and after intake of the test solution in all subjects. The 12-week intake of FDWG significantly increased the chloride value and decreased the levels of aspartate aminotransferase, γ-glutamyl transpeptidase, total protein, lactate dehydrogenase, and blood glucose compared to the first day of intake. In the FDW group, chloride was significantly increased and the hematocrit, red blood cells, total protein, albumin, and blood glucose were significantly decreased after 12 weeks compared to the first day of intake. These significant changes remained within the normal ranges. The total cholesterol levels were decreased from 192.2 ± 7.0 mg/dl to 183.9 ± 5.8 mg/dl in the FDWG subgroup and from 210.1 ± 8.3 mg/dl to 199.8 ± 6.5 mg/dl in the FDW subgroup, although these changes were not significant. There were no significant differences between the FDWG and FDW subgroups at 12 weeks.
Table 2.
Results of hematology, blood chemistry, and urinalysis before (week 0) and after (week 12) the intake of fermented drinking water containing vinegar and dried bonito with (FDWG) or without (FDW) GABA.
| FDWG subgroup |
FDW subgroup |
|||
|---|---|---|---|---|
| Before | After | Before | After | |
| Hematology | ||||
| Red blood cells (×104/µl) | 474.4 ± 7.9 | 464.2 ± 9.8 | 464.7 ± 10.3 | 457.0 ± 11.6* |
| White blood cells (/µl) | 5244.4 ± 336.8 | 5222.2 ± 369.6 | 5666.7 ± 258.2 | 5672.0 ± 260.6 |
| Platelets (×104/µl) | 22.9 ± 1.2 | 22.0 ± 1.1 | 25.0 ± 1.4 | 25.1 ± 1.3 |
| Hemoglobin (g/dl) | 14.1 ± 0.3 | 13.9 ± 0.3 | 14.4 ± 0.3 | 14.2 ± 0.3 |
| Hematocrit (%) | 42.8 ± 0.7 | 42.1 ± 0.8 | 42.8 ± 0.7 | 42.0 ± 0.9** |
| Blood chemistry variables | ||||
| Aspartate aminotransferase (IU/l) | 22.9 ± 1.3 | 20.6 ± 0.9* | 20.7 ± 0.9 | 21.9 ± 1.0 |
| Alanine aminotransferase (IU/l) | 21.6 ± 2.7 | 18.4 ± 1.6 | 21.8 ± 1.9 | 23.1 ± 2.1 |
| γ-glutamyl transpeptidase (IU/l) | 37.1 ± 7.0 | 28.1 ± 4.4* | 42.6 ± 7.1 | 40.7 ± 6.5 |
| Alkali phosphatase (IU/l) | 212.3 ± 10.9 | 205.5 ± 12.1 | 197.6 ± 11.4 | 199.4 ± 11.5 |
| Blood urea nitrogen (mg/dl) | 14.2 ± 0.7 | 13.8 ± 0.8 | 13.6 ± 0.9 | 13.3 ± 1.0 |
| Creatinine (mg/dl) | 0.7 ± 0.03 | 0.7 ± 0.03 | 0.7 ± 0.03 | 0.7 ± 0.03 |
| Total cholesterol (mg/dl) | 192.2 ± 7.0 | 183.9 ± 5.8 | 210.1 ± 8.3 | 199.8 ± 6.5 |
| High density lipoprotein cholesterol (mg/dl) | 58.3 ± 4.0 | 56.3 ± 4.0 | 63.1 ± 4.0 | 59.7 ± 3.9 |
| Low density lipoprotein cholesterol (mg/dl) | 116.3 ± 6.3 | 110.9 ± 4.9 | 128.2 ± 6.4 | 121.2 ± 4.6 |
| Triglycerides (mg/dl) | 99.8 ± 16.7 | 92.1 ± 11.6 | 144.2 ± 32.4 | 131.3 ± 19.7 |
| Total protein (g/dl) | 7.1 ± 0.1 | 7.0 ± 0.1* | 7.1 ± 0.1 | 6.9 ± 0.1** |
| Albumin (g/dl) | 4.4 ± 0.1 | 4.3 ± 0.1 | 4.4 ± 0.02 | 4.3 ± 0.1* |
| Albumin-globulin ratio | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.04 | 1.6 ± 0.04 |
