Abstract
Objective
Fast low angle shot (FLASH) and double echo steady state (DESS) MRI sequences were recently cross-calibrated for quantification of cartilage morphology at 3 Tesla. In this pilot study for the Osteoarthritis Initiative we compare their test-retest precision and sensitivity to longitudinal change.
Method
9 participants with mild to moderate clinical OA were imaged at baseline, year 1 and year 2. Coronal 1.5mm FLASH and sagittal 0.7mm DESS sequences were acquired; 1.5mm coronal multiplanar reformats (MPR) were obtained from the DESS. Patellar, femoral and tibial cartilage plates were quantified in paired fashion, with blinding to time point.
Results
In the weight-bearing femorotibial joint, average precision errors across plates were 1.8% for FLASH, 2.6% for DESS, and 3.0% for MPR-DESS. Volume loss at year 1 was not significant; at year 2 the average change across the femorotibial cartilage plates was −1.7% for FLASH, −2.8% for DESS, and −0.3% for MPR-DESS. Volume change in the lateral tibia (−5.5%; p<0.03), and in the medial (−2.9%; p<0.04) and lateral femorotibial compartment (−3.8%; p<0.03) were significant for DESS.
Conclusion
FLASH, MPR-DESS and DESS all displayed adequate test-retest precision. Although the comparison between protocols is limited by the small number of participants and by the relatively small longitudinal change in cartilage morphology in this pilot study, the data suggest that significant change can be detected with MRI in a small sample of OA subjects over 2 years.
Keywords: Cartilage, Magnetic Resonance Imaging, Osteoarthritis, Cartilage, Biomarker
Magnetic resonance imaging (MRI) of knee articular cartilage provides valuable information on the status and progression of structural changes of articular tissues in osteoarthritis (OA) and shows particular promise for evaluating the efficacy of disease modifying OA drugs (DMOADs) 1–6. Because changes in cartilage morphology over time are relatively small in OA, high resolution MR acquisitions and quantitative image analysis technologies have been applied, in order to accurately quantify subtle changes throughout joint cartilages over relatively short periods 5. These studies have reported changes of 0% to 7% per annum in various cohorts 5,7–10. The Osteoarthritis Initiative (OAI), a program jointly sponsored by the National Institute of Health (NIH), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), and the pharmaceutical industry, is targeted at identifying reproducible and sensitive biomarkers for identifying incident and progressive knee OA, including quantitative measurement of cartilage morphology and composition. Whereas various MR sequences are acquired in the OAI to monitor cartilage changes with time, it is currently unclear which of these sequences is best suited for this purpose.
Previous work using fat-suppressed or water-excited (we) spoiled gradient-recalled echo (SPGR) or fast low angle shot (FLASH) sequences has shown that cartilage morphology can be accurately measured at 1.5 Tesla (T) 5, and that using newer 3T magnets the test-retest reproducibility errors can be reduced 11. However, SPGR/FLASH has a number of limitations, including the relatively low contrast between the cartilage surface and adjacent tissues and the limited spatial resolution (1.0 to 1.5 mm slice thickness) that can be achieved at reasonable contrast-to-noise ratios and acquisition times 5. Double echo steady state with water excitation (DESSwe) 12 may potentially overcome these limitations, because of the higher fluid-to-cartilage contrast 13 and the lower partial volume effects that can be achieved with thinner slices at near-isotropic resolution.
A previous pilot study for the OAI demonstrated that sagittal double echo steady state (sagDESS) imaging and coronal multiplanar reconstructions of sagDESS (corMPR-DESS) produce results consistent with those of corFLASH at 3 Tesla 14, and that the test-retest precision (reproducibility) was similar between these sequences, when a non-paired analyses were performed 14. This study design reflected conditions of cross-sectional, but not those of longitudinal studies, where data sets are usually processed in pairs. The objective of the current pilot study for the OAI was therefore to compare the test-retest precision of the above MR sequences (sagDESS, corMPR-DESS, and corFLASH) when the data are processed in paired fashion, and to examine their sensitivity of cartilage morphometry to change over 1 and 2 years, respectively.
METHODS
Study participants and MR imaging
Nine subjects with mild to moderate knee OA (4 men, 5 women, age 52.2±9.3 years, body mass index 33.9±5.2 kg/m2) participated in the study. 8 participants were examined twice at baseline (BL), year 1 (Y1) and year 2 (Y2); one participant was examined at baseline and Y2 only. Six of these subjects were enrolled in the OAI and underwent radiography 15 during their screening visit: 2 were Kellgren Lawrence grade 16 1, 3 grade 2, and 1 grade 3. All participants suffered from knee pain, aching or stiffness on the majority of days within one 1 of the last 12 months or had a clinical diagnosis of knee OA.
Test-retest acquisitions were performed at all time points, with subjects walking for 10 min between repeat exams. Images were acquired at two sites using 3T MR systems (Siemens Magnetom Trio, Erlangen, Germany) and quadrature transmit-receive knee coils (USA Instruments, Aurora, OH). A sagittal DESS (0.7mm slice thickness, in-plane resolution = 0.37mm × 0.46mm interpolated to 0.37mm × 0.37mm, acquisition time = 10 min 23 sec) and a double oblique coronal FLASH (1.5mm slice thickness, in-plane resolution = 0.31mm × 0.31mm, acquisition time = 8 min 30 sec.) were acquired during each exam, both using water excitation 14. Other acquisition parameters for the corFLASHwe were: 20ms repletion time (TR), 7.6ms echo time (TE), 12° flip angle (FA), 80 slices, 160mm field of view (FOV), and those for the sagittal DESS 16.3ms TR, 4.7ms TE, 25° FA, 160 slices, 140mm FOV 14. Double oblique coronal multiplanar reformats (corMPR-DESS) with 1.5 mm slice thickness were obtained from the sagDESS. The other imaging parameters and the acquisition procedure have been described in detail previously 14. The study protocol, amendments, and informed consent documentation were reviewed and approved by the local institutional review boards 14.
The images were anonymized and the image analysis center blinded to acquisition date, but not to subject identification. Two experienced readers with formal training in cartilage analysis (M.K., M.S.) manually segmented the Y1 versus BL images (quadruples), and one reader (M.K.) the Y2 versus BL images (pairs). Y1 and Y2 analysis were performed separately, because the Y1 and BL data were delivered prior to acquisition of the Y2 data.
The following cartilage plates were analyzed in all images: medial tibia (MT), central (weight bearing) medial femur (cMF), lateral tibia (LT), and central lateral femur (cLF) 14,17. In the sagDESS sequence, the patella (P) and posterior femoral condyles (pMF and pLF) were analyzed in addition. Segmentation involved manual tracing the total area of subchondral bone (tAB) and the area of the cartilage surface (AC) using proprietary software (Chondrometrics GmbH, Ainring, Germany) 11,14. The cartilage volume (VC) and the mean cartilage thickness averaged over the cartilaginous part of the subchondral bone area (ThCcAB) were then determined in addition to tAB and AC. Abbreviations of anatomical regions and measurement variables used in this article are defined in the nomenclature proposal for quantitative cartilage imaging 17. For cartilage volume (VC) and thickness (ThCcAB), aggregate values of MT/cMF and of LT/cLF were computed for the medial weight-bearing femorotibial (MFTC) and lateral femorotibial (LFTC) compartment, respectively 9.
Precision errors were determined by computing the root-mean-square (RMS) coefficient of variation (CV%) 18 for the test-retest BL (8x2) and Y1 measurements (8x2), and for all paired measurements (16x2). The change over 1 year was evaluated by subtracting the mean of the two Y1 measurements from the mean of the BL measurements [(Y1–BL)/BL*100]. The mean and standard deviation (SD) of the individual percent changes was reported, and systematic differences were tested for statistical significance using a paired t-test. The change over 2 years was evaluated by comparing the Y2 with the BL measurements in all 9 subjects [(Y2–BL)/BL*100], one BL and Y2 acquisition being analyzed in each subject.
RESULTS
When the RMS CV% values were averaged across the four plates of the weight-bearing femorotibial compartment (Table 1), the precision errors (paired analysis) for cartilage volume (VC) measurements were 1.8% for corFLASH, 2.6% for sagDESS, and 3.0% for corMPR-DESS. No obvious differences were apparent between precision errors at BL and at Y1. Paired precision errors for VC in the other cartilage plates (sagDESS) were 4.8% for the patella (P), 3.3% for the posterior medial femoral condyle (pMF), and 4.8% for the lateral posterior femoral condyle (pLF). Paired precision errors for cartilage thickness (ThCcAB) were similar to those for cartilage volume (VC). Precision errors for surface areas (AC and tAB) were generally below 1.7%, except for the lateral tibia (LT) with corMPR-DESS (2.7% and 2.5%, respectively).
Table 1.
Test-retest precision for cororonal FLASH, coronal MPR-DESS (MPR), and sagittal DESS (DESS) analyzed in a paired manner with blinding to time point in 8 participants; RMS CV% for all 4 acquisitions, for paired baseline acquisitions, and for paired year 1 (Y1) follow up acquisitions.