| Total bilirubin (mg/dl) | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| Lactate dehydrogenase (IU/l) | 186.9 ± 5.2 | 176.1 ± 5.0* | 193.9 ± 7.2 | 189.1 ± 8.9 |
| Blood glucose (mg/dl) | 91.4 ± 2.1 | 88.4 ± 1.9* | 98.6 ± 3.1 | 92.7 ± 3.2** |
| HbA1c (%) | 4.9 ± 0.1 | 4.9 ± 0.1 | 5.1 ± 0.2 | 5.0 ± 0.1 |
| Uric acid (mg/dl) | 5.0 ± 0.3 | 5.2 ± 0.3 | 5.4 ± 0.3 | 5.6 ± 0.4 |
| Na+ (mEq/l) | 141.8 ± 0.4 | 142.7 ± 0.4 | 142.3 ± 0.3 | 142.7 ± 0.5 |
| K+ (mEq/l) | 4.2 ± 0.1 | 4.2 ± 0.1 | 4.3 ± 0.1 | 4.3 ± 0.1 |
| Cl− (mEq/l) | 104.0 ± 0.4 | 105.1 ± 0.5** | 103.9 ± 0.5 | 105.2 ± 0.5* |
| Ca2+ (mEq/l) | 9.3 ± 0.1 | 9.3 ± 0.1 | 9.3 ± 0.1 | 9.3 ± 0.1 |
| Mg2+ (mEq/l) | 2.3 ± 0.04 | 2.4 ± 0.1 | 2.3 ± 0.04 | 2.3 ± 0.04 |
| Urinalysis | ||||
| Urine pH | 6.3 ± 0.1 | 6.1 ± 0.1* | 6.3 ± 0.1 | 6.3 ± 0.2 |
| Glucosuria | Not detected | Not detected | Not detected | Not detected |
| Proteinuria | Not detected | Not detected | Not detected | Not detected |
Where appropriate, data are presented as the mean ± standard error of the mean for the 18 subjects who received FDWG or the 18 subjects who received FDW. The FDWG subgroup did not differ significantly (p<0.05; unpaired t test) with respect to any characteristic compared with the FDW subgroup. *p<0.05 (paired t test) compared to the 0-week value. **p<0.01 (paired t test) compared to the 0-week value.
The pH of urine was significantly decreased in the FDWG subgroup after 12 weeks compared to the first day of intake. However, these changes were within the normal range. There were no abnormal findings regarding the levels of protein in urine, urinary sugar, or urobilinogen in urine.
Discussion
BP reduction is effective in preventing stroke and other vascular events, including heart failure [19–21]. The purpose of this study was to examine the effects of GABA in FDWG and its safety. In this study, 90 ml of FDWG taken daily for 12 weeks lowered SBP and DBP compared to the 0-week value in mildly hypertensive patients without affecting HR, but FDW (vinegar and dried bonito without GABA) also exhibited these effects. Several constituents of FDWG and FDW have been reported to lower BP. A positive association between vinegar and BP has been demonstrated [2–3]. It has also been reported that the ingestion of peptides from dried bonito decreases BP in spontaneously hypertensive rats [4]. Our previous study showed that only an improvement in lifestyle habits had no effect on BP reduction over 12 weeks (data not shown). The BP-lowering effect of FDW was due to the presence of vinegar and peptides in dried bonito. Our study clarified that FDWG intake tended to be slightly more effective against lowering BP than FDW intake, but the effects were not significantly different. An additional study with a large number of volunteers is needed to clarify the effects of GABA on BP in the future.