| CV% all | CV% baseline | CV% follow up (Y1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| FLASH | MPR | DESS | FLASH | MPR | DESS | FLASH | MPR | DESS | |
| Cartilage volume (VC) | |||||||||
| MT | 1.8% | 3.5% | 2.0% | 1.9% | 3.7% | 2.2% | 1.7% | 3.3% | 1.7% |
| cMF | 2.2% | 2.5% | 2.9% | 1.8% | 2.7% | 2.3% | 2.6% | 2.3% | 3.4% |
| MFTC | 1.5% | 2.9% | 1.7% | 1.1% | 3.2% | 1.6% | 1.9% | 2.4% | 1.7% |
| LT | 1.1% | 3.0% | 2.6% | 1.2% | 3.9% | 2.4% | 1.1% | 1.8% | 2.7% |
| cLF | 2.2% | 3.1% | 3.0% | 2.2% | 3.2% | 2.6% | 2.2% | 3.0% | 3.4% |
| LFTC | 0.9% | 2.0% | 2.3% | 0.9% | 2.2% | 1.8% | 0.9% | 1.8% | 2.6% |
| P | 4.8% | 2.7% | 6.2% | ||||||
| pMF | 3.3% | 2.3% | 4.0% | ||||||
| pLF | 4.8% | 3.0% | 6.0% | ||||||
|
| |||||||||
| Mean cartilage thickness without denuded areas (ThCcAB.Me) | |||||||||
| MT | 1.6% | 2.5% | 1.9% | 1.1% | 2.4% | 2.4% | 2.0% | 2.6% | 3.4% |
| cMF | 2.3% | 1.9% | 2,5% | 2.1% | 1.9% | 1.8% | 2.5% | 1.9% | 3.0% |
| MFTC | 1.3% | 1.7% | 1.4% | 1.5% | 1.9% | 1.4% | 1.3% | 1.5% | 1.5% |
| LT | 1.1% | 1.9% | 1.8% | 1.3% | 2.4% | 1.6% | 1.0% | 1.0% | 2.0% |
| cLF | 1.9% | 2.5% | 2.8% | 1.9% | 2.4% | 2.9% | 1.9% | 2.6% | 2.8% |
| LFTC | 1.0% | 1.4% | 1.5% | 1.2% | 1.4% | 1.1% | 0.9% | 1.3% | 1.9% |
| P | 3.6% | 2.7% | 4.4% | ||||||
| pMF | 2.8% | 1.8% | 3.6% | ||||||
| pLF | 3.5% | 1.5% | 4.8% | ||||||
|
| |||||||||
| Area of cartilage surface (AC) | |||||||||
| MT | 1.5% | 1.4% | 1.5% | 1.7% | 1.7% | 1.o% | 1.4% | 0.8% | 1.9% |
| cMF | 0.9% | 1.2% | 2.2% | 0.9% | 1.3% | 2.1% | 1.0% | 1.1% | 2.3% |
| LT | 0.9% | 2.7% | 3.1% | 0.9% | 3.7% | 2.8% | 0.8% | 0.7% | 3.3% |
| cLF | 1.0% | 1.1% | 1.7% | 1.0% | 1.3% | 1.8% | 1.0% | 0.8% | 1.6% |
| P | 2.3% | 1.7% | 2.8% | ||||||
| pMF | 1.6% | 1.6% | 1.6% | ||||||
| pLF | 2.8% | 3.0% | 2.7% | ||||||
|
| |||||||||
| Total area of subchondral bone (tAB) | |||||||||
| MT | 1.2% | 1.4% | 1.2% | 1.0% | 1.6% | 0.7% | 1.4% | 1.2% | 1.6% |
| cMF | 1.0% | 1.0% | 2.0% | 1.0% | 1.2% | 1.9% | 1.1% | 0.9% | 2.1% |
| LT | 1.1% | 2.5% | 2.5% | 1.2% | 3.5% | 2.3% | 1.0% | 0.9% | 2.7% |
| cLF | 0.8% | 1.1% | 1.5% | 0.9% | 1.3% | 1.5% | 0.8% | 0.7% | 1.6% |
| P | 1.6% | 1.1% | 1.9% | ||||||
| pMF | 1.4% | 1.1% | 1.7% | ||||||
| pLF | 2.2% | 2.4% | 2.1% | ||||||
for abbreviations see Table 2.
No significant cartilage loss was observed over the first year (Table 2). At Y2, the average loss of cartilage volume (VC) across the 4 weight-bearing femorotibial plates was −1.7% for corFLASH, −2.8% for sagDESS, and −0.3% for corMPR-DESS. The change in VC in the lateral tibia (−5.5±6.4%; p=0.03), the medial femorotibial compartments (−2.9±3.8%; p=0.04), and the lateral femortibial compartments (−3.8±4.1%; p=0.03) was significant for sagDESS. The reduction in cartilage thickness (ThCcAB) was significant in the medial femorotibial compartment (MFTC) and in the lateral tibia (LT), and that of the area of the cartilage surface (AC) in the patella (sagDESS). No significant changes were observed over two years with other sequences or parameters (Table 2).
Table 2.
Longitudinal change (mean of individual % change values, standard deviation [SD] of individual % values, and significance level of change [paired t-test]) in cartilage morphology over 1 year (Y1) and 2 years (Y2), respectively, for coronal FLASH, corMPR-DESS (MPR), and sagittal DESS (DESS); paired image analysis with blinding to time point.
| Year 1 change (n = 8) | Year 2 change (n = 9) | ||||||
|---|---|---|---|---|---|---|---|
| % | SD | p-value | % | SD | p-value | ||
| Cartilage volume (VC) | |||||||
| MT | FLASH | +0.6% | 4.4% | 0.96 | −2.3% | 3.9% | 0.14 |
| MPR | −2.2% | 2.8% | 0.09 | −0.7% | 3.4% | 0.38 | |
| DESS | +0.1 | 4.0% | 0.90 | −2.8% | 5.7% | 0.11 | |
| cMF | FLASH | −0.2% | 3.7% | 0.97 | −1.6% | 5.6% | 0.54 |
| MPR | −0.9% | 3.4% | 0.50 | +0.6% | 4.6% | 0.88 | |
| DESS | −2.1% | 6.8% | 0.33 | −2.5% | 4.8% | 0.18 | |
| MFTC | FLASH | +0.4% | 3.6% | 0.98 | −1.9% | 4.1% | 0.17 |
| MPR | −1.8% | 2.8% | 0.14 | −0.2% | 3.4% | 0.64 | |
| DESS | −0.7% | 4.5% | 0.63 | −2.9% | 3.8% | 0.04 | |
| LT | FLASH | −0.7% | 5.5% | 0.71 | −3.4% | 5.0% | 0.08 |
| MPR | −1.4% | 4.1% | 0.21 | −2.0% | 4.4% | 0.15 | |
| DESS | −4.0% | 9.1% | 0.24 | −5.5% | 6.4% | 0.03 | |
| cLF | FLASH | +1.4% | 2.7% | 0.26 | +0.4% | 8.6% | 0.99 |
| MPR | −0.1% | 3.2% | 0.75 | +1.0% | 5.6% | 0.53 | |
| DESS | −0.1% | 5.9% | 0.76 | −0.5% | 4.0% | 0.61 | |
| LFTC | FLASH | +0.1% | 3.6% | 0.99 | −2.0% | 5.1% | 0.21 |
| MPR | −0.9% | 2.9% | 0.25 | −0.9% | 4.0% | 0.37 | |
| DESS | −2.6% | 5.8% | 0.24 | −3.8% | 4.1% | 0.03 | |
| P | DESS | −10% | 20.6% | 0.17 | −3.4% | 5.9% | 0.16 |
| pMF | DESS | +1.3% | 7.0% | 0.74 | −0.9% | 5.2% | 0.81 |
| pLF | DESS | −0.8% | 8.25% | 0.74 | −4.0% | 8.4% | 0.14 |
| Mean cartilage thickness without denuded areas (ThCcAB.Me) | |||||||
| MT | FLASH | +1.3% | 4.2% | 0.46 | −1.5% | 3.4% | 0.19 |
| MPR | −1.9% | 2.6% | 0.09 | −0.9% | 3.0% | 0.43 | |
| DESS | −0.4 | 3.4% | 0.72 | −2.6% | 4.2% | 0.07 | |
| cMF | FLASH | −0.5% | 3.1% | 0.80 | −1.2% | 5.1% | 0.53 |
| MPR | −1.9% | 2.6% | 0.09 | +0.6% | 2.9% | 0.79 | |
| DESS | −1.4% | 4.0% | 0.28 | −1.6% | 3.8% | 0.18 | |
| MFTC | FLASH | +0.5% | 3.1% | 0.72 | −1.3% | 3.5% | 0.24 |
| MPR | −1.3% | 2.7% | 0.21 | −0.2% | 2.0% | 0.76 | |
| DESS | −0.9% | 3.2% | 0.37 | −2.3% | 2.5% | 0.02 | |
| LT | FLASH | −0.9% | 5.1% | 0.60 | −2.5% | 3.7% | 0.06 |
| MPR | −1.8% | 3.9% | 0.21 | −2.2% | 3.0% | 0.06 | |
| DESS | −2.7% | 5.2% | 0.19 | −3.8% | 4.5% | 0.03 | |
| cLF | FLASH | +1.2% | 2.2% | 0.15 | −1.2% | 9.7% | 0.58 |
| MPR | −0.3% | 2.5% | 0.66 | +0.3% | 4.7% | 0.86 | |
| DESS | −1.6% | 4.4% | 0.28 | −0.9% | 4.0% | 0.53 | |
| LFTC | FLASH | ±0.0% | 2.6% | 0.96 | −2.0% | 4.8% | 0.21 |
| MPR | −1.1% | 2.2% | 0.21 | −0.9% | 2.7% | 0.27 | |
| DESS | −2.0% | 2.7% | 0.10 | −2.4% | 3.8% | 0.09 | |
| P | DESS | −2.1% | 10.7% | 0.52 | −0.8% | 6.6% | 0.65 |
| pMF | DESS | −0.1% | 6.8% | 0.95 | −0.9% | 5.4% | 0.67 |
| pLF | DESS | −2.1% | 5.5% | 0.32 | −3.2% | 5.8% | 0.12 |
|
| |||||||
| Area of cartilage surface (AC) | |||||||
| MT | FLASH | −0.4% | 1.7% | 0.47 | −0.9% | 2.4% | 0.30 |
| MPR | +0.0% | 1.6% | 0.93 | +0.8% | 2.7% | 0.56 | |
| DESS | +0.1% | 4.0% | 0.90 | −0.5% | 2.7% | 0.50 | |
| cMF | FLASH | +0.3% | 1.8% | 0.64 | −0.1% | 4.1% | 0.96 |
| MPR | +0.1% | 1.5% | 0.87 | −0.1% | 4.4% | 0.98 | |
| DESS | −0.2% | 7.7% | 0.91 | −1.1% | 2.6% | 0.30 | |
| LT | FLASH | +0.0% | 1.3% | 0.97 | −1.3% | 4.5% | 0.39 |
| MPR | −0.3% | 2.4% | 0.86 | −0.5% | 3.2% | 0.58 | |
| DESS | −1.5% | 5.8% | 0.42 | −2.4% | 4.0% | 0.11 | |
| cLF | FLASH | +0.1% | 1.4% | 1.00 | +1.5% | 4.1% | 0.28 |
| MPR | −0.1% | 1.3% | 0.81 | +0.7% | 2.6% | 0.40 | |
| DESS | +1.5% | 3.8% | 0.25 | −0.6% | 5.0% | 0.69 | |
| P | DESS | −8.5% | 17.4% | 0.23 | −2.9% | 2.9% | 0.03 |
| pMF | DESS | +1,5% | 3.4% | 0.29 | −0.0% | 1.5% | 0.92 |