Kajimoto et al. reported the hypotensive effect of drink containing 15 g/100 ml of vinegar [3]. Kawasaki et al. reported the antihypertensive effect of vegetable drink with 500 mg/195 ml of peptide derived from sardine protein [17]. We have chosen vinegar at a concentration of 2.07 g/90 ml (15% of above report) because of 1) considering other ingredients in FDWG which would be expected to have a hypotensive effect and 2) considering taste of test drinks. For similar reason, we have chosen dried bonito at a concentration of 0.15 g/90 ml (60% of above report). The concentration of GABA in FDWG was determined 70 mg/90 ml, which would be expected to have a greater effect on BP than fermented milk containing GABA (approximately 7-fold higher than that in the above study [15]). Therefore we predicted that serum GABA concentration which had not measured in this study might be increased by FDWG intake, because Inoue et al. reported the hypotensive effect of GABA at much less dose than we used [15]. However there were no significant differences between FDWG and FDW in group-H. Inoue et al. reported the hypotensive effect of GABA in hypertensives (average of baseline SBP was 155.1 ± 2.8 mmHg) [15]. One of the causes why there were no significant differences between FDWG and FDW in group-H might be due to the average baseline of SBP in group-H in this study (136.0 ± 1.9 mmHg) which was lower than above study [15].
Kajimoto et al. reported that the effect of GABA on BP change was greater in hypertensive than in mildly hypertensive patients [13]. This report was confirmed by our results that no significant changes of BP were observed during the intake of FDWG in normotensive subjects. The BP remained below the baseline level for 4 weeks after ceasing FDWG or FDW intake. The maintenance of a decreased BP after the discontinuation of the long-term administration of antihypertensive substances such as peptide [22] and GABA [14] has been observed in humans. The present results are in agreement with those of previous studies [14, 22], which may indicate the safety of FDWG. Indeed, there was no rebound phenomenon or excessive hypotension after the discontinuation of FDWG in this study.
Regarding hematological and blood chemistry variables, the total cholesterol values had fallen from the baseline value after 12 weeks in both FDWG and FDW subgroups in all subjects, but these changes were non-significant in this small-scale study (n = 36). Blood glucose was significantly decreased after 12 weeks in the FDWG and FDW subgroups in all subjects. It has been reported that a drink containing vinegar was beneficial in reducing serum total cholesterol and blood glucose levels in humans [23, 24]. This was comparable with our results.
In this study, no marked changes were observed in any of the hematologic or clinical chemistry indexes measured. Additionally, no other side effects were demonstrated in any of the subjects. Because FDWG exhibited no effects on serum cholesterol, blood glucose, or triacylglycerol concentrations, which were seen with diuretic and β-blockers [25–28], vinegar, dried bonito and GABA might be safe for patients with hypertension accompanied by hyperlipemia or diabetes.
Study limitations
One potential limitation of this study could be the fact that the BP decreased markedly in the FDW subgroup, complicating the interpretation of the findings. The next limitation is the weak statistical power due to the small sample size, through which we could not effectively evaluate BP attenuation or GABA safety. Moreover, the average value of BP between FDWG and FDW subgroup on baseline was not equivalent because of random grouping. Each group should split into two subgroups considering the equability of average values of BP. Additionally, measuring the plasma GABA or plasma catecholamine concentrations would have possibly been a valuable addition to the study. Also, it would have been interesting to measure the growth hormone level, since its regulation by GABA supplementation remains controversial.
Conclusions
The results of the present study suggest that vinegar and dried bonito with or without GABA might exhibit a BP-lowering effect in mildly hypertensive patients.
Acknowledgments
We thank Sayaka Mito, Hiroko Simazaki, Punniyakoti T. Veeraveedu, Rajarajan A. Thandavarayan, Flori R. Sari, Wawaimuli Arozal, and Takako Sano for help with writing this manuscript. This research was supported by grants from the Yujin Memorial Grant, the Ministry of Education, Science, Sports, and Culture of Japan, and the Promotion and Mutual Aid Corporation for Private Schools of Japan.
Conflict of interest
We have no conflicts of interest.