| pLF | DESS | +1.3% | 4.6% | 0.44 | −1.4% | 4.6% | 0.29 |
MT = medial tibia, cMF = central (weight-bearing) portion of the medial femoral condyle, MFTC = aggregate values in the medial femorotibial compartment; LT = lateral tibia, cLF = central (weight-bearing) portion of the lateral femoral condyle, LFTC = aggregate values in the lateral femorotibial compartment; P = patella, pMF = posterior aspect of the medial femoral condyle; pLF = posterior aspect of the lateral femoral condyle 14,17
DISCUSSION
This pilot study for the Osteoarthritis Initiative (OAI) extends previous work on cross-validating DESS image contrast for quantitative analysis of cartilage morphology 14. The DESS sequence was compared with SPGR/FLASH, because the latter represents the currently accepted and validated standard for quantitative MRI of cartilage morphology 5,6,19.
A limitation of this study clearly is the small sample size. However, this is the first study to apply a paired analysis design at two time points (quadruple images) to the analysis of precision errors of cartilage morphometry, the first to compare DESS and FLASH in a longitudinal study, and the first to compare change in aggregate values of cartilage morphology in the medial (MFTC) and lateral (LFCT) femorotibial compartment to single femorotibial cartilage plates longitudinally. Although this pilot study only involved few OA patients, the OAI is acquiring longitudinal data on more than 4500 subjects over 5 years, and these data should become available for analysis in the near future.
Previous findings 14 indicate that corFLASH, sagDESS, and corMPR-DESS display similar precision in the analysis of cartilage morphology, when being read in an unpaired, completely blinded manner. These precision errors examine the differences in image contrast 14, but are not directly applicable for use in longitudinal studies where baseline and follow-up images are usually processed as pairs. Other studies investigating test-retest-precision have been confined to one time point only 14, but this approach may introduce bias towards conformance of segmentation in repeat data sets. The advantage of the current design, in which quadruples were processed (with blinding to BL and Y1 time points) is that the readers were aware that change may have occurred between acquisitions, so that this bias was minimized. In contrast with our previous unpaired analysis 14 results, the paired analysis precision errors of the sagDESS, and in particular that of the corMPR-DESS were generally higher, than those with the corFLASH. Precision errors of the corFLASH were at lower end of those reported in the literature 5.
The changes seen at Y1 and Y2 were small, given that annual rates of cartilage volume loss of up to 7% have been reported in the literature 5,7–10. This may be due to the patients having relatively mild OA. Interestingly, the sagDESS tended to display higher sensitivity to change (ratio of % loss to its SD) than corFLASH, and corFLASH higher sensitivity than corMPR-DESS. Given that the image contrast of corMPR-DESS and sagDESS is similar and that previous studies have revealed lower precision errors of coronal FLASH in the weight-bearing femorotibial joint compared with sagittal FLASH 5,20,21, we assume that the potentially higher sensitivity of sagDESS compared to cor FLASH and corMPR-DESS is most likely due to the higher spatial resolution (lower slice thickness) rather than to the different image orientation and contrast. While exciting, these initial results are based on a small data set and must be confirmed in a larger cohort. Also, the potential advantages of increased spatial resolution are often offset by increases in both image acquisition time and, in particular, the segmentation time, which doubles if the number of slices doubles.
At this stage, the technique applied here is not intended for use in diagnosing osteoarthritis in a single patient, in particular because the predictive value of imaging outcomes for clinical endpoints (e.g. indicating for total knee arthroplasty) has so far only been reported in one relatively small study 22. Establishing the relationship between imaging endpoints (such as changes in cartilage morphology) and clinical endpoints, however, is one of the goals of the OA Initiative. The technique presented here has particularly high potential for the evaluation of DMOADs in clinical trials. At this point, however, it is still unclear how much of a change in cartilage morphology (and how much of a modulation of this change by a DMOAD) is clinically significant.
While the focus of this work was on the comparison of FLASH and DESS for the analysis of cartilage morphology, other MRI-based methods for rating changes in OA have also been described: Semi-quantitative scoring of conventional proton density-, T1-, and T2-weighted MR images has been used to rate alterations of cartilage and other articular tissues 23, but their responsiveness to changes has been reported to be relatively low 24. While conventional proton density-, T1-, and T2-weighted MR images are acquired in the OAI, these cannot be used to quantify changes of cartilage morphology, because of the lower spatial resolution and the presence of susceptibility artifact at the bone cartilage interface 4,5. For the purpose of analysis of cartilage composition (specifically collage content, collagen structure and hydration), T2 mapping has been included as part of the OAI acquisition protocol 25, but no longitudinal changes in OA have so far been reported. Compositional techniques for analysis of cartilage proteoglycan content, such as dGEMRIC and T1rho have also been developed 6,26, but have not been included in the OAI acquisition protocol.
One of the most difficult tasks in the segmentation process of cartilage morphology is to accurately identify the contact zone between femorotibial cartilage, since the contrast is often very low where the femorotibial cartilage plates are in direct contact. The variation introduced by this difficulty can be reduced by additionally analyzing aggregate measures of cartilage volume (VC) and thickness (ThC) in the medial (MFTC) and lateral femorotibial compartment (LFTC) 9. Our results indicate that morphometric measurements in MFTC and LFTC not only tend to be more reproducible, but also more sensitive to change than individual femorotibial plates (MT, cMF, LT, cLF). Although the comparison between protocols is limited by the relatively small longitudinal change in cartilage morphology in this pilot study and the small number of participants, the data suggest that significant change can be detected with MRI in a small sample of OA subjects over 2 years. The results on longitudinal change should be confirmed in a larger sample, and the OAI will provide the opportunity to do so in the future.
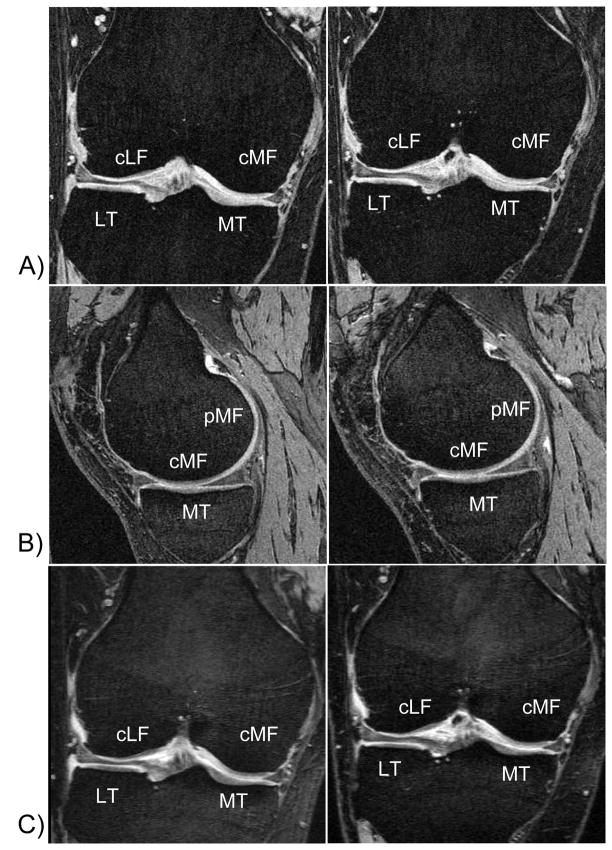
Figure 1.
MR images showing a baseline (left column) and year 2 acquisitions (right column) in one of the patients studied. The images highlight the challenge in delineating the articular surfaces of the tibial and femoral cartilages in the femoro-tibial contact areas:
A) coronal double oblique FLASH with water excitation (1.5mm slice thickness),
B) sagittal DESS with water excitation (0.7mm slice thickness through the medial femorotibial compartment),
C) coronal double oblique multiplanar reconstruction of the sagittal DESS (1.5mm slice thickness).