Abbreviations
- BP
Blood pressure
- GABA
γ-Aminobutyric acid
- FDWG
Fermented drinking water containing vinegar and dried bonito with GABA
- FDW
Fermented drinking water containing vinegar and dried bonito without GABA
- HR
Heart rate
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
References
- 1.Japanese Society of Hypertension. Japanese Society of Hypertension guidelines for the management of hypertension (JSH2004) Hypertens. Res. 2006;29:S1–S105. doi: 10.1291/hypres.29.s1. [DOI] [PubMed] [Google Scholar]
- 2.Kondo S., Tayama K., Tsukamoto Y., Ikeda K., Yamori Y. Antihypertensive effects of acetic acid and vinegar on spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2001;65:2690–2694. doi: 10.1271/bbb.65.2690. [DOI] [PubMed] [Google Scholar]
- 3.Kajimoto O., Ohshima Y., Tayama K., Hirata H., Nishimura A., Tsukamoto Y. Hypotensive effects of drinks containing vinegar on high normal blood pressure and mild hypertensive subjects. Journal of Nutritional Food. 2003;6:51–68. [Google Scholar]
- 4.Fujita H., Yoshikawa M. LKPNM: a prodrug-type ACE-inhibitory peptide derived from fish protein. Immunopharmacology. 1999;44:123–127. doi: 10.1016/s0162-3109(99)00118-6. [DOI] [PubMed] [Google Scholar]
- 5.Tsushida T., Murai T. Conversion of glutamic Acid to γ-aminobutyric acid in tea leaves under anaerobic conditions. Agric. Biol. Chem. 1987;51:2865–2871. [Google Scholar]
- 6.Streeter J.G., Thompson J.F. In vivo and in vitro studies on γ-aminobutyric acid metabolism with the radish plant (Paphanus stivus L.) Plant Physiol. 1972;49:579–584. doi: 10.1104/pp.49.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leitch B., Laurent G. GABAergic synapses in the antennal lobe and mushroom body of the locust olfactory system. J. Comp. Neurol. 1996;372:487–514. doi: 10.1002/(SICI)1096-9861(19960902)372:4<487::AID-CNE1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Curtis D.R., Johnston G.A. Amino acid transmitters in the mammalian centrial nervous system. Ergeb. Physiol. 1974;69:97–188. doi: 10.1007/3-540-06498-2_3. [DOI] [PubMed] [Google Scholar]
- 9.Hardt J., Larsson L.I., Hougaard D.M. Immunocytochemical evidence suggesting that diamine oxidase catalyzes biosynthesis of γ-aminobutyric acid in antropyloric gastrin cells. J. Histochem. Cytochem. 2000;48:839–846. doi: 10.1177/002215540004800612. [DOI] [PubMed] [Google Scholar]
- 10.Hayakawa K., Kimura M., Kamata K. Mechanism underlying γ-aminobutyric acid-induced antihypertensive effect in spontaneously hypertensive rats. Eur. J. Pharmacol. 2002;438:107–113. doi: 10.1016/s0014-2999(02)01294-3. [DOI] [PubMed] [Google Scholar]
- 11.Kimura M., Hayakawa K., Sansawa H. Involvement of γ-aminobutyric acid (GABA) B receptors in the hypotensive effect of systemically administered GABA in spontaneously hypertensive rats. Jpn. J. Pharmacol. 2002;89:388–394. doi: 10.1254/jjp.89.388. [DOI] [PubMed] [Google Scholar]
- 12.Hayakawa K., Kimura M., Yamori Y. Role of the renal nerves in gamma-aminobutyric acid-induced antihypertensive effect in spontaneously hypertensive rats. Eur. J. Pharmacol. 2005;52:120–125. doi: 10.1016/j.ejphar.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Kajimoto O., Hirata H., Nishimura A. Hypotensive action of novel fermented milk containing γ-aminobutyric acid (GABA) in subjects with mild or moderate hypertension. Journal of Nutritional Food. 2003;6:51–64. [Google Scholar]