MT = medial tibia, LT = lateral tibia, cMF = central (weight-bearing) portion of the medial femoral condyle; pMF = posterior portion of the medial femoral condyle; cLF = central (weight-bearing) portion of the lateral femoral condyle; pMF = posterior portion of the medial femoral condyle
Acknowledgments
Funding Source: National Institute of Health (NIH) National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)
The Osteoarthritis Initiative (OAI) and this pilot study are conducted by the National Institute of Arthritis and Musculoskeletal and Skin Diseases in collaboration with the OAI Investigators and Consultants. This manuscript has been reviewed by the OAI Publications committee for scientific content and data interpretation. The research reported in this article was supported in part by contracts N01-AR-2-2261, N01-AR-2-2262 and N01-AR-2-2258 from NIAMS.
We are grateful to the Ohio State University team, particularly Kim Toussant, and to the Center for Primary Care and Prevention team at Memorial Hospital of Rhode Island for recruitment of the study subjects, and to Larry Martin RTR(MR) and Lynn Fanella RTR(MR) for acquiring the MR images.
Footnotes
Competing interest statement: None of the authors has a competing interest with regard to publication of the study, because no organisation may gain or lose financially from the results of conclusions published here. Felix Eckstein works as a consultant for Pfizer Inc., GlaxoSmithKline, and Virtualscopics Inc. He is CEO of Chondrometrics GmbH, a company providing MR image analysis services. Martin Hudelmaier has a part time appointment with Chondrometrics GmbH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peterfy CG. Imaging of the disease process. Curr Opin Rheumatol. 2002;14:590–6. doi: 10.1097/00002281-200209000-00020. [DOI] [PubMed] [Google Scholar]
- 2.Peterfy CG. Role of MR imaging in clinical research studies. Semin Musculoskelet Radiol. 2001;5:365–78. doi: 10.1055/s-2001-19045. [DOI] [PubMed] [Google Scholar]
- 3.Gray ML, Eckstein F, Peterfy C, Dahlberg L, Kim YJ, Sorensen AG. Toward imaging biomarkers for osteoarthritis. Clin Orthop. 2004:S175–S181. doi: 10.1097/01.blo.0000144972.50849.d9. [DOI] [PubMed] [Google Scholar]
- 4.Peterfy CG, Gold G, Eckstein F, Cicuttini F, Dardzinski B, Stevens R. MRI protocols for whole-organ assessment of the knee in osteoarthritis. Osteoarthritis Cartilage. 2006;14(Suppl 1):95–111. doi: 10.1016/j.joca.2006.02.029. Epub;%2006 Jun 5.:95–111. [DOI] [PubMed] [Google Scholar]
- 5.Eckstein F, Cicuttini F, Raynauld JP, Waterton JC, Peterfy C. Magnetic resonance imaging (MRI) of articular cartilage in knee osteoarthritis (OA): morphological assessment . Osteoarthritis Cartilage. 2006;14(Suppl 1):46–75. doi: 10.1016/j.joca.2006.02.026. Epub;%2006 May;%19.:46–75. [DOI] [PubMed] [Google Scholar]
- 6.Eckstein F, Burstein D, Link TM. Quantitative MRI of cartilage and bone: degenerative changes in osteoarthritis. NMR Biomed. 2006;19:822–54. doi: 10.1002/nbm.1063. [DOI] [PubMed] [Google Scholar]
- 7.Wluka AE, Stuckey S, Snaddon J, Cicuttini FM. The determinants of change in tibial cartilage volume in osteoarthritic knees. Arthritis Rheum. 2002;46:2065–72. doi: 10.1002/art.10460. [DOI] [PubMed] [Google Scholar]
- 8.Cicuttini FM, Wluka AE, Wang Y, Stuckey SL. Longitudinal study of changes in tibial and femoral cartilage in knee osteoarthritis. Arthritis Rheum. 2004;50:94–7. doi: 10.1002/art.11483. [DOI] [PubMed] [Google Scholar]
- 9.Raynauld JP, Martel-Pelletier J, Berthiaume MJ, Labonte F, Beaudoin G, de Guise JA, et al. Quantitative magnetic resonance imaging evaluation of knee osteoarthritis progression over two years and correlation with clinical symptoms and radiologic changes. Arthritis Rheum. 2004;50:476–87. doi: 10.1002/art.20000. [DOI] [PubMed] [Google Scholar]
- 10.Raynauld JP, Martel-Pelletier J, Berthiaume MJ, Beaudoin G, Choquette D, Haraoui B, et al. Long term evaluation of disease progression through the quantitative magnetic resonance imaging of symptomatic knee osteoarthritis patients: correlation with clinical symptoms and radiographic changes. Arthritis Res Ther. 2006;8:R21. doi: 10.1186/ar1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckstein F, Charles HC, Buck RJ, Kraus VB, Remmers AE, Hudelmaier M, et al. Accuracy and precision of quantitative assessment of cartilage morphology by magnetic resonance imaging at 3.0T. Arthritis Rheum. 2005;52:3132–6. doi: 10.1002/art.21348. [DOI] [PubMed] [Google Scholar]
- 12.Hardy PA, Recht MP, Piraino D, Thomasson D. Optimization of a dual echo in the steady state (DESS) free-precession sequence for imaging cartilage. J Magn Reson Imaging. 1996;6:329–35. doi: 10.1002/jmri.1880060212. [DOI] [PubMed] [Google Scholar]
- 13.Mosher TJ, Pruett SW. Magnetic resonance imaging of superficial cartilage lesions: role of contrast in lesion detection. J Magn Reson Imaging. 1999;10:178–82. doi: 10.1002/(sici)1522-2586(199908)10:2<178::aid-jmri11>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 14.Eckstein F, Hudelmaier M, Wirth W, Kiefer B, Jackson R, Yu J, et al. Double echo steady state magnetic resonance imaging of knee articular cartilage at 3 Tesla: a pilot study for the Osteoarthritis Initiative. Ann Rheum Dis. 2006;65:433–41. doi: 10.1136/ard.2005.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kothari M, Guermazi A, von Ingersleben G, Miaux Y, Sieffert M, Block JE, et al. Fixed-flexion radiography of the knee provides reproducible joint space width measurements in osteoarthritis. Eur Radiol. 2004;14:1568–73. doi: 10.1007/s00330-004-2312-6. [DOI] [PubMed] [Google Scholar]
- 16.Peterfy C, Li J, Zaim S, Duryea J, Lynch J, Miaux Y, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003;32:128–32. doi: 10.1007/s00256-002-0603-z. [DOI] [PubMed] [Google Scholar]
- 17.Eckstein F, Ateshian G, Burgkart R, Burstein D, Cicuttini F, Dardzinski B, et al. Proposal for a nomenclature for Magnetic Resonance Imaging based measures of articular cartilage in osteoarthritis . Osteoarthritis Cartilage. 2006 May 24; doi: 10.1016/j.joca.2006.03.005. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Glüer CC, Blake G, Lu Y, Blunt BA, Jergas M, Genant HK. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos Int. 1995;5:262–70. doi: 10.1007/BF01774016. [DOI] [PubMed] [Google Scholar]
- 19.Graichen H, Eisenhart-Rothe R, Vogl T, Englmeier KH, Eckstein F. Quantitative assessment of cartilage status in osteoarthritis by quantitative magnetic resonance imaging: technical validation for use in analysis of cartilage volume and further morphologic parameters. Arthritis Rheum. 2004;50:811–6. doi: 10.1002/art.20191. [DOI] [PubMed] [Google Scholar]
- 20.Eckstein F, Heudorfer L, Faber SC, Burgkart R, Englmeier KH, Reiser M. Long-term and resegmentation precision of quantitative cartilage MR imaging (qMRI) Osteoarthritis Cartilage. 2002;10:922–8. doi: 10.1053/joca.2002.0844. [DOI] [PubMed] [Google Scholar]
- 21.Glaser C, Burgkart R, Kutschera A, Englmeier KH, Reiser M, Eckstein F. Femoro-tibial cartilage metrics from coronal MR image data: Technique, test-retest reproducibility, and findings in osteoarthritis. Magn Reson Med. 2003;50:1229–36. doi: 10.1002/mrm.10648. [DOI] [PubMed] [Google Scholar]
- 22.Cicuttini FM, Jones G, Forbes A, Wluka AE. Rate of cartilage loss at two years predicts subsequent total knee arthroplasty: a prospective study. Ann Rheum Dis. 2004;63:1124–7. doi: 10.1136/ard.2004.021253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Hunter DJ, Conaghan PG, Peterfy CG, Bloch D, Guermazi A, Woodworth T, et al. Responsiveness, effect size, and smallest detectable difference of Magnetic Resonance Imaging in knee osteoarthritis . Osteoarthritis Cartilage. 2006;14(Suppl 1):112–5. doi: 10.1016/j.joca.2006.02.027. Epub;%2006 May 5.:112–5. [DOI] [PubMed] [Google Scholar]
- 25.Gold GE, Burstein D, Dardzinski B, Lang P, Boada F, Mosher T. MRI of articular cartilage in OA: novel pulse sequences and compositional/functional markers. Osteoarthritis Cartilage. 2006;14(Suppl 1):76–86. doi: 10.1016/j.joca.2006.03.010. Epub;%2006 May 23.:76–86. [DOI] [PubMed] [Google Scholar]
- 26.Burstein D, Gray M. New MRI techniques for imaging cartilage. J Bone Joint Surg Am. 2003;85-A(Suppl 2):70–7. doi: 10.2106/00004623-200300002-00009. [DOI] [PubMed] [Google Scholar]