- 14.Matsubara F., Ueno H., Kentaro T., Tadano K., Suyama T., Imaizumi K., Suzuki T., Magata K., Kikuchi N. Effects of GABA supplementation on blood pressure and safety in adults with mild hypertension. Jpn. Pharmacol. Ther. 2002;30:963–972. [Google Scholar]
- 15.Inoue K., Shirai T., Ochiai H., Kasao M., Hayakawa K., Kimura M., Sansawa H. Blood-pressure-lowering effect of a novel fermented milk containing gamma-aminobutyric acid (GABA) in mild hypertensives. Eur. J. Clin. Nutr. 2003;57:490–495. doi: 10.1038/sj.ejcn.1601555. [DOI] [PubMed] [Google Scholar]
- 16.Higuchi T., Hayashi H., Abe K. Exchange of glutamate and gamma-aminobutyrate in a Lactobacillus strain. J. Bacteriol. 1997;179:3362–3364. doi: 10.1128/jb.179.10.3362-3364.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawasaki T., Jun C.J., Fukushima Y., Kegai K., Seki E., Osajima K., Itoh K., Matsui T., Matsumoto K. Antihypertensive effect and safety evaluation of vegetable drink with peptides derived from sardine protein hydrolysates on mild hypertensive, high-normal and normal blood pressure subjects. Fukuoka Igaku Zasshi. 2002;93:208–218. [PubMed] [Google Scholar]
- 18.Lewis R.J., Bessen H.A. Statistical concepts and methods for the reader of clinical studies in emergency medicine. J. Emerg. Med. 1991;9:221–232. doi: 10.1016/0736-4679(91)90417-e. [DOI] [PubMed] [Google Scholar]
- 19.Lawes C.M., Bennett D.A., Feigin V.L., Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke. 2004;35:1024–1033. [PubMed] [Google Scholar]
- 20.Lalande S., Johnson B.D. Diastolic dysfunction: a link between hypertension and heart failure. Drugs Today (Barc) 2008;44:503–513. doi: 10.1358/dot.2008.44.7.1221662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papadopoulos D.P., Papademetriou V. Aggressive blood pressure control and stroke prevention: role of calcium channel blockers. J. Hypertens. 2008;26:844–852. doi: 10.1097/HJH.0b013e3282f4d1d5. [DOI] [PubMed] [Google Scholar]
- 22.Hata Y., Yamamoto M., Ohni M., Nakajima K., Nakamura Y., Takano T. A placebo-controlled study of the effect of sour milk on blood pressure in hypertensive subjects. Am. J. Clin. Nutr. 1996;94:767–771. doi: 10.1093/ajcn/64.5.767. [DOI] [PubMed] [Google Scholar]
- 23.Fushimi T., Oshima Y., Kishi M., Nishimura A., Kajimoto O., Tsukamoto Y. Effects of a drink containing vinegar on serum total cholesterol and assessment of its safety. Journal of Nutritional Food. 2005;8:13–26. [Google Scholar]
- 24.Hlebowicz J., Darwiche G., Björgell O., Almér L.O. Effect of apple cider vinegar on delayed gastric emptying in patients with type diabetes mellitus: a pilot study. BMC Gastroenterol. 2007;7:46. doi: 10.1186/1471-230X-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnesen E., Thelle D.S., Førde O.H., Mjøs O.D. Serum lipids and glucose concentrations in subjects using antihypertensive drugs: Finnmark 1977. J. Epidemiol. Community Health. 1983;37:141–144. doi: 10.1136/jech.37.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hollenberg N.K. Management of hypertension: considerations involving cardiovascular risk reduction. J. Cardiovasc. Pharmacol. 1990;15:S73–S78. [PubMed] [Google Scholar]
- 27.Ames R.P. Negative effects of diuretic drugs on metabolic risk factors for coronary heart disease: possible alternative drug therapies. Am. J. Cardiol. 1983;51:632–638. doi: 10.1016/s0002-9149(83)80200-8. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura H. Effects of antihypertensive drugs on plasma lipids. Am. J. Cardiol. 1987;60:E24–E28. doi: 10.1016/0002-9149(87)90538-8. [DOI] [PubMed] [Google Scholar]